Abstract
Objective
To evaluate whether change in fixed location measures of radiographic joint space width (JSW) and in cartilage thickness by MRI predict knee replacement.
Methods
Knees replaced between 36-60 months follow-up (M) in the Osteoarthritis Initiative were each matched with one control by age, sex, and radiographic status. Radiographic JSW was determined from fixed flexion radiographs, and subregional femorotibial cartilage thickness from 3 Tesla MRI. Changes between the annual visit before replacement (T0) and 2 years before T0 (T-2) were compared using conditional logistic regression.
Results
One hundred and nineteen knees from 102 participants (55.5% women; age 64.2±8.7 [mean±SD]) were studied. Fixed location JSW change at 22.5% from medial to lateral differed more between replaced and control knees (case-control [cc] OR=1.57; 95%CI: 1.23,2.01) than minimum medial JSW change (ccOR=1.38; 95%CI: 1.11,1.71). Medial femorotibial cartilage loss displayed discrimination similar to minimum JSW, and central tibial cartilage loss similar to fixed location JSW. Location-independent thinning and thickening scores were both elevated prior to knee replacement.
Conclusions
Discrimination of structural progression between knee pre-placement cases versus controls was stronger for fixed-location than for minimum radiographic JSW. MRI displayed similar discrimination to radiography and suggested greater simultaneous cartilage thickening and loss prior to knee replacement.
Keywords: Magnetic Resonance Imaging, Radiographic Joint Space Width (JSW), Knee Osteoarthritis, Clinical Validation, Measurement Performance
Introduction
Osteoarthritis is the most common form of arthritis, with the knee most commonly affected. The lifetime risk of knee osteoarthritis is 14%, and almost 10% of the US population has a diagnosis of knee osteoarthritis at an age of 60 [1]. Knee osteoarthritis substantially impacts the remaining quality-adjusted life-years of persons aged 50-84 [2]. Patients with knee osteoarthritis show elevated utilization of health care including diagnostic imaging with much of the expense for therapy being caused by knee replacement surgery; the number of knees replaced in the U.S. has doubled over the last decade; over half the patients diagnosed with knee osteoarthritis eventually undergo knee replacement [3].
Currently, no structure-modifying agent has been approved for the treatment of osteoarthritis. Radiological imaging represents the most direct way of evaluating structural progression, with conventional radiography and magnetic resonance imaging (MRI) being most often utilized [4,5]. Regulatory guidance for approval of disease modifying osteoarthritis drugs (DMOADs) requests reduction of structural pathology to be accompanied by benefits in clinical outcomes. Ideally, radiological imaging biomarkers used in clinical trials should thus not only reliably indicate structural progression, but also predict relevant clinical outcomes such as knee replacement [5,6].
Reduction in radiographic joint space width (JSW) is recognized as standard for demonstrating structural benefits in knee osteoarthritis by the Food and Drug Administration and other regulatory bodies. Minimum JSW was shown to predict joint replacement in the hip [7] and knee [8,9]. However, “fixed location measures” of femorotibial JSW were recently demonstrated to be more sensitive in detecting structural change in knee osteoarthritis than minimum JSW [10,11]; yet, fixed-location measures have not been related to the risk of knee replacement.
Quantitative measures of cartilage loss by MRI were shown to be more sensitive to change than radiographs [5,6] and to predict knee replacement [6,12-14], in particular in “fast clinical progressors” with less advanced radiographic disease stages at baseline [14,15]. However, only a small study compared MRI with radiography in context of predicting knee replacement and relied on an outdated radiographic acquisition method [16]. Finally, recent work using MRI highlighted that spatial patterns of femorotibial cartilage loss vary substantially between subjects, depending on individual sets of risk factors [17,18]. MRI has been used to determine the (maximum) rate of cartilage loss, independent of spatial location [18,19], by ordering the rates of subregional femorotibial cartilage thickness change by magnitude in each knee. This approach was shown more sensitive in discriminating rates of cartilage loss between radiographic strata [18,19], but it has not been studied to what extent these location-independent approaches are related to knee replacement.
The purpose of this study was therefore to evaluate with what level of accuracy fixed location measures of radiographic JSW, region-specific MRI, and location-independent MRI predict knee replacement as clinical outcome, compared with minimum radiographic JSW
Methods
Study Design
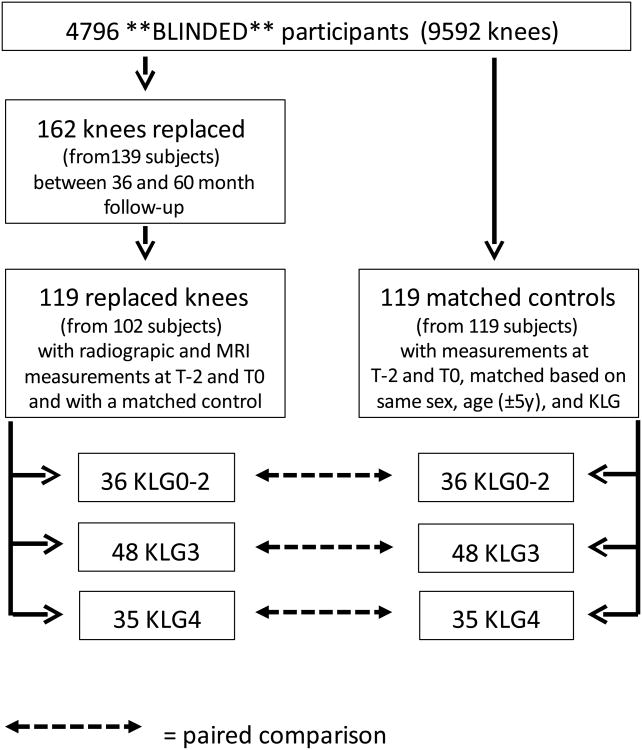
This case-control study is ancillary to the Osteoarthritis Initiative (OAI), an ongoing prospective, multi-centre cohort study (http://www.oai.ucsf.edu/) designed to identify and validate imaging, biochemical and genetic biomarkers for the onset and/or progression of knee osteoarthritis. The Osteoarthritis Initiative was conducted in compliance with the ethical principles derived from the Declaration of Helsinki, in compliance with local Institutional Review Board, informed consent regulations, and International Conference on Harmonization Good Clinical Practices Guidelines. The Osteoarthritis Initiative, its imaging protocol, and quality assurance metrics over 8 years have been reported [20-22]: both knees of 4,796 participants (Fig. 1) were studied using fixed flexion radiography and 3Tesla MRI at baseline 12, 24, 36, and 48 month follow-up (M) [21]; there was also a clinical visit, without imaging, at 60M. Participants were interviewed about having received a knee replacement in the preceding year and this was confirmed by radiography or from hospital records, when the former was not available.
Figure 1. Flow chart demonstrating inclusion of knee replacement cases and matched controls for the current study.
The sample studied here, i.e. the Osteoarthritis Initiative participants who received a knee replacement, and one control for each; Fig. 1) was described previously, with the 2-year observation interval of MRI-based cartilage loss prior to knee replacement (T-2→T0) being most discriminative between cases and controls [15]. To be eligible as a case, a knee replacement had to be confirmed at 36, 48, or 60M and fixed flexion X-rays acceptable for radiographic JSW analysis, and MRI acquisitions had to be available for T-2 and T0. When both knees of one participant were replaced, both were included. Control knees were selected from Osteoarthritis Initiative participants without knee replacement between baseline and 60M (Fig. 1); if the contra-lateral knee received a knee replacement during the study, knees did not qualify as controls. Controls had to have fixed flexion radiographs and MRIs available at time points corresponding with those of knees replaced (T-2 and T0) and were matched 1:1 to the cases by sex, age (±5years), and radiographic disease stage (Fig. 1). The matching for radiographic disease stage was done by using release 0.4 from the Osteoarthritis Initiative, i.e. the central readings by three expert radiologists or rheumatologists at Boston University (https://oai.epiucsf.org/datarelease/SASDocs/kXR_SQ_BU_descrip.pdf) at the baseline visit. These readings used the traditional KLG classification as well as Osteoarthritis Research Society International (OARSI) osteophyte and JSN scores. The matching was performed using the following KLG strata0--2, 3, or 4). In a second (post-hoc) step, only case-control pairs were included in whom the same compartment (medial or lateral or both) showed evidence of radiographic joint space narrowing (JSN).
Radiography
The radiographic JSW measurement relied on fixed flexion radiographs acquired using a SynaFlexer™ frame (Bioclinica, Newtown, PA)[21]. Minimum and fixed location JSW measures in the medial femorotibial compartment were performed by one of the authors (J.D.) using automated software [10,23] (Fig. 2A). The software determines a line tangential to the femoral condyles to represent the x-axis of the coordinate system. The medial and lateral borders of the knee are then marked manually, tangential to the largest prominence of the femoral epicondyles to determine location-specific positions in the joint from 0.0 (0%) to 1.0 (100%) (Fig. 2A). Medial compartment fixed location JSW(x) measurements were obtained between 0.15 (15%) and 0.30 (30%) in the medial femorotibial compartment). The radiographs were read viewing all time points (including the visits other than T0 and T-2) simultaneously but with the reader blinded to the correct order.
Figure 2.
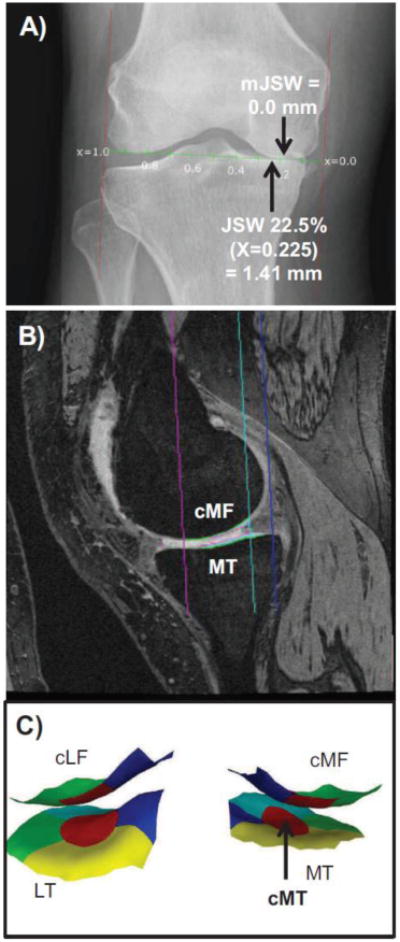
A) Illustration showing the radiography-based measurement of the minimal joint space width (mJSW) and of the joint space width at the central fixed location JSW at 22.5% from medial to lateral based on the femoral epicondyles (x=0.225).
B) Illustration showing the sagittal DESS MRI-based measurement in the medial femorotibial compartment: MT = medial tibia; cMF=weight-bearing medial femur C) Illustration (3D reconstruction) showing the central (red), external (greem), internal (blue), anterior (turquoise), and posterior subregions (yellow) computed in the medial (MT) and lateral tibia (LT) and in the central, weight-bearing part if the medial (cMF) and lateral (cLF) femoral condyle (only central, external, and internal subregions). In the current study, the central medial tibia (cMT) was used for statistical analysis.
Region-specific MRI
MR image analysis relied on the oblique sagittal double-echo steady-state (DESS) sequence with water excitation [21] (Fig. 2B). Segmentation of the femorotibial cartilages was performed at one centre (BLINDED). T-2 and T0 images were processed as pairs by one of 12 readers, but with blinding to case/control status and to image acquisition order [15]. All segmentations were quality controlled by one of two experts (S.M.; F.E). The mean cartilage thickness (ThCtAB.Me) was computed in the medial and in the lateral femorotibial compartment, and in 5 tibial (central, external, internal, anterior, posterior) and 3 medial and lateral femoral subregions (central, external, internal)[24] (Fig. 2C). Cartilage thickness change was computed as an absolute value (μm).
Location-independent MRI
Based on the above 16 subregions, location-independent cartilage thickness change was determined using the extended ordered value (OV) approach [19]: Ordered value 1 represented the subregion with the greatest rate of cartilage thinning in each knee, ordered value 2 the subregion with the second strongest thinning, and so forth, and ordered value 16 the subregion with the least thinning or with the greatest rate of thickening [19]. In addition, novel summary measures of subregional cartilage thickness change were computed [25]: these included the total subregional cartilage thinning score; i.e. the sum of all negative cartilage thickness changes across as many of the 16 subregions in which cartilage loss occurred in each knee), the total subregional cartilage thickening score (the sum of all positive cartilage thickness changes), and the total subregional cartilage change score (the sum or all 16 subregional cartilage thickness changes independent of direction).
Statistical Analysis
All tests were performed using SAS software (version 9.2, SAS Institute, Cary, NC). Minimum radiographic JSW (mJSW) in the medial compartment was considered the benchmark structural outcome, because it represents the accepted imaging endpoint in context of structure modification in knee osteoarthritis. Fixed location JSW at 22.5% from the medial to lateral edge of the femoral condyle (x=0.225, Fig. 2A) was used as comparative radiographic measure, because it was previously found to be the most responsive location in knee osteoarthritis -related JSW change [10,11]. Medial femorotibial compartment cartilage thickness change was used as a global measure of region-specific MRI analysis (Fig. 2B), because it summarizes change across the entire medial femorotibial compartment. Central medial tibial (cMT) cartilage thickness was used as a subregional measure, because it was previously identified as most discriminative between knees replaced and matched controls [14] (Fig. 2C). Extended OVs [19] and total subregional thinning, thickening, and change scores were used as location-independent MRI measures of cartilage change. Raw differences between the rates of change in knees replaced and non-replaced controls were compared using paired t-tests (Fig. 1). After standardizing the variables to facilitate comparisons, case-control conditional logistic regression odds ratios (ccOR) were calculated using generalized estimating equation models with an independent working correlation and a robust sandwich estimator to account for the correlation of knees within an individual [14,15]. Robustness of these comparisons was evaluated by adjusting for the effects of baseline BMI and pain at T-2 (ccORbp) [15] since previous studies have revealed associations of cartilage loss with BMI and pain [26,27]. No adjustment for multiple comparisons was made, because the study was exploratory and because measures are expected to be highly correlated to each other. Given previous observations of superior discrimination between case/control pairs with “early” radiographic disease status at baseline [14,15], sensitivity analyses were conducted using a stratum of KLG 0-2 knees, and further sensitivity analyses were performed excluding case/control pairs with a mismatch in the location (medial/lateral) of baseline JSN.
Results
Sample description
162 knees of 139 Osteoarthritis Initiative participants received a femorotibial knee replacement between 36 and 60M (Fig. 1) 54 at 36M, 46 at 48M, and 62 at 60M). 119 knees from 102 participants (55.5% women; age 64.2±8.7 [mean±SD]; BMI 29.4±4.5) had radiographic JSW and MRI readings at T-2 and T0, and a matched control (also 55.5% women; age 63.9±8.4; BMI 30.0±4.45; Fig. 1). Of the 119 case and control knees, 36 were KLG0-2, 48 KLG3, and 35 KLG4; Fig. 1)
Radiography
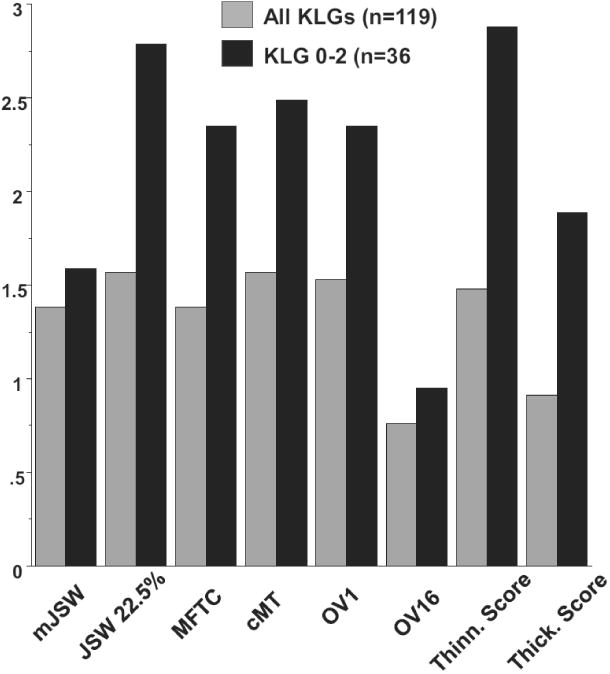
Minimum JSW change over 2 years prior to surgery was substantially and significantly greater in cases with knee replacement (p=0.0058, paired t-test) than in controls (Table 1; Fig. 2); the ccOR was 1.38 (95% confidence interval [CI] 1.11;1.71) and the ccpbOR 1.45 (95% CI 1.15;1.83). Change in fixed location JSW at 22.5% from medial to lateral (x=0.225) also differed strongly and significantly between matched case-control pairs (p=0.0001) and displayed greater ORs than minimum JSW (ccOR=1.57; ccpbOR=1.64; Table 1, Fig. 2).
Table 1.
Change in radiographic joint space width (JSW) and in medial femorotibial compartment (MFCT) and central tibia (cMT) cartilage thickness over a period of two years (T-2 →T0) in knees from **BLINDED** prior to knee replacement (KR; n=119) and in non-KR control knees (n=119) matched 1:1 by Kellgren-Lawrence grade (KLG), sex, and age. Values in μm.
| KR Cases Mean ± SD |
Controls Mean ± SD |
Paired T P-value |
Cc P-value |
ccOR [95% CI] |
ccbp P-value |
ccbp_OR [95% CI] |
|
|---|---|---|---|---|---|---|---|
| mJSW | -415±1008 | -133±621 | 0.0058 | 0.0039 | 1.38 [1.11,1.71] | 0.0015 | 1.45 [1.15,1.83] |
| JSW 22.5% | -610±1059 | -162±650 | 0.0001 | 0.0003 | 1.57 [1.23,2.01] | 0.0003 | 1.64 [1.26,2.15] |
| MFTC | -223±372 | -95±189 | 0.001 | 0.0003 | 1.38 [1.16,1.64] | 0.0002 | 1.4 [1.17,1.66] |
| cMT | -178±249 | -57±155 | <.0001 | <.0001 | 1.57 [1.27,1.95] | <.0001 | 1.6 [1.29,1.99] |
|
| |||||||
| OV1 | -513±490 | -289±174 | <.0001 | <.0001 | 1.53 [1.25,1.87] | <.0001 | 1.56 [1.26,1.92] |
| OV2 | -368±376 | -194±124 | <.0001 | <.0001 | 1.57 [1.3,1.9] | <.0001 | 1.61 [1.32,1.96] |
| OV3 | -291±349 | -150±109 | <.0001 | <.0001 | 1.51 [1.24,1.82] | <.0001 | 1.53 [1.26,1.87] |
| OV4 | -234±279 | -111±78 | <.0001 | <.0001 | 1.5 [1.24,1.8] | <.0001 | 1.52 [1.25,1.84] |
| OV5 | -184±233 | -88±73 | <.0001 | <.0001 | 1.47 [1.23,1.75] | <.0001 | 1.49 [1.24,1.79] |
| OV6 | -145±191 | -68±69 | 0.0001 | <.0001 | 1.48 [1.24,1.75] | <.0001 | 1.5 [1.26,1.78] |
| OV7 | -100±154 | -49±62 | 0.0016 | 0.0003 | 1.43 [1.18,1.74] | 0.0002 | 1.44 [1.19,1.75] |
| OV8 | -72±108 | -34±60 | 0.0016 | 0.0003 | 1.4 [1.17,1.67] | 0.0002 | 1.4 [1.18,1.68] |
| OV9 | -46±80 | -21±59 | 0.0088 | 0.0036 | 1.35 [1.1,1.65] | 0.0029 | 1.35 [1.11,1.66] |
| OV10 | -27±77 | -6±59 | 0.0212 | 0.0093 | 1.3 [1.07,1.59] | 0.0071 | 1.32 [1.08,1.61] |
| OV11 | -1±67 | 10±58 | 0.1559 | 0.1052 | 1.19 [0.96,1.48] | 0.0927 | 1.2 [0.97,1.5] |
| OV12 | 23±62 | 28±57 | 0.5074 | 0.4781 | 1.09 [0.86,1.38] | 0.4551 | 1.1 [0.86,1.4] |
| OV13 | 46±62 | 41±58 | 0.5648 | 0.5645 | 0.93 [0.72,1.2] | 0.5797 | 0.93 [0.72,1.2] |
| OV14 | 75±67 | 60±62 | 0.0715 | 0.0855 | 0.8 [0.61,1.03] | 0.0705 | 0.79 [0.62,1.02] |
| OV15 | 110±78 | 95±74 | 0.1317 | 0.1474 | 0.82 [0.63,1.07] | 0.0988 | 0.81 [0.63,1.04] |
| OV16 | 184±103 | 154±91 | 0.022 | 0.0153 | 0.76 [0.61,0.95] | 0.0092 | 0.75 [0.6,0.93] |
|
| |||||||
| Thinn. Score | -2066±2277 | -1111±766 | <.0001 | <.0001 | 1.48 [1.24,1.75] | <.0001 | 1.51 [1.27,1.8] |
| Thick.Score Change Score | 524±377 2590±2182 | 489±402 1600±610 | 0.4846 <.0001 | 0.4724 <.0001 | 0.91 [0.7,1.18] 0.65 [0.54,0.79] | 0.4537 <.0001 | 0.9 [0.69,1.18] 0.63 [0.52,0.77] |
SD standard deviation; CI = confidence interval; ccOR = case-control conditional logistic regression odds ratios adjusting for matching variables and bilateral knees; ccORbp = ccOR adjusting for the above and additionally for the effects of BMI and pain at baseline (T-2); mJSW = minimum joint space width in the medial femorotibial compartment, measured using fixed flexion radiographs; JSW225 = joint space width measured at a fixed location(22.5% from medial to lateral) in the medial femorotibial compartment, measured using fixed flexion radiographs; MFTC = total medial femorotibial compartment cartilage loss, as measured quantitative by MRI; cMT = central medial tibial cartilage loss, as measured quantitative by MRI; OV = ordered values = cartilage loss throughout 16 femorotibial subregions, sorted individually by magnitude (i.e. 1-16); Thinn. Score = total subregional thinning score = sum of all negative cartilage thickness changes across as many of the 16 subregions in which cartilage loss occurred in each knee, Thick. Score = total subregional cartilage thickening score = the sum of all positive cartilage thickness changes; Change Score = total subregional cartilage change score = sum or all 16 subregional cartilage thickness changes, independent of direction
MRI
MRI cartilage loss in the medial femorotibial compartment displayed similar discrimination between knees replaced and non-replaced controls (paired t-test p=0.001 and ccOR=1.38) as did minimum JSW (Table 1; Fig. 2). Central tibial cartilage loss showed higher odds ratios than the entire medial femorotibial compartment (p<0.0001 and ccOR=1.57), and similar discrimination to fixed location JSW (Table 1; Fig. 2).
As location-independent measures, OV1-OV10 discriminated significantly between matched pairs (range p=0.02 to p<0.0001), with greater cartilage loss observed in cases with knee replacement than in non-replaced controls, and with the greatest ccOR observed for OV2 (1.57; Table 1). OV13-16 suggested slightly greater subregional cartilage thickening in knee replacements than in controls, but the difference only reached significance for OV16 (p=0.02; ccOR=0.76). The total subregional thinning score was greater in knee replacements than in controls (p<0.0001; ccOR=1.48), whereas the difference in the total thickening score failed to reach statistical significance (Table 1, Fig. 2). The total subregional change score discriminated significantly between case-control pairs (p<0.0001; ccOR=0.65).
Sensitivity analyses
When restricting analysis to case-control pairs with less advanced radiographic disease stage at baseline (KLG0-2), the ORs for all imaging measures were greater than for the full sample; however, the relative performance of these measures was similar (Table 2). However, cartilage loss appeared to dominate in this group of “fast clinical progressors”, as subregional cartilage thickening (OVs 13-16, and total subregional thickening score) was less in knees replaced than in matched controls (Table 2).
Table 2.
Change in radiographic joint space width (JSW) and in medial femorotibial compartment (MFCT) and central tibia (cMT) cartilage thickness over a period of two years (T-2 →T0) in knees from **BLINDED** participants with baseline Kellgren Lawrence Grade (KLG) 0-2 who received a knee replacement (KR; n=36), and in non-KR control knees (n=36) matched 1:1 by KLG, sex, and age. Values in μm.
| KR Cases Mean ± SD |
Controls Mean ± SD |
Paired T P-value |
cc P-value |
ccOR [95% CI] |
ccbp P-value |
ccbp_OR [95% CI] |
|
|---|---|---|---|---|---|---|---|
| mJSW | -748±1339 | -224±451 | 0.026 | 0.0142 | 1.59 [1.1,2.31] | 0.0484 | 1.82 [1,3.31] |
| JSW 22.5% | -938±1364 | -200±444 | 0.0033 | 0.0023 | 2.79 [1.44,5.39] | 0.0016 | 2.78 [1.47,5.24] |
| MFTC | -240±495 | 8±136 | 0.0046 | 0.0073 | 2.35 [1.26,4.39] | 0.0028 | 2.93 [1.45,5.91] |
| cMT | -170±299 | 15±111 | 0.0009 | 0.0143 | 2.49 [1.2,5.15] | 0.0012 | 2.59 [1.46,4.61] |
| OV1 | -726±748 | -196±99 | 0.0002 | 0.0091 | 2.35 [1.24,4.46] | 0.0013 | 2.48 [1.43,4.32] |
| OV2 | -499±562 | -138±75 | 0.0006 | 0.0414 | 2.34 [1.03,5.3] | 0.0015 | 2.37 [1.39,4.03] |
| OV3 | -407±543 | -103±66 | 0.0023 | 0.1292 | 2.18 [0.8,5.99] | 0.0739 | 2.58 [0.91,7.32] |
| OV4 | -337±434 | -78±63 | 0.0015 | 0.1152 | 2.03 [0.84,4.89] | 0.0551 | 2.19 [0.98,4.87] |
| OV5 | -263±359 | -49±52 | 0.0013 | 0.0674 | 3.66 [0.91,14.67] | 0.0166 | 3.93 [1.28,12.07] |
| OV6 | -205±292 | -33±55 | 0.0015 | 0.0345 | 3.38 [1.09,10.43] | 0.0005 | 4.99 [2.02,12.29] |
| OV7 | -149±248 | -18±48 | 0.0037 | 0.0166 | 3.3 [1.24,8.79] | 0.0002 | 4.02 [1.95,8.29] |
| OV8 | -105±155 | -5±47 | 0.0007 | 0.0081 | 2.97 [1.33,6.63] | 0.001 | 3.58 [1.68,7.62] |
| OV9 | -63±98 | 9±48 | 0.0002 | 0.0044 | 3.27 [1.45,7.39] | 0.0026 | 3.98 [1.62,9.79] |
| OV10 | -36±92 | 24±53 | 0.001 | 0.0102 | 2.62 [1.26,5.46] | 0.0037 | 3.09 [1.44,6.64] |
| OV11 | -9±82 | 38±54 | 0.0042 | 0.0133 | 2.2 [1.18,4.12] | 0.0125 | 2.18 [1.18,4.02] |
| OV12 | 18±73 | 56±56 | 0.0078 | 0.0277 | 2.06 [1.08,3.94] | 0.0504 | 1.87 [1,3.49] |
| OV13 | 44±65 | 69±56 | 0.0587 | 0.0858 | 1.72 [0.93,3.21] | 0.1135 | 1.61 [0.89,2.89] |
| OV14 | 71±67 | 92±65 | 0.1703 | 0.1274 | 1.43 [0.9,2.26] | 0.2609 | 1.33 [0.81,2.21] |
| OV15 | 100±73 | 126±85 | 0.1641 | 0.0823 | 1.43 [0.96,2.13] | 0.1803 | 1.39 [0.86,2.27] |
| OV16 | 191±98 | 185±112 | 0.814 | 0.8058 | 0.95 [0.66,1.39] | 0.7865 | 0.93 [0.54,1.59] |
|
| |||||||
| Thinn. Score | -2883±3519 | -712±407 | 0.0009 | 0.0977 | 2.88 [0.82,10.08] | 0.0463 | 3.84 [1.02,14.44] |
| Thick. Score | 508±269 | 692±484 | 0.0316 | 0.0276 | 1.89 [1.07,3.33] | 0.0507 | 1.82 [1,3.3] |
| Change Score | 3391±3399 | 1403±337 | 0.0018 | 0.0005 | 0.63 [0.48,0.81] | 0.0008 | 0.6 [0.45,0.81] |
SD standard deviation; CI = confidence interval; ccOR = case-control conditional logistic regression odds ratios adjusting for matching variables and bilateral knees; ccORbp = ccOR adjusting for the above and additionally for the effects of BMI and pain at baseline (T-2); mJSW = minimum joint space width in the medial femorotibial compartment, measured using fixed flexion radiographs; JSW225 = joint space width measured at a fixed location(22.5% from medial to lateral) in the medial femorotibial compartment, measured using fixed flexion radiographs; MFTC = total medial femorotibial compartment cartilage loss, as measured quantitative by MRI; cMT = central medial tibial cartilage loss, as measured quantitative by MRI; OV = ordered values = cartilage loss throughout 16 femorotibial subregions, sorted individually by magnitude (i.e. 1-16); Thinn. Score = total subregional thinning score = sum of all negative cartilage thickness changes across as many of the 16 subregions in which cartilage loss occurred in each knee, Thick. Score = total subregional cartilage thickening score = the sum of all positive cartilage thickness changes; Change Score = total subregional cartilage change score = sum or all 16 subregional cartilage thickness changes, independent of direction
When restricting analysis to the 70 case control pairs in which the location of the baseline JSN was observed in the same (medial or lateral) compartment, both the ORs and the relative performance of the measures were similar to the full sample (Table 3). However, when accounting for JSN location, subregional thickening was found to be significantly greater in OV14-16 of knees replaced than in non-replaced controls, despite the smaller sample (Table 3).
Table 3.
Change in radiographic joint space width (JSW) and in medial femorotibial compartment (MFCT) and central tibia (cMT) cartilage thickness over a period of two years (T-2 →T0) in knees from **BLINDED** participants prior to knee replacement (KR; n=70) and in non-KR control knees (n=70) matched 1:1 by Kellgren-Lawrence grade (KLG) sex, and age, in which the location of the baseline joint space narrowing (JSN) was observed in the same (medial or lateral) femorotibial compartment.
| KR Cases Mean ± SD |
Controls Mean ± SD |
Paired T P-value |
cc P-value |
ccOR [95% CI] |
ccbp P-value |
ccbp_OR [95% CI] |
|
|---|---|---|---|---|---|---|---|
| mJSW | -428±1009 | -99±602 | 0.018 | 0.0141 | 1.41 [1.07,1.86] | 0.0021 | 1.58 [1.18,2.11] |
| JSW 22.5% | -630±1053 | -165±588 | 0.003 | 0.0047 | 1.51 [1.14,2.01] | 0.0019 | 1.56 [1.18,2.06] |
|
| |||||||
| MFTC | -238±346 | -129±192 | 0.0257 | 0.0103 | 1.32 [1.07,1.64] | 0.0037 | 1.35 [1.1,1.65] |
| cMT | -204±221 | -94±163 | 0.0016 | 0.0009 | 1.58 [1.2,2.07] | 0.0023 | 1.66 [1.2,2.29] |
|
| |||||||
| OV1 | -462±357 | -307±182 | 0.0021 | 0.0029 | 1.51 [1.15,1.98] | 0.0019 | 1.59 [1.19,2.13] |
| OV2 | -324±252 | -200±136 | 0.0004 | 0.0006 | 1.6 [1.22,2.09] | 0.0015 | 1.72 [1.23,2.4] |
| OV3 | -246±213 | -163±123 | 0.005 | 0.003 | 1.44 [1.13,1.84] | 0.0053 | 1.52 [1.13,2.05] |
| OV4 | -188±161 | -119±80 | 0.0023 | 0.0012 | 1.46 [1.16,1.84] | 0.0019 | 1.55 [1.18,2.04] |
| OV5 | -154±144 | -94±75 | 0.0041 | 0.0019 | 1.41 [1.14,1.76] | 0.0017 | 1.48 [1.16,1.89] |
| OV6 | -121±127 | -72±70 | 0.0082 | 0.0019 | 1.38 [1.13,1.7] | 0.0008 | 1.44 [1.16,1.77] |
| OV7 | -79±90 | -54±63 | 0.0668 | 0.0395 | 1.28 [1.01,1.63] | 0.0147 | 1.33 [1.06,1.67] |
| OV8 | -57±87 | -39±61 | 0.1545 | 0.0935 | 1.22 [0.97,1.54] | 0.033 | 1.28 [1.02,1.59] |
| OV9 | -36±77 | -26±60 | 0.4196 | 0.3525 | 1.12 [0.88,1.43] | 0.1991 | 1.16 [0.92,1.46] |
| OV10 | -14±74 | -11±60 | 0.7601 | 0.7395 | 1.04 [0.81,1.35] | 0.5165 | 1.09 [0.85,1.39] |
| OV11 | 13±67 | 6±58 | 0.5175 | 0.4913 | 0.9 [0.68,1.2] | 0.6932 | 0.95 [0.71,1.25] |
| OV12 | 32±66 | 25±57 | 0.4578 | 0.4338 | 0.89 [0.67,1.19] | 0.6844 | 0.94 [0.7,1.26] |
| OV13 | 52±69 | 38±58 | 0.2184 | 0.2023 | 0.83 [0.63,1.1] | 0.3101 | 0.87 [0.66,1.14] |
| OV14 | 86±79 | 55±62 | 0.0185 | 0.0166 | 0.71 [0.54,0.94] | 0.0178 | 0.72 [0.55,0.94] |
| OV15 | 126±94 | 91±78 | 0.0236 | 0.0333 | 0.72 [0.53,0.97] | 0.0259 | 0.72 [0.54,0.96] |
| OV16 | 195±129 | 150±96 | 0.0307 | 0.0148 | 0.75 [0.59,0.94] | 0.0108 | 0.73 [0.57,0.93] |
|
| |||||||
| Thinn. Score | -1768±1442 | -1180±835 | 0.0044 | 0.0014 | 1.43 [1.15,1.79] | 0.001 | 1.52 [1.19,1.96] |
| Thick. Score | 593±515 | 459±389 | 0.1071 | 0.0904 | 0.79 [0.6,1.04] | 0.0971 | 0.79 [0.6,1.04] |
| Change Score | 2360±1335 | 1638±670 | 0.0002 | 0.0003 | 0.61 [0.47,0.8] | 0.0001 | 0.54 [0.4,0.74 |
For abbrêviations please see Table 1
Discussion
In this study we have explored for the first time the relative performance of fixed location and minimum radiographic JSW, and that of region-specific and location-independent MRI measures of cartilage thickness change, in predicting femorotibial knee replacement as a clinical outcome. The reliability of the fixed location measurements [23] and that of subregional MRI measurements has been described previously [24], and a face-to-face comparison of their responsiveness (i.e. sensitivity to change in knee osteoarthritis) has also been presented [11]. Change in fixed location radiographic JSW differed more between knees replaced and non-replaced controls than that in minimum medial JSW, a measure recognized as structural endpoint for disease modifying osteoarthritis drug intervention trials by regulatory agencies. The “feasibility” of both measurements (fixed-location radiographic JSW measurement and minimum JSW measurement) is very similar, since they are both based on the delineated joint margins from the same automated measurement technique [23] using the very same radiographic acquisition. Since the “responsiveness” of fixed location JSW in knee osteoarthritis has previously been shown to be greater than that of minimum JSW [11], the current results suggest that fixed location measurements of radiographic JSW are superior to minimum JSW and should preferably be used in future studies. MRI measurement of central tibial cartilage thickness change showed similar discrimination between knee replacements and controls to fixed location JSW. Location-independent measures of femorotibial cartilage change suggested “perturbation” of cartilage thickness prior to knee replacement, with greater rates of subregional thickening and loss occurring simultaneously than in non-replaced controls.
Despite the great clinical success of knee replacement, the criteria on which surgery is performed are not uniform. Apart from symptom and radiographic status, surgical indication depends on willingness, comorbidity, access to health care, socio-economic status, etc. Yet, knee replacement represents a “hard” outcome and a socioeconomic reality and thus a clinical endpoint against which an imaging biomarker and the effect of disease modifying osteoarthritis drugs should be evaluated [6,14]. A limitation of the current study is that, albeit controls did not undergo knee replacement up to 60M, they may have been replaced later. Also, controls may have been in need for knee replacement, but did not receive it for reasons mentioned above. Future studies that may use a validated “virtual” knee replacement (vTKR) indication as a clinical outcome may circumvent such classification issues and potentially improve the discrimination between cases and controls. Further, the current study focused on quantitative measures of radiographic change and cartilage loss, while a recent study examined the ability of other features of structural pathology for predicting knee replacement. [28].
Only one prior study compared radiographic JSW and cartilage volume change with respect to clinical outcome [16]. The authors showed a trend towards a significant relationship between 2-year change in medial femorotibial compartment volume and knee replacement at year 4 (OR=9.0, p=0.07), but no relationship for minimum JSW change (OR=1.1, p=0.92). In that study, however, radiographs were acquired in full knee extension. Further, only 28 of 113 subjects had radiographs taken with sufficient quality to support JSW measures, with the statistical analysis based on 5 knees with replacement [16]. Our current results contradict the above findings in that, when fixed flexion radiographs are used, radiographic JSW appears to discriminate similarly between knees replaced and non-replaced controls as MRI-based cartilage thickness measures. Further, fixed-location measurements appeared to be superior to minimum JSW.
The ORs observed in the current study are smaller than those reported above [16], and than those in another study (113 participants, 18 with knee replacement) focusing on MRI cartilage volume change alone [12]. However, in the current study cases and controls were matched for age, sex, and for baseline radiographic status (KLG): Since knees with advanced radiographic knee osteoarthritis exhibit substantially larger rates of cartilage loss than those at an earlier stage [19] and also are more likely to receive knee replacement in the intermediate future, it is obvious that ORs are substantially lower using the matched case-control design, since they reflect the differences observed “over and above” radiographic baseline status rather than the total difference between knees in case cohort studies.
Studying knee radiographs acquired in full extension, Bruyere et al. [8] reported cut-offs of 0.5 to 0.8mm in minimum JSW change over 3 years to discriminate between knees who received a knee replacement up to 8 years follow-up (n=16) versus those who did not. Change in mean JSW, in contrast, was not predictive of knee replacement [8]. The results of our current study extend these findings in several important ways: it uses a nested matched case-control study design, confirming that differences in change in minimum JSW exist between knees replaced and controls, even after matching for baseline KLG status; it studies a non-fluoroscopic radiographic acquisition technique now commonly used in clinical trials; it suggests that with fixed flexion radiography, fixed location JSW change at 22.5% from medial to lateral (x=0.225) is superior in discriminating between knees replaced vs. matched controls than minimum JSW; and it shows that medial cartilage loss by MRI has similar ability of predicting knee replacement as a clinical outcome as the radiographic measures currently accepted for disease modification by regulatory agencies. The latter finding is important, because radiographs are generally part of the decision making in an indication to knee replacement surgery, whereas quantitative MRI cartilage loss represents an independent measure.
Location-independent MRI-based measures of femorotibial cartilage thickness change, including OVs and novel sum scores, appear to exhibit a similar ability of discriminating between knees replaced and controls as the most discriminative region/location-specific MRI (cMT) and radiographic measure (JSW at x=0.225). Although apparently not superior in predicting knee replacement, these measures were shown to be statistically superior in discriminating rates of cartilage loss between radiographic strata [18,19] than region-specific approaches of MRI-based cartilage loss and radiographic JSW change. Further, these location-independent measures preclude the need to define a specific cartilage region of interest a priori, tailored to the study inclusion criteria such as medial or lateral compartment involvement, and they can help to assess subregional cartilage thickness change in either direction (loss or swelling) independently. The current study provides evidence that not only cartilage loss, but also subregional thickness gain was greater over 2 years prior to knee replacement than in matched controls. Greater simultaneous subregional cartilage thickness gain and loss also have been recently reported after anterior cruciate ligament injury [25] and may describe a state of cartilage “perturbation”, during which cartilage loss in some locations is accompanied by cartilage swelling or hypertrophy in others. Such observations are unique to the use of location-independent MRI and are obscured when only region-specific measurements are performed by MRI or radiography [25].
In conclusion, discrimination of structural progression rates between knees replaced versus controls were greater for fixed-location radiographic JSW than for minimum medial JSW. MRI-measures of cartilage thickness change displayed similar discrimination between knee replacements and non-replaced controls to radiography and suggested “perturbation” of cartilage thickness prior to knee replacement, with greater rates of subregional thickening and loss occurring simultaneously than in non-replaced controls. Drugs that attempt to modify the structural changes that lead to knee replacement may thus have to stabilize cartilage by preventing both cartilage loss, and cartilage thickening, due to swelling or hypertrophy.
Figure 3.
- Minimum radiographic joint space width in the medial compartment (mJSW)
- Fixed location radiographic JSW at 22.5% from medial to lateral (x=0.225)
- MRI-based cartilage thickness loss in the medial femorotibial compartment (MFTC)
- MRI-based cartilage thickness loss in the central medial tibia (cMT)
- MRI-based cartilage thickness loss in the subregion with the greatest loss (OV1)
- MRI-based cartilage thickness gain in the subregion with the greatest gain (OV16)
- Sum scores of subregion cartilage thinning (Thinn. Score)
- Sum scores of subregion cartilage thickening (Thick. Score)
Key Points.
Fixed-location JSW predicts surgical knee replacement more strongly than minimum JSW.
MRI predicts knee replacement with similar accuracy as radiographic JSW.
MRI reveals greater cartilage thinning and thickening prior to knee replacement.
Acknowledgments
The authors thank the following operators at Chondrometrics GmbH: Gudrun Goldmann, Linda Jakobi, Manuela Kunz, Tanja Killer, Dr. Susanne Maschek, Jana Matthes, Tina Matthes, Sabine Mühlsimer, Julia Niedermeier, Annette Thebis, Dr. Barbara Wehr and Dr. Gabriele Zeitelhack for the segmentation of the magnetic resonance imaging data. Susanne Maschek is to be thanked for quality control readings of the segmentations.
Further, the authors would like to thank the readers of the fixed flexion radiographs at Boston University for the central KL grading, the OAI investigators, clinic staff and OAI participants at each of the OAI clinical centers for their contributions in acquiring the publicly available clinical and imaging data, the team at the OAI coordinating center, particularly John Lynch, Maurice Dockrell, and Jason Maeda, for their help in selecting images and verifying the knee replacements radiographically, and Stephanie Green and Hilary Peterson at Pittsburgh for administrative support.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The scientific guarantor of this publication is Dr. C. Kent Kwoh. This work was supported by by the OAI, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
The image analysis of this study was partly funded by a contract with the University of Pittsburgh (Pivotal OAI MRI Analyses POMA: NIH/NHLBI Contract No. HHSN2682010000 21C), by a vendor contract from the OAI coordinating center at University of California, San Francisco (N01-AR-2-2258), by an ancillary grant to the OAI held by Northwestern University (NIH/NIAMS R01 AR052918 [Sharma]) and by Novartis Pharma AG (Basel, Switzerland) and MerckKGaA (Darmstadt, Germany).
The statistical data analysis was funded by a contract with the University of Pittsburgh (Pivotal OAI MRI Analyses POMA: NIH/NHLBI Contract No. HHSN2682010000 21C) and the University of Pittsburgh Multidisciplinary Clinical Research Center (MCRC) for Rheumatic and Musculoskeletal Diseases (P60 AR054731).
The sponsors were not directly involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The statistical analysis of the data was conducted by an independent statistical team at an academic institution (the University of Pittsburgh) which was independent of the commercial sponsors. No compensation or funding from a commercial sponsor was received for conducting the statistical analyses.
Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Some study subjects or cohorts have been previously reported in been previously published in:
Trajectory of cartilage loss within 4 years of knee replacement--a nested case-control study from the Osteoarthritis Initiative. Eckstein F, Boudreau RM, Wang Z, Hannon MJ, Wirth W, Cotofana S, Guermazi A, Roemer F, Nevitt M, John MR, Ladel C, Sharma L, Hunter DJ, Kwoh CK; OAI investigators.
Osteoarthritis Cartilage. 2014 Oct;22(10):1542-9. doi: 10.1016/j.joca.2014.04.016. Epub 2014 Apr 30.
The authors of this manuscript declare relationships with the following companies: Chondrometrics GmbH, MerckSerono, Mariel Therapeutics, Medtronic, Pfizer, Eli Lilly, Glaxo Smith Kline, Centocor R&D, Wyeth, Novartis, Abbvie, Stryker, Synarc, Ampio, Boston Imaging Core Lab (BICL), Ortho-Trophix, Genzyme, TissueGene,Novartis Pharma AG, Merck KGaA, Allergan. Methodology: retrospective, case-control study diagnostic or prognostic study / observational, multicenter study.
References
- 1.Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, Rome BN, Chen SP, Hunter DJ, Suter LG, Jordan JM, Katz JN. Lifetime Risk and Age at Diagnosis of Symptomatic Knee Osteoarthritis in the US. Arthritis Care Res (Hoboken) 2013;65(5):703–711. doi: 10.1002/acr.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, Jordan JM, Hunter DJ, Suter LG, Weinstein AM, Paltiel AD, Katz JN. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154(4):217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz JN, Brophy RH, Chaisson CE, de CL, Cole BJ, Dahm DL, Donnell-Fink LA, Guermazi A, Haas AK, Jones MH, Levy BA, Mandl LA, Martin SD, Marx RG, Miniaci A, Matava MJ, Palmisano J, Reinke EK, Richardson BE, Rome BN, Safran-Norton CE, Skoniecki DJ, Solomon DH, Smith MV, Spindler KP, Stuart MJ, Wright J, Wright RW, Losina E. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368(18):1675–1684. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roemer FW, Eckstein F, Hayashi D, Guermazi A. The role of imaging in osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):31–60. doi: 10.1016/j.berh.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein F, Guermazi A, Gold G, Duryea J, Hellio Le Graverand MP, Wirth W, Miller CG. Imaging of cartilage and bone: promises and pitfalls in clinical trials of osteoarthritis. Osteoarthritis Cartilage. 2014;22(10):1516–1532. doi: 10.1016/j.joca.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelletier JP, Cooper C, Peterfy C, Reginster JY, Brandi ML, Bruyere O, Chapurlat R, Cicuttini F, Conaghan PG, Doherty M, Genant H, Giacovelli G, Hochberg MC, Hunter DJ, Kanis JA, Kloppenburg M, Laredo JD, McAlindon T, Nevitt M, Raynauld JP, Rizzoli R, Zilkens C, Roemer FW, Martel-Pelletier J, Guermazi A. What is the predictive value of MRI for the occurrence of knee replacement surgery in knee osteoarthritis? Ann Rheum Dis. 2013;72(10):1594–1604. doi: 10.1136/annrheumdis-2013-203631. [DOI] [PubMed] [Google Scholar]
- 7.Chu Miow LD, Reichmann WM, Gossec L, Losina E, Conaghan PG, Maillefert JF. Validity and responsiveness of radiographic joint space width metric measurement in hip osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2011;19(5):543–549. doi: 10.1016/j.joca.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruyere O, Richy F, Reginster JY. Three year joint space narrowing predicts long term incidence of knee surgery in patients with osteoarthritis: an eight year prospective follow up study. Ann Rheum Dis. 2005;64(12):1727–1730. doi: 10.1136/ard.2005.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyere O, Pavelka K, Rovati LC, Gatterova J, Giacovelli G, Olejarova M, Deroisy R, Reginster JY. Total joint replacement after glucosamine sulphate treatment in knee osteoarthritis: results of a mean 8-year observation of patients from two previous 3-year, randomised, placebo-controlled trials. Osteoarthritis Cartilage. 2008;16(2):254–260. doi: 10.1016/j.joca.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, Le Graverand MP, Luchi M, Beals CR, Hunter DJ. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010;62(7):932–937. doi: 10.1002/acr.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth W, Duryea J, Hellio Le Graverand MP, John MR, Nevitt M, Buck RJ, Eckstein F. Direct comparison of fixed flexion, radiography and MRI in knee osteoarthritis: responsiveness data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21(1):117–125. doi: 10.1016/j.joca.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicuttini FM, Jones G, Forbes A, Wluka AE. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: a prospective study. Ann Rheum Dis. 2004;63(9):1124–1127. doi: 10.1136/ard.2004.021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raynauld JP, Martel-Pelletier J, Haraoui B, Choquette D, Dorais M, Wildi LM, Abram F, Pelletier JP. Risk factors predictive of joint replacement in a 2-year multicentre clinical trial in knee osteoarthritis using MRI: results from over 6 years of observation. Ann Rheum Dis. 2011;70(8):1382–1388. doi: 10.1136/ard.2010.146407. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Kwoh CK, Boudreau R, Wang Z, Hannon M, Cotofana S, Hudelmaier M, Wirth W, Guermazi A, Nevitt M, John MR, Hunter DJ for the OAI investigators. Quantitative magnetic resonance imaging measures of cartilage predict knee replacement - a case-control study from the Osteoarthritis Initiative. Ann Rheum Dis. 2013;72:707–714. doi: 10.1136/annrheumdis-2011-201164. [DOI] [PubMed] [Google Scholar]
- 15.Eckstein F, Boudreau RM, Wang Z, Hannon MJ, Wirth W, Cotofana S, Guermazi A, Roemer F, Nevitt M, John MR, Ladel C, Sharma L, Hunter DJ, Kwoh CK. Trajectory of cartilage loss within 4 years of knee replacement - a nested case-control study from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2014;22(10):1542–1549. doi: 10.1016/j.joca.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cicuttini F, Hankin J, Jones G, Wluka A. Comparison of conventional standing knee radiographs and magnetic resonance imaging in assessing progression of tibiofemoral joint osteoarthritis. Osteoarthritis Cartilage. 2005;13(8):722–727. doi: 10.1016/j.joca.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Chang A, Moisio K, Chmiel JS, Eckstein F, Guermazi A, Almagor O, Cahue S, Wirth W, Prasad P, Sharma L. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann Rheum Dis. 2011;70(1):74–79. doi: 10.1136/ard.2010.130278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck RJ, Wirth W, Dreher D, Nevitt M, Eckstein F. Frequency and spatial distribution of cartilage thickness change in knee osteoarthritis and its relation to clinical and radiographic covariates - data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2013;21(1):102–109. doi: 10.1016/j.joca.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Wirth W, Buck R, Nevitt M, Le Graverand MP, Benichou O, Dreher D, Davies RY, Lee JH, Picha K, Gimona A, Maschek S, Hudelmaier M, Eckstein F. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography--data from the OA initiative. Osteoarthritis Cartilage. 2011;19(6):689–699. doi: 10.1016/j.joca.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckstein F, Kwoh CK, Link TM. Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205310. [DOI] [PubMed] [Google Scholar]
- 22.Schneider E, NessAiver M. The Osteoarthritis Initiative (OAI) magnetic resonance imaging quality assurance update. Osteoarthritis Cartilage. 2013;21(1):110–116. doi: 10.1016/j.joca.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann G, Hunter D, Nevitt M, Chibnik LB, Kwoh K, Chen H, Harris T, Satterfield S, Duryea J. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage. 2009;17(6):761–765. doi: 10.1016/j.joca.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein F, Wirth W, Lohmander LS, Hudelmaier MI, Frobell RB. Five-year follow-up of knee joint cartilage thickness changes after acute anterior cruciate ligament rupture. Arthritis Rheumatol. 2014 doi: 10.1002/art.38881. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, Beary JF, Cline GA, Meyer JM, Martel-Pelletier J. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saunders J, Ding C, Cicuttini F, Jones G. Radiographic osteoarthritis and pain are independent predictors of knee cartilage loss: a prospective study. Intern Med J. 2011:10–5994. doi: 10.1111/j.1445-5994.2011.02438.x. [DOI] [PubMed] [Google Scholar]
- 28.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Wang Z, Boudreau RM, John MR, Nevitt MC, Guermazi A. Can Structural Joint Damage Measured with MR Imaging Be Used to Predict Knee Replacement in the Following Year? Radiology. 2014:140991. doi: 10.1148/radiol.14140991. [DOI] [PMC free article] [PubMed] [Google Scholar]