Abstract
The gut microbiota is compartmentalized in the intestinal lumen and induces local immune responses, but it remains unknown whether the gut microbiota can induce systemic response and contribute to systemic immunity. We report that selective gut symbiotic gram-negative bacteria were able to disseminate systemically to induce immunoglobulin G (IgG) response, which primarily targeted gram-negative bacterial antigens and conferred protection against systemic infections by E. coli and Salmonella by directly coating bacteria to promote killing by phagocytes. T cells and Toll-like receptor 4 on B cells were important in the generation of microbiota-specific IgG. We identified murein lipoprotein (MLP), a highly conserved gram-negative outer membrane protein, as a major antigen that induced systemic IgG homeostatically in both mice and humans. Administration of anti-MLP IgG conferred crucial protection against systemic Salmonella infection. Thus, our findings reveal an important function for the gut microbiota in combating systemic infection through the induction of protective IgG.
INTRODUCTION
Bloodstream infection is a major cause of morbidity and mortality despite advances in antimicrobial treatment (Angus et al., 2001). More than 750,000 cases of sepsis occur annually in the United State (Angus et al., 2001). Bacteremia due to gram-negative symbiotic bacilli, most commonly Escherichia coli, accounts for 25%–50% of all bloodstream infections globally and poses a serious challenge due to increasing antibiotic resistance (Kang et al., 2004). The gut microbiota plays critical roles in the development of the immune system (Kamada et al., 2013b). For example, gut microbiota-induced local responses, such as secretory immunoglobulin A (IgA) and local T helper 17 (Th17) cells and regulatory T cells, contribute to gut homeostasis and/or protection against enteric pathogens (Macpherson et al., 2012; Peterson et al., 2007). However, little is known regarding whether the gut microbiota provides direct protection against systemic infections such as sepsis. Most of the bacteria responsible for extra-intestinal infections are of enteric origin (MacFie, 2004; Sedman et al., 1994), which are manifested due to disrupted mucosal barrier function after the use of chemotherapeutics, irradiation, or long-term antibiotics (Alverdy and Chang, 2008). It remains unclear under homeostatic conditions whether the immune system has acquired defense mechanisms to directly combat systemic attacks from bacteria that have inhabited our intestinal tract since birth.
As an essential link between adaptive immunity and effector functions of innate immunity, antigen-specific antibodies offer direct and specific protection against pathogens. Polyclonal intravenous immunoglobulins (IVIGs) are frequently used in critically ill septic patients, due to their unique functions in eradication of microbes and neutralization of endotoxins (Angus et al., 2001; Shankar-Hari et al., 2012). However, multiple clinical trials of IVIGs in sepsis did not demonstrate consistent beneficial effects of IVIGs, perhaps because the IVIGs used in these studies lacked specificities against sepsis-causing microbes. Emerging studies have now supported additional benefits in using IVIGs prepared from donors who have antibodies that can target microbes related to causative bacteria in sepsis (Alejandria et al., 2013; Almansa et al., 2015). However, the specificities of protective antibodies in sepsis and their origin remain undefined. Because many bacterial antigens are highly conserved in both symbiotic and pathogenic bacteria, we hypothesized that the gut microbiota is a source of antigens that drive protective antibodies against translocated symbiotic bacteria or invasive enteric pathogens in systemic infection.
In the gut, dendritic cells (DCs) have been shown to extend dendrites through epithelial cells and directly sample bacteria in proximity to the luminal side of the epithelium (Rescigno et al., 2001), and commensal-loaded DCs induce selective and local IgA response in gut-associated lymphoid tissues (GALTs) (Macpherson and Uhr, 2004). Mucosal IgA then further prevents symbiotic bacteria from breaching the epithelial barrier by blocking their access to epithelial receptors (Mantis et al., 2011). Additionally, physical barriers such as the epithelium and mucus layer prevent translocation of symbiotic bacteria to extra-intestinal organs, thereby limiting the systemic response to symbiotic bacteria, such as IgG response (Macpherson and McCoy, 2013; Macpherson and Uhr, 2004). However, bacterial translocation to extra-intestinal organs has been reported in mice and healthy humans (Haas et al., 2011; Sedman et al., 1994). Currently, it remains poorly understood whether there is a systemic IgG response to symbiotic bacteria under homeostatic conditions. In this study, we demonstrated in mice that under homeostatic conditions, a small subset of primarily gram-negative symbiotic bacteria from the gut was capable of disseminating systemically to induce systemic IgG, which conferred critical protection against systemic infections by symbiotic bacteria and pathogens through recognition of conserved antigens. We also identified murein lipoprotein (MLP), an outer membrane protein conserved in gram-negative symbiotic bacteria and pathogens, as a major microbiota-derived antigen that drives protective IgG in mice.
RESULTS
Homeostatic Induction of Systemic IgG to Gram-Negative Symbiotic Bacteria
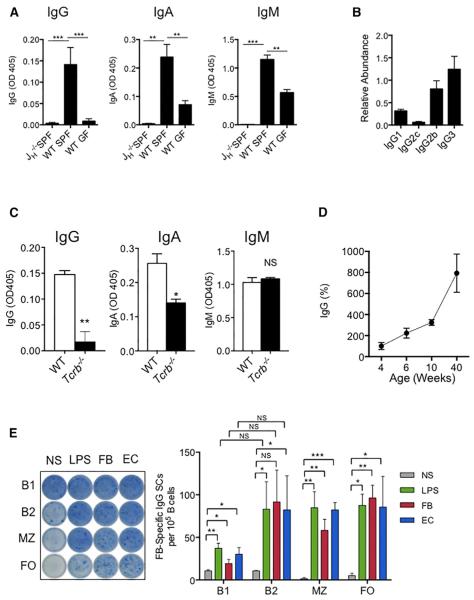
To determine whether there is pre-existing systemic IgG against symbiotic bacteria in mice under homeostatic conditions, we measured serum IgG, IgA, and IgM against fecal bacteria by ELISA in naive wild-type (WT) specific-pathogen-free (SPF) or germ-free (GF) mice, and in immunoglobulin- and B cell-deficient JH−/− SPF mice. Appreciable concentrations of fecal bacteria-specific IgG, IgA, and IgM were detected in sera of WT naive SPF mice, at levels significantly higher than that in JH−/− and WT naive GF mice of the same age (Figure 1A). Symbiotic bacteria-specific IgG included IgG3, IgG2b, IgG1, and IgG2c in decreasing abundance (Figure 1B). The concentrations of IgG and IgA against symbiotic bacteria were reduced by about 90% and 50%, respectively, in αβ T cell-deficient Tcrb−/− mice, suggesting that the induction of symbiotic bacteria-specific IgG is dependent on T cells but that T cells were dispensable for innate IgM production (Figure 1C). Furthermore, the concentration of serum IgG reactive to fecal bacteria increased by 8-fold as the mice aged from 4 weeks to 40 weeks (Figure 1D). To elucidate the types of B cells involved in generating microbiota-specific IgG, we performed an ELISpot assay with peritoneal B1 and B2 cells and with splenic marginal zone (MZ) and follicular (FO) B cells from WT SPF mice. All four types of B cells exhibited the ability to produce IgG that recognized heat-killed fecal bacteria, after stimulation by LPS, or heat-killed fecal bacteria or E. coli ex vivo for 72 hr (Figures 1E and S1). B1 and MZ cells are associated with production of T-cell-independent IgG3 (Cerutti et al., 2013). Therefore, B2 and FO cells were probably responsible for the IgG1 and IgG2b with specificities against symbiotic bacteria (Figure 1B).
Figure 1. Gut Microbiota Induces Antigen-Specific IgG in the Steady State.
(A) ELISA of serum IgG, IgA, and IgM against fecal bacteria (FB) in naive SPF JH−/− and WT mice and GF WT mice. 6–10 mice were used for each genotype.
(B) ELISA of serum IgG1, IgG2c, IgG2b, and IgG3 against fecal bacteria in 6- to 8-week-old naive SPF WT mice. Six WT mice were used.
(C) ELISA of serum IgG, IgA, and IgM against fecal bacteria in 6- to 8-week-old WT and Tcrb−/− naive mice. 6–10 mice were used for each genotype.
(D) ELISA of serum IgG against fecal bacteria of 4-, 6-, 10-, and 40-week-old mice.
(E) Peritoneal B1 and B2 cells and splenic marginal zone (MZ) and follicular (FO) B cells were stimulated ex vivo with LPS, heat-killed fecal bacteria, or E. coli for 3 days, and cells producing IgG that recognized fecal bacteria were detected by ELISpot.
Data represent two to three independent experiments. Error bars indicate SD. *p < 0.05, **p < 0.01,
***p < 0.001. See also Figure S1.
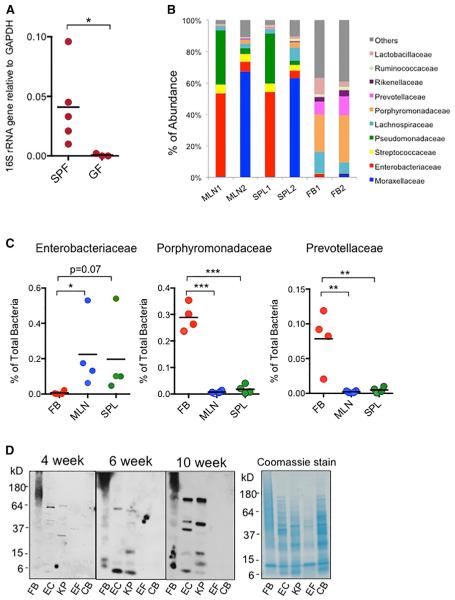
The presence of serum IgG that was able to target gut symbiotic bacteria suggested that some gut bacteria or bacterial products might be able to circulate systemically in spite of intact intestinal barriers. Therefore, to investigate how symbiotic bacteria from the gut induce systemic IgG response under homeostatic conditions, we first detected and confirmed the presence of bacterial 16S rRNA gene in the spleens (Figure 2A) and mesenteric lymph nodes (MLNs) (not shown) of WT SPF mice, which was absent in these organs from GF mice. Additionally, Illumina sequencing of the bacterial DNA from the spleen, MLNs, and fecal bacteria from the same naive WT SPF mice revealed vastly different compositions of bacteria in the spleens and MLNs in comparison to the bacterial population in the feces. In particular, gram-negative bacterial families such as Enterobacteriaceae and Moraxellaceae were the predominant families in the spleen and MLNs but were of very low abundance in the fecal population. On the other hand, there were very minimal concentrations of gram-negative Porphyromonadaceae and Prevotellaceae in the spleen and MLNs despite high abundance of these bacteria in the fecal population (Figures 2B and 2C). Consistently, immunoblotting analyses revealed that IgG in sera of WT naive mice recognized antigens from fecal bacteria, symbiotic gram-negative E. coli and Klebsiella pneumonia from the Enterobacteriaceae family, but not gram-positive Enteroccocus faecalis and Clostridium bifermentans (Figure 2D). Taken together, our data suggest that under homeostatic conditions, a selective group of gram-negative symbiotic bacteria might be able to breach the intestinal epithelial barrier and disseminate systemically to induce IgG response.
Figure 2. Spontaneous Systemic Dissemination of Gram-Negative Symbiotic Bacteria.
(A) qPCR of bacterial 16S rRNA gene in the spleens of SPF and GF WT mice. Each dot represents one mouse.
(B) Compositions of bacterial communities in mesenteric lymph nodes (MLNs), spleens (SPL), and fecal bacteria (FB) of same WT SPF mice.
(C) Abundance of gram-negative Enter-obacteriaceae, Porphyromondaceae, and Pre-votellaceae in MLNs, spleens, and feces from WT SPF mice. Each dot represents one mouse.
(D) Immunoblotting of bacterial antigens from fecal bacteria (FB), Escherichia coli (EC), Klebsiella pneumoniae (KP), Enterococcus faecalis (EF), and Clostridium bifermentans (CB) that were recognized by IgG in the serum of a 4-, 6-, or 10-week-old SPF WT mouse. A SDS-PAGE gel was separately stained with Coomassie blue to indicate total amounts of cell lysates loaded.
Data are representative of two to three independent experiments. Error bars indicate SD.
*p < 0.05, **p < 0.01, ***p < 0.001.
Protective Role of Pre-existing Symbiotic-Specific IgG in DSS-Induced Bacteremia
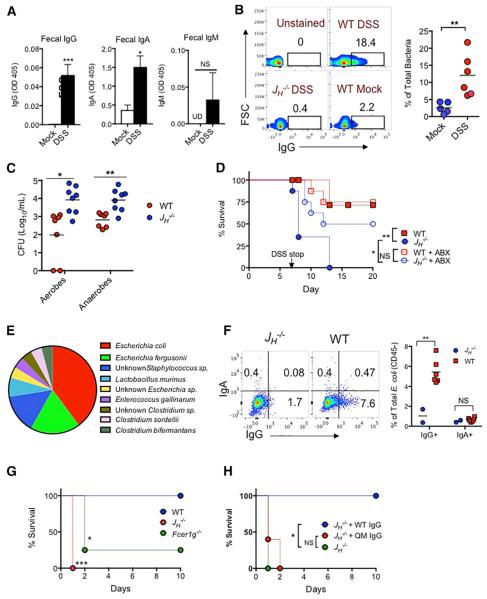
Gut microbiota-induced IgA has been extensively studied (Geuking et al., 2012), but the role of gut microbiota-induced IgG is yet to be defined. We first assessed the role of pre-existing microbiota-specific IgG in bacteremia induced by dextran sodium sulfate (DSS). WT SPF mice were given 2.5% DSS in drinking water for 7 days, which is known to damage the colonic epithelium and subsequently leads to bacteremia (Hofer et al., 2010). Despite no changes in serum concentrations of microbiota-specific IgG, IgA, and IgM in DSS-treated mice (Figure S2A), fecal concentrations of symbiotic bacteria-specific IgG and IgA were increased in DSS-treated WT mice compared to that in naive mice (Figure 3A). In addition, flow cytometric analysis of fecal bacteria taken directly from mice revealed that there was a small population (~2%) of fecal bacteria with a detectable IgG coating in mock-treated mice, and the population of IgG-coated fecal bacteria increased significantly to more than 10% on day 7 in DSS-treated mice (Figure 3B), suggesting that coating by microbiota-specific IgG might be a mechanism to prevent bacterial translocation when the epithelium is disrupted. To determine whether pre-existing microbiota-specific IgG is protective, bacteremia was induced by DSS in immunoglobulin-deficient JH−/− and WT mice that had been co-housed for at least 4 weeks. Colonic inflammation in WT and JH−/− mice appeared similar on day 5 (Figure S3B). However, compared to WT mice, JH−/− mice exhibited increased numbers of aerobic and anaerobic bacteria in the blood (Figure 3C), spleen, and liver (Figure S2C), as well as increased mortality, which was reduced by administration of broad-spectrum antibiotics prior to and during DSS treatment (Figure 3D). Previous studies have reported conflicting results on the role of IgA in the regulation of the gut microbiota (Suzuki et al., 2004; Thoene-Reineke et al., 2014; Wei et al., 2011). However, we did not observe perturbed gut microbiota in JH−/− mice after 4 weeks of co-housing with WT mice prior to DSS treatment (Figure S2D). To identify translocated bacteria in DSS-treated JH−/− mice that might be associated with mortality of these mice, we sequenced >30 bacterial isolates from the blood, spleen, and liver of day 7 DSS-treated JH−/− mice. Gram-negative E. coli and E. fergusonii were the predominant translocated symbiotic bacteria on day 7 after DSS treatment, together accounting for more than 60% of total isolates (Figure 3E). Subsequently, WT and JH−/− mice were challenged by intraperitoneal injection with one of the E. coli isolates, referred to as ECM6L4 hereafter. ECM6L4 was recovered from the peritoneum after 6 hr and analyzed for surface IgG and IgA coating. More than 7% of ECM6L4 bacteria were coated by IgG, but no IgA coating was observed in WT mice (Figure 3F), suggesting rapid recognition of ECM6L4 by pre-existing IgG in WT mice. Importantly, 100% of JH−/− mice and >70% of FcγR-deficient mice (Fcer1g−/−), which lack the FcR g chain required for assembly and signal transduction for activating all FcgRs, succumbed to infection by ECM6L4 within 24–48 hr, whereas all WT mice remained alive for longer than 10 days (Figure 3G). All JH−/− mice were rescued by transfer of purified serum IgG from WT naive mice, but not by purified IgG from 6-week-old quasi-monoclonal (QM) mice with very contracted B cell repertoire (Figure 3H; Cascalho et al., 1996, 1997). QM mice displayed no microbiota-specific serum IgG despite normal concentrations of total serum IgG, IgA, and IgM, and importantly, increased mortality after i.p. infection with ECM6L4 as seen in JH−/− mice (Figures S1E–S1G). Collectively, these results demonstrate a vital role for pre-existing microbiota-specific IgG in protecting mice from systemic infection by translocated symbiotic bacteria from the intestine.
Figure 3. Gut Microbiota-Induced IgG Confers Protection against DSS-Induced Bacteremia.
(A) ELISA of fecal IgG, IgA, and IgM against fecal bacteria in feces of naive (mock) or day 7 DSS-treated mice (mock = 5 mice; day 7 DSS = 7 mice).
(B) Flow cytometry for IgG on fecal bacteria from naive (mock) or day 7 DSS-treated mice.
(C) WT and JH−/− mice were treated with DSS for 7 days and CFU of aerobes and anaerobes in the blood were determined. Each dot represents one mouse.
(D) Survival of WT and JH−/− mice, treated with or without antibiotics, after administration of 2.5% DSS in drinking water for 7 days.
(E) Percentages of bacterial isolates from spleens, livers, and blood of day 7 DSS-treated JH−/− mice.
(F) WT and JH−/− mice were i.p. injected with 5 × 107 CFU of ECM6L4 (an isolate from a DSS-treated JH−/− mouse), and E. coli was recovered from the peritoneum 6 hr after infection and analyzed for IgG or IgA coating by FACS.
(G) Survival of WT, JH−/−, and Fcer1g−/− mice after i.p. injection with 107 CFU of M6L4 (WT n = 9; JH−/− n = 8; Fcer1g−/− n = 8).
(H) Survival of untreated JH−/− mice or JH−/− mice that were administered 400 mg of purified serum IgG from WT or QM mice 24 hr prior to i.p. infection with 107 CFU of ECM6L4 (JH−/− n= 6; JH−/− +WT IgG n = 7; JH−/− +QM IgG n = 6).
Data represent two to three independent experiments. Error bars indicate SD. *p < 0.05, **p < 0.01,
***p < 0.001. See also Figure S2.
TLR4 Signaling Regulates the Generation of Microbiota-Specific IgG
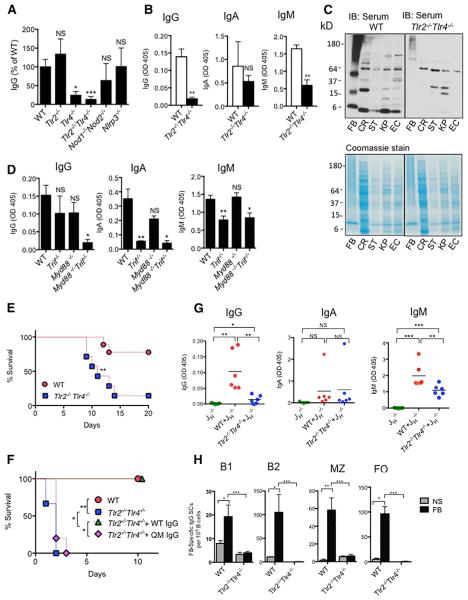
To identify pattern-recognition receptor(s) that are involved in sensing symbiotic bacteria and induction of IgG antibodies, we screened mice with deficiencies in the toll-like receptor pathway (TLR)—Tlr2−/−, Tlr4−/−, Tlr2−/−Tlr4−/−, and Nod1−/−Nod2−/− and Nlrp3−/− mice—for serum IgG against fecal bacteria. The concentrations of IgG reactive with fecal bacteria were substantially reduced in Tlr4−/− and Tlr2−/−Tlr4−/− mice, at about 20% and 10% of WT concentrations, respectively (Figures 4A and 4B). However, the total concentration of serum IgG in Tlr2−/−Tlr4−/− mice was comparable to that in WT mice (Figure S3A). Immunoblotting with bacterial lysates using sera from Tlr2−/−Tlr4−/− mice also showed reduced IgG in Tlr2−/−Tlr4−/− sera that recognized antigens produced by fecal bacteria, symbiotic bacteria E. coli and K. pneumoniae, as well as the gram-negative enteric pathogens Salmonella enterica serovar Typhimurium (Salmonella) and Citrobacter rodentium (Figure 4C). A significant reduction in microbiota-specific IgG was also observed in Myd88−/−Trif−/− mice but not Myd88−/− or Trif−/− mice, suggesting redundancy between these two signaling pathways in mediating generation of IgG response to symbiotic bacteria (Figure 4D). Similar to JH−/− mice, Tlr2−/−Tlr4−/− mice exhibited higher susceptibility to DSS treatment and i.p. infection with ECM6L4, and ECM6L4-treated Tlr2−/−Tlr4−/− mice were completely rescued by transfer of purified WT IgG (Figures 4E and 4F). An increasing number of studies have elucidated the engagement of TLRs in modulating responses in cells of the adaptive immune cells (Caramalho et al., 2003; Kubinak and Round, 2012; Rawlings et al., 2012). Therefore, we next assessed the importance of TLRs in B cells for homeostatic IgG response to symbiotic bacteria by generating mixed bone marrow (BM) chimeras with donor BM cells from Tlr2−/−Tlr4−/− and JH−/− mice. B-cell-specific deletion of TLR2 and TLR4 in these mice was verified 8 weeks after BM transplantation (Figures S3B–S3D). Serum concentrations of microbiota-specific IgG, but not IgA or IgM, were specifically diminished in BM chimeras with B-cell-specific deletion of TLR2 and TLR4, compared to the concentrations of these antibodies in BM chimeras transplanted with mixed WT and JH−/− BM cells that had WT B cells (Figure 4G). Consistently, after ex vivo stimulation by heat-killed fecal bacteria, very few peritoneal B1 and B2 B cells, as well as splenic MZ and FO cells, from untreated Tlr2−/−Tlr4−/− mice secreted microbiota-specific IgG, in sharp contrast to that seen in these B cell subsets from WT mice (Figure 4F). Taken together, our data demonstrate that TLR4 is a critical sensor of symbiotic bacteria in the induction of IgG against the microbiota under homeostatic conditions and that TLR4 on B cells plays an indispensable role in this process.
Figure 4. TLR4 Signaling Is Required for Induction of Microbiota-Specific IgG.
(A) Serum concentrations of IgG against fecal bacteria in age-matched WT, Tlr2−/−, Tlr2−/−Tlr4−/−, Nod1−/−Nod2−/−, and Nlrp3−/− mice (n = 4–8 per genotype).
(B) Serum concentrations of IgG, IgA, and IgM against fecal bacteria in age-matched and cohoused WT and Tlr2−/−Tlr4−/− mice (n = 8–10 per genotype).
(C) Immunoblotting for IgG in sera from age-matched WT and Tlr2−/−Tlr4−/− mice that bound to gram-negative C. rodentium (CR), Salmonella (ST), E. coli (EC), and K. pneumoniae (KP). Coomassie-stained SDS-PAGE gels loaded with lysates are shown.
(D) Serum concentrations of IgG, IgA, and IgM against fecal bacteria in age-matched and co-housed WT, Myd88−/−, Trif−/−, and Myd88−/−Trif−/− mice.
(E) Survival of WT and Tlr2−/−Tlr4−/− mice and of Tlr2−/−Tlr4−/− mice after treatment of 2.5% DSS for 7 days (WT n = 9; Tlr2−/−Tlr4−/− n = 7).
(F) Survival of WT and Tlr2−/−Tlr4−/− mice and of Tlr2−/−Tlr4−/− mice that were administered 400 mg of purified serum IgG from WT or QM mice 24 hr prior to i.p. infection with ECM6L4 (WT n = 5; Tlr2−/−Tlr4−/− n= 6; Tlr2−/−Tlr4−/− +WT IgG n = 6; Tlr2−/−Tlr4−/− +QM IgG n = 5).
(G) Serum concentrations of IgG, IgA, and IgM against fecal bacteria in co-housed bone marrow chimeras that were transplanted with JH−/−, WT+ JH−/−, or Tlr2−/− Tlr4−/− + JH−/− bone marrow cells.
(H) Peritoneal B1 and B2 cells, and splenic marginal zone (MZ) and follicular (FO) B cells from WT or Tlr2−/−Tlr4−/− mice were stimulated ex vivo with heat-killed fecal bacteria for 72 hr before analysis by ELISpot to determine the number of cells secreting IgG that recognized fecal bacteria. Data represent two to three independent experiments. Error bars indicate SD. *p < 0.05, **p < 0.01, ***p < 0.001. See also Figure S3.
Gram-Negative Murein Lipoprotein Is a Major Microbiota-Derived Antigen
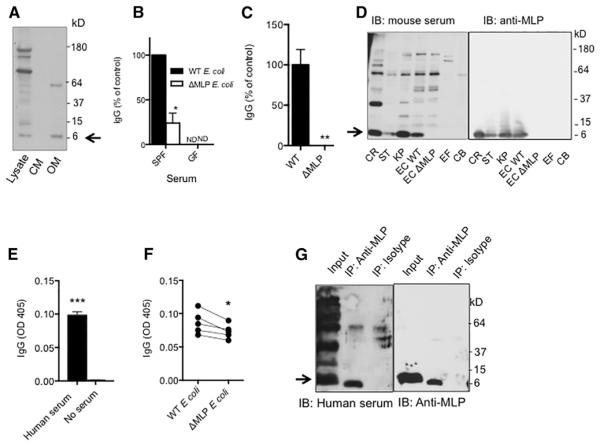
Identifying gram-negative antigens that drive homeostatic IgG response to symbiotic bacteria would provide insight as to how to harness the homeostatic symbiotic-specific IgG in treating infection. Given the therapeutic potential of targeting membrane antigens, we first fractionated WT E. coli (K-12) lysates and determined whether there were outer membrane proteins that were recognized by serum IgG from adult mice. Two major proteins of about 40 kD and 7 kD in the outer membrane fraction were recognized by serum IgG (Figure 5A), whereas the 7 kD protein appeared to be a major IgG-targeting antigen observed previously in 6- and 10-week-old mice (Figure 2D). Murein lipoprotein (MLP), a highly conserved outer membrane protein of ~7 kD expressed abundantly in gram-negative enterobacteria, was detected in the plasma of septic patients (Hellman et al., 2000; Suzuki et al., 1978). To determine whether naive WT SPF mice generated anti-MLP IgG homeostatically, reactivity of IgG in sera of 6- to 8-week-old mice against WT E. coli (K-12) and a K-12 strain of E. coli lacking MLP (ΔMLP) was measured by ELISA. The amount of IgG reactive to ΔMLP E. coli was markedly reduced to about 30% of that to WT E. coli (Figure 5B), suggesting the presence of anti-MLP IgG in sera from naive WT mice. MLP is shed from gram-negative bacteria (Hellman and Warren, 2001). Consistent with this, robust IgG binding was seen when ELISA plates were coated with the supernatant of over-night cultures of WT but not ΔMLP E. coli strain (Figure 5C). Immunoblotting for IgG in sera from 6- to 7-week-old WT mice revealed a major protein close to 7 kD in all lanes with gram-negative bacterial lysates, including lysates from pathogens C. rodentium and Salmonella. However, this band was absent in lanes with lysates from ΔMLP E. coli or gram-positive E. faecalis and C. bifermentans (Figure 5D), thus confirming the presence of anti-MLP IgG in naive WT mice. IgA or IgM against MLP was not observed in mouse sera by immunoblotting (Figure S4A), and serum anti-MLP IgG appeared to belong mainly to the IgG2b subclass (Figure S4B). In addition, we observed significant IgG against fecal bacteria in sera from healthy humans (Figure 5E). There was markedly reduced binding of human serum IgG to ΔMLP E. coli compared to the binding to WT E. coli (Figure 5F). Importantly, the presence of anti-MLP IgG in human serum was confirmed by the observation of human IgG binding to immunoprecipitated MLP from WT E. coli lysates (Figure 5G). MLP was previously reported to activate TLR2 and induce production of proinflammatory cytokines (Shin et al., 2011). Consistently, WT BMDCs stimulated with heat-killed ΔMLP E. coli produced about 30%–50% less IL-1b, TNF-a, and IL-10 compared to BMDCs stimulated with heat-killed WT E. coli (Figure S4C). However, MLP appeared dispensable for E. coli to stimulate B cells, because similar proliferation of isolated splenic B cells was observed after stimulation with heat-killed WT or ΔMLP E. coli (Figure S4D). Together, our data demonstrate that gram-negative outer membrane MLP is a major microbiota-derived antigen that can be targeted by homeostatic IgG in both mice and humans.
Figure 5. Gram-Negative Murein Lipoprotein Is a Major Microbiota-Derived Antigen to Induce Steady-State IgG Response.
(A) Immunoblotting for serum IgG from a 10-week-old WT naive mice that bound to E. coli total cell lysate, cytosolic membrane proteins (CM), and outer membrane proteins (OM).
(B) Reactivity of serum IgG in WT SPF or GF mice to WT or MLP-deficient E. coli (ΔMLP). Eight WT SPF mice were used; three GF mice were used.
(C) Reactivity of serum IgG in a WT SPF mouse to particles in culture supernatants from WT or ΔMLP E. coli. Eight WT SPF mice were used.
(D) Immunoblotting for IgG in the serum from a 6-week-old WT SPF mouse that bound to bacterial antigens from gram-negative C. rodentium (CR), Salmonella (ST), E. coli (EC), and K. pneumoniae (KP) and gram-positive E. faecalis (EF) and C. bifermentans (CB). Immunoblotting was performed separately for MLP using an anti-MLP monoclonal antibody.
(E) ELISA of human serum IgG against fecal bacteria (n = 5).
(F) Binding of human serum IgG to WT or MLP-deficient E. coli. Each dot represents one person.
(G) Immunoprecipitation of MLP from WT E. coli lysate by anti-MLP or isotype and immunoblotting with human serum or anti-MLP. Data represent two to three independent experiments. Error bars indicate SD. *p < 0.05, **p < 0.01, ***p < 0.001. See also Figure S4.
Protective Role of Anti-MLP IgG in Systemic Infection by E. coli
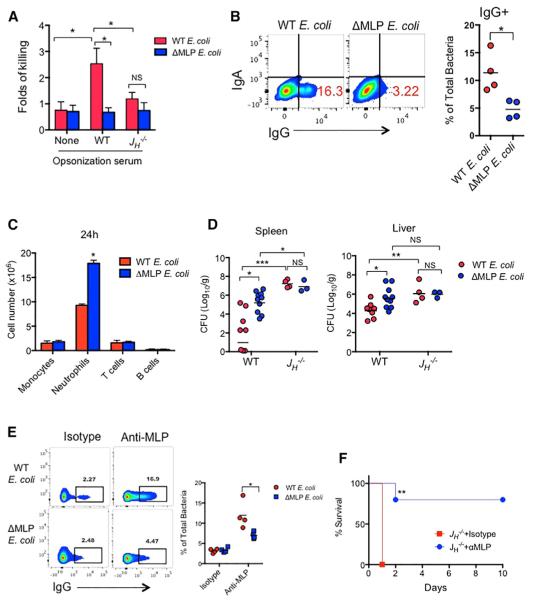
To address whether pre-existing anti-MLP IgG protects against systemic infection, we first assessed whether the presence of MLP affected the killing of bacteria opsonized by heat-inactivated mouse serum. We observed that in vitro opsonization with WT serum enhanced killing of WT E. coli but not ΔMLP E. coli by neutrophils, and the difference in killing of these two strains of E. coli was absent when JH−/− serum was used for opsonization (Figure 6A). To assess the physiological relevance of pre-existing anti-MLP IgG, we i.p. injected 6- to 8-week-old mice with 5 × 107 CFU of WT or ΔMLP E. coli, and the presence of bacteria coated with IgG was assessed by flow cytometry. At 6 hr after injection, the percentage of IgG coating was higher on WT E. coli than on ΔMLP E. coli (Figure 6B). Furthermore, WT mice infected with 107 CFU of ΔMLP E. coli showed more neutrophils in the peritoneum 24 hr after i.p. infection (Figure 6C), probably because of impaired clearance of ΔMLP E. coli in these mice, because higher burdens of ΔMLP E. coli in the spleen and liver were observed in these mice at 24 hr after infection (Figure 6D). Higher concentrations of CXCL1 were detected in both sera (Figure S5) and peritoneal fluid (not shown) of mice infected with ΔMLP E. coli, probably reflecting enhanced inflammation due to delayed clearance of ΔMLP E. coli in these mice. In addition, immunoglobulin-deficient JH−/− mice showed overall higher burdens of both strains of E. coli than WT mice, but there was no difference in the clearance between WT and ΔMLP E. coli in JH−/− mice (Figure 6D). We next determined whether a monoclonal IgG antibody against MLP conferred protection against ECM6L4, which induced 100% mortality of J −/− mice upon systemic infection (Figure 3G). Incubation of monoclonal anti-MLP IgG with E. coli in vitro resulted in robust IgG coating of WT but not ΔMLP E. coli (Figure 6E). Furthermore, i.p. administration of monoclonal anti-MLP IgG 24 hr prior to i.p. injection with 107 CFU of ECM6L4 significantly reduced the mortality of JH−/− mice from 100% to only ~20% (Figure 6F), thus demonstrating a role for anti-MLP IgG in protecting mice against systemic E. coli infection.
Figure 6. Anti-MLP IgG Promotes Killing of E. coli In Vitro and In Vivo.
(A) Neutrophil killing of WT or ΔMLP E. coli that were opsonized with PBS, WT, or JH−/− serum (fold of killing over killing of non-opsonized E. coli).
(B) 5 × 107 CFU WT or ΔMLP E. coli were i.p. injected into 8-week-old WT mice and harvested 6 hr later to analyze surface IgG or IgA by flow cytometry.
(C and D) WT mice of 6–8 weeks were i.p. injected with 5 × 107 CFU WT and or ΔMLP E. coli. The absolute numbers of monocytes/macrophages (CD11b+LY6C+LY6Glo), neutrophils (CD11b+LY6Ghi), T cells (CD3+), and B cells (B220+) were determined by flow cytometry at 24 hr after infection (C). Bacterial numbers of E. coli in spleens and livers were determined at 24 hr (D). Each dot represents one mouse.
(E) WT and ΔMLP E. coli were incubated with anti-MLP or isotype-matched control IgG for 30 min. The bacterial numbers of IgG-coated bacteria were determined by flow cytometry.
(F) Survival of JH−/− mice that were administered 1 mg of monoclonal anti-MLP IgG or isotype 24 hr prior to i.p. infection with 107 of ECM6L4 (isotype n = 7; anti-MLP n = 10).
Data represent two to three independent experiments. Error bars indicate SD. *p < 0.05, **p < 0.01, ***p < 0.001. See also Figure S5.
Protective Role of Anti-MLP Antibodies in Systemic Salmonella Infection
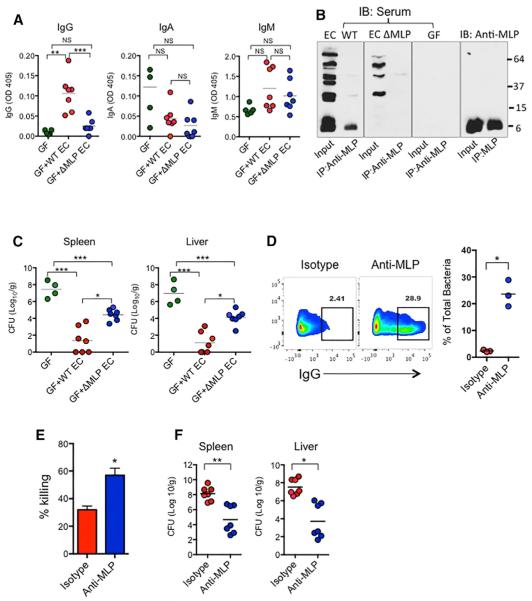
Because MLP is also expressed in pathogenic gram-negative bacteria, such as C. rodentium and Salmonella (Figure 5D), we hypothesized that pre-existing anti-MLP IgG also plays a protective role in systemic infection by gram-negative pathogens such as Salmonella. To first assess the immunogenicity of MLP and the significance of that in the context of pathogenic infection, we first immunized GF mice by i.p. injection with 107 CFU of WT or ΔMLP E. coli for 2–3 weeks to allow induction of anti-MLP IgG. The mice immunized with WT E. coli produced significantly higher concentrations of serum IgG against fecal bacteria than mice with ΔMLP E. coli, but the concentrations of IgA and IgM against fecal bacteria in these two groups were comparable and not significantly increased compared to the concentrations in GF mice (Figure 7A). The presence and absence of anti-MLP IgG in mice immunized with WT or ΔMLP E. coli, respectively, were also confirmed by immunoblotting for IgG in the sera of immunized mice and GF mice that bound to immunoprecipitated MLP from WT E. coli cell lysate (Figure 7B). The mice were then challenged with 104 CFU of Salmonella M525P i.p. Pre-immunization, with either WT or ΔMLP E. coli, significantly reduced Salmonella burdens in the spleens and livers compared to that in non-immunized GF mice. Importantly, lower burdens of Salmonella in these tissues were found in mice pre-immunized with WT E. coli than with ΔMLP E. coli (Figure 7C). Additionally, we observed binding of anti-MLP IgG to Salmonella in vitro (Figure 7D), and pre-incubation of Salmonella with anti-MLP IgG led to enhanced killing of Salmonella by neutrophils in vitro (Figure 7E). To further confirm the protective role of anti-MLP IgG, we treated 6- to 8-week-old WT SPF mice with 1 mg of monoclonal anti-MLP IgG or isotype by i.p. injection 24 hr prior to sublethal i.p. infection with Salmonella. On day 3 after infection, mice pre-treated with anti-MLP IgG had about 10% more monocytes (CD11b+LY6C+LY6Glo) in the peritoneum than mice pre-treated with isotype (not shown) but otherwise had no apparent exaggerated inflammatory response, as indicated by comparable concentrations of local and systemic inflammatory cytokines and chemokines (Figures S6A and S6B). Significantly lower CFUs of Salmonella were found in both spleens and livers of mice pre-treated with anti-MLP IgG (Figure 7F). Together, our data demonstrated robust immunogenicity of MLP and a role for anti-MLP IgG in protecting against systemic infection by MLP-expressing pathogens.
Figure 7. IgG against Murein Lipoprotein Confers Protection against Salmonella Infection.
(A–C) GF mice were i.p. administered PBS or 107 WT or ΔMLP E. coli. 2 weeks later, the concentrations of serum IgG, IgA, and IgM against fecal bacteria were determined by ELISA (A), and the presence of anti-MLP IgG in the sera of these mice were also confirmed by immunoprecipitation of MLP from WT E. coli lysates and immunoblotted using sera from these mice or monoclonal anti-MLP (B). 2–3 weeks after administration of PBS or 107 CFU of WT and or ΔMLP E. coli, GF mice were i.p. infected with 104 CFU of Salmonella M525P and the levels in spleens and livers were determined at 72 hr (C).
(D and E) Salmonella were incubated with anti-MLP IgG or isotype for 30 min, and then assessed for surface IgG coating (D) and killing by neutrophils in vitro (E). 6- to 8-week-old WT mice were i.p. administered 1 mg of anti-MLP IgG or isotype for 24 hr before i.p. infection with 4 × 104 ST M525P. CFU of ST M525P in spleens and livers were determined at 72 hr.
Data represent two to three independent experiments. Error bars indicate SD. *p < 0.05, **p < 0.01,
***p < 0.001. See also Figure S6.
DISCUSSION
Although bacteria residing in our gastrointestinal tract vastly outnumber host cells by 10-fold, host-microbial symbiosis is maintained, largely because bacteria are effectively compartmentalized in our gastrointestinal tract. Many vital functions provided by the gut microbiota, including digestion of complex polysaccharides and resistance to pathogen colonization, are achieved while the gut microbiota remains in the intestinal lumen (Kamada et al., 2013a). The intestinal epithelium, mucosal mucus layer, and an army of immune cells in the lamina propria on the basal side of the epithelium represent a concerted effort to prevent dissemination of symbiotic bacteria from the intestine and to maintain immunological ignorance toward symbiotic bacteria. This conventional view is supported by rare detection of live commensal bacteria in extra-intestinal organs under homeostatic conditions in immunocompetent hosts. A T-cell-independent primitive intestinal IgA response to symbiotic bacteria is induced locally in the intestine (Macpherson et al., 2000). On the other hand, it remains largely unknown whether under normal homeostatic conditions the gut microbiota is capable of inducing systemic IgG immune response. Circulating IgG against symbiotic bacteria has been reported in healthy humans and at higher concentrations in patients with Crohn’s disease and diabetes (Benckert et al., 2011; Harmsen et al., 2012; Mohammed et al., 2012). The higher IgG response to symbiotic bacteria in these patients could be a result of leaky guts and increased bacterial translocation, and at the same time might serve as a compensatory protective mechanism, given that as shown in our study, pre-existing microbiota-specific IgG plays a protective role in systemic infection. From an evolutionary point of view, the systemic IgG response, albeit at low concentrations under steady-state conditions, might be an important mechanism to ensure confinement of the gut microbiota in the gastrointestinal tract and thereby preserve host-microbial symbiosis. Additionally, the pre-existing microbiota-specific IgG recognizes conserved antigens on pathogens to mediate elimination of pathogens. Therefore, induction of systemic microbiota-specific IgG might represent a previously unappreciated yet critical function of the gut microbiota to maintain host homeostasis.
A recent study reported on the ability of natural IgG to bind both gram-negative and gram-positive bacteria indirectly after the bacteria are opsonized with ficolin and mannan-binding lectin (MBL), two ancestral lectins of the innate immune system (Panda et al., 2013; Puga and Cerutti, 2013). There are two major characteristics about the spontaneous symbiotic bacteria-specific IgG discovered in our study that distinguish it from natural IgG. First, whereas the interaction of natural IgG with bacteria is indirect and dependent on host innate immune factors, microbiota-specific IgG displays remarkable specificities against proteins of bacterial origin, as we have demonstrated by immunoblotting, and can directly interact with bacteria through recognition of the bacterial antigen, such as MLP. The high specificity of microbiota-specific IgG is also supported by its absence in GF mice. Second, natural IgG is generally produced by innate-like B1 and marginal zone B cells in a T-cell-independent manner. However, as shown by our ELISpot data, in addition to B1 and marginal zone B cells, follicular B cells also possess the capacity to secret IgG that targets fecal bacteria. This observation is in line with our finding that the subclasses of microbiota-specific IgG include IgG1 and IgG2b, in addition to IgG3, which is primarily produced by innate-like B1 and marginal zone B cells (Cerutti et al., 2013). Additionally, generation of at least some of the microbiota-specific IgG by follicular B cells is consistent with our finding of an important role of T cells in generating microbiota-specific IgG.
Our observation of increased susceptibility of Tlr2−/−Tlr4−/− mice to DSS treatment is consistent with previous reports of increased DSS-induced mortality in Myd88−/− and Tlr4−/− mice and in mice with B cell-specific Myd88 deficiency (Kirkland et al., 2012; Rakoff-Nahoum et al., 2004). The indispensible role of TLR4 on B cells in the generation of microbiota-specific IgG, as shown by our data, illustrates another example of the importance of TLR in B cells for microbial antibody response, which has been supported by numerous studies (Bekeredjian-Ding and Jego, 2009; Kirkland et al., 2012). For example, TLRs in B cells have been shown to be critical for class switch recombination, plasma cell differentiation, and antigen presentation. It remains unclear how TLR4 on B cells contributes to the generation of microbiota-specific IgG, or where B cells sense microbial products via TLR4. There are B cells in the gut lamina propria and Peyer’s patches, which might be where TLR4-mediated microbial priming of B cells occurs initially, followed by circulation of these B cells systemically. However, we also detected bacterial DNA in the spleens of SPF WT mice, suggesting possible TLR4-mediated B cell priming in systemic organs such as the spleen. These two scenarios might not be mutually exclusive. Of note, the important role of TLR4 in the generation of microbiota-specific IgG is consistent with the observation of primarily gram-negative bacteria being recognized by the pre-existing microbiota-specific IgG in mice.
It remains unclear how symbiotic bacteria are able to breach the epithelium and induce IgG response homeostatically. One possibility is that the symbiotic bacteria involved in inducing homeostatic IgG response express additional virulent factors, such as seen in pathobionts, that allow a small number of these bacteria to disseminate to the bloodstream and activate systemic immune response. Pathobionts are resident microbes with pathogenic potential, and many have been described to possess distinct properties to allow adherence to and invasion of the mucosal epithelium (Chow et al., 2011). Some pathobionts employ mechanisms to resist killing by phagocytes, such as resistance to complement as recently described (Hasegawa et al., 2014). Systemic dissemination of a small number of pathobionts, although transient and insufficient to cause disease, might be sufficient to mount a systemic IgG response and generate memory against them. Mounting evidence has emerged to point to Peyer’s patches localized in the small intestine as the portal of entry for AIECs (Barnich et al., 2007) and pathogens (Clark et al., 1998; Lelouard et al., 2012). This is in agreement with the observation of primary gram-negative Enterobacteriaceae such as E. coli translocated in the spleens and also targeted by serum IgG, given that Enterobacteriaceae are found mostly in the small intestine. More work is required to delineate the bacterial species that give rise to the homeostatic protective IgG.
In infants, the first colonizers in the gastrointestinal tract are facultative aerobes such as Proteobacteria within the Enterobacteriaceae family, which deplete oxygen to create a new environment suitable for colonization of strict anaerobes such as Bacteroides, Clostridium, and Bifidobacterium spp. (Rodríguez et al., 2015). Therefore, our discovery of MLP, a highly conserved gram-negative outer membrane protein found in abundance in Enterobacteriaceae such as E. coli, as a primary antigen for systemic IgG response in younger mice might coincide with these bacteria being among the first colonizers. In addition, geographic location of these bacteria, namely in the small intestine with Peyer’s patches and M cells, might also contribute to the relative readiness of Proteobacteria to disseminate to systemic organs. MLP is highly conserved, and anti-MLP IgG is found in both humans and mice. Importantly, our data demonstrate the effectiveness of anti-MLP IgG in protecting mice from systemic infection by both E. coli and Salmonella. MLP was previously found at high concentrations in the bloodstream of septic patients, probably as a result of shedding from the causative bacteria (Hellman and Warren, 2001). Because MLP itself can, as previously shown, activate TLR2 to induce production of proinflammatory cytokines (Liang et al., 2005), circulating MLP in the bloodstream might further exacerbate the fatal ‘‘cytokine storm’’ in sepsis. Hence, in addition to its role in eliminating MLP-expressing bacteria, anti-MLP IgG might also be involved in neutralizing excessive proinflammatory MLP in gram-negative sepsis and contribute to protection. Therefore, the potential of anti-MLP IgG in treating gram-negative sepsis should be further explored.
Our data showed low concentrations of symbiotic bacteria-specific IgG in younger mice, which increased steadily as the mice aged. Conversely, microbiota-specific IgG in human infants and toddlers might not reach optimal concentrations to confer best protection, which might partly explain the heightened susceptibility of young children to infection. Therefore, microbiota-specific IgG could be transported from the mother to the fetus through the placenta or to the infant through breast milk to provide passive immunity in the developing fetus or infant. The dependence of the gut microbiota for proper induction of IgG against potentially harmful symbiotic bacteria and pathogens also highlights the importance of having balanced gut microbiota especially in early years of life. In fact, a study showed that infants who developed sepsis began life with low microbial diversity and acquired a predominance of Staphylococci, whereas healthy infants had more diverse gut microbiota with predominance of Clostridium, Klebsiella, and Verilonella (Madan et al., 2012). In light of these studies and our findings, excessive use of antibiotics in young children might potentially delay or impair proper development of IgG response and immune memory against symbiotic bacteria and have a profound impact on later life. Therefore, our results underscore the importance of having a balanced microbial community in the intestine in early life.
EXPERIMENTAL PROCEDURES
Animals
WT SPF C57BL/6J mice were originally purchased from Jackson Laboratory and bred in-house. Tlr2−/−Tlr4−/−, Tlr4−/−, and Tlr2−/− mice were kindly provided by Shizuo Akira (Osaka University, Japan). JH−/− and QM (JH−/− × HOM) mice were kindly provided by Marilia Cascalho (Univ. of Mich-igan) (Cascalho et al., 1996, 1997). All mice were on the C57BL/6 background. Germ-free C57BL/6J mice were also originally obtained from Jackson Laboratory, re-derived into GF conditions, and bred and maintained GF in the University of Michigan GF Mouse Facility. GF mice were housed in double isolators and were free of all bacteria, fungi, viruses, and parasites. Sterility was verified by regular interval aerobic and anaerobic cultures as well as gram stains of feces and bedding. All animal studies were approved by the University of Michigan Committee on the Care and Use of Animals.
ELISA
For measurement of commensal bacteria-specific IgG, IgM, and IgA, fecal bacteria were isolated from naive WT SPF mice (8–10 weeks old) used to coat ELISA plates. Fecal bacteria from ~200 mg fecal pellets (~5–7 pellets) were isolated by homogenization in sterile PBS, filtered through a 40 μm cell strainer, and separated from debris/mouse cells by removing the pellet after centrifugation at 1,000 rpm for 5 min. Isolated fecal bacteria were washed twice, heat-killed at 85° C for 1 hr, and resuspended in 10 ml coating buffer, and 100 μL was added to each well of a 96-well ELISA plate for overnight coating at 4° C. Wells were blocked with 1% (w/v) BSA in PBS for 2 hr at room temperature. Mouse sera were diluted at 1:20 and 1:100 and incubated overnight at 4° C for detection of IgG, IgM, and IgA. Anti-mouse IgG and HRP substrate were purchased from Bethyl Laboratories. Anti-mouse IgA and IgM were from Southern Biotech. To measure luminal and fecal IgG and IgA, small intestine content or feces was resuspended with sterile PBS (100 mg/mL) and filtered through a 40 μm cell strainer to remove debris, and 200 μL was added to each well. The same antibodies were used for quantification of total IgG, IgA, and IgM concentrations, where captured mouse IgG, IgA, and IgM and reference serum were purchased from Bethyl Laboratories. ELISAs for cytokines (IL-1β, IL-6, TNF-α, IL-23, IL-12/23 p40) were performed by the University of Michigan ELISA Core.
Immunoprecipitation and Immunoblotting
109 CFU of WT E. coli (K-12) were lysed in 1 mL of lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% (v/v) NP-40, and 1 mM PMSF [pH 7.5]) and sonicated. 200 μL of cell-free supernatant was first incubated with 20 μL of Protein G resin (50% slurry) (GenScript) at 4° C for 1 hr, and supernatant was then incubated with anti-MLP (5 μg/mL) overnight at 4° C before incubation with 20 μL of Protein G resin at 4° C for 2 hr. After that, Protein G resin was pelleted, washed five times with cold lysis buffer, and resuspended in 30 μL of lysis buffer for immunoblotting. Proteins were separated by 4%–20% precast gels (Mini-PROTEAN TGX Gels; Bio-Rad) and transferred to PVDF membranes by electroblotting (BioRad), and membranes were immunoblotted with WT mouse serum (1: 200), human serum (1:400), or anti-mouse monoclonal MLP (1:2,000) overnight at 4° C. Proteins were detected with goat anti-mouse IgG, goat anti-mouse IgM, goat anti-human IgG (Jackson ImmunoResearch Laboratories), or goat anti-mouse IgA (Santa Cruz Biotechnology) and enhanced chemiluminescent substrate (Thermo Scientific).
DSS-Induced Bacteremia and Taxonomic Identification of Bacteria
WT and J −/− mice co-housed for at least 4 weeks were administered 2.5% dextran sodium sulfate (DSS) (MP Biomedicals) in drinking water for 7 days and then switched to regular water. Body weights and stool consistency were checked daily, and inflammation and colonic damage were examined by histology of colons from day 5 mice. In some experiments, 2 days prior to DSS treatment, mice were given an antibiotic cocktail (500 μg ampicillin, 250 μg vancomycin, 250 μg metronidazole, 250 μg gentamycin, and 500 μg neomycin) by oral gavage daily for the entire duration of experiments (about 20 days). At day 7 after DSS administration, aerobes and anaerobes were cultured from the blood, spleens, and livers on brain-heart infusion (BHI) plates. Bacterial DNAs of a total of 32 isolates from day 7 JH−/− blood, spleen, and liver homogenates were purified, and 16S rRNA gene was amplified by PCR using universal primers 8F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′-CGG TTA CCT TGT TAC GAC TT-3′) (PCR Kit: Expand High Fidelity PCR System, Roche). The amplicons were sequenced by Sanger sequencing (University of Michigan DNA Sequencing Core), and identities of isolates were determined by BLAST (NCBI BLAST) of amplicon sequences.
Flow Cytometry
To analyze IgG or IgA coating fecal bacteria ex vivo, two to three mouse fecal pellets from the same mouse were homogenized in cold sterile PBS (100 μg/mL) thoroughly, filtered through a 40 mm cell strainer, and centrifuged at 900 × g for 5 min to remove debris and mouse cells in the pellet. Fecal bacteria in the supernatant were washed several times with cold PBS, pelleted by centrifugation at 3,700 × g for 10 min, and stained for mouse IgG, IgA, and CD45 with FITC-anti mouse IgG (eBioscience), PE-anti mouse IgA (eBioscience), and APC-anti mouse CD45 (eBioscience; 30-F11). Stained bacteria were then washed with FACS buffer once, resuspended with FACS buffer containing DAPI, and analyzed by FACSAriaIII (BD Bioscience). Data were analyzed by Flow Jo (Tree Star).
Statistical Analyses
Statistical analysis was performed with Prism v.6.0 (GraphPad). Comparisons between two groups were determined by Student’s t test. Data with abnormal distribution or with fewer than eight samples were analyzed by the Mann-Whitney test. For multiple comparisons, one-way ANOVA was used. For survival assays, comparisons were performed with the Log-rank test. A p value of less than 0.05 was considered significant.
Supplementary Material
Highlights.
Gut microbiota induces homeostatic IgG response to commensal antigens
Homeostatic commensal-specific IgG antibodies recognize gram-negative antigens
Commensal-specific IgG opsonizes pathogens to mediate clearance
Gram-negative murein lipoprotein (MLP) is a major commensal antigen for IgG response
In Brief.
It is not clear whether gut microbiota can induce systemic responses. Nunez and colleagues find that the gut microbiota induces the generation of IgG antibodies under homeostatic conditions. These IgG antibodies mainly recognize gram-negative commensal antigens and confer protection against systemic infection by targeting conserved antigens on pathogens.
ACKNOWLEDGMENTS
We thank N. Kamada for suggestions and critical reading of the manuscript, W.V. Giannobile (University of Michigan School of Dentistry) for providing human serum samples, and L. Burmeister for mouse care and husbandry. We appreciate assistance from Joel Whitfield from the University of Michigan ELISA Core, the Flow Cytometry Core, the Gnotobiotic Animal Facility, and the Host Microbiome Initiative at the University of Michigan Medical School. D.C. was an exchange student from the PDSE program of the CAPES agency from Brazil. M.Y.Z. was supported by NIH training grants T32DK094775 and T32HL007517 and received additional research support from the University of Michigan Center for Gastrointestinal Research (NIH 5P30DK034933). This work was supported by NIH grants R01 GM59694 (H.S.W.), 5R21AI117561 (M.C.), and DK091191 and DK095782 (G.N.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2016.02.006.
AUTHOR CONTRIBUTIONS
M.Y.Z. and G.N. conceived the study, designed experiments, and wrote the manuscript. M.Y.Z. did most of the experiments, and D.C. helped with some experiments. S.V. helped with hybridomas producing anti-MLP antibodies. J.H., H.S.W., and M.C. provided critical material for the studies. N.I. provided technical assistance and analyzed the data. G.N. supervised all aspects of the study.
REFERENCES
- Alejandria MM, Lansang MA, Dans LF, Mantaring JB., 3rd Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst. Rev. 2013;9:CD001090. doi: 10.1002/14651858.CD001090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almansa R, Tamayo E, Andaluz-Ojeda D, Nogales L, Blanco J, Eiros JM, Gomez-Herreras JI, Bermejo-Martin JF. The original sins of clinical trials with intravenous immunoglobulins in sepsis. Crit. Care. 2015;19:90. doi: 10.1186/s13054-015-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J. Leukoc. Biol. 2008;83:461–466. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J. Clin. Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekeredjian-Ding I, Jego G. Toll-like receptors–sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, Wardemann H. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J. Clin. Invest. 2011;121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascalho M, Ma A, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- Cascalho M, Wong J, Wabl M. VH gene replacement in hyper-selected B cells of the quasimonoclonal mouse. J. Immunol. 1997;159:5795–5801. [PubMed] [Google Scholar]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J, Tang H, Mazmanian SK. Pathobionts of the gastroin-testinal microbiota and inflammatory disease. Curr. Opin. Immunol. 2011;23:473–480. doi: 10.1016/j.coi.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect. Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuking MB, McCoy KD, Macpherson AJ. The function of secretory IgA in the context of the intestinal continuum of adaptive immune responses in host-microbial mutualism. Semin. Immunol. 2012;24:36–42. doi: 10.1016/j.smim.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Haas A, Zimmermann K, Graw F, Slack E, Rusert P, Ledergerber B, Bossart W, Weber R, Thurnheer MC, Battegay M, et al. Swiss HIV Cohort Study Systemic antibody responses to gut commensal bacteria during chronic HIV-1 infection. Gut. 2011;60:1506–1519. doi: 10.1136/gut.2010.224774. [DOI] [PubMed] [Google Scholar]
- Harmsen HJ, Pouwels SD, Funke A, Bos NA, Dijkstra G. Crohn’s disease patients have more IgG-binding fecal bacteria than controls. Clin. Vaccine Immunol. 2012;19:515–521. doi: 10.1128/CVI.05517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Yada S, Liu MZ, Kamada N, Muñoz-Planillo R, Do N, Núñez G, Inohara N. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity. 2014;41:620–632. doi: 10.1016/j.immuni.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman J, Warren HS. Outer membrane protein A (OmpA), peptidoglycan-associated lipoprotein (PAL), and murein lipoprotein (MLP) are released in experimental Gram-negative sepsis. J. Endotoxin Res. 2001;7:69–72. [PubMed] [Google Scholar]
- Hellman J, Loiselle PM, Tehan MM, Allaire JE, Boyle LA, Kurnick JT, Andrews DM, Sik Kim K, Warren HS. Outer membrane protein A, peptidoglycan-associated lipoprotein, and murein lipoprotein are released by Escherichia coli bacteria into serum. Infect. Immun. 2000;68:2566–2572. doi: 10.1128/iai.68.5.2566-2572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer U, Schlaepfer E, Baenziger S, Nischang M, Regenass S, Schwendener R, Kempf W, Nadal D, Speck RF. Inadequate clearance of translocated bacterial products in HIV-infected humanized mice. PLoS Pathog. 2010;6:e1000867. doi: 10.1371/journal.ppat.1000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013a;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013b;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob. Agents Chemother. 2004;48:4574–4581. doi: 10.1128/AAC.48.12.4574-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland D, Benson A, Mirpuri J, Pifer R, Hou B, DeFranco AL, Yarovinsky F. B cell-intrinsic MyD88 signaling prevents the lethal dissemination of commensal bacteria during colonic damage. Immunity. 2012;36:228–238. doi: 10.1016/j.immuni.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinak JL, Round JL. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog. 2012;8:e1002785. doi: 10.1371/journal.ppat.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel JP. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology. 2012;142:592–601. doi: 10.1053/j.gastro.2011.11.039. e3. [DOI] [PubMed] [Google Scholar]
- Liang MD, Bagchi A, Warren HS, Tehan MM, Trigilio JA, Beasley-Topliffe LK, Tesini BL, Lazzaroni JC, Fenton MJ, Hellman J. Bacterial peptidoglycan-associated lipoprotein: a naturally occurring toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J. Infect. Dis. 2005;191:939–948. doi: 10.1086/427815. [DOI] [PubMed] [Google Scholar]
- MacFie J. Current status of bacterial translocation as a cause of surgical sepsis. Br. Med. Bull. 2004;71:1–11. doi: 10.1093/bmb/ldh029. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, McCoy KD. Stratification and compartmental- isation of immunoglobulin responses to commensal intestinal microbes. Semin. Immunol. 2013;25:358–363. doi: 10.1016/j.smim.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Geuking MB, Slack E, Hapfelmeier S, McCoy KD. The habitat, double life, citizenship, and forgetfulness of IgA. Immunol. Rev. 2012;245:132–146. doi: 10.1111/j.1600-065X.2011.01072.x. [DOI] [PubMed] [Google Scholar]
- Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH, Sogin ML, Foster JA, Edwards WH, Palumbo P, Hibberd PL. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 2012;97:F456–F462. doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed N, Tang L, Jahangiri A, de Villiers W, Eckhardt E. Elevated IgG levels against specific bacterial antigens in obese patients with diabetes and in mice with diet-induced obesity and glucose intolerance. Metabolism. 2012;61:1211–1214. doi: 10.1016/j.metabol.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Zhang J, Tan NS, Ho B, Ding JL. Natural IgG antibodies provide innate protection against ficolin-opsonized bacteria. EMBO J. 2013;32:2905–2919. doi: 10.1038/emboj.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Puga I, Cerutti A. Protection by natural IgG: a sweet partnership with soluble lectins does the trick! EMBO J. 2013;32:2897–2899. doi: 10.1038/emboj.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat. Rev. Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015;26:26050. doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman PC, Macfie J, Sagar P, Mitchell CJ, May J, Mancey-Jones B, Johnstone D. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643–649. doi: 10.1016/0016-5085(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Shankar-Hari M, Spencer J, Sewell WA, Rowan KM, Singer M. Bench-to-bedside review: Immunoglobulin therapy for sepsis - biological plausibility from a critical care perspective. Crit. Care. 2012;16:206. doi: 10.1186/cc10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HS, Xu F, Bagchi A, Herrup E, Prakash A, Valentine C, Kulkarni H, Wilhelmsen K, Warren S, Hellman J. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J. Immunol. 2011;186:1119–1130. doi: 10.4049/jimmunol.1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Nishimura Y, Yasuda S, Nishimura A, Yamada M, Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol. Gen. Genet. 1978;167:1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. USA. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoene-Reineke C, Fischer A, Friese C, Briesemeister D, Göbel UB, Kammertoens T, Bereswill S, Heimesaat MM. Composition of intestinal microbiota in immune-deficient mice kept in three different housing conditions. PLoS ONE. 2014;9:e113406. doi: 10.1371/journal.pone.0113406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat. Immunol. 2011;12:264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.