Abstract
Colonoscopy is an invaluable tool for screening and diagnosis of many colonic diseases. For most colonoscopies, moderate sedation is used during the procedure. However, insufflation of the colon produces a nociceptive stimulus that is usually accompanied by facial grimacing/groaning while under sedation. The objective of the current study was to evaluate whether a nociceptive signal elicited by colonic insufflation could be measured from the brain. Seventeen otherwise healthy patients (age 54.8±9.1; 6 female) undergoing routine colonoscopy (i.e., no history of significant medical conditions) were monitored using near-infrared spectroscopy (NIRS). Moderate sedation was produced using standard clinical protocols for midazolam and meperidine, titrated to effect. NIRS data captured during the procedure was analyzed offline to evaluate the brains’ responses to nociceptive stimuli evoked by the insufflation events (defined by physician or observing patients’ facial responses). Analysis of NIRS data revealed a specific, reproducible prefrontal cortex activity corresponding to times when patients grimaced. The pattern of the activation is similar to that previously observed during nociceptive stimuli in awake healthy individuals, suggesting that this approach may be used to evaluate brain activity evoked by nociceptive stimuli under sedation, when there is incomplete analgesia. While some patients report recollection of procedural pain following the procedure, the effects of repeated nociceptive stimuli in surgical patients may contribute to postoperative changes including chronic pain. The results from this study indicate that NIRS may be a suitable technology for continuous nociceptive afferent monitoring in patients undergoing sedation and could have applications under sedation or anesthesia.
Keywords: prefrontal cortex, near-infrared spectroscopy, sedation, insufflation, analgesia
Introduction
Colonoscopy is commonly performed under sedation to allay anxiety and reduce/eliminate pain or discomfort during the procedure. The combination of anxiolytic and analgesic drugs administered is variable, and the best technique is still debatable [16]. During colonoscopy, patient movement or grimacing during insufflation is considered to be a manifestation of nociceptive/pain activation. The most common patient complaints following colonoscopy are abdominal distension and pain [44]. Pain at discharge following colonoscopy may be underreported as a result of patient expectations [20], cognitive impairment from the residual effects of the sedative and/or analgesic agents [38], and gender differences [47], all of which have been stated to affect patient report of intraoperative awareness. Some authors have suggested that colonoscopies be performed only with analgesic agents and omitting sedatives which may produce amnesia for the procedure without contributing to analgesic effect [14; 35]. Although most patients do not have subsequent conscious recall of pain experienced during the procedure when performed with sedation, up to 25% reportedly recall their procedural pain [12; 20; 40]. Recall of pain seems to be based on peak intensity of pain and the intensity during the last few minutes of the procedure [41; 42]. The implications of such data are that while patients may not express pain during a procedural event, nociceptive pathways may still be activated as a result of these stimuli, causing local or central neuronal changes in brain networks (e.g., central sensitization) and that may lead to postoperative pain or a chronic pain state [6; 7; 30].
There is currently a lack of an objective measure of pain that could assist clinicians to evaluate a patient's analgesic state during procedures. A number of approaches have been utilized to minimize pain and discomfort during and after colonoscopy, including use of CO2 for insufflation, improving the analgesic regimen, providing technical aids, and patient controlled sedation [8] or analgesia [15]. As the interval from administration of pharmacologic agents to commencement of the colonoscopy is variable, sufficient time may not be given for the drugs to exert their effects. In addition, not all patients display signs of discomfort or pain, and it is likely that some experience significant pain. Hence, an objective measure for pain may contribute to performance of the procedure under optimal conditions with adjustment of the analgesic regime as necessary. Routine colonoscopy provides an ideal model to evaluate whether a pain signal occurs under sedation for a number of reasons: (1) a specific known mechanism produces the pain –stretching of the colon during insufflation; (2) there are usually associated patient behaviors – grimacing, moaning or movement; and (3) patients are frequently healthy and on no medications.
The limited environmental requirements of near-infrared spectroscopy (NIRS) allows for its application in operating rooms as well as at the bedside, making it ideal for clinical applications. In this study we monitored patients using NIRS, while they underwent routine colonoscopy, in order to analyze brain activity during various procedural events. We hypothesized that a specific signal, previously observed in awake, healthy volunteers experiencing pain stimuli, may be observed that correlated to pain induced by stretching of the colon during insufflation. Such a signal would permit development of a more objective approach to providing analgesia for colonoscopy.
Materials and Methods
Clinical Procedures
Colonoscopy is a very common medical procedure that is frequently performed under moderate sedation. Moderate sedation/analgesia, as defined by the American Society of Anesthesiologists, is a drug-induced depression of consciousness during which patients respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation [2]. No interventions are required to maintain a patient airway, spontaneous ventilation is adequate, and cardiovascular function is usually maintained [2]. The procedure for colonoscopy was standard and has been described elsewhere [19], with adjunct medications for anesthesia and pain control. Meperidine is an analgesic that may diminish the pain signal, however this is dose dependent [48]. Midazolam is an anesthetic with minimal affects on nociceptive signals, although it may inhibit pain pathways involved in anxiety/emotional processing [37].
Patients
Seventeen healthy adults undergoing routine screening colonoscopy were studied. Written informed consent was obtained from all subjects according to the guidelines established by the Massachusetts General Hospital Institutional Review Board who reviewed and approved this study. The following inclusion criteria were used: 25-70 years of age and right-handed. The following exclusion criteria were used: significant medical history (e.g. diabetes, infectious disease, neurological conditions affecting the CNS) aside from need for a routine endoscopy requirement, smoking history, or subject's scalp or hair does not permit sufficient optical light detection. The study conformed to the Helsinki agreement for experimental studies in Human Subjects.
All patients underwent a similar approach in terms of medication used, however the total dose varied between patients. All patients received midazolam (2.5 to 6mg), 12 patients received meperidine (50-100mg), 1 patient received fentanyl (50 mcg instead of midazolam/meperidine), 1 patients received 150 mcg fentanyl instead of meperidine. Of the 17 patients studied, 4 were excluded from the analysis. In 2 subjects (11.76%) no signal was recorded due to equipment errors, and in 2 subjects (11.76%) patient motion at the time of pain related events resulted in poor signal quality. The remaining 13 data sets, with a total of 40 stimuli after motion artifact removal, (71.4 %) were used for analysis (7 males, 54.5±7.0 years-old (mean±SD), 12 White and 1 Asian). While heart rate and blood pressure can be affected by nociception [32], the group average heart rate increased from pre- to post-insufflation by a non-significant amount (62.57 to 63.14 beats per minute, p = 0.58, paired t-test).
NIRS Measures
NIRS is a portable, non-invasive, inexpensive method of monitoring cerebral hemodynamic activity at moderate depths (surface cortices) [26]. NIRS is able to characterize the changes in concentrations of both oxygenated (HbO) and deoxygenated hemoglobin (HbR), which combined indicate change in total blood volume (HbT). These hemodynamic fluctuations are the result of vascular dilation to increase cerebral blood flow to active areas of the brain. Therefore changes in HbO and HbT volume positively correlates with neuron activity.
Data Collection
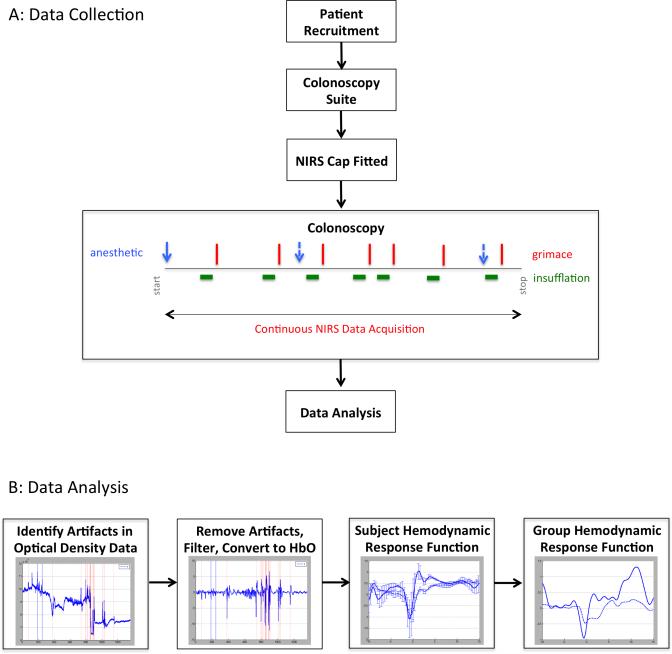
Subjects were fitted with a TechEn CW6 NIRS imaging system (TechEn, Milford MA) and data was recorded for the duration of the procedure. Auxiliary channels in the data acquisition system were utilized to mark events of interest, i.e. subject grimacing, subject motion, and injection of anesthetic agents (Figure 1(a)).
Figure 1. Data collection and analysis.
(a) Data was collected in healthy patients undergoing routine screening colonoscopy. Data was recorded using a NIRS imaging system and events related to the procedure were marked in the data. (b) Data was analyzed using Homer2 and averaged across the subject population at every pain event marked.
Data Analysis
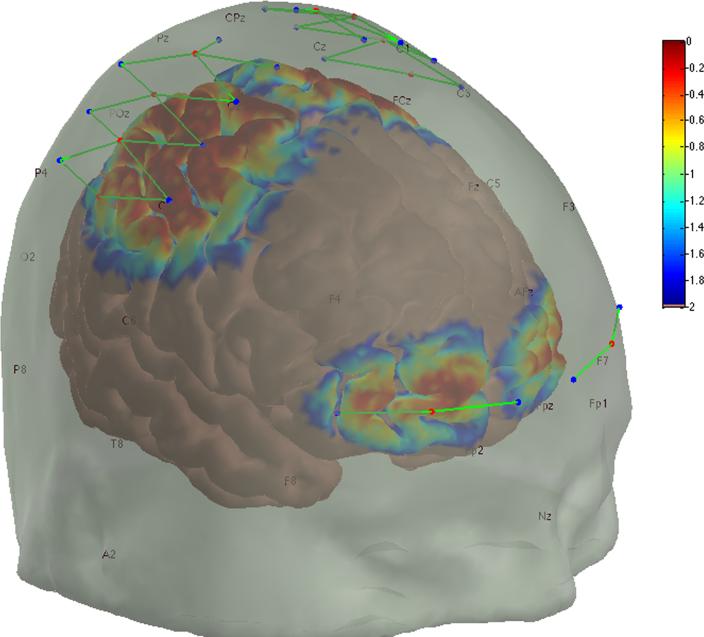
NIRS data was analyzed using Homer2 (available at http://www.nmr.mgh.harvard.edu/PMI/resources/homer2/home.htm) and as previously published [18]. Briefly, signal intensity was converted to optical density, checked for artifacts using the automated Homer2 process hmrMotionArtifact with the settings tMotion: 0.5, tMask: 1, STDEVthresh: 50, AMPthresh: 10 and enStimRejection_tRange: [−10, 10]; bandpass filtered 0.016-0.5 Hertz, converted to chromophore concentrations; and the oxygenated and deoxygenated hemoglobin concentrations were group averaged using the Homer2 block averaging technique hmrBlockAvg, with tRange: [−10, 10], synchronized through marks placed in the data at the time patients grimaced, which were used to locate the decrease in the frontal channel signal that occurs following stimulus onset (Figure 1(b)). The probe design used was analyzed using the Homer2 tool AtlasViewer to determine the probe's sensitivity to our brain regions of interest (Figure 2). Based on these results we are confident that our system was positioned to primarily measure brain activity over the prefrontal and sensorimotor cortices.
Figure 2. NIRS probe and sensitivity profile.
The fNIRS sensor array light sources (red dots), detectors (blue dots), and sensor channels (green lines) are shown at their intended locations. However, some shifting may occur for each subject, but has been shown to be sufficiently minimal in other studies where subject specific optode placements were measured. The sensitivity of the probe to detecting brain hemodynamics is shown as a temperature plot ranging from 1.00 (red) to 0.01 (blue) times the maximum sensitivity.
Results
NIRS Response to Insufflation
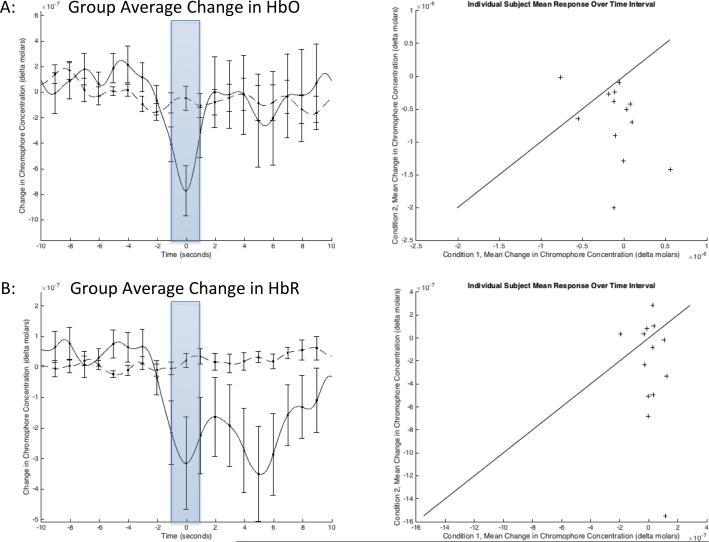
The mean hemodynamic response across the prefrontal cortex is presented in Figure 3. The sensors over the medial sensorimotor cortex recorded the largest change in oxygenation and blood volume, but were unable to obtain adequate signal quality in enough subjects to reach statistical significance. These results agree with the processing of painful stimuli in both the sensorimotor and prefrontal cortices and correlates to observations made in other studies [3; 50].
Figure 3. Average hemodynamic activity across the prefrontal cortex.
Group hemodynamic response functions observed between two sources and four detectors across the prefrontal cortex. (a) The solid line represents the change in oxygenated hemoglobin (HbO) during insufflation of the bowel, while the dashed line represents change in HbO during rest, with standard error bars for both conditions. The average value over the interval [−1, 1] (shaded) was used to perform paired t-tests (p = 0.0169, n = 13). (b) The change in deoxygenated hemoglobin (HbR) during the same stimuli resulted in a non-significant change from the resting condition (p = 0.0803, n = 13). The scatter plots on the right include a line with a slope of one and zero offset to help illustrate the trend in the data.
Frontal Response
In the prefrontal cortex, the lateralized decrease in HbO centered at time zero, and rapid return to normal levels a few seconds later, is indicative of a pain response. Figure 3 depicts the specific decrease in oxygenated hemoglobin, at approximately 1.2±3.0 seconds prior to the grimace response, indicative of deactivation of medial prefrontal cortex neurons following the probable initiation of the pain stimulus. This is based on the approximately ten-second time-to-negative-peak observed in studies where pain was applied with known timing [3]. Marking the data with the timing of the grimace response instead of the start of insufflation was chosen because the time at which insufflation becomes painful is inconsistent, while grimacing should immediately follow experiencing an unpleasant sensation and precede the peak of the decrease in HbO.
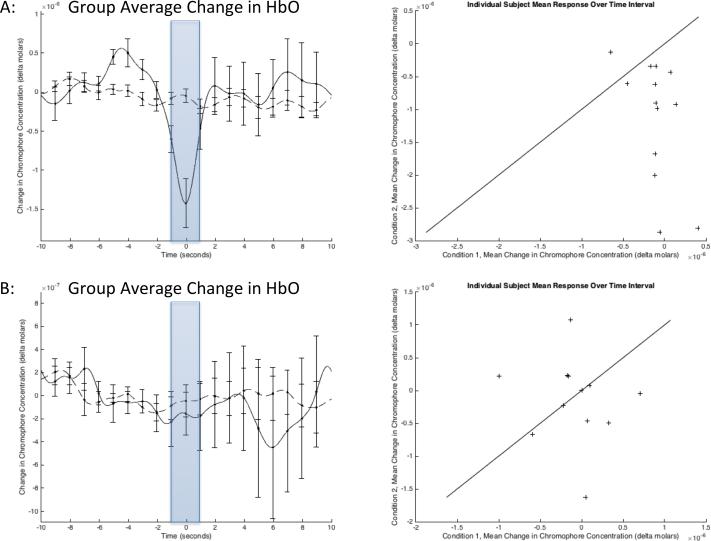
To evaluate the statistical significance of this response, semi-random points were selected in the data during periods that the subjects were at rest to produce a “normal” condition to test against. Using paired t-test the average change in HbO over the interval [−1, 1] seconds, across all frontal channels, was determined to be significant (p = 0.0169, n = 13, Figure 3(a)), while the change in HbR was non-significant (p = 0.0803, n = 13, Figure 3(b)). Separating the channels into right and left hemispheres reveals that the response on the two left channels is driving this result (p = 0.0053, n = 13, Figure 4(a)) while the response on the right channels is non-significant (p = 0.8094, n = 11, Figure 4(b)). The difference between the insufflation response and normal condition was less significant on a channel-wise basis, likely because of inconsistency in probe positioning and exclusion of individual channels due to noise.
Figure 4. Average change in oxygenated hemoglobin for left and right frontal cortices.
Group-average change in oxygenated hemoglobin for the right and left hemispheres separately. The average value over the interval [−1, 1] (shaded) was used to perform paired t-tests. (a) The signals from the two left channels are the largest contributors to the insufflation response (p = 0.0053, n = 13). (b) The response observed on the right channels alone is non-significant (p = 0.8094, n = 11). The scatter plots on the right include a line with a slope of one and zero offset to help illustrate the trend in the data.
The group average hemodynamic response function observed in the prefrontal cortex (Figure 3) resembles results obtained from previous near-infrared studies monitoring the brain during controlled painful stimulation [3]. The time offset is decidedly the result of the different methods of marking the stimulus onset, with the significant delay caused by the reaction time of a sedated patient corresponding to a negative-time shift in the hemodynamic response. The increase in signal intensity, relative to previously reported results, is believed to be indicative of increased pain intensity and/or stimulus duration when compared to the controlled stimulus study.
Sensorimotor Response
Due to the limited number of subjects with low-noise channels in the sensorimotor cortex, the hemodynamic response observed did not reach statistical significance (p = 0.2166, n = 3). One reason for this is that the representation of visceral pain is lateral and beyond the region of detection for the NIRS setup used in these experiments. The signal observed in this study likely corresponds to a motor/premotor activation.
Discussion
Our results show a signal alteration in brain neuronal activity that corresponds with the timing of insufflation of the colon and facial grimacing, suggesting that the brain measures observed are related to nociceptive inputs. Importantly, the NIRS signal observed is similar to that produced by painful stimuli in awake, healthy subjects, recorded over the same region. Having a metric for pain during the procedure would provide a number of potential benefits including: (a) a readout of the duration and number of nociceptive signals reaching the brain during these and similar procedures requiring sedation; and (b) an approach to develop standards of care including the type of sedation used for the procedure. In addition, the model may serve as a method for measurement of nociceptive signals in surgical patients who receive general anesthesia.
Pain in Colonoscopy and Sedation
Stretching of the luminal nociceptor fibers in the colon activates high threshold nociceptors [36]. Visceral pain has a number of features including: it is diffuse and poorly localized, it is referred to other locations, and it is accompanied with motor and autonomic reflexes (e.g. nausea, vomiting, and lower-back muscle tension) [9; 10]. Nociceptive information travels predominantly via the splanchnic nerve in the distal gut to second order neurons in the spinal cord and to supraspinal structures (e.g. thalamus, cortex) where the perception of visceral pain is created. Numerous studies have evaluated distention of the colon, including functional imaging studies, in order to understand brain processing of visceral pain in humans [4; 29; 45]. Visceral pain activation, as determined by functional magnetic resonance imaging, is observed in a number of regions including the frontal areas [21].
NIRS Signal During Colonoscopy
As noted in the results, we observed a robust signal over the frontal cortex. The signal correlated with the observation of grimacing (A sharp contortion of the face expressive of pain, contempt, or disgust- www.thefreedictionary.com) in the patients. Accordingly, there is a motor component in conjunction with pain. Motor responses in the brain, however, do not involve the prefrontal cortex and hence the observed signal should likely arise from pain processing.
The observed signal in frontal cortex is similar to that following phasic thermal and electrical stimulation [3], further suggesting that the signal is not modality specific but seems to be representative of pain processing. Based on the results obtained in this study, it is likely that afferent pain signals are being processed in the brain of patients undergoing colonoscopy, even with moderate sedation. Intense pain events can sensitize the brain to future stimuli, resulting in hyperalgesia or allodynia and diminished responses to post-operative analgesics [49] or rarely, set up brain processes that contribute to long-term pain recall following colonoscopy. This sensitization will exacerbate mild pain events or cause non-painful events that the patient experiences in the future to be perceived as painful. Additionally, the expectation that a colonoscopy will be an uncomfortable procedure, can contribute to poor outcomes [31].
Frontal Cortex and Pain
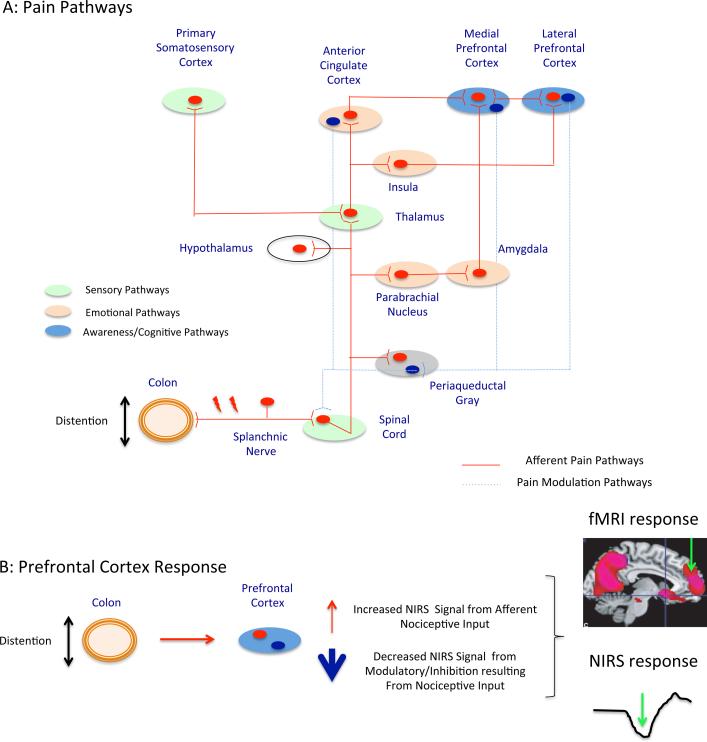
The frontal lobes are involved in a number of process including conscious awareness [13; 23; 24], cognitive processing and integration of context specific functions such as fear [34] and thus are involved in executive functions [1]. Figure 5(a) illustrates a simplified drawing of the afferent pain pathways to the frontal areas. The figure also shows how these regions send efferent projections to regions such as the periaqueductal gray to modulate (inhibit under healthy conditions) nociceptive activation of cells in the dorsal horn of the spinal cord. Conceptually, this provides a model for the activation profile we observe in the frontal regions of the signal we observe based on a balance between increased activation (resulting from afferent nociceptive input) and decreased activation (resulting from inhibitory processes in these regions). In support of complex interactions whereby pain may inhibit activation in frontal areas, research in a non-human animal model has indicated that “glutamatergic afferents from the amygdala monosynaptically innervate GABAergic interneurons in the [medial prefrontal cortex], which synapse on layer V pyramidal cells and control pyramidal cell output” and therefore medial prefrontal cortex deactivation during pain stimulus can be the result of “amygdala-driven feedforward inhibition of pyramidal cells” [25]. Human study has revealed that gastric fundus distention produces a deactivated network relative to baseline, which includes medial prefrontal cortex and subgenual anterior cingulate [46]. Thus, the activation-deactivation profile may produce changes that we measure using NIRS (Figure 5(b)).
Figure 5. Propagation of nociceptive signal and pain processing in the brain.
(a) Pain pathways associated with activation of sensorimotor cortex and deactivation of prefrontal cortex. (b) Pain processing results in both activation and deactivation of specific prefrontal cortex regions.
Pain Detection at the Individual Level
The ideal process for using this technique in pain monitoring would be to identify pain related brain activation with sufficient sensitivity at the individual level. Accomplishing this is within the means of current NIRS imaging equipment through the use of more advanced signal processing, including the use of short separation channels, which are used to remove background systemic noise through regression [18]. Through this process a series of painful events experienced by a single patient, or even a single event, may be deterministic for the detection of pain. Additionally, there may be increased sensitivity when both prefrontal and sensorimotor are reliably measured in each patient.
Model to Evaluate Brain Responses to Pain Under Sedation
Twenty-two percent of the subjects surveyed (n = 9) had explicit recall of their pain during the procedure. The recall relates to the level of the anesthetic effects on amnesia and level of analgesia. Subjects were all provided a similar anesthetic regimen “titrated to effect”. The selection of agents is also important. For example, in patient controlled anesthesia for colonoscopy, propofol does not provide effective pain relief, but the combination of propofol and alfentanil (an opioid analgesic) does [22]. Propofol was not used in this patient group, because it can only be administered (at least at our institution) with full anesthesiology coverage, which was not available, but midazolam and meperidine were utilized in all patients. Presumably, the fact that some patients had recall of their colonoscopy indicates a variation in response to the drugs based on difference in the interactions of clinical evaluation, pharmacogemonic [11; 33], or sex differences. As noted above, individuals may therefore require different doses of drugs to achieve a level of effective sedation. Independent of sedation levels, our data suggests that nociceptive signals are not effectively blocked by the anesthetic regimen used. Effective blockade of nociceptive signaling needs to be dependent on the intensity, duration, and repetition of afferent signals [6]. Repeated stimuli have accumulative affects on neuronal circuits [39]. While a patient may not express pain during a procedural event, nociceptive pathways may still be activated as the result of the stimuli, which can cause local or central sensitization to occur, resulting in postoperative pain or the formation of chronic pain. Use of approaches that may include rapidly acting patient controlled approaches (viz., rapidly acting opioids such as remifentanil), may help improve pain control during colonoscopy [15]. The use of an objective measure may allow for studies to define optimal approaches.
Caveats
Hemodynamic effects initiated by neuronal activity are considerably smaller in magnitude than the background variations in blood oxygenation and volume caused by systemic activities (e.g. cardiac rhythm, respiration, and blood pressure oscillation). The results presented in this study rely on the averaging of many events into a single group hemodynamic response function, which reduces the background noise through averaging. In order to identify single events, a regression technique such as using short separation optodes [18], in addition to our sensor array, would be necessary. Additionally, near-infrared spectroscopy is dependent on consistent contact with the medium being imaged. Excessive patient movement can cause both loss of contact with the scalp as well as movement within the cerebral fluid. Loss of contact results in sudden changes in received light intensity.
These results do not take into account any changes that occur due to the influence of pharmacologic agents on hemodynamic response to stimuli or brain function in general [17; 27; 28; 43]. In moderate sedation with propofol, a decrease in backward cortico-cortical connectivity from frontal areas has been observed [5]. Similarly, propofol produces significant frontal area decreases when measured using functional magnetic resonance imaging [51]. Thus the use of sedative hypnotics may produce some alteration in frontal lobe function, but not inhibit the responses to pain. Cortical activity monitored in healthy, awake individuals through NIRS may not provide a direct comparison to individuals undergoing screening colonoscopy, because a pain signal observed in patients under sedation or anesthesia may exhibit variations in amplitude or time of onset of activity, as well as other factors not present in the control data.
Conclusions
Through the application of NIRS, a pain-monitoring device is being developed to provide continuous monitoring of neural activity related to pain throughout procedures. The use of such a tool in anesthesia would provide a closed-loop control of analgesic load, which should improve patient intraoperative comfort and may decrease postoperative pain. Additionally, an objective measure of pain, or identification of pain processing in the brain, could be used to evaluate the effectiveness of pharmaceuticals for conditions such as irritable bowel syndrome. Future studies will evaluate the clinical effectiveness of this tool at improving patient care. We hope that this improvement to patient comfort will change the public perception of this procedure, which would in turn improve patient outcomes.
Acknowledgements
We would like to thank Margaret Grant for her contribution to data collection on this project. This work was supported by the Center for Integration of Medicine and Innovative Technology, the National Institutes of Health through R01-GM104986, and the Mayday Foundation.
Institution: This study was performed at Massachusetts General Hospital (Boston, MA).
Footnotes
Author Contributions: Study concept and design (LB, EG, PK, DB); acquisition of data (LB, MG, PK); analysis and interpretation of data (LB, CMA, MG, DAB, MAY, DB); drafting of the manuscript (LB, CMA, EG, DB, BK, PK); critical revision of the manuscript for important intellectual content (LB, CMA, DAB, EG, MAY, BK, PK, DB); statistical analysis (LB, CMA, DAB, MAY, DB); obtained funding (LB, DB).
Conflict of Interest Statements
DAB is an inventor of Continuous-Wave Near Infrared Spectroscopy technology licensed to TechEn, a company whose medical pursuits focuses on non-invasive, optical brain monitoring. DAB's interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. No other author has a conflict of interest with this research.
References
- 1.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review. 2006;16(1):17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists Task Force on S, Analgesia by N-A Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96(4):1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Becerra L, Harris W, Joseph D, Huppert T, Boas DA, Borsook D. Diffuse optical tomography of pain and tactile stimulation: activation in cortical sensory and emotional systems. Neuroimage. 2008;41(2):252–259. doi: 10.1016/j.neuroimage.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein CN, Frankenstein UN, Rawsthorne P, Pitz M, Summers R, McIntyre MC. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. The American journal of gastroenterology. 2002;97(2):319–327. doi: 10.1111/j.1572-0241.2002.05464.x. [DOI] [PubMed] [Google Scholar]
- 5.Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, Ledoux D, Bonhomme V, Brichant JF, Tononi G, Laureys S, Friston K. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(20):7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borsook D, George E, Kussman B, Becerra L. Anesthesia and perioperative stress: consequences on neural networks and postoperative behaviors. Progress in neurobiology. 2010;92(4):601–612. doi: 10.1016/j.pneurobio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically Induced Neuropathic Pain: Understanding the Perioperative Process. Annals of surgery. 2012 doi: 10.1097/SLA.0b013e3182701a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bright E, Roseveare C, Dalgleish D, Kimble J, Elliott J, Shepherd H. Patient-controlled sedation for colonoscopy: a randomized trial comparing patient-controlled administration of propofol and alfentanil with physician-administered midazolam and pethidine. Endoscopy. 2003;35(8):683–687. doi: 10.1055/s-2003-41519. [DOI] [PubMed] [Google Scholar]
- 9.Cervero F, Laird JM. Visceral pain. Lancet. 1999;353(9170):2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 10.Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. Journal of neurobiology. 2004;61(1):45–54. doi: 10.1002/neu.20084. [DOI] [PubMed] [Google Scholar]
- 11.Chidambaran V, Ngamprasertwong P, Vinks AA, Sadhasivam S. Pharmacogenetics and anesthetic drugs. Current clinical pharmacology. 2012;7(2):78–101. doi: 10.2174/157488412800228866. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LB, Hightower CD, Wood DA, Miller KM, Aisenberg J. Moderate level sedation during endoscopy: a prospective study using low-dose propofol, meperidine/fentanyl, and midazolam. Gastrointestinal endoscopy. 2004;59(7):795–803. doi: 10.1016/s0016-5107(04)00349-9. [DOI] [PubMed] [Google Scholar]
- 13.Demertzi A, Soddu A, Faymonville ME, Bahri MA, Gosseries O, Vanhaudenhuyse A, Phillips C, Maquet P, Noirhomme Q, Luxen A, Laureys S. Hypnotic modulation of resting state fMRI default mode and extrinsic network connectivity. Progress in brain research. 2011;193:309–322. doi: 10.1016/B978-0-444-53839-0.00020-X. [DOI] [PubMed] [Google Scholar]
- 14.Eberl S, Preckel B, Fockens P, Hollmann MW. Analgesia without sedatives during colonoscopies: worth considering? Techniques in coloproctology. 2012;16(4):271–276. doi: 10.1007/s10151-012-0834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanti L, Agostoni M, Gemma M, Gambino G, Facciorusso A, Guslandi M, Torri G, Testoni PA. Remifentanil vs. meperidine for patient-controlled analgesia during colonoscopy: a randomized double-blind trial. The American journal of gastroenterology. 2009;104(5):1119–1124. doi: 10.1038/ajg.2009.53. [DOI] [PubMed] [Google Scholar]
- 16.Fanti L, Testoni PA. Sedation and analgesia in gastrointestinal endoscopy: what's new? World journal of gastroenterology : WJG. 2010;16(20):2451–2457. doi: 10.3748/wjg.v16.i20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiset P, Paus T, Daloze T, Plourde G, Meuret P, Bonhomme V, Hajj-Ali N, Backman SB, Evans AC. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(13):5506–5513. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagnon L, Perdue K, Greve DN, Goldenholz D, Kaskhedikar G, Boas DA. Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling. NeuroImage. 2011;56(3):1362–1371. doi: 10.1016/j.neuroimage.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garborg KK, Løberg M, Matre J, Holme O, Kalager M, Hoff G, Bretthauer M. Reduced pain during screening colonoscopy with an ultrathin colonoscope: a randomized controlled trial. Endoscopy. 2012;44(8):740–746. doi: 10.1055/s-0032-1309755. [DOI] [PubMed] [Google Scholar]
- 20.Gavaruzzi T, Carnaghi A, Lotto L, Rumiati R, Meggiato T, Polato F, De Lazzari F. Recalling pain experienced during a colonoscopy: pain expectation and variability. British journal of health psychology. 2010;15(Pt 2):253–264. doi: 10.1348/135910709X458305. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi T, Kano M, Rikimaru H, Kanazawa M, Itoh M, Yanai K, Fukudo S. Brain activity during distention of the descending colon in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(3):299–309. doi: 10.1111/j.1365-2982.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 22.Heiman DR, Tolliver BA, Weis FR, O'Brien BL, DiPalma JA. Patient-controlled anesthesia for colonoscopy using propofol: results of a pilot study. Southern medical journal. 1998;91(6):560–564. doi: 10.1097/00007611-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain's default mode network during deep sleep. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishai A. Seeing with the mind's eye: top-down, bottom-up, and conscious awareness. F1000 biology reports. 2010:2. doi: 10.3410/B2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji G, Neugebauer V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. Journal of neurophysiology. 2011;106(5):2642–2652. doi: 10.1152/jn.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198(4323):1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 27.Kaisti KK, Langsjo JW, Aalto S, Oikonen V, Sipila H, Teras M, Hinkka S, Metsahonkala L, Scheinin H. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99(3):603–613. doi: 10.1097/00000542-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Kaisti KK, Metsahonkala L, Teras M, Oikonen V, Aalto S, Jaaskelainen S, Hinkka S, Scheinin H. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology. 2002;96(6):1358–1370. doi: 10.1097/00000542-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Kanazawa M, Hamaguchi T, Watanabe S, Terui T, Mine H, Kano M, Fukudo S. Site-specific differences in central processing of visceral stimuli from the rectum and the descending colon in men. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22(2):173–180, e153. doi: 10.1111/j.1365-2982.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 30.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 31.Khan RS, Ahmed K, Blakeway E, Skapinakis P, Nihoyannopoulos L, Macleod K, Sevdalis N, Ashrafian H, Platt M, Darzi A, Athanasiou T. Catastrophizing: a predictive factor for postoperative pain. American journal of surgery. 2011;201(1):122–131. doi: 10.1016/j.amjsurg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Kratz T, Dette F, Schmitt J, Wiesmann T, Wulf H, Zoremba M. Impact of regional femoral nerve block during general anesthesia for hip arthoplasty on blood pressure, heart rate and pain control: A randomized controlled study. Technol Health Care. 2015 doi: 10.3233/THC-150898. [DOI] [PubMed] [Google Scholar]
- 33.Landau R, Bollag LA, Kraft JC. Pharmacogenetics and anaesthesia: the value of genetic profiling. Anaesthesia. 2012;67(2):165–179. doi: 10.1111/j.1365-2044.2011.06918.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu CC, Crone NE, Franaszczuk PJ, Cheng DT, Schretlen DS, Lenz FA. Fear conditioning is associated with dynamic directed functional interactions between and within the human amygdala, hippocampus, and frontal lobe. Neuroscience. 2011;189:359–369. doi: 10.1016/j.neuroscience.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loberg M, Furholm S, Hoff I, Aabakken L, Hoff G, Bretthauer M. Nitrous oxide for analgesia in colonoscopy without sedation. Gastrointestinal endoscopy. 2011;74(6):1347–1353. doi: 10.1016/j.gie.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 36.Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. The Journal of physiology. 1999;518(Pt 1):271–282. doi: 10.1111/j.1469-7793.1999.0271r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;(182):335–360. doi: 10.1007/978-3-540-74806-9_16. [DOI] [PubMed] [Google Scholar]
- 38.Padmanabhan U, Leslie K, Eer AS, Maruff P, Silbert BS. Early cognitive impairment after sedation for colonoscopy: the effect of adding midazolam and/or fentanyl to propofol. Anesthesia and analgesia. 2009;109(5):1448–1455. doi: 10.1213/ane.0b013e3181a6ad31. [DOI] [PubMed] [Google Scholar]
- 39.Petersen-Felix S, Arendt-Nielsen L, Bak P, Fischer M, Bjerring P, Zbinden AM. The effects of isoflurane on repeated nociceptive stimuli (central temporal summation) Pain. 1996;64(2):277–281. doi: 10.1016/0304-3959(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 40.Poincloux L, Laquiere A, Bazin JE, Monzy F, Artigues F, Bonny C, Abergel A, Dapoigny M, Bommelaer G. A randomized controlled trial of endoscopist vs. anaesthetist-administered sedation for colonoscopy. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011;43(7):553–558. doi: 10.1016/j.dld.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Redelmeier DA, Kahneman D. Patients’ memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66(1):3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- 42.Redelmeier DA, Katz J, Kahneman D. Memories of colonoscopy: a randomized trial. Pain. 2003;104(1-2):187–194. doi: 10.1016/s0304-3959(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 43.Schlunzen L, Juul N, Hansen KV, Cold GE. Regional cerebral blood flow and glucose metabolism during propofol anaesthesia in healthy subjects studied with positron emission tomography. Acta anaesthesiologica Scandinavica. 2012;56(2):248–255. doi: 10.1111/j.1399-6576.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- 44.Senore C, Ederle A, Fantin A, Andreoni B, Bisanti L, Grazzini G, Zappa M, Ferrero F, Marutti A, Giuliani O, Armaroli P, Segnan N. Acceptability and side-effects of colonoscopy and sigmoidoscopy in a screening setting. Journal of medical screening. 2011;18(3):128–134. doi: 10.1258/jms.2011.010135. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, Watanabe S, Hamaguchi T, Mine H, Terui T, Kanazawa M, Oohisa N, Maruyama M, Yambe T, Itoh M, Fukudo S. Brain activation associated with changes in heart rate, heart rate variability, and plasma catecholamines during rectal distention. Psychosomatic medicine. 2009;71(6):619–626. doi: 10.1097/PSY.0b013e31819b69ca. [DOI] [PubMed] [Google Scholar]
- 46.van Oudenhove L, Vandenberghe J, Dupont P, Geeraerts B, Vos R, Bormans G, van Laere K, Fischler B, Demyttenaere K, Janssens J, Tack J. Cortical deactivations during gastric fundus distension in health: visceral pain-specific response or attenuation of ‘default mode’ brain function? A H2 15O-PET study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21(3):259–271. doi: 10.1111/j.1365-2982.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- 47.Viiala CH, Olynyk JK. Outcomes after 10 years of a community-based flexible sigmoidoscopy screening program for colorectal carcinoma. The Medical journal of Australia. 2007;187(5):274–277. doi: 10.5694/j.1326-5377.2007.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 48.Wolff M, Olschewski A, Vogel W, Hempelmann G. Meperidine suppresses the excitability of spinal dorsal horn neurons. Anesthesiology. 2004;100(4):947–955. doi: 10.1097/00000542-200404000-00027. [DOI] [PubMed] [Google Scholar]
- 49.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yücel MA, Aasted CM, Petkov MP, Borsook D, Boas DA, Becerra L. Specificity of hemodynamic brain responses to painful stimuli: a functional near-infrared spectroscopy study. Sci Rep. 2015;5:9469. doi: 10.1038/srep09469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Wang W, Zhao Z, Ge Y, Zhang J, Yu D, Chai W, Wu S, Xu L. The action sites of propofol in the normal human brain revealed by functional magnetic resonance imaging. Anatomical record. 2010;293(12):1985–1990. doi: 10.1002/ar.21069. [DOI] [PubMed] [Google Scholar]