Abstract
Introduction
Inflammatory breast cancer (IBC) is an aggressive and rare cancer with a poor prognosis and a need for novel targeted therapeutic strategies. Preclinical IBC data demonstrates strong activation of the PI3K/mTOR and JAK/STAT pathways, expression of inflammatory cytokines and tumor associated macrophages (TAMs).
Methods
Archival tumor tissue from three disease types (IBC treated with neoadjuvant chemotherapy (NAC) (n=45); invasive ductal carcinoma (IDC) treated with NAC (n=24; ‘treated IDC’); and untreated IDC (n=27; ‘untreated IDC’)) was analyzed for the expression of biomarkers pS6 (mTOR), pJAK2, pSTAT3, IL6, CD68 (monocytes, macrophages) and CD163 (TAMs). Surrounding non-tumor tissue was also analyzed.
Results
Biomarker levels and surrogate activity by site-specific phosphorylation were demonstrated in the tumor tissue of all three disease types but were highest in IBC and treated IDC and lowest in untreated IDC for pS6, pJAK2, pSTAT3 and IL6. Of 37 IBC patients with complete biomarker data available, 100% were pS6 positive and 95% were pJAK2 positive. In non-tumor tissue, biomarker levels were observed in all groups but were generally highest in untreated IDC and lowest in IBC, except for JAK2.
Conclusions
IBC and treated IDC display similar levels of mTOR and JAK2 biomarker activation, suggesting a potential mechanism of resistance after NAC. Biomarker levels in surrounding non-tumor tissue suggest that the stroma may be activated by chemotherapy and resembles the oncogenic tumor-promoting environment. Activation of both pS6 and pJAK2 in IBC may support dual targeting of mTOR and JAK/STAT pathways, and the need for prospective studies to investigate combinatorial targeted therapies in IBC.
Keywords: JAK2, Stat3, mTOR, inflammatory breast cancer, neoadjuvant chemotherapy
INTRODUCTION
Inflammatory breast cancer (IBC) is an uncommon and aggressive form of breast cancer accounting for approximately 1–5% of all breast cancers1, 2. The diagnosis is made clinically when patients present with sudden onset of erythema, inflammation, tenderness and warmth involving more than one-third of the breast with a duration of no more than 6 months1. The hallmark and lethality of IBC is the formation of tumor cell emboli: non-adherent cell clusters that spread rapidly within blood and dermal lymphatic vessels3. Despite multi-modality treatment with chemotherapy, surgery and radiation therapy, the prognosis for IBC is poor, with a 5-year median overall survival (OS) of 30–50%, underscoring a clear unmet need for more effective and molecularly targeted strategies1, 2.
Understandably, there has been considerable interest in investigating the underlying molecular pathways of IBC in an attempt to identify potential therapeutic targets. The human epidermal growth factor receptor (HER2/neu) was one of the first targets studied1. The benefit of anti-HER2 therapy in IBC was established by the randomized phase 3, NeOAdjuvant Herceptin (NOAH) trial4. An increase in 3 year event-free survival (EFS) was found when adding the monoclonal anti-HER2 antibody trastuzumab to neoadjuvant chemotherapy, continued for one year post-surgery, in patients with locally advanced breast cancer (LABC) that included a distinct cohort of IBC patients4. Other trials evaluating trastuzumab in HER2-positive LABC have included small cohorts of patients with IBC, and also showed benefit of anti-HER2 therapy5–7. Lapatinib, a dual HER2 and epidermal growth factor receptor (EGFR) inhibitor demonstrated efficacy in IBC, both as a single agent and in combination with a taxane1, 8, 9. As IBC demonstrates extensive angiolymphatic invasion, anti-angiogenesis targets, including bevacizumab, have been studied with mixed results10–15.
Biologic features that make IBC so aggressive include but are not limited to p53 mutations, overexpression of E-cadherin and RhoC GTPase, increased cytoplasmic MUC-1, loss of WISP3 gene, overexpression of translation initiation factor, eIF4GI, that drives Ecadherin and pro-invasive p120 catenin expression, and NF-κB expression, a regulator of cytokines interleukin-6 (IL-6) and IL-83, 16–20. These cytokines signal through the Janus kinase (JAK)/Signal transducer and activator of transcription (STAT) pathway, which is involved in cell proliferation, differentiation, and apoptosis21. IL-6 and IL-8 also play a role in the epithelial to mesenchymal transition (EMT)22, and may contribute to the aggressiveness of IBC. IBC cells are enriched in the basal/mesenchymal CD44+/CD24− cancer stem cell phenotype23, consistent with IL-6/IL-8 activity. The IL-6/JAK2/STAT3 pathway is required for the growth of basal/mesenchymal-type cells, which have tumor initiating properties and invasive features24. Despite all these findings, efforts to identify an IBC-specific molecular signature have been limited due to small sample sizes and low statistical power, the molecular heterogeneity of the disease, and technological differences related to the use of different genome-wide assay platforms25, 26.
We have focused on identifying molecular drivers associated with IBC tumor growth, invasion and metastasis. We previously identified hyperactivation of the phosphatidylinositide-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway, a key regulator of cell proliferation and survival in breast cancer, as also being important in IBC cell lines and xenografts3, 27. Studies have shown that mTOR signaling is increased in HER2-amplified IBC compared to non-IBC breast cancer28. We found that eIF4GI, a translation initiation factor that is regulated by mTOR, is overexpressed in IBC tumor biopsies, the SUM149 IBC cell line, mouse xenograft patient IBC models, and promotes greater formation and invasive activity of IBC tumor emboli3, 29.
In addition to mTOR activation, a statistically significant increase in macrophage infiltration has been demonstrated in IBC when compared to non-IBC tissues30. Tumor associated macrophages (TAM) in particular, exhibit pro-tumor like properties including increased tumor progression, enhanced angiogenesis, cell migration and invasion31. These differ from M1 macrophages which are classically activated macrophages and are involved in enhancing the production of oxygen radicals, IL-6, IL-12 and potentiating innate immune responses32. TAMs also promote the release of inflammatory cytokines, including IL-6, which activates the JAK/STAT pathway21. Phosphorylated STAT3 was shown to be activated in 85 percent of IBC patient tissues, and a phase I/II clinical trial is underway evaluating ruxolitinib, an oral JAK 1/2 inhibitor, in combination with neoadjuvant chemotherapy in triple negative IBC23, 33. However, the baseline levels of mTOR and JAK/STAT signaling pathways in both IBC and invasive ductal carcinoma (IDC) remains poorly studied. In this study we therefore examined baseline activities of these pathways, in both the tumor and the surrounding non-tumor tissues, and the effect of conventional chemotherapy, in order to determine whether they constitute potentially favorable targets for co-treatment with JAK1/2 and/or mTOR inhibitors in IDC and/or IBC.
While activation of mTOR and JAK/STAT pathways has been described as independent events in IBC, cross-talk between these signaling pathways has been shown, and they respond reciprocally to inhibition34. In triple negative breast cancer models, treatment with a dual PI3K/mTOR inhibitor, BEZ235 hyperactivates the JAK2/STAT5 pathway, AKT, and expression of IL-8. This in turn inhibits cell death and dampens the response to PI3K/mTOR inhibition. This feedback loop is abrogated by JAK2 inhibition, and dual inhibition of both PI3K/mTOR and JAK2 synergistically reduces tumor growth and metastasis and increases overall survival in animals35. The existing preclinical data suggest a need to explore a potential role for the PI3K/mTOR and JAK/STAT pathways, activation of TAMs, and the expression of inflammatory cytokines in the pathogenesis of IBC, and in response to conventional chemotherapy. We therefore investigated the involvement of these pathways in patient tissues of IBC and both treated and untreated stage II/III IDC.
METHODS
Patient cohorts and tissues
We obtained archival tumor tissue specimens with prior Institutional Review Board approval for patients ≥ 18 years of age with (1) IBC treated with neoadjuvant therapy; (2) stage II/III invasive ductal carcinoma (IDC) treated with neoadjuvant therapy; and (3) untreated stage II/III IDC breast cancer from three institutions (Table 1A).
Table 1.
| A. Sources by Disease Type | |||
|---|---|---|---|
| IBC | Treated IDC | Untreated IDC | |
| Institution | George Washington (GW) University School of Public Health and Health Services (Voluntary IBC registry) and New York University (NYU) Langone Medical Center | NYU Langone Medical Center | Yale University School of Medicine |
| Number of samples (N) | 45 (35 from GW; 10 from NYU) | 24 | 27 |
| Diagnosis Dates | 1999–2013 (1999–2009: GW; 2002–2013: NYU) | 2004–2011 | 2001–2005 |
| B. Antibodies and Scoring Methods | ||||
|---|---|---|---|---|
| Antibody | Origin/Reference | Scoring | Ordinal or Continuous Variable |
|
| Negative | Positive | |||
| pS6 | Cell Signaling/4857 | 0 | 1+, 2+, 3+ | Ordinal |
| pJAK2 | Abcam/ab32101 | 0 | 1+, 2+, 3+ | Ordinal |
| pSTAT3 | Cell Signaling/4113 | 0 | 1+, 2+ | Ordinal |
| IL-6 | R&D/MAB2061 | 0 | 1+, 2+ | Ordinal |
| CD68 | Dako/M0876 | Scored as 0–150 cells/HPF | Continuous | |
| CD163 | NCL-CD163 | Scored as 0–99 cells/HPF | Continuous | |
/HPF: per high power field 63× objective
Eligibility criteria
IBC patients were required to have a clinical presentation of IBC as evidenced by inflammation (erythema, tenderness, warmth, peau d’orange appearance) involving at least one third of the breast, prior neoadjuvant chemotherapy with an anthracycline and/or taxane-based chemotherapy regimen, and residual disease at the time of definitive surgery. Patients who had achieved a pathologic complete response (pCR) with no residual disease at time of definitive surgery and patients with de novo metastatic disease with no primary tumor were excluded because of the inability in obtaining a definitive biopsy from the primary lesion. Cases were obtained from the voluntary IBC registry at George Washington University (GWU) established in 2002 and from New York University (NYU) Langone Medical Center. Patients from both institutions met the same criteria for diagnosis of IBC. For the GWU cases, disease status was confirmed through patient medical records, patient interviews from the IBC registry and pathology records. For the NYU cases, a pathology database of all available mastectomy specimens was queried to identify cases between 2002–2013 that had a clinical description of “IBC” or pathologic findings of dermal lymphatic involvement consistent with IBC. Clinical charts were reviewed for the cases identified via the pathology database to ensure patient eligibility.
Treated stage II/III IDC patients (treated IDC) were required to have clinical stage II or III IDC breast cancer, prior neoadjuvant chemotherapy with an anthracycline and/or taxane-based chemotherapy regimen, and residual disease at the time of definitive surgery. Patients who had achieved a pCR and had no residual disease at the time of definitive surgery were excluded. We obtained these cases from the same NYU pathology database used for the IBC cases with dates of diagnosis from 2004–2011. Clinical charts were reviewed to ensure patient eligibility.
Untreated stage II/III IDC patients (untreated IDC) were required to have pathologic stage II or III IDC breast cancer, definitive surgery with lumpectomy or mastectomy, and could not have received any prior neoadjuvant chemotherapy. The specimens were obtained from Yale University School of Medicine.
Each tumor specimen included surrounding non-tumor tissue. For all cases, clinical and survival data were obtained including age, date of clinical and pathological diagnosis, estrogen receptor (ER), progesterone receptor (PR), HER2 status, treatment history including details of systemic therapy, if available (neoadjuvant and adjuvant chemotherapy), endocrine therapy, anti-HER2 directed therapy (Supplement 1), radiation therapy, date and type of surgery, date of recurrence, date of last follow up and survival status.
Immunohistochemistry and scoring
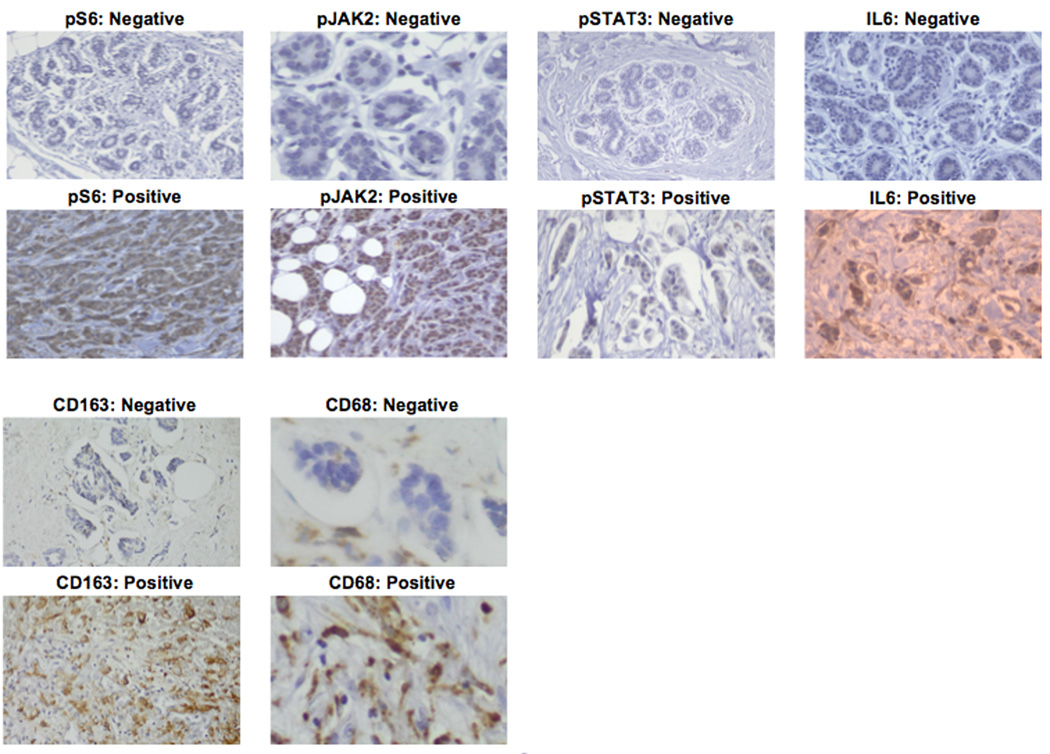
All specimens were analyzed using immunohistochemistry (IHC) and scored by three independent pathologists. Paraffin-embedded ovarian sections were warmed and serially de-paraffinized in Xylene and ethanol, introduced into an antigen unmasking solution, blocked and sections incubated overnight at 4°C with antibodies as indicated in Table 1B. Antigen retrieval requirements were first determined and then primary antibody was serially diluted to determine the optimum dilution/concentration. Briefly, sections were deparaffinized in xylene (3 changes), rehydrated through graded alcohols (3 changes 100% ethanol, 3 changes 95% ethanol) and rinsed in distilled water. Heat induced epitope retrieval was performed in 10 mM citrate buffer pH 6.0 in a 1200-Watt microwave oven at 90% power. Sections were allowed to cool for 30 min and then rinsed in distilled water. Antibody incubations and detection were carried out on a NEXes platform (Ventana Medical Systems Tucson, AZ USA) using Ventana’s buffer and detection system unless otherwise noted. Expression of activated phosphorylated mTOR was carried out using surrogate activating phosphorylation of rS6 (P-Ser240/244); Activating phosphorylation nuclear JAK2 and STAT3 (pJAK2, Tyr1007/8; pSTAT3, Tyr705), CD68 (monocytes/macrophages), CD163 (TAMs) and JAK2/STAT3-mediated cytokine expression represented by IL-6. (Figure 1). Control studies included staining in the absence of primary antibody, and titration of primary antibodies to determine minimum amount required for strongest signal to noise ratio. Specimens were scored using traditional categorical standards that reflect the range of responses and allowed the middle quantile to represent the median of the data spread, assuring a full dynamic range36. Quartiles were therefore used for scoring pS6 and pJAK2 which have a larger dynamic range, and tertiles for pSTAT3 and IL-6. Since macrophage infiltration is quantified by cell number, analysis of CD68 and CD163 used continuous scoring. Specimens were scored as 0, 1, 2, 3+ for pS6 and pJAK2, and 0, 1, 2+ for pSTAT3 and IL-6. CD68 and CD163 were scored as continuous variables (0–150 cells/per high power field (HPF, 63× objective, 10× eye piece objective) for CD68 and 0–99 cells/HPF for CD163, respectively). The cut-off for staining had to include at least 5% of the cells in a given population and took into consideration the percentage of positive cells as follows. For quartile scoring: 0 <5%; +1 ≥6% but <33%; +2 >33% but <65%; +3 >66%. For tertile staining: 0 <5%; +1 ≥6% but <50%; +2 ≥50%. Antibodies and scoring methods are listed in Table 1B.
Figure 1.
Representative immunohistochemical stains (positive and negative) for each biomarker.
Statistical methods
Patient characteristics and biomarker scores were summarized by disease groups using descriptive statistics including medians and ranges for continuous variables and frequency distributions for categorical and ordinal variables. Recurrence-free survival (RFS) and overall survival (OS) were measured from the clinical diagnosis date. RFS and OS were analyzed using the Kaplan–Meier method with point estimates and 95% confidence intervals (CIs) calculated from Kaplan–Meier curves. Numbers of recurrences and deaths were reported. Differences in biomarker levels and cytokine/protein expression were evaluated among the three disease types. For biomarkers with ordinal measurements, we used Fisher’s Exact Test; for biomarkers with continuous measurements, the Kruskal-Wallis non parametric analysis of variance test was used. To evaluate differences in biomarker expression levels of the surrounding non-tumor tissue among the three disease types, similar methods were used. For each marker, in each analysis, pairwise differences were tested when the overall difference from the analysis of variance was significant (p ≤0.05) using a Bonferroni adjusted p-value of 0.0167 for 3 pairwise comparisons.
Differences in levels in tumor versus surrounding non-tumor tissue were compared among the three disease types. We defined a change score to adjust for surrounding non-tumor tissue expression for those markers with ordinal outcomes. Three values were defined for this score: tumor tissue levels < surrounding tissue levels, tumor tissue levels = surrounding tissue levels, and tumor tissue levels > surrounding tissue levels. Fisher’s Exact Tests were used to test for differences in the distributions of this score among the three disease types. Again, pairwise differences between groups were tested using a Bonferroni adjusted p value as described above. For biomarkers with continuous measurements, we calculated the actual difference between the tumor tissue and matched surrounding non-tumor tissue; differences were evaluated using the Kruskal-Wallis non parametric analysis of variance test.
RESULTS
We evaluated mastectomy specimens from patients with IBC (N=45), lumpectomy or mastectomy specimens from patients with treated IDC (N=24) who received neoadjuvant chemotherapy (+/− adjuvant chemotherapy), and untreated IDC (N=27). Patient tissues from all groups were stained for pS6, pJAK2, pSTAT3, CD68, CD163 and IL-6. ER and HER2 status and treatment history were collected for each group as descriptive data and are shown in Supplement 1. All of the patients who received chemotherapy were treated with an anthracycline and/or taxane based regimen (with the exception of one patient whose chemotherapy regimen is unknown). The majority of patients who were ER and HER2 positive received endocrine and anti-HER2 therapy, respectively. Patient age at diagnosis, overall survival (OS) and recurrence-free survival (RFS) by disease group are shown in Table 2. Younger age at diagnosis in IBC compared to IDC, either treated or untreated, is consistent with the bimodal pregnancy-associated and in part younger age at presentation for IBC than that seen for IDC. The high recurrence rate and poorer overall survival for IBC than treated or untreated IDC is also consistent and typifies IBC.
Table 2.
Patient Age, RFS, and OS by Disease Type
| Clinical Variables | IBC | Treated IDC | Untreated IDC |
|---|---|---|---|
| Number of patients (N) | 45 | 24 | 27 |
| Median age in years at diagnosis (range) | 47.3 (31.8–73.8) | 51.5 (33.1–72.4) | 58.6 (39.0–94.1) |
| Median RFS (months) | 31.2 months (95% CI 22.0–68.9 months) | 78.2 months (95% CI 42.0 months-not yet reached) | 87.0 months (95% CI 34.5 months-not yet reached) |
| Median OS (months) | 85.5 months (95% CI 55.1 months-not yet reached) (median f/u time 74.7 months) | Not yet reached (median f/u time 46.4 months) | Not yet reached (median f/u time 95.4 months) |
| Number of Recurrences* | 27/45 | 7/24 | 11/27 |
| Number of Deaths | 21/45 | 3/24 | 9/27 |
Number of recurrences includes new cancer diagnosis. RFS, relapse-free survival; OS, overall survival; CI, confidence interval; f/u, follow up
Biomarker analysis
Biomarker analysis for tumor tissue among disease types
Biomarker levels and/or expression in the IBC, treated IDC and untreated IDC tumor specimens are summarized in Table 3A. There are statistically significant differences in levels of pS6, pJAK2, STAT3, IL6, CD68 and CD163 among the three disease types (p<0.0174 for all markers). Table 3B provides the p-values for all pairwise comparisons. While pS6 expression levels were similar between IBC (88.4%, 2+ or 3+) and treated IDC (91.3%, 2+ or 3+), untreated IDC had lower pS6 levels than the IBC and treated IDC patients (37.0%, 2+; 63.0%, 0 or 1+) (p<0.0001). The same pattern is also observed in levels of pJAK2 and pSTAT3. For pJAK2, while levels were similar between IBC (95.2%, 1+ or 2+) and treated IDC (91.7%, 1+ or 2+; 4.2%, 3+), untreated IDC had lower levels (80.0%, 0; 20.0%, 1+) (p<0.0001). For pSTAT3, 55.0% of IBC tumors had 1+ or 2+ level with 45% of tumors having 0 level. In treated IDC, 62.5% had 1+ or 2+ expression with 37.5% of tumors having 0 level. This is in contrast to untreated IDC where 92.3% of tumors had 0 level (p=0.0001). IL-6 expression was highest in the IBC (95.2%, 1+ or 2+) and treated IDC (87.5%, 1+ or 2+) groups and lowest in the untreated IDC group (15.4%, 0; 84.6%, 1+) (p=0.0174). In contrast, untreated IDC tumors had the highest CD68 level (median 80, range: 30–105) followed by treated IDC (median 57.5, range: 10–100) and then IBC (median 40, range: 4–100) (p<0.001). For CD163 level, both treated IDC and untreated IDC tumors had a mean level of 40 (range: 18–75 and range: 6–90, respectively). IBC cases had lower CD163 level with a median of 28 (range: 3–80).
Table 3.
| A: Surrounding Tumor Tissue Markers by Disease Type | |||||
|---|---|---|---|---|---|
| IBC Cases (N=45) |
Treated IDC Cases (N=24) |
Untreated IDC Cases (N=27) |
P-Value | ||
| pS6 | 0 | 0 | 0 | 4 (14.8%) | <0.0001^ |
| 1+ | 5 (11.6%) | 2 (8.7%) | 13 (48.2%) | ||
| 2+ | 37 (86.1%) | 19 (82.6%) | 10 (37%) | ||
| 3+ | 1 (2.3%) | 2 (8.7%) | 0 | ||
| No normal tissue | |||||
| Analysis N/A | 2 | 1 | |||
| pJAK2 | 0 | 2 (4.8%) | 1 (4.2%) | 20 (80%) | <0.0001^ |
| 1+ | 21 (50.0%) | 9 (37.5%) | 5 (20%) | ||
| 2+ | 19 (45.2%) | 13 (54.2%) | 0 | ||
| 3+ | 0 | 1 (4.2%) | 0 | ||
| No normal tissue | |||||
| Analysis N/A | 3 | 2 | |||
| pSTAT3 | 0 | 18 (45.0%) | 9 (37.5%) | 24 (92.3%) | 0.0001^ |
| 1+ | 19 (47.5%) | 13 (54.2%) | 2 (7.7%) | ||
| 2+ | 3 (7.5%) | 2 (8.3%) | 0 | ||
| No normal tissue | |||||
| Analysis N/A | 5 | 1 | |||
| IL6 | 0 | 2 (4.8%) | 3 (12.5%) | 4 (15.4%) | 0.0174^ |
| 1+ | 30 (71.4%) | 15 (62.5%) | 22 (84.6%) | ||
| 2+ | 10 (23.8%) | 6 (25%) | 0 | ||
| No normal tissue | |||||
| Analysis N/A | 3 | 1 | |||
| CD68* | Median (range) | 40 (4–100) | 57.5 (10–100) | 80 (30–105) | <.0001^^ |
| No Tumor Available | 5 | ||||
| No Tissue Available | |||||
| CD163* | Median (range) | 28 (3–80) | 40 (18–75) | 40 (6–90) | 0.0013^^ |
| No Tumor Available | 4 | ||||
| No Tissue Available | |||||
| B: Pairwise Comparisons of Expression Levels in Tumor Tissue – P-Values (Pairwise Comparisons of Table 3A) | |||
|---|---|---|---|
| IBC vs Treated IDC |
IBC vs Untreated IDC |
Treated IDC vs Untreated IDC |
|
| pS6^ | 0.4889 | <.0001 | 0.0002 |
| pJAK2^ | 0.4945 | <.0001 | <.0001 |
| pSTAT3^ | 0.8569 | 0.0002 | <.0001 |
| IL6^ | 0.4915 | 0.0065 | 0.0226 |
| CD68^^ | 0.0051 | <.0001 | 0.0005 |
| CD163^^ | 0.0017 | 0.0034 | 0.7539 |
Fisher's Exact Test
Kruskal-Wallis non parametric analysis of variance test
p<0.0167 is considered significant
Fisher’s Exact Test
Wilcoxon Rank Sum Test
Biomarker analysis for the surrounding non-tumor tissue between disease types
Biomarker expression levels in the IBC, treated IDC and untreated IDC surrounding non-tumor specimens are summarized in Table 4A. There are statistically significant differences in expression levels of pS6, pJAK2, pSTAT3 and IL6 in the surrounding non-tumor tissue among the three disease types. Pairwise comparisons for all analyses are summarized in Table 4B. Of note, there were no statistically significant differences in biomarker scores within groups as a result of institutional source of specimens.
Table 4.
| A: Surrounding Non-Tumor Tissue Markers by Disease Type | |||||
|---|---|---|---|---|---|
| IBC Matched Non-Tumor Tissue (N=45) |
Treated IDC Matched Non-Tumor Tissue (N=24) |
Untreated IDC Matched Non-Tumor Tissue (N=27) |
P-Value | ||
| pS6 | 0 | 7 (21.9%) | 0 | 0 | <0.0001^ |
| 1+ | 22 (68.8%) | 19 (82.6%) | 2 (7.4%) | ||
| 2+ | 3 (9.4%) | 4 (17.4%) | 25 (92.6%) | ||
| 3+ | 0 | 0 | 0 | ||
| No normal tissue | 11 | 1 | |||
| Analysis N/A | 2 | ||||
| pJAK2 | 0 | 20 (60.6%) | 1 (4.2%) | 10 (52.6%) | <0.0001^ |
| 1+ | 10 (30.3%) | 9 (37.5%) | 9 (47.4%) | ||
| 2+ | 2 (6.1%) | 14 (58.3%) | 0 | ||
| 3+ | 1 (3.0%) | 0 | |||
| No normal tissue | 9 | 8 | |||
| Analysis N/A | 3 | ||||
| pSTAT3 | 0 | 20 (66.7%) | 10 (45.5%) | 2 (4.3%) | <0.0001^ |
| 1+ | 4 (13.3%) | 11 (50%) | 12 (85.7%) | ||
| 2+ | 6 (20.0%) | 1 (4.6%) | 0 | ||
| No normal tissue | 10 | 2 | 12 | ||
| Analysis N/A | 5 | 1 | |||
| IL6 | 0 | 16 (50%) | 6 (25%) | 1 (6.3%) | 0.0069^ |
| 1+ | 14 (43.8%) | 16 (66.7%) | 15 (93.8%) | ||
| 2+ | 2 (6.3%) | 2 (8.3%) | 0 | ||
| No normal tissue | 10 | 10 | |||
| Analysis N/A | 3 | 1 | |||
| CD68* | Median (range) | 15 (0–150) | 15 (5–60) | 20 (7–40) | 0.0739^^ |
| No Tumor Available | |||||
| No Tissue Available | 5 | ||||
| CD163* | Median (range) | 12 (2–40) | 15.5 (5–40) | 15 (4–35) | 0.1487^^ |
| No Tumor Available | |||||
| No Tissue Available | 4 | ||||
| B: Pairwise Comparisons of Expression Levels in Surrounding non-Tumor Tissue – P-Values (Pairwise Comparisons of Table 4A) | |||
|---|---|---|---|
| IBC vs Treated IDC |
IBC vs Untreated IDC |
Treated IDC vs Untreated IDC |
|
| pS6^ | 0.0408 | <0.0001 | <0.0001 |
| pJAK2^ | <0.0001 | 0.5383 | <0.0001 |
| pSTAT3^ | <0.0001 | <0.0001 | 0.0007 |
| IL6^ | 0.1580 | 0.0018 | 0.1753 |
| CD68^^ | 0.1554 | 0.0294 | 0.5225 |
| CD163^^ | 0.0729 | 0.1651 | 0.7318 |
Fisher's Exact Test
Kruskal-Wallis non parametric analysis of variance test
p<0.0167 is considered significant
Fisher’s Exact Test
Wilcoxon Rank Sum Test
pS6 levels were highest in untreated IDC (92.6%, 2+) in contrast to treated IDC (Table 4A) where the majority (82.6%) had 1+ levels, and in IBC (21.9%, 0; 68.8%, 1+; 9.4%, 3+) (p<0.0001). pJAK2 levels were higher in both treated IDC and untreated IDC. Sixty-one percent of IBC surrounding non-tumor tissue lacked any pJAK2 level and 30.3% were 1+. In contrast, 95.8% of treated IDC were 1+ or 2+ (p<0.0001). For untreated IDC, 52.6% lacked any detectable level and 47.4% were 1+. pSTAT3 was highest in untreated IDC, followed by treated IDC and then IBC. There was no pSTAT3 expression in 66.7% of IBC surrounding non-tumor tissue, with the remaining 33.3% being 1+ or 2+. For treated IDC, 54.6% had 1+ or 2+ expression and 45.4% lacked any detectable level. For untreated IDC, the majority (85.7%) were 1+ (p<0.0001).
IL6 expression was highest in the untreated IDC group. Ninety-four percent and 91.7% of IBC and treated IDC non-tumor tissue had 0 or 1+ levels, respectively, while 93.8% of IDC non-tumor tissue showed 1+ levels.
Monocyte/macrophage and TAMs levels in the surrounding non-tumor tissue did not differ among the three groups for CD68 (p=0.0739) and for CD163 (p=0.1487). Median CD68 expression was 15, 15, and 20 for IBC, treated IDC and untreated IDC, respectively. Median CD163 expression was 12, 15.5 and 15 for IBC, treated IDC and untreated IDC, respectively.
Biomarker analysis of tumor versus surrounding tissue among disease types
The direction of the differences between tumor and matched surrounding non-tumor tissue are shown in Table 5 by disease type. There were differences across disease types in the tumor versus matched surrounding non-tumor tissue score for pS6, pJAK2, pSTAT3, IL6 and CD163. The majority of IBC cases demonstrated higher biomarker levels in the tumor compared to the surrounding non-tumor tissue for pS6 (81.3%), pJAK2 (63.6%) and IL6 (53.1%). Only 33.3% of IBC cases had higher pSTAT3 levels in the tumor, whereas, 46.7% had equal levels and 20% had less in the tumor compared to surrounding tissue.
Table 5.
Expression Levels in Tumor Tissue Compared to Matched Surrounding Non-Tumor Tissue
| Tumor Vs Surrounding Tissue |
IBC (N=45) | Treated IDC (N=24) |
Untreated IDC (N=27) |
P-Value | |
|---|---|---|---|---|---|
| pS6 | < | 0 | 0 | 15 (55.6%) | <0.0001^ |
| = | 6 (18.8%) | 6 (26.1%) | 12 (44.4%) | ||
| > | 26 (81.3%) | 17 (73.9%) | 0 | ||
| missing | 13 | 1 | 0 | ||
| pJAK2 | < | 3 (9.1%) | 5 (20.8%) | 7 (38.9%) | <0.0001^ |
| = | 9 (27.3%) | 13 (54.2%) | 11 (61.1%) | ||
| > | 21 (63.6%) | 6 (25%) | 0 | ||
| missing | 12 | 0 | 9 | ||
| pSTAT3 | < | 6 (20%) | 16 (72.7%) | 10 (71.4%) | 0.0002^ |
| = | 14 (46.7%) | 5 (22.7%) | 4 (28.6%) | ||
| > | 10 (33.3%) | 1 (4.6%) | 0 | ||
| missing | 15 | 2 | 13 | ||
| IL6 | < | 0 | 1 (4.2%) | 0 | 0.0039^ |
| = | 15 (46.9%) | 15 (62.5%) | 15 (93.8%) | ||
| > | 17 (53.1%) | 8 (33.3%) | 1 (6.3%) | ||
| missing | 13 | 0 | 11 | ||
| CD68 | Median (Tumor-Surrounding Tissue) & Range | 24 (−90 –75) | 32.5 (2–85) | 60 (10–90) | <0.0001^^ |
| CD163 | Median (Tumor-Surrounding Tissue) & Range | 15 (−3–55) | 25 (6–58) | 25 (−10 –63) | 0.0048^^ |
Kruskal-Wallis non parametric analysis of variance test
Higher levels of the biomarker in treated IDC patients in the tumor compared to the surrounding non-tumor tissue was only seen for pS6 (73.9%). For pJAK2 and IL-6, 54.2% and 62.5% of cases demonstrated equal levels, respectively. For pSTAT3, 72.7% of cases had higher levels in surrounding non-tumor tissue compared to the tumor itself. In the untreated IDC group, there was actually higher levels in surrounding non-tumor tissue compared to the tumor tissue for pS6 (55.6%) and pSTAT3 (71.4%). The majority of cases for pJAK2 and IL6 had equal levels (61.1% and 93.8%, respectively).
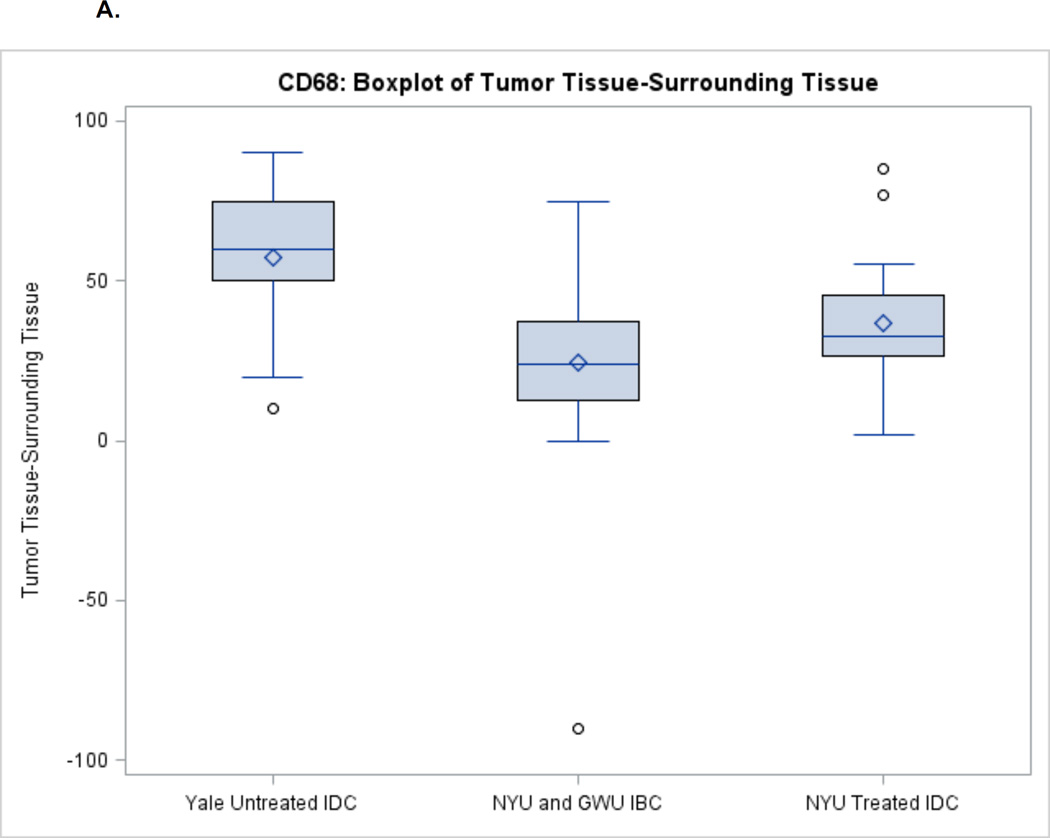
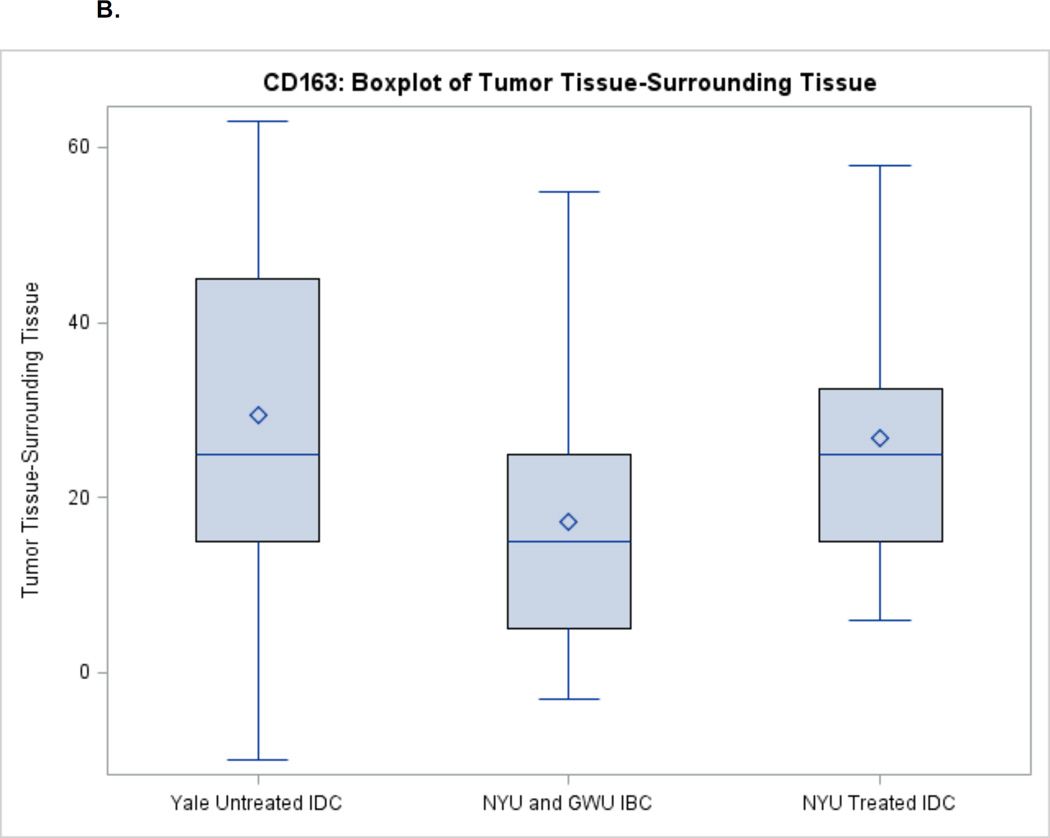
For TAMs, there was higher invasion in the tumor among all disease types (Figures 2A–2B). Untreated IDC had the largest difference in CD68 levels between tumors and the surrounding tissue (median 60) followed by treated IDC (median 32.5) and IBC (median 24). For CD163, there was a similar difference in levels between tumor and surrounding tissue for treated and untreated IDC (median 25) followed by IBC (median 15).
Figure 2.
A. CD68 Expression between Tumor and Surrounding Non-Tumor Tissue
B. CD163 Expression between Tumor and Surrounding Non-Tumor Tissue
DISCUSSION
This is the first retrospective study designed to evaluate activation of the PI3K/mTOR and JAK/STAT pathways, infiltration of monocytes, macrophages and TAMs, and representative inflammatory cytokines in IBC compared to treated and untreated IDC. We show that the PI3K/mTOR and JAK/STAT pathways are strongly activated in IBC patient tumor tissues, along with strong expression of the pro-invasive cytokine IL-6, and infiltrating TAMs. In IBC, there was a much lower level of activation or infiltration in surrounding non-tumor stromal tissues compared to tumor tissues. Notably, STAT3 mRNA has previously been shown to be increased in IBC in a transcriptomic study using datasets from the World IBC Consortium37. We also evaluated these biomarkers in treated and untreated IDC specimens for activating phosphorylation. While we found mTOR and JAK2/STAT3 activation in the untreated IDC tumors, it was at lower levels of activation (surrogate phosphorylation) than in the IBC and treated IDC specimens.
With the exception of TAMs, there was no statistical difference in biomarkers between treated IDC tumor tissue compared to IBC tumor tissue. Additionally, in some cases, biomarker levels in surrounding non-tumor tissue showed a significant level of activation similar to that of adjacent tumor tissue. Biomarker levels in the surrounding non-tumor tissue were for the most part highest in untreated IDC, with the exception of TAMs.
The findings above led us to ask two broad questions: (1) Does strong activation of mTOR and JAK2/STAT3 pathways, despite neoadjuvant chemotherapy, suggest a mechanism of resistance; and (2) Why is the surrounding “non-tumor” tissue activated in these pathways, and is this adjacent tissue truly ‘benign?’ Activation of mTOR and JAK2/STAT3 were similar between IBC and treated IDC tumor tissues based on pS6, pJAK2, pSTAT3 and IL6 expression, whereas it was much lower in the untreated IDC cohort, suggesting a potential mechanism of resistance. While this is likely the case, we do not have serial pre-and post-therapy biopsies for each patient to confirm these findings. In support of the mechanism that elevated activation of mTOR and JAK2/STAT3 is evidence for selected chemoresistance, JAK2 amplifications have been found in 10% of triple negative breast cancers (TNBC) in patients treated with neoadjuvant chemotherapy, commensurate with increased IL-6 expression and metastatic progression. Data from the Cancer Genome Atlas (TCGA) demonstrated that JAK2 gains and amplifications are more frequent in TNBC patients treated with neoadjuvant chemotherapy when compared to primary untreated basal-like breast cancers and further increase upon metastatic progression. In the TCGA, JAK2 amplifications are associated with pSTAT3 activation38. Additionally, doxorubicin has been shown to potentiate STAT1 activation in breast cancer cells39, 40 and docetaxel-resistant tumors upregulate several genes, including PI3K/AKT/mTOR pathway39, 41, 42. The majority of patients in our IBC and treated IDC cohorts received anthracycline and taxane-based chemotherapy, consistent with these results.
There is a growing body of evidence suggesting that the surrounding non-tumor tissue which appears morphologically normal is not truly benign as it demonstrates activation of prooncogenic signaling pathways43–47. While the stroma normally functions as the main barrier against tumorigenesis, tumor and immune cells can convert it to a tumor-promoting environment48. Tumor-associated normal epithelial stromal cells, immune cells, and vascular cells promote tumor growth and disease progression, and also play a role in drug resistance49. In this regard, we observed significantly increased activation of mTOR, JAK2, and STAT3 in the surrounding non-tumor tissue, which was highest in untreated IDC compared to treated IDC and IBC. STAT3 activation has previously been demonstrated in the cytoplasm of the non-tumor areas of breast cancer specimens50, 51. Our findings highlight the clear interplay between tumor and the surrounding tumor milieu. Additionally, this data demonstrates that future efforts to target these biomarkers may prove less toxic in IBC, where we would expect less effect on the so called ‘normal’ tissue.
We also sought to evaluate the role of TAMs in tumor pathogenesis. We found that there were more TAMs in the untreated IDC cohort compared to the IBC and treated IDC cohorts. This phenomenon has been previously described and several studies have investigated the effects of cytotoxic chemotherapy in subverting the pro-tumorigenic activities of macrophages32. Cyclophosphamide has been shown to promote the skewing of TAMs into M1 macrophages32. Neoadjuvant treatment can increase macrophage and other immune cell death, perhaps accounting for the decreased presence of TAMs after neoadjuvant chemotherapy that we observed.
We investigated the interplay between the mTOR and JAK2/STAT3 pathways in IBC. Preclinical data supports dual targeting of the PI3K/mTOR and JAK/STAT pathways in TNBC35. In our IBC cohort, of 37 IBC patients with complete biomarker data available, 100% were pS6 positive and 95% were JAK2 positive. The co-expression of both biomarkers in IBC patient tissues certainly supports a cross-activating link, which may have important therapeutic implications, as trials of JAK2 inhibitors and PI3K/mTOR inhibitors are ongoing in clinical practice for treatment of breast cancer33, 52.
We acknowledge the limitations of this study. This is a retrospective analysis with a small sample size of a disease (IBC) with a rare incidence. Patients were treated at multiple different hospitals, and while they generally received similar treatments, we could not control the choice, length or duration of treatment due to variable clinical practices, which have evolved in the last decade. For instance, HER2-positive patients who were diagnosed prior to 2006 did not receive adjuvant trastuzumab, which is now the standard of care. The study was not powered to test for any correlations between ER/HER2 status and biomarker expression. There may have been intratumoral heterogeneity present and sampling of non-tumor areas can be quite complex. For example, there is evidence for greater TAM infiltration further from the tumor and there may be differences in the way that the macrophages activate the stroma. In conclusion, we show that there is increased activation of mTOR and JAK2/STAT3 pathways and increased levels of IL-6 in IBC and treated IDC when compared to untreated IDC. These findings suggest that a potential mechanism of resistance may be selected following neoadjuvant chemotherapy. These data also support the view that morphologically normal surrounding non-tumor tissue can be significantly activated in pro-transforming pathways. Our findings warrant further prospective investigation to explore combinatorial strategies of targeted therapies in IDC and IBC.
Supplementary Material
CLINICAL PRACTICE POINTS.
Inflammatory breast cancer (IBC) is a rare and aggressive form of breast cancer that carries a poor prognosis.
Preclinical data has demonstrated activation of the PI3K/mTOR and JAK/STAT pathways in IBC, along with expression of inflammatory cytokines and tumor associated macrophages (TAMs).
This retrospective study analyzed pS6 (mTOR), pJAK2 (JAK2), pSTAT3 (STAT3), IL-6 (cytokine), CD68 (monocytes, macrophages) and CD163 (TAMs) biomarker expression in archival tumor tissue from IBC, invasive ductal carcinoma (IDC) treated with neoadjuvant chemotherapy and untreated IDC as well as from surrounding non-tumor tissue.
Of the 37 IBC patients with complete biomarker data available, 100% were pS6 positive and 95% were pJAK2 positive. With the exception of macrophages, biomarker expression was generally highest in IBC and treated IDC, suggesting a potential mechanism of resistance after neoadjuvant chemotherapy.
Biomarker activation was also demonstrated in the surrounding non-tumor tissue, suggesting that the stroma may resemble the tumor oncogenic environment.
Our data along with the published preclinical data supports a role for prospective studies investigating combinations of targeted agents in this patient population.
ACKNOWLEDGMENTS
This work was supported by grants from the Breast Cancer Research Foundation (RJS), the Avon Foundation for Women (RJS, KJ), the Marcus Foundation (KJ), and the Cancer Center Support Grant NIH/NCI 5 P30 CA16087 (JDG).
Footnotes
Conflicts of Interest: None
Komal Jhaveri: Work done at New York University Langone Medical Center
Eleonora Teplinsky: Work done at New York University Langone Medical Center
References
- 1.Yamauchi H, Woodward WA, Valero V, et al. Inflammatory breast cancer: what we know and what we need to learn. The oncologist. 2012;17:891–899. doi: 10.1634/theoncologist.2012-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertucci F, Finetti P, Birnbaum D, Viens P. Gene expression profiling of inflammatory breast cancer. Cancer. 2010;116:2783–2793. doi: 10.1002/cncr.25165. [DOI] [PubMed] [Google Scholar]
- 3.Silvera D, Arju R, Darvishian F, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nature cell biology. 2009;11:903–908. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 4.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 5.Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:1831–1838. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 6.Van Pelt AE, Mohsin S, Elledge RM, et al. Neoadjuvant trastuzumab and docetaxel in breast cancer: preliminary results. Clinical breast cancer. 2003;4:348–353. doi: 10.3816/cbc.2003.n.040. [DOI] [PubMed] [Google Scholar]
- 7.Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman B, Trudeau M, Awada A, et al. Lapatinib monotherapy in patients with HER2-overexpressing relapsed or refractory inflammatory breast cancer: final results and survival of the expanded HER2+ cohort in EGF103009, a phase II study. The lancet oncology. 2009;10:581–588. doi: 10.1016/S1470-2045(09)70087-7. [DOI] [PubMed] [Google Scholar]
- 9.Boussen H, Cristofanilli M, Zaks T, DeSilvio M, Salazar V, Spector N. Phase II study to evaluate the efficacy and safety of neoadjuvant lapatinib plus paclitaxel in patients with inflammatory breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3248–3255. doi: 10.1200/JCO.2009.21.8594. [DOI] [PubMed] [Google Scholar]
- 10.Colpaert CG, Vermeulen PB, Benoy I, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. British journal of cancer. 2003;88:718–725. doi: 10.1038/sj.bjc.6600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen PB, van Golen KL, Dirix LY. Angiogenesis, lymphangiogenesis, growth pattern, and tumor emboli in inflammatory breast cancer: a review of the current knowledge. Cancer. 2010;116:2748–2754. doi: 10.1002/cncr.25169. [DOI] [PubMed] [Google Scholar]
- 12.Van der Auwera I, Van Laere SJ, Van den Eynden GG, et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:7965–7971. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- 13.Pierga JY, Petit T, Delozier T, et al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. The lancet oncology. 2012;13:375–384. doi: 10.1016/S1470-2045(12)70049-9. [DOI] [PubMed] [Google Scholar]
- 14.Gianni L, Romieu GH, Lichinitser M, et al. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJSS, Buyse M, Martin M, Geyer CE, Im Y-H, Pienkowski T, Kim S-B, Robert NJ, Steger G, Crown J, Verma S, Eiermann W, Constantino JP, Im S-A, Mamounas EP, Schwartzberg L, Paterson A, Mackey JR, Provencher L, Press MF, Thirlwell M, Bee-Munteanu V, Henschel V, Crepelle-Flechais A, Wolmark N. Primary results from BETH, a phase 3 controlled study of adjuvant chemotherapy and trastuzumab +/– bevacizumab in patients with HER2-positive, node-positive or high risk node-negative breast cancer; 2013 San Antonio Breast Cancer Symposium; San Antonio, Texas. 2013. [Google Scholar]
- 16.Turpin E, Bieche I, Bertheau P, et al. Increased incidence of ERBB2 overexpression and TP53 mutation in inflammatory breast cancer. Oncogene. 2002;21:7593–7597. doi: 10.1038/sj.onc.1205932. [DOI] [PubMed] [Google Scholar]
- 17.Levine PH, Portera CC, Hoffman HJ, et al. Evaluation of lymphangiogenic factors, vascular endothelial growth factor D and E-cadherin in distinguishing inflammatory from locally advanced breast cancer. Clinical breast cancer. 2012;12:232–239. doi: 10.1016/j.clbc.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrakchi R, Khadimallah I, Ouerhani S, et al. Expression of WISP3 and RhoC genes at mRNA and protein levels in inflammatory and noninflammatory breast cancer in Tunisian patients. Cancer investigation. 2010;28:399–407. doi: 10.3109/07357900903405926. [DOI] [PubMed] [Google Scholar]
- 19.Lerebours F, Vacher S, Andrieu C, et al. NF-kappa B genes have a major role in inflammatory breast cancer. BMC cancer. 2008;8:41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charafe-Jauffret E, Tarpin C, Bardou VJ, et al. Immunophenotypic analysis of inflammatory breast cancers: identification of an 'inflammatory signature'. The Journal of pathology. 2004;202:265–273. doi: 10.1002/path.1515. [DOI] [PubMed] [Google Scholar]
- 21.Quintas-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overmoyer VA BA, Shu S, Peluffo G, Park SY, Nakhlis F, Bellon JR, Yeh ED, Jacene HA, Hirshfield-Bartek J, Polyak K. JAK2/STAT3 activity in inflammatory breast cancer supports the investigation of JAK2 therapeutic targeting. Cancer Res. 2012;72 Abstract nr P4-06-01. [Google Scholar]
- 24.Marotta LL, Almendro V, Marusyk A, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. The Journal of clinical investigation. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertucci F, Finetti P, Vermeulen P, et al. Genomic profiling of inflammatory breast cancer: A review. Breast. 2014 doi: 10.1016/j.breast.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi H, Cristofanilli M, Nakamura S, Hortobagyi GN, Ueno NT. Molecular targets for treatment of inflammatory breast cancer. Nature reviews. Clinical oncology. 2009;6:387–394. doi: 10.1038/nrclinonc.2009.73. [DOI] [PubMed] [Google Scholar]
- 27.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. The New England journal of medicine. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwamoto T, Bianchini G, Qi Y, et al. Different gene expressions are associated with the different molecular subtypes of inflammatory breast cancer. Breast cancer research and treatment. 2011;125:785–795. doi: 10.1007/s10549-010-1280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvera D, Schneider RJ. Inflammatory breast cancer cells are constitutively adapted to hypoxia. Cell cycle. 2009;8:3091–3096. doi: 10.4161/cc.8.19.9637. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed MM, El-Ghonaimy EA, Nouh MA, Schneider RJ, Sloane BF, El-Shinawi M. Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. The international journal of biochemistry & cell biology. 2014;46:138–147. doi: 10.1016/j.biocel.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer letters. 2013;332:3–10. doi: 10.1016/j.canlet.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell death and differentiation. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clinicaltrials.gov. Phase II Study of Combination Ruxolitinib (INCB018242) With Preoperative Chemotherapy for Triple Negative Inflammatory Breast Cancer Following Completion of a Phase I Combination Study in Recurrent/Metastatic Breast Cancer ( NCT02041429) 2014
- 34.Britschgi A, Radimerski T, Bentires-Alj M. Targeting PI3K, HER2 and the IL-8/JAK2 axis in metastatic breast cancer: Which combination makes the whole greater than the sum of its parts? Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2013;16:68–72. doi: 10.1016/j.drup.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Britschgi A, Andraos R, Brinkhaus H, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer cell. 2012;22:796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Hyndman RJ, Fan Y. Sample quantiles in statistical packages. American Statistician. 1996;50:361–365. [Google Scholar]
- 37.Van Laere SJ, Ueno NT, Finetti P, et al. Uncovering the molecular secrets of inflammatory breast cancer biology: an integrated analysis of three distinct affymetrix gene expression datasets. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4685–4696. doi: 10.1158/1078-0432.CCR-12-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balko JMGJ, Schwarz L, Sanders ME, Wang K, Harris LN, Lin NU, Miller VA, Stephens PJ, Yelensky R, Pinto JA, Gomez H, Arteaga CL. JAK2 amplifications are enriched in triple negative breast cancers after neoadjuvant chemotherapy and predict poor prognosis; 2013 San Antonio Breast Cancer Symposium; San Antonio, TX. 2013. [Google Scholar]
- 39.Lee SC, Xu X, Lim YW, et al. Chemotherapy-induced tumor gene expression changes in human breast cancers. Pharmacogenetics and genomics. 2009;19:181–192. doi: 10.1097/FPC.0b013e32831ebb5d. [DOI] [PubMed] [Google Scholar]
- 40.Thomas M, Finnegan CE, Rogers KM, et al. STAT1: a modulator of chemotherapy-induced apoptosis. Cancer Res. 2004;64:8357–8364. doi: 10.1158/0008-5472.CAN-04-1864. [DOI] [PubMed] [Google Scholar]
- 41.Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. The Journal of biological chemistry. 2006;281:25903–25914. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang JC, Wooten EC, Tsimelzon A, et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:1169–1177. doi: 10.1200/JCO.2005.03.156. [DOI] [PubMed] [Google Scholar]
- 43.Boersma BJ, Reimers M, Yi M, et al. A stromal gene signature associated with inflammatory breast cancer. International journal of cancer. Journal international du cancer. 2008;122:1324–1332. doi: 10.1002/ijc.23237. [DOI] [PubMed] [Google Scholar]
- 44.Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nature medicine. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 45.Casey T, Bond J, Tighe S, et al. Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast cancer research and treatment. 2009;114:47–62. doi: 10.1007/s10549-008-9982-8. [DOI] [PubMed] [Google Scholar]
- 46.Farmer P, Bonnefoi H, Anderle P, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nature medicine. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 47.Martin DN, Boersma BJ, Yi M, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PloS one. 2009;4:e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 49.Yoshihara K, Shahmoradgoli M, Martinez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nature communications. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dolled-Filhart M, Camp RL, Kowalski DP, Smith BL, Rimm DL. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:594–600. [PubMed] [Google Scholar]
- 51.Berclaz G, Altermatt HJ, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. International journal of oncology. 2001;19:1155–1160. doi: 10.3892/ijo.19.6.1155. [DOI] [PubMed] [Google Scholar]
- 52. Clinicaltrials.gov. A Phase III Study of BKM120 With Fulvestrant in Patients With HR+,HER2-, AI Treated, Locally Advanced or Metastatic Breast Cancer Who Progressed on or After mTORi (BELLE-3) ( NCT01633060) 2014 Clinicaltrials.gov.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.