Abstract
Data emerging from the past 10 years have consolidated the rationale for investigating the use of aspirin as a chemopreventive agent; however, the mechanisms leading to its anti-cancer effects are still being elucidated. We hypothesized that aspirin’s chemopreventive actions may involve cell cycle regulation through modulation of the levels or activity of cyclin A2/cyclin dependent kinase-2 (CDK2). In this study, HT-29 and other diverse panel of cancer cells were used to demonstrate that both aspirin and its primary metabolite, salicylic acid, decreased cyclin A2 (CCNA2) and CDK2 protein and mRNA levels. The down regulatory effect of either drugs on cyclin A2 levels was prevented by pretreatment with lactacystin, an inhibitor of proteasomes, suggesting the involvement of 26S proteasomes. In-vitro kinase assays showed that lysates from cells treated with salicylic acid had lower levels of CDK2 activity. Importantly, three independent experiments revealed that salicylic acid directly binds to CDK2. Firstly, inclusion of salicylic acid in naïve cell lysates, or in recombinant CDK2 preparations, increased the ability of the anti-CDK2 antibody to immunoprecipitate CDK2, suggesting that salicylic acid may directly bind and alter its conformation. Secondly, in 8-anilino-1-naphthalene-sulfonate (ANS)-CDK2 fluorescence assays, pre-incubation of CDK2 with salicylic acid, dose-dependently quenched the fluorescence due to ANS. Thirdly, computational analysis using molecular docking studies identified Asp145 and Lys33 as the potential sites of salicylic acid interactions with CDK2. These results demonstrate that aspirin and salicylic acid down-regulate cyclin A2/CDK2 proteins in multiple cancer cell lines, suggesting a novel target and mechanism of action in chemoprevention.
Implications
Biochemical and structural studies indicate that the anti-proliferative actions of aspirin are mediated through cyclin A2/CDK2.
Keywords: Chemoprevention, Colorectal cancer, CDKs and CDK inhibitors, Cell cycle regulation, Cyclins, Aspirin, Salicylic acid
Introduction
Evidence from epidemiological studies have demonstrated a significant correlation between regular aspirin use and reduced cancer incidence and mortality. This inverse correlation is now established for the cancers of the colon [1-3], breast, prostate, lung and skin [4-7]. Animal and human studies also support a role for aspirin in chemoprevention. Aspirin suppresses aberrant crypt foci formation in colorectal cancer patients [8]. Its use after the colorectal and breast cancer diagnosis was associated with a decreased risk and increased patient survival [9, 10]. The evidence that aspirin prevents cancer is compelling; however, the underlying mechanisms leading to its anticancer effect is enigmatic as numerous protein targets and pathways have been suggested. Which of these primarily contributes to its anti-cancer effects is not clear; however, one widely accepted mechanism is the inhibition of COX-1 and COX2 by acetylation leading to decreased prostaglandin synthesis [2]. Interestingly, several COX-independent pathways have been proposed [2, 11]. We and others have demonstrated that aspirin acetylates multiple cellular proteins, which further gives it the potential to affect simultaneously multiple cellular pathways [12-17]. Additional mechanisms described include inhibition of NF-κB [18], inhibition of Wnt/β-catenin pathway [19], down-regulation of Sp transcription factors [20], inhibition of mTOR signaling [21] among others. Thus, aspirin affects multiple pathways rather than one single target; thus, the broadly-specific nature of its action may be the key to its chemopreventive properties.
Aspirin is mainly absorbed intact in the gastrointestinal tract and later hydrolyzed to acetate and salicylate ions in the plasma, liver and within the cell. The plasma concentration of salicylic acid obtained from the hydrolysis of analgesic and anti-inflammatory doses of aspirin can vary from 0.5 to 2.5 mM [11], and its half-life is ~4.5 h. Some studies showed that similar to aspirin, salicylic acid also exhibits potent anti-proliferative and antitumor activity in-vitro and in-vivo [20, 22, 23]. Thus, the contribution of the salicylic acid to aspirin’s anticancer effects cannot be discounted.
Cyclins control the progression of cells through the cell cycle by physically interacting and activating cyclin dependent kinase (CDK) enzymes [24]. The cell cycle is regulated by multiple cyclins such as A, B, D and E; CDKs such as 1, 2, 4 and 6; and CDK inhibitors such as p16, p21 and p27. Several isoforms also exist for cyclin family members; for example, humans contain two distinct types of cyclin A: cyclin A1, the embryonic specific form; and cyclin A2, the somatic form. Cyclin A2 can activate two different CDKs: CDK1 and CDK2 [25]. Its levels are low during the G1 phase, increases at the onset of S phase, and remains high during G2 and early mitosis. By associating with CDK2 during the S phase, it regulates DNA synthesis through phosphorylation of proteins involved in DNA replication. Cyclin A2 is also important during the G2 to M phase transition [26]. During early mitosis it associates with CDK1 and drives chromosome condensation and nuclear envelope breakdown [27]. It is degraded during pro-metaphase through ubiquitination by the anaphase promoting complex/cyclosome (APC/C) [28].
In this paper, we focused our study on cyclin A2 and its binding partner CDK2 because, firstly, they regulate DNA synthesis during the S phase; secondly, both proteins are de-regulated or up-regulated in breast, liver and lung cancers [29-33]. In addition, there has been significant interest in targeting cell cycle through inhibition of CDK2 activity as a strategy to treat cancer [34-36]. Since aspirin is known to inhibit cell proliferation, we hypothesized that its anti-cancer effects may involve down-regulation of cyclin A2 / CDK2 proteins or their mRNA levels or both. Our goal in this research paper was to study the effect of aspirin and salicylic acid on cyclin A2/CDK2 in multiple cancer cell lines representing cancers of various tissues such as colon, breast, lung, skin, prostate and ovary, which would also establish the universality of the observation. Here, we report that cyclin A2 and CDK2 are novel targets of aspirin and salicylic acid, as both drugs caused their down-regulation in a concentration-dependent fashion in the human colon cancer HT-29 and also in 10 other cancer cell lines. Aspirin- and salicylic acid-mediated decrease in cyclin A2 protein levels required a lactacystin sensitive protease. Both drugs caused a decrease in exogenously expressed DDK-tagged cyclin A2 levels. Moreover, cells treated with aspirin and salicylic acid had reduced amounts of cyclin A2 and CDK2 mRNA levels. The decrease in cyclin A2/CDK2 protein levels was associated with a decrease in CDK2 kinase activity. Through anti-CDK2 antibody immunoprecipitations, molecular docking studies and CDK2-ANS (8-anilino-1-naphthalene sulfonate) fluorescence assay, we show that salicylic acid binds and interacts with Asp145 and Lys33 in the CDK2 protein. Our results show that aspirin and salicylic acid regulate cyclin A2 gene expression at the transcriptional/post-transcriptional and post-translational levels. We suggest that down-regulation of the cyclin A2/CDK2 mRNA and protein levels may represent one important mechanism by which aspirin exerts its anti-cancer effect via the formation of salicylic acid.
Materials and Methods
Materials
Cell lines
HCT 116, HT-29, SW480 (Human colon cancer cells), SK-MEL-28, SK-MEL-5 (Human skin melanoma cells), MDA-MB-231, MCF7 (Human Breast cancer cells); NCI-H226 (Human lung cancer cells); OVCAR-3 (Human Ovarian cancer cells); PC-3 (Human prostate cancer cells); and B16-F10 (Mouse Melanoma cells) were purchased from American Type Culture Collection (ATCC). Authentication of cell lines were done by ATCC through their DNA-STR profile.
Reagents
Aspirin, salicylic acid, trypsin-EDTA solution were purchased from Sigma, SuperSignal™ West Pico Chemiluminescent Substrate and protease inhibitor tablets from Thermo Scientific; lactacystin, Immobilon membranes, H1 Histones from EMD Millipore; FuGENE HD Transfection Reagent from Promega; protein G agarose, Halt™ Phosphatase Inhibitor Cocktail, GeneJET Gel Extraction Kit, Mlu I and EcoR I restriction enzymes from Life technologies; TRIzol reagent from Ambion; 32P γ-ATP and 32P α-dCTP were from MP Biochemical; Random Primer DNA labelling Kit from Clontech; Zeta probe blotting membranes from Bio-Rad and all other chemicals were obtained from Thermo Fischer Scientific.
Recombinant proteins, plasmid DNA and antibodies
Recombinant human CDK2 from Prospec; Myc-DDK-tagged (C-terminus) human cyclin A2 (CCNA2), and anti-DDK antibody were obtained from OriGene; Anti-cyclin A2 and anti-β actin antibodies from Cell Signaling Technology; anti-Cyclin A2 antibody was from Abcam, Anti- CDK2 antibody from EMD Millipore or Santa Cruz Biotechnologies; goat anti-rabbit and goat anti-mouse antibodies were obtained from Bio-Rad.
Methods
Cell Culture
The cells were grown in appropriate ATCC recommended medium containing 10% FBS for 24-48 h before adding aspirin or salicylic acid for indicated times.
Cell proliferation
Approximately 100,000 cells were seeded per 100 mm plates containing 10% FBS and grown overnight. Drugs were added at various concentrations and incubated for 48h. The floating cells (if any) were collected from the conditioned media, and pooled with the trypsinized adhered cells, and counted in the Nexcelom Cellometer Auto T4 cell counter. The viability of the cells was determined by trypan blue staining.
Total cell lysate preparation, immunoprecipitation, and Western Blotting
Cells were treated with aspirin or salicylic acid at different concentrations for the indicated time and washed with phosphate buffered saline (PBS). Cells were scraped in lysis buffer (10mM Tris-Cl pH 7.4, 150 mM NaCl, 15% glycerol, 1% Triton X-100 with protease inhibitors). Samples containing 50μg of proteins were separated by an 8 or 10% polyacrylamide gel electrophoresis (PAGE) and immunoblotted with respective antibodies. For immunoprecipitations, 500 μg of the total proteins isolated from cells, or 300 ng of the recombinant CDK2 protein, were diluted to 1 ml of lysis buffer, immunoprecipitated with anti-CDK2 antibodies overnight at 4°C, the immune complex was captured by adding 35 μls of protein G agarose for 3h. The immunocomplexes were washed 3 times with PBS, and dissolved in SDS-sample buffer. The samples were analyzed on a SDS-PAGE, immunoblotted with either anti-cyclin A2 antibody or anti-CDK2 antibody. Immunoreactive bands were detected using chemiluminescence reagents. The intensities of bands were determined using NIH ImageJ software.
Expression of recombinant DDK-tagged proteins
Cells were seeded on a 100 mm plate at 50% confluency overnight and transfected with 3 μg of recombinant cyclin A2 or CDK2 plasmids using FuGENE HD Transfection Reagent (Promega Inc). The cells were incubated for 24 h-48 h to allow for the expression of recombinant proteins. Cell lysates were prepared and immunoblotted with either anti-DDK antibodies, or anti-cyclin A2 or anti-CDK2 antibodies.
Northern blot analysis
The pCMV6-Entry vector carrying full-length cyclin A2 and CDK2 plasmid DNA was digested with MluI and EcoRI to release the cDNA insert, and the DNA purified. Total RNA was extracted from cells untreated or treated cells with drugs using TRIzol reagent, and Northern blot analysis was carried out as previously described [14]. The blots were washed with 0.1× SSC buffer containing 0.1% SDS for 1h at 65°C, dried and exposed to X-ray film.
In-vitro CDK assay
The CDK assay was performed according to the previously published method [37]. In brief, 500 μg of the protein from cell lysates were diluted with 1 ml of the lysis buffer and immunoprecipitated using anti-CDK2 antibody followed by the addition of protein G agarose as described above. After washing three times with lysis buffer, the immunocomplexes were washed twice with lysis buffer containing no Triton X-100 and once with kinase buffer (40 mM Tris-HCl pH 8.0, 5mM MgCl2, and 5% glycerol). The final pellet was suspended in kinase buffer containing 20 μM ATP, 2 μci of γ 32P ATP, 5μg of H1 Histone, 0.5× phosphatase inhibitor, and incubated for 30min at 37°C. The reaction was stopped by the addition of SDS-sample buffer, and loaded on a 10% SDS-PAGE, gel stained with Coomassie blue, dried and exposed to X-ray film.
Molecular docking studies
An in-silico approach was adopted to identify potential target inhibitors through molecular docking studies. In an attempt to understand the ligand-protein interactions in terms of binding affinity, aspirin and salicylic acid were subjected to docking with CDK2 using AutoDockVina. The small-molecule topology generator Dundee PRODRG2 server [38] was used for ligand optimization. The crystallographic three-dimensional structures of selected target proteins (PDB ID: 1FIN (2.30Å) were retrieved from the Protein Data Bank (PDB) http://www.pdb.org. The human Cyclin A2 (PDB ID: 1FIN B chain), CDK2 (PDB ID: 1AQ1) and cyclin A2/CDK2 complex (PDB ID: 1FIN A, B chain) protein molecule was selected for energy minimization using Gromacs 3.3.1 package with the GROMOS96 force field [39]. These molecules were used as the receptor for virtual small molecule docking with the ligand aspirin and salicylic acid using AutoDockVina. Python molecular viewer with AutoDock Tools were used for conversion to pdbqt format, required by AutoDockVina.
CDK2/ANS fluorescence assay
The CDK2/ANS assay is based on the fluorescence emitted from the interaction of ANS within the allosteric pocket of CDK2 [40]. For the assays, the previously recommended concentrations of ANS and CDK2 at 50 μM and 1.6 μM (0.5mg/ml) respectively, was used. Commercially obtained recombinant CDK2 protein was mixed with ANS in a total volume of 50 μls in a 96 well plate, and the fluorescence was measured at excitation and emission wavelengths of 405 and 460 nm using a Spectramax M2 spectrophotometer. Alternatively, recombinant CDK2 was first pre-incubated with salicylic acid at different concentrations before the addition of ANS, and then the fluorescence was measured.
Statistical analysis
All experiments were repeated 3-6 times independently of each other. One-way ANOVA followed by Tukey’s range tests were adopted to compare group differences to control and significance was defined as P<0.05.
Results
Aspirin and salicylic acid decrease cell proliferation in HT-29, SK-MEL-28, and MDA-MB-231 cells
Previous studies have shown that treatment of cells with aspirin and salicylic acid, at concentrations ranging from 2.5 mM to 10 mM, induced a profound reduction in cell proliferation in HT-29 and other cancer cells [20, 41, 42]; but, a comparison at lower concentrations were not reported. In this experiment, we chose HT-29 (colon), SK-MEL-28 (skin) and MDA-MB-231 (breast) cancer cells to evaluate the effect of aspirin and salicylic acid on proliferation rate at the concentrations ranging from 0.25 to 2.5 mM. Cells were treated separately with both drugs for 48h, trypsinized and counted. We observed that aspirin and salicylic acid progressively reduced the cell number particularly from 0.5 mM to 2.5 mM (supplemental Fig. 1). The cell viability was unaffected at all concentrations of the drugs tested. These results show that both drugs are effective in reducing the cell proliferation rate upon exposure for 48h, without affecting the viability.
Effect of aspirin and salicylic acid on cell cycle regulatory proteins
We hypothesized that aspirin and salicylic acid may exert their anti-proliferative effects through modulation of cell cycle regulatory proteins. Therefore, we sought to determine whether these drugs would affect the levels of cyclins A, B, D and E; CDKs 1, 2, 4 and 6; and CDK inhibitors p16, p21 and p27. To address this, HT-29 cells were left untreated or treated with aspirin or salicylic acid at various concentrations for 24h. Cell lysates were prepared and immunoblotted with various anti-cyclin, anti-CDK and anti-CDK inhibitor antibodies. We observed that both aspirin and salicylic acid down regulated the levels of cyclins A2, B1 and D3; and CDKs 1, 2, 4 and 6. Interestingly, both drugs up-regulated the levels of cyclin E1 as well as CDK inhibitors, p27 and p21. The levels of p16 were not detected in these experiments possibly reflecting the lower expression. The data on the effect of aspirin and salicylic acid on cyclin A2 in HT-29 cells is shown in Fig. 1A and B. The results obtained on CDK2 is discussed elsewhere in Fig. 4 (see below). The data on cyclins B1, E1 and D3 are shown in supplemental Fig. 2; CDKs 1, 4 and 6 in supplemental Fig. 3 and CDK inhibitors p21 and p27 in supplemental Fig. 4. It is clear from these results that aspirin exposure to HT-29 cells causes differential regulation of cell cycle regulatory proteins. Among these identified protein targets, we focused mainly on cyclin A2 and CDK2 in the present study because: a) they play an important role in the regulation of DNA synthesis during cell cycle progression; b) they are de-regulated or up-regulated in several cancers such as breast, liver and lung [29-33]. We hypothesized that aspirin and salicylic acid may primarily target cyclin A2/CDK2 to cause the cell cycle arrest, which has been previously reported by other investigators [20, 41, 42].
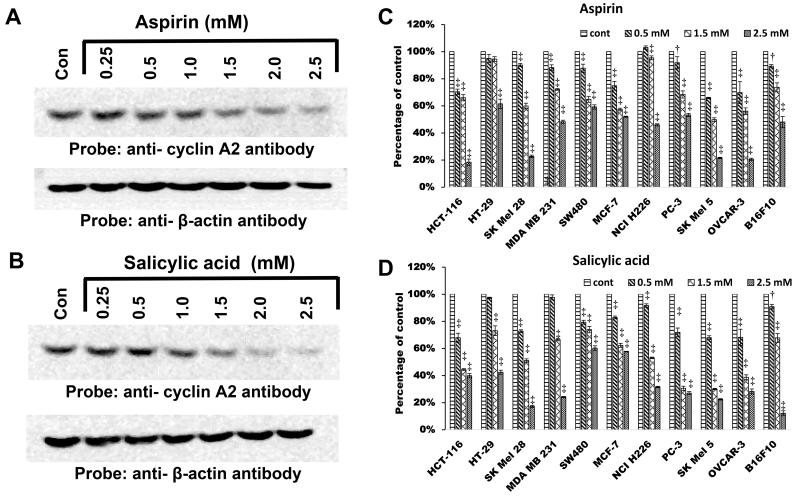
Figure 1.
Aspirin and salicylic acid down-regulate cyclin A2 protein levels in multiple cell lines. A and B respectively represent the effect of aspirin and salicylic acid on cyclin A2 protein levels in HT-29 cells. C and D represent comparison of the effect of aspirin and salicylic acid in multiple cancer cell lines. For C and D, the intensity of bands in various western blots were quantified and expressed as percentage of control. P-value < 0.001‡, <0.01†
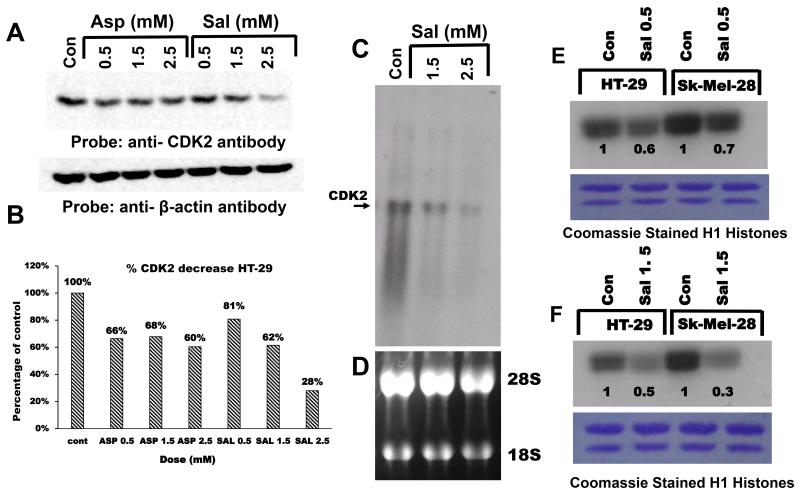
Figure 4.
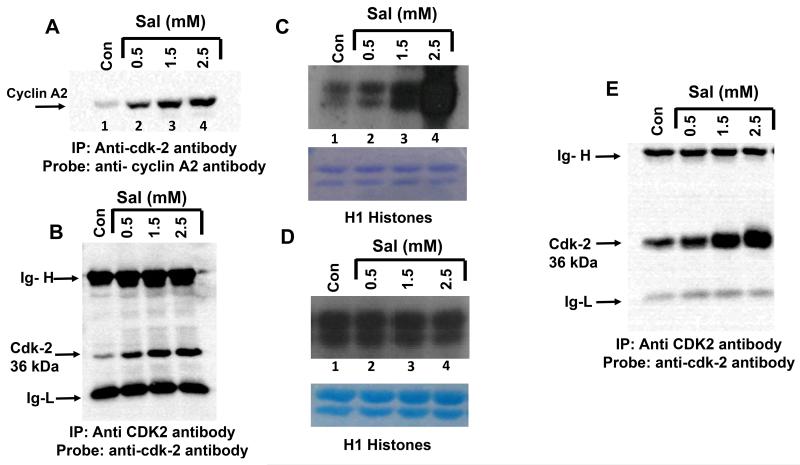
Aspirin/salicylic acid down-regulate CDK2 protein/mRNA levels and activity. A, aspirin and salicylic acid down-regulate CDK2 protein in HT-29 cells. B, quantification of the band in blot A, expressed as percentage control. C, Northern blot analysis of CDK2 in response to salicylic acid treatment in HT-29 cells. D, ethidium bromide stained ribosomal RNA pattern of blot C. E and F, respectively represent CDK2 activity at two different concentrations (0.5 mM and 1.5 mM) in HT-29 and SK-MEL-28 cells, numbers on the blot represent intensities expressed as percentage of control. The lower panel shows the Coomassie blue stained histones following electrophoresis (see the text for details).
Aspirin and salicylic acid decrease cyclin A2 levels in multiple cell lines
We compared the ability of aspirin and salicylic acid at 3 different concentrations (0.5, 1.5 and 2.5 mM) to down-regulate cyclin A2 levels in 11 different cancer cell lines representing human colon (HCT 116, HT-29, SW480); breast (MDA-MB-231, MCF7), skin (SK-MEL-28, SK-MEL-5); Lung (NCI-H226); prostate (PC-3); OVCAR-3 (ovary), and also in mouse skin melanoma (B16-F10) cells. Figure 1C and 1D respectively demonstrates the comparison of the down-regulatory effect of aspirin and salicylic acid on cyclin A2 levels in these cell lines. It is clear from Figs. 1C and D that the decrease in cyclin A2 was greater at higher concentrations of the drugs, although the sensitivity of cells towards drug treatment differed. Most dramatic down-regulation was observed in HCT 116, MCF7, SK-MEL-5 and OVCAR-3 cells at all drug concentrations tested.
Lactacystin completely prevents aspirin and salicylic acid-mediated down-regulation of cyclin A2 levels
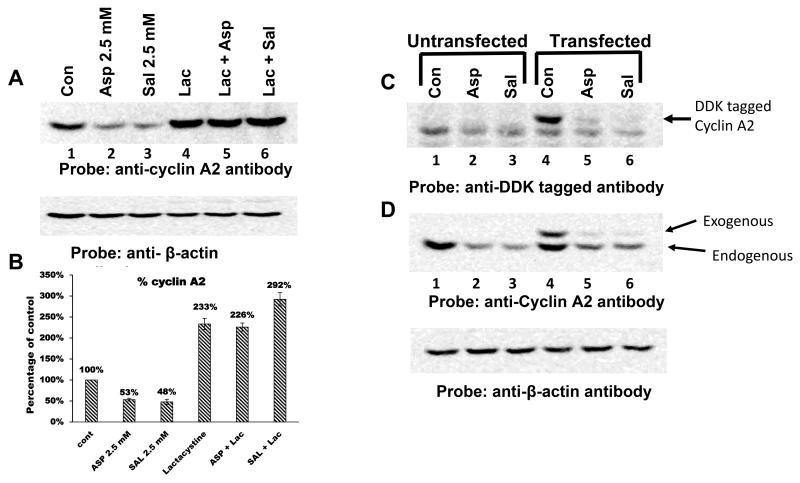
Cyclin A2 naturally undergoes degradation via ubiquitin-proteasomal pathway [28]. During pro-metaphase, it is ubiquitinated by the APC/C and this tags cyclin A2 for degradation by the 26S proteasomes [28]. To determine a role for the proteasomal pathway, we tested the ability of lactacystin, a 26S proteasomal inhibitor [43], to prevent aspirin and salicylic acid-mediated decrease in cyclin A2 levels. Cells were left untreated or first treated with lactacystin (10 μM) for 1h, then aspirin or salicylic acid (2.5 mM) were added for 24h. Cell lysates were prepared and immunoblotted with the anti-cyclin A2 antibody. Figure 2A demonstrates that lactacystin pretreatment completely prevented the degradation of cyclin A2 caused by aspirin and salicylic acid. Quantification of these bands showed that aspirin and salicylic acid decreased the cyclin A2 levels by 47% and 52% respectively (Fig. 2B). Lactacystin treatment alone stabilized the cyclin A2 protein levels; it was 2.3-fold higher compared to untreated control. However, in the presence of lactacystin, both drugs failed to cause the down-regulation of the cyclin A2. This suggests that, 26S proteasomes are involved in aspirin and salicylic acid-mediated down-regulatory effects.
Figure 2.
Down-regulation of cyclin A2 by aspirin and salicylic acid is mediated by 26S proteasomal pathway. A, effect of lactacystin on the ability of aspirin and salicylic acid to decrease cyclin A2 protein levels in HT-29 cells. B, represents the quantification of the bands in blot A. C and D, aspirin and salicylic acid down-regulate exogenously expressed DDK-tagged cyclin A2 protein. C and D, respectively represent immunoblots probed with anti-DDK tagged antibody and anti-cyclin A2 antibody. Positions of the exogenous and endogenous cyclin A2 were shown by arrows (see the text for details).
Aspirin and salicylic acid decrease exogenously expressed, DDK-tagged, cyclin A2 protein levels
To investigate whether aspirin and salicylic acid could decrease the exogenously expressed DDK-tagged cyclin A2 protein levels, HT-29 cells were left untransfected or transfected with DDK-tagged full-length cyclin A2 cDNA cloned in the pCMV6 vector. After transfections, cells were incubated for 24 h to allow for the expression DDK-tagged cyclin A2. Following this, cells were either left untreated or treated with drugs at a concentrations of 2.5 mM for 24 h. Cell lysates were prepared and immunoblotted with anti-DDK or anti-cyclin A2 antibodies. Figure 2C demonstrates that anti-DDK antibody detected the expression of the DDK-tagged cyclin A2 protein in transfected cells (lane 4). Interestingly, both drugs decreased the DDK-tagged cyclin A2 (lanes 5 and 6). When the samples were immunoblotted with anti-cyclin A2 antibody, the levels of exogenously expressed, DDK tagged cyclin A2, as well as the endogenous cyclin A2 protein, were decreased following aspirin or salicylic acid treatment (Fig. 2D). Reprobing the blot of Figure 2C with β-actin antibody showed equal amounts of the protein in all lanes. Thus, both aspirin and salicylic acid caused a decrease in the endogenous as well as exogenous cyclin A2 protein levels.
Aspirin and salicylic acid decrease cyclin A2 mRNA levels
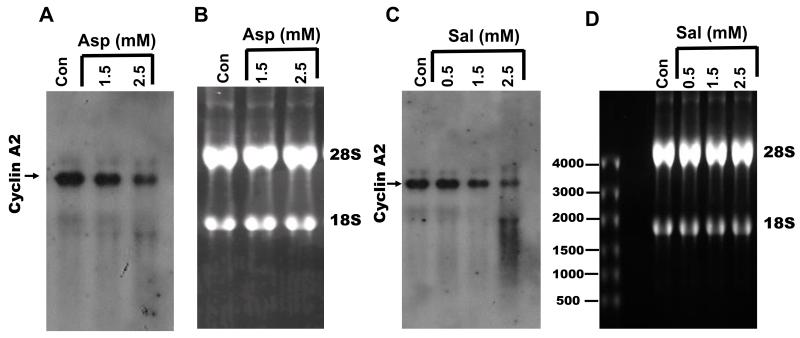
To investigate if aspirin and salicylic acid regulate cyclin A2 expression at the transcriptional/post-transcriptional level, we measured cyclin A2 mRNA levels in HT-29 cells treated with drugs following 24 h treatment. Cells were left untreated or treated with aspirin or salicylic acid at different concentrations for 24h; total RNA was prepared and analyzed for cyclin A2 mRNA in Northern blots. Figure 3A and C demonstrates that in untreated control cells (lane 1), abundant cyclin A2 mRNA was detected; both aspirin and salicylic acid respectively caused a significant decrease in cyclin A2 mRNA levels at 1.5 mM and 2.5 mM concentrations. Figure 3B and D shows the ethidium bromide stained pattern of the ribosomal 28S and 18S RNA, representing the blot in Fig. 3A and C, which shows equal RNA loading in all lanes. These results show that the observed decrease in cyclin A2 protein levels following treatment with drugs is at least in part due to decreased presence of cyclin A2 mRNA levels.
Figure 3.
Aspirin (A) and salicylic acid (C) decrease cyclin A2 mRNA levels in a concentration dependent fashion. B and D respectively represents, the ethidium bromide stained ribosomal RNA pattern of blots in A and C (see the text for details).
Aspirin and salicylic acid down-regulate CDK2 protein and mRNA levels in HT-29 cells
We then determined whether exposure of cells to aspirin and salicylic acid modulates CDK2 protein and mRNA levels. For this, cells were left untreated or treated with aspirin or salicylic acid at various concentrations, total lysates or mRNAs were prepared and analyzed by Western blots and Northern blots respectively. Figures 4A and B, demonstrates that aspirin and salicylic acid decreased the CDK2 protein levels at all concentrations tested. Figure 4C demonstrates that salicylic acid caused a reduction in CDK2 mRNA levels. Similar results were obtained in SK-MEL-28 and other cancer cell lines (data not shown).
Salicylic acid decreases CDK2 activity in HT-29 and SK-MEL-28 cells
It is clear from the results described above that both aspirin and salicylic acid decrease cellular cyclin A2 (Fig. 1) and CDK2 (Fig. 4A) levels. To investigate if this is associated with a corresponding reduction in the CDK2 activity, we carried out an in-vitro kinase assay to measure the CDK2 activity using anti-CDK2 immunoprecipitates isolated from cells treated with salicylic acid. Samples representing Fig. 4A (control, 0.5 and 1.5 mM) were immunoprecipitated with anti-CDK2 antibodies, and the immunocomplexes subjected to an in-vitro kinase assays using radiolabeled γ-32P ATP and H1-histones as substrates. Figure 4E and F demonstrates that CDK2 kinase activity is significantly reduced in cells treated with salicylic acid as measured by the ability of CDK2 to phosphorylate H1 histones. The amount of histones were similar in all lanes (Fig.4E and F, lower panel).
Inclusion of salicylic acid during immunoprecipitation enhances the ability of anti-CDK2 antibody to immunoprecipitate CDK2 in HT-29 naïve total cell lysates
It has been shown that cyclin A2 binds to CDK2 to form a hetero-dimer, and regulates CDK2 activity. The cell culture (in vivo) experiments described in Fig. 1 and Fig. 4A respectively demonstrate that exposure of cells to aspirin / salicylic acid down-regulates cyclin A2 and CDK2 protein levels. In order to gain insight into the mechanisms of down-regulation, we hypothesized that salicylic acid may directly bind to CDK2 causing a conformation change. Such a scenario is also supported by reports in literature which suggest that salicylic acid indeed interacts with cellular proteins [18, 44, 45]. We further hypothesized that, salicylic acid bound CDK2 may still associate with cyclin A2 to form a triad of CDK2/salicylic acid/cyclin A2 complex, the formation of this un-natural complex may lead to degradation of these proteins by the 26S proteasomes. To address this, we prepared lysates from HT-29 cells that were not treated with aspirin or salicylic acid (naïve cell lysates). We pre-incubated these naïve cell lysates with different concentration of salicylic acid and then tested the ability of anti-CDK2 antibodies to bind and immunoprecipitate CDK2 protein. We reasoned that salicylic acid-induced changes in CDK2 conformation may affect the ability of anti-CDK2 antibody to bind (due to changes in the accessibility of the epitope) and immunoprecipitate CDK2. Therefore, measuring the levels of CDK2 and cyclin A2 (cyclinA2 naturally associates with CDK2) in the anti-CDK2 antibody immunoprecipitates would suggest how salicylic acid affects CDK2 protein recognition by anti-CDK2 antibody. This approach is described in supplemental Fig. 5 (flow chart).
The association of cyclin A2 with CDK2 was determined by first immunoprecipitating the samples with the anti-CDK2 antibody (mouse monoclonal), followed by reprobing the blots with anti-cyclin A2 antibody (rabbit monoclonal). This approach was used to avoid detection of immunoglobulin heavy chain (Ig-H) in anti-cyclin A2 immunoblots of the anti-CDK2 immunoprecipitates, as Ig-H and cyclin A2 have a similar molecular weight of 54-56 kDa. The untreated control cell lysate was divided into 4 aliquots, each containing 500 μg of protein in a volume of 1 ml immunoprecipitation buffer. One aliquot was left untreated, and to the other three aliquots, salicylic acid was added at different concentrations (0.5, 1.5 and 2.5 mM) for 1 hour at RT. Pre-incubation of lysates with salicylic acid was performed to allow for the potential binding (if any) of salicylic acid to CDK2. The CDK2 protein was immunoprecipitated by adding monoclonal anti-CDK2 antibody, immunocomplexes were immunoblotted with rabbit anti-cyclin A2 antibody (see supplemental Fig. 5). Consistent with the literature, anti-CDK2 antibody immunoprecipitates from untreated control lysates (no incubation with salicylic acid), contained cyclin A2 protein, suggesting that it naturally associates with CDK2 (Figure 5A, lane 1). We observed that, with increasing salicylic acid concentration (pre-incubated samples), greater amount of cyclin A2 was detected in the anti-CDK2 immunoprecipitates (Fig. 5A lanes 2-4). Reprobing the blot in Fig. 5A with anti-CDK2 antibody showed that, in samples pre-incubated with salicylic acid, greater amount of CDK2 protein (33 kDa) was also immunoprecipitated by anti-CDK2 antibodies (Fig. 5B). There was no significant change in the pH of the immunoprecipitation buffer before and after the addition of salicylic acid and therefore, increased CDK2 immunoprecipitation does not appear to be due to changes in the buffer pH. It was also not due to a non-specific adsorption to protein G agarose (data not shown). The Ig heavy chain (Ig-H) and light chain (Ig-L) levels remained the same in Fig. 5B, confirming equal amount of anti-CDK2 antibody addition to the immunoprecipitation reactions. These results provided the first clues on the ability of salicylic acid to bind to CDK2, and possibly alter its conformation.
Figure 5.
The inclusion of salicylic acid increases the ability of anti-CDK2 antibody to immunoprecipitate CDK2 from naïve cell lysates and recombinant CDK2 protein. A, anti-cyclin A2 antibody (cat. Number ab32386; Abcam) immunoblots of anti-CDK2 immunoprecipitates (cat. Number-05-596, EMD Millipore), showing the presence of increased levels of cyclin A2 with increased concentration of salicylic acid. In this blot, the area that has cyclin A2 bands are only shown. B, anti-CDK2 antibody immunoblots of the anti-CDK2 immunoprecipitates, shows the presence of increased levels of CDK2 with increased concentrations of salicylic acid. C, shows the results of the in-vitro kinase assay performed on anti-CDK2 immunoprecipitates in an experiment similar to figure 5A. For the experiment in 5C, salicylic acid was pre-incubated with lysate before immunoprecipitation, but not included in the kinase assay. D, shows the results of in-vitro kinase assay performed on anti-CDK2 immunoprecipitates. For the experiments in Fig. 5D, lysates were not pre-incubated with salicylic acid before immunoprecipitation, but was included during the kinase assay. E, anti-CDK2 immunoblot of anti-CDK2 immunoprecipitate of recombinant CDK2, immunoprecipitation was carried out in the presence of increasing concentration of salicylic acid. Ig-H, immunoglobulin heavy chain, Ig-L immunoglobulin light chain.
Intrigued with this observation, we repeated the immunoprecipitations described in Fig. 5A and B several times, and in lysates isolated from multiple cell lines (data not shown) to ensure reproducibility. We next determined whether the CDK2 present in the immunoprecipitated samples of Fig. 5A is catalytically active. For this, the experiment was performed similar to the one described in Fig.5A, wherein 3 different concentrations of salicylic acid was added to the naïve cell lysates for 1 h before immunoprecipitation with the anti-CDK2 antibody. Following immunoprecipitation, the immunocomplexes were subjected to an in-vitro kinase assay using radiolabeled 32P-γ-ATP and H1 histone as a substrate, as described for Fig. 4E. Figure 5C shows a correlation between the presence of increasing concentration of salicylic acid during immunoprecipitation reactions and increased phosphorylation of H1 histones in the in-vitro kinase assay. H1 Histone phosphorylation progressively increased when salicylic acid was included in the immunoprecipitation reactions at 0.5, 1.5 and 2.5 mM (lanes 2, 3 and 4). The lower panel in Fig. 5C shows that all lanes contained similar amounts of H1 histones. These results suggest that the increased amount of H1 phosphorylation observed in kinase assay in Fig. 5C probably reflects the greater amount of CDK2/cyclin A2 protein present in the anti-CDK2 immunoprecipitates, and that CDK2 in the immunoprecipitate is catalytically active.
Inclusion of salicylic acid during in-vitro kinase assay does not affect the CDK2 activity
The experiments described in Fig.5C did not contain any salicylic acid added during the kinase assay, and therefore, it was of interest to determine if inclusion of salicylic acid during kinase assay reaction would affect the H1 histone phosphorylation. For this, naive cell lysates were immunoprecipitated with anti-CDK2 antibody (without pre-incubation with salicylic acid), immunoprecipitates were subjected to an in-vitro kinase assays in the absence or presence of different concentrations of salicylic acid (0.5, 1.5 and 2.5 mM). Figure 5D demonstrates that inclusion of salicylic acid during the kinase assay had no effect on the ability of CDK2 to phosphorylate H1 histones at all concentrations tested.
Salicylic acid increases the ability of anti-CDK2 antibodies to bind to the purified recombinant CDK2 protein
In order to determine if salicylic acid can increase the ability of the anti-CDK2 antibody to recognize/bind directly to CDK2, the experiments performed in Figure 5A was repeated except that, commercially obtained purified CDK2 was used instead of total cell lysates. Three hundred ng of the recombinant CDK2 protein was mixed in 1 ml of immunoprecipitation buffer and incubated in the absence or presence of salicylic acid at different concentrations (0.5, 1.5 and 2.5mM) for 1h at RT. Following this, the anti-CDK2 antibody was added overnight, antigen-antibody complexes collected, and immunoblotted with the anti-CDK2 antibody. As shown in Fig. 5E, salicylic acid dose dependently increased the ability of anti-CDK2 antibody to bind and immunoprecipitate recombinant CDK2. The amount of the Ig-H chain and Ig-L chains were equal in all lanes. The increased CDK2 immunoprecipitation observed was not due to a change in the pH of the immunoprecipitation reaction as addition of 0.5 mM salicylic acid, for example, to 1 ml of the immunoprecipitation buffer caused only a marginal decrease in the pH (7.4 Vs 7.18). The isoelectric pH of the unmodified CDK2 is 8.8, and therefore, the increased immunoprecipitation of CDK2 observed in Fig. 5E in the presence of salicylic acid is not due to non-specific protein precipitation related to the isoelectric point.
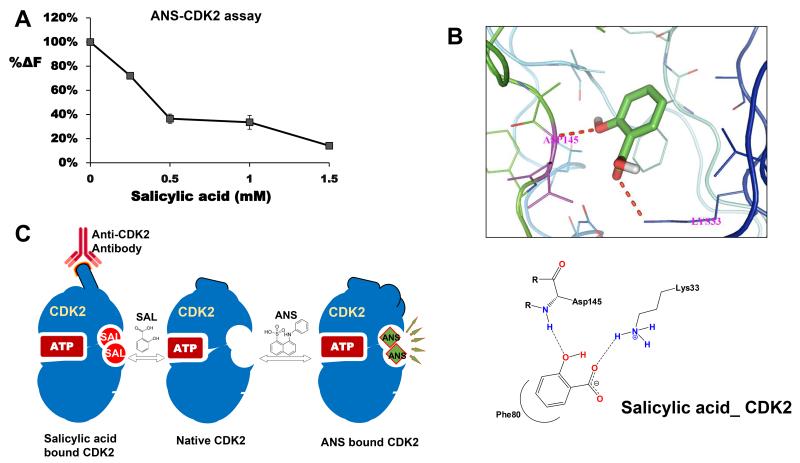
Pre-incubation of salicylic acid with CDK2 decreases fluorescence due to ANS
8-anilino-1-naphthalene sulfonate (ANS) is an extrinsic fluorophore demonstrated to interact with CDK2 at an allosteric site, leading to a change in the conformation and also increase in fluorescence [40, 46]. Based on the results obtained in the immunoprecipitation experiments (Fig. 5B and E), we hypothesized that salicylic acid may physically interact with CDK2, causing a conformational change, this would affect the binding of ANS to CDK2 leading to decreased fluorescence. To address this, ANS (50 μM) was added to recombinant CDK2 (1.6 μM), or CDK2 (1.6 μM) which was pre-incubated with salicylic acid at different concentrations, and the fluorescence was measured. Figure 6A demonstrates that pre-incubation of CDK2 with salicylic acid dose-dependently quenched the fluorescence due to ANS. This suggests that salicylic acid is likely to bind to CDK2 protein, supporting the results obtained in immunoprecipitation reactions (Figs. 5A, B and E).
Figure 6.
A, effect of pre-incubation of salicylic acid with CDK2 on fluorescence due to ANS. CDK2 (1.6 μM) was incubated with ANS (50 μM) alone or with salicylic acid at different concentrations, fluorescence measured as described in the text. Salicylic acid mediated decrease in fluorescence was compared with fluorescence due to ANS/CDK2. The decrease in fluorescence was expressed as a percentage of control; B, is the molecular docking studies showing interactions of salicylic acid with CDK2; C, a model showing potential salicylic acid binding to CDK2. We predict that salicylic acid binds to an allosteric site on CDK2, similar to a site described for ANS binding to CDK2. Binding of salicylic acid to CDK2 changes the conformation; increases the ability of anti-CDK2 antibody to immunoprecipitate CDK2 due to a better exposure of the epitope. Binding of salicylic acid to CDK2 would also quench the fluorescence due to ANS. We predict that potential allosteric inhibitors could be developed by screening new salicylic acid derivatives with allosteric binding potential and inhibition of CDK2 activity.
Molecular docking studies show potential interactions of salicylic acid with CDK2 and cyclin A2
Molecular docking is used to predict binding modes and free energy calculations between the ligand and the receptor [47]. We used AutoDockVina to understand the interactions between aspirin/salicylic acid with CDK2/cyclin A2. The binding free energy and hydrogen bond lengths were determined to check the ability of aspirin and salicylic acid to dock separately with CDK2, cyclin A2 or with CDK2/cyclin A2 complex. The results of the docking studies are shown in Table-1 and supplemental Figs 6A-E. The free binding energy values for the interactions between aspirin or salicylic acid with CDK2 were similar (−5.8 Kcal/mol). The energy value was much greater when salicylic acid interacted with cyclin A2 monomer (−6.8 Kcal/mol), or with cyclin A2/CDK2 complex (−6.1 Kcal/mol), as compared to aspirin’s interactions with cyclin A2 monomer (−6.2 Kcal/mol), or with the complex (−5.2 Kcal/mol). Since negative energy values indicate a more favorable binding of ligands with receptor molecules, our data suggests that salicylic acid has a better binding affinity to cyclin A2 than aspirin. Among the potential interactions shown in Table-1 (also see supplemental Fig. 6), salicylic acid interactions with CDK2 through Asp 145 and Lys 33 is a very significant one (Fig. 6B), as it corroborates the results obtained in the immunoprecipitation experiments (Fig. 5A, B, E) and ANS-CDK2 fluorescence assay (Fig. 6A), which independently suggest that salicylic acid binds to CDK2.
Table 1. Molecular docking studies on aspirin and salicylic acid with CDK2, cyclin A2 and with the CDK2/cyclinA2 complex.
Free energy binding values and hydrogen bond lengths for the interaction of salicylic acid and aspirin with CDK2, cyclin A2 and CDK2/cyclin-A2 complex (see text for details).
| Protein | Ligand | Binding Affinity Kcal/mol. |
Amino acids | Bond length (Å) |
Note |
|---|---|---|---|---|---|
| CDK2 | Aspirin | −5.8 | LYS33 | 3.2 | Interacts with −COOH |
| Salicylic acid | −5.8 | ASP145, LYS33 | 2.4, 2.6 | Interacts with −OH and −COOH |
|
| Cyclin A2 | Aspirin | −6.2 | LYS194 | 3.2 | Interacts with −COOH |
| Salicylic acid | −6.8 | ASN237, ASP240, LYS194 |
2.1, 2.1, 3.1 | Interacts with −OH and −COOH |
|
|
CDK2/Cyclin A2
Complex |
Aspirin | −5.2 | LYS194 (B chain) | 3.2 | Interacts with −COOH to cyclin A2 only |
| Salicylic acid | −6.1 | ASN237, ASP240, LYS194 (B chain) |
2.0, 2.4, 2.6 | Interacts with −OH and −COOH to cyclin A2 only |
Discussion
Aspirin has attracted considerable attention as a potential drug in the chemoprevention of epithelial cancers. However, there is an extensive debate regarding the molecular pathways by which it exerts its anti-cancer effects. Aspirin contains acetyl and salicylate groups both of which have their own targets, and are believed to contribute to its chemopreventive actions. Studies from our laboratory [12-14, 17], and others [15, 16] showed that, besides COX, it can acetylate numerous other proteins. While the identification of the aspirin-mediated acetylation targets has recently gained momentum [15, 16] after our initial reports [12] [14], identification of direct binding targets for salicylic acid has been under-explored [13]. Till date, salicylic acid has been shown to bind directly and interact with three cellular proteins in human cells: IκB kinase (IKK) β, a component of the NF-κB complex [18], AMP-activated protein kinase [44] and High Mobility Group Box1 proteins [45]. We hypothesized that, salicylic acid being a small molecule with hydroxyl (−OH) and carboxyl (−COOH) functional groups, potentially could directly bind/interact with additional cellular proteins and affect their functions.
In the present study, we report several novel observations including a mechanism by which aspirin and salicylic acid may exert their anti-cancer effects in epithelial cell types. We report the identification of cyclin A2/CDK2 as novel targets of aspirin and salicylic acid in multiple cancer cell lines. We demonstrated that both drugs decrease cyclin A2 and CDK2 protein as well as their mRNA, in a concentration-dependent fashion. The down-regulatory effect of both drugs on cyclin A2 protein was sensitive to pretreatment with lactacystin, suggesting that 26S proteasomal enzymes are involved. It is to be noted that cyclin A2 protein naturally undergoes degradation mediated by 26S proteasomal pathway [28] and our observation, therefore, is consistent with the known pathway of cyclin A2 degradation. The decrease in cyclin A2/CDK2 levels in aspirin/salicylic acid treated cells was associated with a corresponding decrease in CDK2 activity, which suggest that the cellular CDK2 activity is likely to be reduced upon drug exposure.
Our findings show that aspirin and salicylic acid regulate cyclin A2 expression at two levels, transcriptional/post-transcriptional, and post-translational levels. At the post-translational level, a lactacystin sensitive cysteine protease activated in response to aspirin or salicylic acid within the cells may cause the direct degradation of cyclin A2 protein. Alternatively, the observed decrease in the levels of cyclin A2 mRNAs in aspirin and salicylic treated cells may be also due to the degradation of a transcription factor(s) (TFs) (mediated by a lactacystin-sensitive protease) involved in cyclin A2 gene transcription. In this context, it is important to note that several TFs, such as c-Myc [48], CREB (cyclic AMP response element binding protein) and CREM (cyclic AMP response element modulators) [49] have been implicated in the transcription of cyclin A2 gene; and which of these TFs are affected by these two drugs requires additional study. In fact, in a recent study we reported the ability of aspirin and salicylic acid to down-regulate c-Myc protein and mRNA in cancer cells [50]. Therefore, it is likely that the decreased levels of cyclin A2 mRNA or CDK2 mRNAs observed in aspirin and salicylic acid treated cells is not a non-specific effect due to a general cell cycle arrest, but most likely due to the down-regulations of TFs.
Results obtained from three independent experiments strongly suggest that salicylic acid interacts with CDK2 possibly at an allosteric site leading to a change in its conformation. First clues for the binding of salicylic acid to CDK2 came from immunoprecipitation studies. We observed the increased ability of anti-CDK2 antibody to immunoprecipitate CDK2 protein in naïve cell lysates when they were pre-incubated with salicylic acid (Figs. 5A and B). The inclusion of salicylic acid also dose-dependently enhanced the ability of anti-CDK2 antibodies to immunoprecipitate purified recombinant CDK2 (Fig. 5E). Secondly, our molecular docking studies suggest that salicylic acid potentially interacts with Asp145 and Lys 33 in CDK2 (Table-1 and Fig. 6B), both of which have been previously identified as being present in its active site [51, 52]. Thirdly, pre-incubation of CDK2 with salicylic acid, dose-dependently quenched the fluorescence due to ANS (Fig. 6A). Interaction of ANS with CDK2 is well characterized and occurs at an allosteric pocket near the ATP binding site, leading to a large conformational change in CDK2 [46], and it has been shown to interact with Asp145 and Lys33 [40, 46]. It is interesting to note that, both ANS and salicylic acid share common amino acid residues Asp 145 and Lys 33, for interactions with CDK2, therefore, it is not surprising that pre-incubation of salicylic acid with CDK2, quenched the fluorescence due to ANS. It has been shown that CDK2 displays significant conformational flexibility and accommodates the binding of highly diverse small molecule ligands [40]. We predict that, similar to ANS, binding of salicylic acid to CDK2 occurs at an allosteric site causing a conformational change, and this would explain greater recognition and immunoprecipitation of CDK2 protein by anti-CDK2 antibody (Figs 5B, 5E; also see Fig. 6C). Further confirmation of Asp145 and Lys33 as salicylic acid binding sites on CDK2 requires mutational and protein crystallization studies.
Endogenous cyclin A2 within cells can exist in the monomeric or dimeric state, bound to CDK2. Our molecular docking studies (Table-1) suggest that in addition to CDK2, aspirin and salicylic acid can potentially interact with cyclin A2 monomeric forms at specific amino acid residues (Supplemental Fig 6B and 6C). However, if cyclin A2/CDK2 dimer has already been formed, it appears that aspirin and salicylic acid can interact only with cyclin A2, but not with CDK2 (Supplemental Fig 6D and 6E). The standard hydrogen bond length between donor and the acceptor atoms is in the order of 2.6 to 3.5 A°, with optimum at 2.8 A° [53]. Based on the hydrogen bond length and the associated negative free energy, salicylic acid showed stronger interaction with binding pockets in CDK2 monomer, cyclin A2 monomer or with cyclin A2 in the cyclin A2/CDK2 hetero-dimer than aspirin. Additional studies involving biochemical and protein crystallization are required to confirm the direct binding of aspirin and salicylic acid with cyclin A2.
The in vitro experiment described in Fig. 5B shows that pre-incubation of naïve cell lysates with salicylic acid increased the ability of anti-CDK2 antibody to bind and immunoprecipitate the CDK2 protein. These anti-CDK2 immunoprecipitates from salicylic acid pre-incubated lysates also contained greater levels of cyclin A2, as cyclin A2 is a natural binding partner with CDK2 (co-precipitation) (Fig. 5A), and showed increased CDK2 activity as measured by H1 histone phosphorylation assay (Fig. 5C). In these kinase experiments, salicylic acid was not included during the kinase reaction. Therefore, the increased CDK2 activity observed in anti-CDK2 immunoprecipitates from salicylic acid pre-incubated samples (Fig. 5C), reflects the greater amounts of cyclin A2/CDK2 protein levels; and thus, not due a stabilization effect of salicylic acid. Interestingly, the inclusion of increasing amounts of salicylic acid during the in-vitro kinase assay performed on the anti-CDK2 immunoprecipitates from naïve cell lysates, had no effect on H1 histone phosphorylation (Fig. 5D). Taken together, these results suggest that binding of salicylic acid to CDK2 most likely changes the conformation leading to increased CDK2 immunoprecipitation; but the occupancy does not affect the CDK2 kinase activity. Efficient phosphorylation of H1 histones in the presence of salicylic acid (Fig. 5D), also suggest that the ATP binding site in CDK2 is unaffected due to interactions with salicylic acid.
Although our in vitro experiments (Figs. 5A/B, 6A) and the molecular docking studies (Fig. 6B; table 1) suggest that salicylic acid binds to CDK2, it is not clear how within the cellular milieu, binding of salicylic acid to CDK2, causes subsequent degradation of cyclin A2 and CDK2 proteins. It is certain that proteasomal pathway is involved (Fig. 2). Inside the cellular milieu, the triad of CDK2/salicylic acid/cyclin A2 complex may be recognized by proteasomal enzymes as an un-natural complex, leading to degradation of both cyclin A2 and CDK2. Alternatively, the triad of CDK2/salicylic-acid/cyclinA2 complex, although still catalytically active, may have an altered substrate specificity. For example, salicylic acid bound CDK2 with an altered conformation and substrate specificity may phosphorylate and activate unique targets / proteasomal enzymes specific for the degradation of cyclin A2/CDK2. This view is supported by reports in literature that conformational changes in flexible parts of the protein have been indeed shown to alter substrate specificity [54]. Investigations into the pathways leading to degradation of cyclin A2/CDK2 proteins following salicylic acid occupancy represent an important extension of this study.
Aspirin’s ability to inhibit cell proliferation or induce cell cycle arrest (G0/G1) has been documented in the literature in many cancer cell lines [41, 42, 55, 56]. In our study, aspirin and salicylic acid down-regulated cyclin A2/CDK2 in 11 different cancer cells representing the cancers of various epithelial tissues (colon, lung, prostate, ovary and skin), which suggest that this is a universal phenomenon and applicable to most cancer cells. Extension of these observations performed in HT-29 cells show that exposure of cells to aspirin and salicylic acid caused down-regulation of cyclins B1 and D3; CDKs 1, 4 and 6; and up-regulation of CDK inhibitors p21 and p27. Down-regulation of many of the important cyclins and CDKs, and up-regulation of CDK inhibitors, would tip the balance strongly towards cell cycle arrest, and will explain the documented ability of aspirin and salicylic acid to cause cell cycle arrest in literature.
In many cancers, CDK2 activity is deregulated [32], and cyclin A2 is over-expressed [29-31, 33]. Therefore, attention is increasingly being focused on cell cycle, as a potential target for therapeutic intervention [35, 57, 58]. In this context, our finding that aspirin and salicylic acid down-regulate cyclin A2 /CDK2 protein and mRNAs in multiple cancer cell lines should initiate new thinking and research on these age-old drugs in cancer treatment. The answer for an effective drug for chemoprevention may lie in revisiting salicylic acid, an ancient drug known for over two millennia in plants for its therapeutic properties. The observation that salicylic acid binds to CDK2 at an allosteric site can be exploited to develop novel anti-cancer drugs, for example, derivatives of salicylic acid can be screened for inhibition of CDK2 activity, or disruption of the cyclin A2/cdk2 complex. Salicylic acid is abundantly present in many plants where it has been shown to protect the cells from infection through induction of cell death [59]. It will be also interesting to determine if salicylic acid-induced cell death in infected leaves involves down-regulation of cyclin A2/CDK2, or other related proteins.
Supplementary Material
Acknowledgements
Support from the Translational Cancer Research Seed Grant, funded as 2010 Research Initiative Center by the State of South Dakota, Faculty Excellence Fund from South Dakota State University (SDSU), Department of Pharmaceutical Sciences, SDSU and from NIH (5RO3CA133061-02) to GJB is gratefully acknowledged. We also gratefully acknowledge Drs. Jane Endicott and Martin Noble of Newcastle University, UK, for the suggestions on the ANS-CDK2 assay. We also thank Dr. Mohit Tyagi, Mr. Chowdhury Abdullah, Ms. Yang Yang and Mr. Mohamed Ismail SDSU, and Dr. Lloyd Alfonso, D’Youville School College of Pharmacy, Buffalo, NY, for helpful discussions.
Footnotes
Disclosure of potential conflicts of interest: Authors have no conflicts of interest to disclose.
Literature Cited
- 1.Kaiser J. Will an Aspirin a Day Keep Cancer Away? Science. 2012;337(6101):1471–1473. doi: 10.1126/science.337.6101.1471. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 3.Chan AT, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res (Phila) 2012;5(2):164–78. doi: 10.1158/1940-6207.CAPR-11-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothwell PM, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. The Lancet. 2012;379(9826):1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 5.Sahasrabuddhe VV, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808–14. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamba CA, et al. Aspirin is associated with lower melanoma risk among postmenopausal Caucasian women: the Women’s Health Initiative. Cancer. 2013;119(8):1562–9. doi: 10.1002/cncr.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veitonmaki T, et al. Use of aspirin, but not other non-steroidal anti-inflammatory drugs is associated with decreased prostate cancer risk at the population level. Eur J Cancer. 2013;49(4):938–45. doi: 10.1016/j.ejca.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Shpitz B, et al. Suppressive effect of aspirin on aberrant crypt foci in patients with colorectal cancer. Gut. 2003;52(11):1598–601. doi: 10.1136/gut.52.11.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–58. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser D, et al. Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study. British journal of cancer. 2014;111(3):623–627. doi: 10.1038/bjc.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dovizio M, et al. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res. 2013;191:39–65. doi: 10.1007/978-3-642-30331-9_3. [DOI] [PubMed] [Google Scholar]
- 12.Marimuthu S, et al. Aspirin acetylates multiple cellular proteins in HCT-116 colon cancer cells: Identification of novel targets. Int J Oncol. 2011;39(5):1273–83. doi: 10.3892/ijo.2011.1113. [DOI] [PubMed] [Google Scholar]
- 13.Alfonso L, et al. Molecular targets of aspirin and cancer prevention. Br J Cancer. 2014 doi: 10.1038/bjc.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfonso LF, et al. Aspirin inhibits camptothecin-induced p21CIP1 levels and potentiates apoptosis in human breast cancer cells. International journal of oncology. 2009;34(3):597–608. doi: 10.3892/ijo_00000185. [DOI] [PubMed] [Google Scholar]
- 15.Bateman LA, et al. An Alkyne–Aspirin Chemical Reporter for the Detection of Aspirin-Dependent Protein Modification in Living Cells. Journal of the American Chemical Society. 2013;135(39):14568–14573. doi: 10.1021/ja408322b. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, et al. Mapping sites of aspirin-induced acetylations in live cells by quantitative acid-cleavable activity-based protein profiling (QA-ABPP) Scientific reports. 2015;5 doi: 10.1038/srep07896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai G, et al. Aspirin acetylates wild type and mutant p53 in colon cancer cells: identification of aspirin acetylated sites on recombinant p53. Tumor Biology. 2015:1–10. doi: 10.1007/s13277-015-4438-3. [DOI] [PubMed] [Google Scholar]
- 18.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265(5174):956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 19.Bos CL, et al. Effect of aspirin on the Wnt/β-catenin pathway is mediated via protein phosphatase 2A. Oncogene. 2006;25(49):6447–6456. doi: 10.1038/sj.onc.1209658. [DOI] [PubMed] [Google Scholar]
- 20.Pathi S, et al. Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors. PLoS One. 2012;7(10):e48208. doi: 10.1371/journal.pone.0048208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Din FV, et al. Aspirin Inhibits mTOR Signaling, Activates AMP-Activated Protein Kinase, and Induces Autophagy in Colorectal Cancer Cells. Gastroenterology. 2012;142(7):1504–1515 e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law BK, et al. Salicylate-induced growth arrest is associated with inhibition of p70s6k and down-regulation of c-myc, cyclin D1, cyclin A, and proliferating cell nuclear antigen. J Biol Chem. 2000;275(49):38261–7. doi: 10.1074/jbc.M005545200. [DOI] [PubMed] [Google Scholar]
- 23.Borthwick GM, et al. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J. 2006;20(12):2009–16. doi: 10.1096/fj.06-5987com. [DOI] [PubMed] [Google Scholar]
- 24.Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24(17):2909–15. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- 25.Pagano M, et al. Cyclin A is required at two points in the human cell cycle. The EMBO journal. 1992;11(3):961. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendris N, et al. Cyclin A2 mutagenesis analysis: a new insight into CDK activation and cellular localization requirements. PLoS One. 2011;6(7):e22879. doi: 10.1371/journal.pone.0022879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong D, et al. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr Biol. 2007;17(1):85–91. doi: 10.1016/j.cub.2006.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7(9):644–56. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 29.Bukholm IR, Bukholm G, Nesland JM. Over-expression of cyclin a is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. International journal of cancer. 2001;93(2):283–287. doi: 10.1002/ijc.1311. [DOI] [PubMed] [Google Scholar]
- 30.Volm M, Koomà R. Cyclin A is associated with an unfavourable outcome in patients with non-small-cell lung carcinomas. British journal of cancer. 1997;75(12):1774. doi: 10.1038/bjc.1997.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi R, et al. Enhanced expression of cyclin E and cyclin A in human hepatocellular carcinomas. Anticancer research. 2000;21(1B):657–662. [PubMed] [Google Scholar]
- 32.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17(1):60–5. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Kanai M, et al. Immunohistochemical detection of sex steroid receptors, cyclins, and cyclin-dependent kinases in the normal and neoplastic squamous epithelia of the uterine cervix. Cancer. 1998;82(9):1709–1719. doi: 10.1002/(sici)1097-0142(19980501)82:9<1709::aid-cncr18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Anscombe E, et al. Identification and Characterization of an Irreversible Inhibitor of CDK2. Chemistry & biology. 2015;22(9):1159–1164. doi: 10.1016/j.chembiol.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zalzali H, et al. CDK2 Transcriptional Repression Is an Essential Effector in p53-Dependent Cellular Senescence—Implications for Therapeutic Intervention. Molecular Cancer Research. 2015;13(1):29–40. doi: 10.1158/1541-7786.MCR-14-0163. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, et al. miR-200c Targets CDK2 and Suppresses Tumorigenesis in Renal Cell Carcinoma. Molecular Cancer Research. 2015 doi: 10.1158/1541-7786.MCR-15-0128. molcanres. 0128.2015. [DOI] [PubMed] [Google Scholar]
- 37.Choi KS, et al. Cdc2 and Cdk2 kinase activated by transforming growth factor-β1 trigger apoptosis through the phosphorylation of retinoblastoma protein in FaO hepatoma cells. Journal of Biological Chemistry. 1999;274(45):31775–31783. doi: 10.1074/jbc.274.45.31775. [DOI] [PubMed] [Google Scholar]
- 38.Komander D, et al. Interactions of LY333531 and other bisindolyl maleimide inhibitors with PDK1. Structure. 2004;12(2):215–226. doi: 10.1016/j.str.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Van Der Spoel D, et al. GROMACS: fast, flexible, and free. Journal of computational chemistry. 2005;26(16):1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 40.Martin MP, et al. A Novel Approach to the Discovery of Small-Molecule Ligands of CDK2. Chembiochem. 2012;13(14):2128–2136. doi: 10.1002/cbic.201200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel A, et al. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clinical Cancer Research. 2003;9(1):383–390. [PubMed] [Google Scholar]
- 42.Luciani MG, Campregher C, Gasche C. Aspirin blocks proliferation in colon cells by inducing a G1 arrest and apoptosis through activation of the checkpoint kinase ATM. Carcinogenesis. 2007;28(10):2207–2217. doi: 10.1093/carcin/bgm101. [DOI] [PubMed] [Google Scholar]
- 43.Fenteany G, Schreiber SL. Lactacystin, proteasome function, and cell fate. Journal of Biological Chemistry. 1998;273(15):8545–8548. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]
- 44.Hawley SA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi HW, et al. Aspirin’s Active Metabolite Salicylic Acid Targets High Mobility Group Box 1 to Modulate Inflammatory Responses. Molecular Medicine. 2015;21(1):526. doi: 10.2119/molmed.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betzi S, et al. Discovery of a potential allosteric ligand binding site in CDK2. ACS chemical biology. 2011;6(5):492–501. doi: 10.1021/cb100410m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bikadi Z, Hazai E. Journal of cheminformatics. 2009;1:15. doi: 10.1186/1758-2946-1-15. Journal of Cheminformatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeller KI, et al. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003;4(10):R69. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desdouets C, et al. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Molecular and Cellular Biology. 1995;15(6):3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ai G, et al. Aspirin and salicylic acid decrease c-Myc expression in cancer cells: a potential role in chemoprevention. Tumor Biology. 2015:1–12. doi: 10.1007/s13277-015-3959-0. [DOI] [PubMed] [Google Scholar]
- 51.Welburn JP, et al. How tyrosine 15 phosphorylation inhibits the activity of cyclin-dependent kinase 2-cyclin A. Journal of Biological Chemistry. 2007;282(5):3173–3181. doi: 10.1074/jbc.M609151200. [DOI] [PubMed] [Google Scholar]
- 52.Lees E. Cyclin dependent kinase regulation. Current opinion in cell biology. 1995;7(6):773–780. doi: 10.1016/0955-0674(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 53.Overington J, et al. Tertiary structural constraints on protein evolutionary diversity: templates, key residues and structure prediction. Proceedings of the Royal Society of London B: Biological Sciences. 1990;241(1301):132–145. doi: 10.1098/rspb.1990.0077. [DOI] [PubMed] [Google Scholar]
- 54.Yu X, Cojocaru V, Wade RC. Conformational diversity and ligand tunnels of mammalian cytochrome P450s. Biotechnology and applied biochemistry. 2013;60(1):134–145. doi: 10.1002/bab.1074. [DOI] [PubMed] [Google Scholar]
- 55.Qiao L, et al. Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol. 1998;55(1):53–64. doi: 10.1016/s0006-2952(97)00400-0. [DOI] [PubMed] [Google Scholar]
- 56.Shiff SJ, et al. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Experimental cell research. 1996;222(1):179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- 57.Davies TG, et al. Structure-based design of a potent purine-based cyclin-dependent kinase inhibitor. Nature Structural & Molecular Biology. 2002;9(10):745–749. doi: 10.1038/nsb842. [DOI] [PubMed] [Google Scholar]
- 58.Deng Y, et al. Modulating the interaction between CDK2 and cyclin A with a quinoline-based inhibitor. Bioorg Med Chem Lett. 2014;24(1):199–203. doi: 10.1016/j.bmcl.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez ME. Programmed Cell Death in Higher Plants. Springer; 2000. Salicylic acid in the machinery of hypersensitive cell death and disease resistance; pp. 185–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.