Abstract
Background
Multinutrient insufficiencies as a consequence of nutritional and economic factors are common in India and other developing countries. We have examined the impact of multi-nutrient insufficiency on markers of one carbon metabolism in the blood, and response to a methionine load in clinically healthy young women.
Design & Methods
Young women from Pune, India (n=10) and Cleveland, USA (n=13) were studied. Blood samples were obtained in the basal state and following an oral methionine load (50mg/kg of body weight in orange juice). Plasma concentrations of vitamin B12, folate and B6 were measured in the basal state. The effect of methionine load on the levels of methionine, total homocysteine, cysteine, glutathione and amino acids was examined.
Results
Indian women were significantly shorter and lighter compared with the American women and had lower plasma concentration of vitamins B12, folate and B6, essential amino acids and glutathione, but higher concentration of total homocysteine. The homocysteine response to methionine load was higher in Indian women. The plasma concentrations of glycine and serine increased in the Indian women after methionine (in juice) load. A significant negative correlation between plasma B6 and homocysteine (r= −0.70), and plasma folate and glycine and serine levels were observed in the Indian group (P<0.05) but not in the American group.
Conclusion
Multi-nutrient insufficiency in the Indian women caused unique changes in markers of whole body protein and one carbon metabolism. These data would be useful in developing nutrient intervention strategies.
Introduction
Folate one-carbon transfers, present ubiquitously in every cell in the body, are key components of cell metabolism. They are involved in transfer of methyl groups for biological methylation reactions including synthesis of nucleotides. In addition to folate, vitamin B12 (B12) and vitamin B6 (B6) along with insulin and glucagon are respectively key co-factors and hormonal regulators of one carbon metabolism in-vivo. Perturbations of one carbon metabolism as a consequence of changes in nutrient status of the individual or of hormonal, and environmental interactions, have been related to birth defects, cancer, metabolic disorders, cardio-vascular disease and to aberrant DNA methylation patterns. Because of their critical role in whole body and cell metabolism, a number of intracellular and circulating biomarkers of nutrient deficiencies related to one carbon metabolism have been identified and validated in order to develop and monitor intervention strategies for the ‘at risk’ populations.1–5 Studies in both human and animal models have examined the impact of micro-nutrient deficiency or insufficiency on biomarkers of one carbon metabolism in the plasma and tissues.1,3 Most of these studies have examined the association between a single nutrient deficiency with the identified biochemical marker/s.1–4 Isolated single nutrient deficiencies although observed in otherwise well-nourished populations, they are uncommon in the undernourished populations, particularly in relation to folate, B12, B6, and protein, the nutrients that impact one carbon metabolism6,7,8.
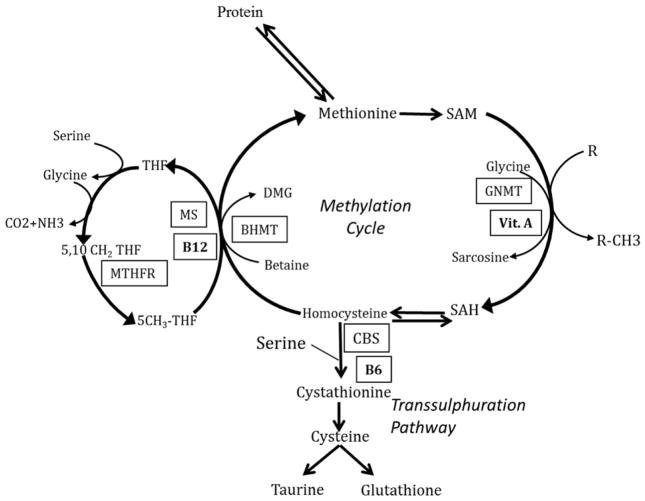
The folate-methionine cycle and its key regulatory cofactors are displayed in Figure 1. As shown, serine and glycine are the major contributors of one carbon (1C) units. In this process, serine is converted reversibly to glycine in a B6 dependent reaction catalysed by serine hydroxymethyl transferase and the 1C unit is transferred to tetrahydrofolate (THF), to from 5,10-methylene tetrahydrofolate. Glycine contributes 1C units via the glycine cleavage system to tetrahydrofolate. Methionine is activated to form s-adenosylmethionine (SAM) by methionine adinosyl transferase and ATP. SAM is the key methyl donor for methylation reactions catalysed by various methyltransferases and in the process is converted to s-adenosyl homocysteine (SAH) and ultimately to homocysteine. SAM also can be converted, in the liver, to SAH by glycine-n-methyl transferase (GNMT). Vitamin A is a transcriptional or translational regulator of GNMT activity. Homocysteine can either be converted back to methionine (remethylation) catalysed by methionine synthase or metabolized to cystathionine and cysteine (transsulfuration). B12 is the cofactor for methionine synthase (5-methyltetrahydrofolate homocysteine methyltransferase) responsible for the transfer of methyl group of 5-methyl tetrahydrofolate to homocysteine to form methionine (remethylation). The two enzymes of the transsulfuration cascade require B6 as a cofactor. Isolated deficiency of the cofactors (B6, B12 or folate) can result in increased levels of the precursor and lower levels of the immediate product. In addition, isocaloric protein restriction in animal models and lower dietary protein intake in humans has been shown to result in increase in plasma levels of homocysteine, serine and glycine9–11. The combined effect of deficiency or insufficiency of these micronutrients and lower protein intake has not been examined. In the present study, we have examined the integrated changes in one carbon metabolism in response to multi-nutrient insufficiency in an otherwise “healthy” group of Indian women and compared with a group of “nutritionally sufficient” American women.
Figure 1. One-carbon metabolism in-vivo in man, described in detail in the text.
- BHMT
- Betaine-homocysteine S-methyltransferase
- CBS
- Cystathionine-β-synthase
- GNMT
- Glycine N-methyltransferase
- MTHFR
- Methylenetetrahydrofolate reductase
- MS
- Methionine Synthase
- R
- Methyl acceptor
- R-CH3
- Methylated compound
- SAH
- S-adenosylhomocysteine
- SAM
- S-adenosylmethionine
- THF
- Tetrahydrofolate
Subjects and Methods
The study participants in India (n=10) were young healthy female staff members of the King Edward Memorial Hospital Research Centre, Pune. Healthy young women in Cleveland, USA (n=13) were recruited by advertisement. The study protocol was approved by the Ethics Committee of the KEM Hospital Research Centre and by the Institutional Review Board of the Cleveland Clinic. Written informed consent was obtained from the participants after fully explaining the procedure.
The subjects reported to the research unit at 7 am following a 12 hour fast. Height and weight were measured as per the standard protocol. An indwelling cannula was placed in an antecubital vein and subjects were allowed to rest for half an hour. After obtaining a basal blood sample, L-methionine (50 mg/kg body weight) was given in orange juice along with a standardized low methionine breakfast (estimated total methionine content ~58mg). We elected to give a lower dose of methionine instead of the usual 100mg/kg body weight, and perform a short 5 hour instead of 6 hour test for the following reasons: (i) Results of the dose response studies show that 50mg/kg can give data similar to the higher dose without compromising the sensitivity of the test12,13 (ii) a short 3 hour test was as good as the standard 6 hour test in identifying subjects with hyperhomocysteinemia14,15, and (iii) our ultimate goal is to do these studies during pregnancy and we were concerned about the potential toxicity of methionine and homocysteine with the higher dose to the mother and the developing conceptus. Blood samples, in EDTA tubes, were drawn at hourly interval for the next 5 hours. Five subjects in the Cleveland group and five in the Indian group received methionine load without the accompanying breakfast. Since there was no significant difference in the response to methionine load in those with or without breakfast the data were combined. Blood samples were centrifuged in cold and the plasma was stored at −80°C for analysis later.
Laboratory analysis
Amino acid concentration in plasma were measured by HPLC using an OPA derivative and a fluorescence detector as described.16 Total homocysteine, total cysteine, and glutathione in the plasma were measured using HPLC.17 Plasma formate levels were measured by an isotope-dilution GC-MS method as described by Lammarre et al.18
Vitamin B6 (Pyridoxal-5-phosphate and Pyridoxal) was measured using commercially available HPLC kit (RECIPE GmbH, Munich, Germany) using post column derivatisation and fluorescence detector. Plasma cobalamin (vitamin B12) and folate were measured by microbiological assay using a colistin sulfate-resistant strain of Lactobacillus leichmanii and a chloramphenicol-resistant strain of Lactobacillus casei respectively19, 20. The coefficients of variation for B12 and folate measurement in the plasma were < 8% and for B6 it was < 5%. High sensitive C-reactive protein (hsCRP) was measured by high-sensitivity ELISA kit (United Biotech, Mountain View, CA, USA) with inter and intra batch coefficient of variation <11%.
Statistical Methods
We performed descriptive statistics and checked for normality of the data. Since data were skewed and the sample size small, we used non parametric methods for statistical analysis. Data are presented as median and 25th and 75th percentiles. Association of homocysteine with B12, folate and B6 was analysed by Spearman’s rank correlation coefficient. Differences in various parameters between Indian and US participants were analyzed by Mann Whitney U test. The incremental (above basal) area under the curve for homocysteine and methionine (basal to 5 hour) was computed using trapezoidal rule. Multiple linear regression analysis (MLRA) was used to compute variation in outcome variable (homocysteine) explained by exposure variables (B6, B12 and folate). Statistical analyses were performed using SPSS 16 (SPSS Inc. Chicago US).
Based on the published literature, the total homocysteine values in Indians is: 23.2 (13.1) and 10.4 (3.6) in western population. Therefore sample size of 8 provides a power of 80% at 5% level of significance and sample size of 11 in each group provides a power of 90% at 5% level of significance. Hence, we have a chosen sample size of 10 in Indians and 13 in US.
Results
Indian women were on average 30 yrs old and predominantly vegetarian. American women were of similar age, and were non-vegetarian in their dietary habits (Table 1). The Indian women were on average 20 cm shorter and 20 kg lighter, though the BMI was not significantly different in the two groups. The plasma levels of B6, folate and B12 in the US group were in the accepted ‘reference’ range. In contrast, the levels of B6, folate and B12 were significantly lower in the Indian subjects. The plasma levels of hsCRP were not different amongst the two groups.
Table 1.
Demographic, nutritional and biochemical characteristics of study subjects
| Indians n=10 | US n=13 | P | |
|---|---|---|---|
| Age-y | 30.2 (24.0, 34.1) | 27.0 (25.0, 30.0) | ns |
| Height-cm | 152.0 (147.7, 160.8) | 171.2 (163.5, 175.3) | 0.001 |
| Weight-kg | 52.6 (45.2, 56.6) | 71.8 (58.0, 89.1) | 0.001 |
| BMI-kg/m2 | 22.0 (18.7, 25.7) | 23.3 (20.6, 30.0) | ns |
| Vitamin B12-pmoles/l | 130.5 (104.1,197.1) | 308.0 (266.0,588.0) | <0.001 |
| Folate- nmoles/l | 19.5 (15.6,21.3) | 30.0 (27.6,40.7) | <0.001 |
| Vitamin B6- nmoles/l | 38.4 (34.4, 50.3) | 114.0 (58.2, 165.4) | 0.001 |
| Vitamin B6 PLP- nmoles/l | 8.9 (8.2, 10.3) | 13.1 (10.7, 18.8) | ns |
| CRP- microg /dl | 121.7 (47.2, 261.0) | 165.1 (76.8, 269.5) | ns |
| Homocysteine-μmol/l | 20.4 (16.4, 24.4) | 7.9 (6.8, 8.9) | <0.001 |
| Cysteine-μmol/l | 332.2 (314.6, 346.8) | 371.6 (328.1, 419.3) | ns |
| Glutathione-μmol/l | 3.8 (2.2, 3.9) | 6.5 (5.4, 7.5) | <0.001 |
Values are 50th (25th – 75th) percentiles. P value by Mann-Whitney test
Reference values: B12 >150pmoles/l, Folate >7nmoles/l, B6 > 21.2 nmoles/l and tHcy >15μmol/l
The plasma levels of amino acids during fasting are displayed in Table 2. As shown, the levels of essential amino acids (valine, phenylalanine, leucine, isoleucine, lysine and methionine) were significantly lower in the Indian women. The levels of histidine and aminobutyric acid also were lower in the Indian women. The concentration of serine and glycine although higher in the Indian group, were not statistically different.
Table 2.
Plasma amino acids values (micromoles/l) during fasting
| Indians | US | |
|---|---|---|
| Glutamate | 33.0 (28.5, 43.5) | 30.0 (21.5, 38.0) |
| Asparagine | 46.5 (25.7, 51.2) | 51.0 (42.5, 63.0) |
| Serine | 121.5 (90.7,146.2) | 107.0 (87.0,123.0) |
| Glutamine | 532.0 (380.5,609.2) | 513.0 (477.0,614.5) |
| Glycine | 259.0 (210.2,348.0) | 208.0 (180.5,238.5) |
| Threonine | 126.5 (83.0,148.2) | 130.0 (107.5,185.0) |
| Histidine | 78.0 (57.0,79.0) | 98.0 (86.5,111.0)*** |
| Alanine | 350.0 (287.2,396.5) | 362.0 (301.0,400.0) |
| Taurine | 33.5 (26.5,41.2) | 43.0 (28.5,48.5) |
| Tyrosine | 55.5(46.5,63.7) | 62.0 (45.0,74.0) |
| Aminobutyric acid | 13.0 (11.0,16.5) | 22.0 (18.5,26.5)*** |
| Arginine | 86.0 (62.5,106.5) | 80.0 (68.5,105.0) |
| Methionine | 12.5 (11.2, 14.7) | 24.0 (19.5, 27.5)*** |
| Valine | 165.0 (149.2,191.2) | 224.0 (194.0,242.5)*** |
| Tryptophan | 34.5 (31.0,39.7) | 51.0 (46.0,60.0)*** |
| Phenylalanine | 50.0 (43.7,53.0) | 60.0 (55.5,63.5)** |
| Isoleucine | 47.5 (40.0,57.0) | 58.0 (55.0,67.5)* |
| Leucine | 90.5 (80.7,102.2) | 107.0 (99.0,115.5)** |
| Ornithine | 52.5 (37.5,58.7) | 60.0 (48.5,81.0) |
| Lysine | 107.5 (71.7,115.0) | 201.0 (139.5,277.5)*** |
Values are 50th (25th – 75th) percentiles. Statistically different compared with the Indian subjects using Mann-Whitney U test
P<0.05,
P<0.01,
P<0.001
In contrast to lower plasma levels of methionine, plasma levels of homocysteine were markedly higher in the Indian women as compared to those in the American women (Indians: 20.4 (16.4, 24.4), Americans: 7.9 (6.8, 8.9) P<0.001). Total plasma glutathione levels (Indians: 3.8 (2.2, 3.9), Americans: 6.5 (5.4, 7.5) P<0.001) were lower in the Indian subjects. Total cysteine concentration in the plasma was similar in the two groups. The levels of formate in the plasma were markedly higher in the Indian women (Indians: 182.9 (167.9, 190.3) micromoles/l, Americans: 39.9(37.2, 48.2) P=0.006).
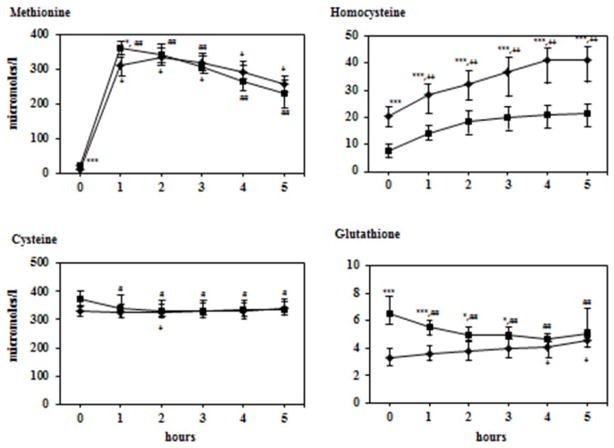
After the oral methionine load, plasma levels of methionine rose, reaching a peak at one hour and then gradually declined over the next four hours. The magnitude of increase in methionine levels from basal to 1 hour was similar in the Indian and American women. As shown in Figure 2, the shape of the methionine curve and the incremental area under the curve were indistinguishable in the two groups. Plasma homocysteine levels rose linearly in both groups. In the American women, it reached a plateau (~ 20μM/l) by two hours and remained unchanged for the next three hours. In contrast, plasma levels of homocysteine continued to increase in the Indian subjects until 4 hours reaching a plateau of ~41μM/l between the 4th and the 5th hour.
Figure 2.
Plasma methionine, homocysteine, cysteine and glutathione response to methionine load. After an overnight fast each subject received methionine 50 mg/kg mixed with orange juice. All data are shown in μmoles/l and represent 50th (25th –75th) percentiles.
Squares: American subjects; diamonds: Indian subjects
Error bars represents 25th and 75th percentiles.
Differences between the two groups were tested using Mann-Whitney test.
*p<0.05, **p<0.01, ***p<0.001
Within group differences from basal were determined using Wilcoxon test.
Difference in US data shown by # sign. Difference in Indian data shown by + sign.
#p<0.05, ##p<0.01, ###p<0.001
+p<0.05, ++p<0.01, +++p<0.001
The rise in homocysteine levels from basal was significantly greater in the Indian subjects at 4 and 5 hours (P<0.01). The incremental area under the curve was not significantly different between the two groups but approached significance following the removal of one outlier (area: 94.9 μM/l.5 hours) in the US group (Indians: 62.03 (43.9, 77.6), Americans: 49.6 (44.7, 50.8) μM/l.5 hours P = 0.06). Plasma levels of total cysteine remained stable in the Indian and American women, following the methionine load. Methionine load caused a small rise in the plasma levels of total glutathione in Indian women but an insignificant increase in the American women (Figure 2).
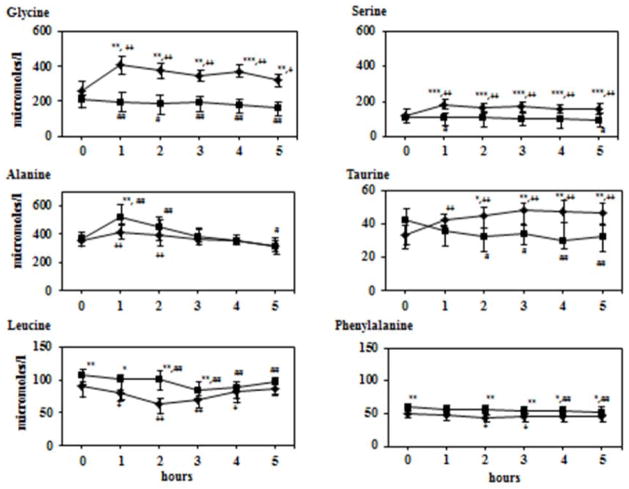
The changes in representative amino acids in the plasma following a methionine load in orange juice are displayed in Figure 3. There was a significant increase in plasma concentration of glycine and serine in the Indian women but not in the nutritionally sufficient American women. The increase in glycine and serine was seen in the Indian women with and without breakfast suggesting that it was due to the carbohydrate load in the orange juice. Plasma concentration of alanine peaked in both groups at 1 hr although the magnitude of increase was less in the Indian women. Plasma levels of taurine showed an increase in Indians and a decrease in the Americans. As anticipated the levels of all essential amino acids decreased following nutritional (breakfast and orange juice) load21. The data of leucine and phenylalanine are displayed in Figure 3.
Figure 3.
Plasma glycine, serine, alanine, taurine, leucine and phenylalanine response to methionine load with orange juice. After an overnight fast each subject received methionine 50 mg/kg mixed with orange juice. Data shown are μmoles/l and represent 50th (25th–75th) percentiles. Squares: American subjects; diamonds: Indian subjects. Error bars represents 25th and 75th percentiles.
Differences between the two groups were tested using Mann-Whitney test :*p<0.05, **p<0.01, ***p<0.001
Within group differences from basal were determined using Wilcoxon test.
Difference in US data shown by # sign. Difference in Indian data shown by + sign.
#p<0.05, ##p<0.01, ###p<0.001
+p<0.05, ++p<0.01, +++p<0.001
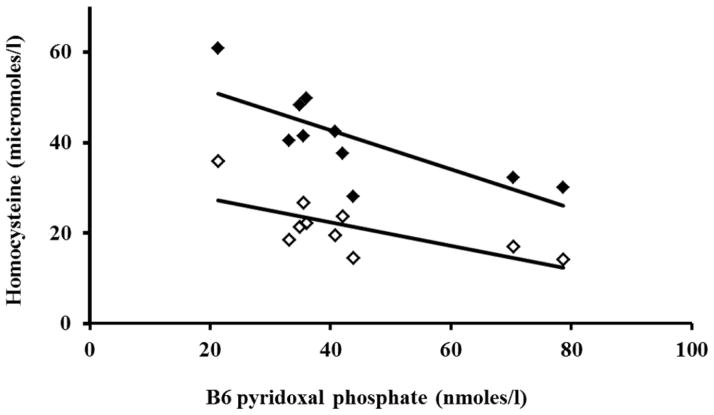
Plasma total homocysteine levels during fasting were not correlated with circulating levels of vitamins B6, folate and B12 and methionine in the American women. In contrast, in the Indian women plasma homocysteine was inversely correlated with vitamin B6 levels both in the fasting state and at five hours after methionine load (Figure 4; basal r=−0.68, P<0.05 and 5 hour r= −0.70, P<0.05). The incremental change in homocysteine concentration was significantly correlated with B12 concentration only in the American group (r= −0.73, P<0.01). Multiple linear regression analysis showed that circulating levels of vitamins B6, folate and B12 explained 26% of the variance in the plasma basal total homocysteine levels in the American women; in the Indian women this figure was 57%. On the other hand, 82% of the difference in the circulating levels of basal total homocysteine in the two groups of women was explained by the difference in the levels of vitamins B6, folate and B12. A significant negative correlation between plasma folate levels and plasma glycine (r= −0.842, P<0.01) and serine (r= −0.697 P<0.05) levels were observed only in the Indian group. There was no correlation between glycine, serine, histidine, methionine and plasma levels of B12 or B6 in either group.
Figure 4.
Correlation between plasma concentration of B6 (Pyridoxal phosphate), and homocysteine in the Indian subjects before (0 hr, open) and after (5 hr, filled) methionine load. Spearman’s rank correlation coefficient: 0 hour r = −0.68 (P<0.05) and 5 hour r = − 0.78 (P<0.01).
Discussion
Our data show that multi-nutrient deficiencies in the Indian women (vitamins regulating one carbon metabolism) resulted in substantially elevated homocysteine concentrations and lower levels of essential amino acids in the plasma. Oral methionine load showed that the nutritionally compromised Indian women could absorb and dispose off methionine equally efficiently as the nutritionally sufficient American women. However, there was a greater increase in plasma homocysteine concentration in the Indian women. Additionally, there was an increase in the plasma serine and glycine concentration in the Indian women only, likely in response to the carbohydrate load (orange juice) administered with methionine.
The present data should be examined in the following context. The dietary habits of Indians from this region are mostly vegetarian with relative lower quantity and quality of protein and lower dietary source of vitamins.22 This was reflected in the markedly low levels of B12 in the Indian women in this study, which has been previously reported in the vegetarians. 22–25 The plasma levels of folate and B6 also were lower in the Indian women. In contrast the American women were all non-vegetarian with higher daily intake of dietary protein. In addition, the folate intake of American subjects was higher due to the mandatory fortification of flour. 26 Given the critical role in one carbon transfers, and as cofactors at specific steps in folate and methionine metabolism, the inadequate intake of these nutrients individually will result in unique changes in one carbon metabolism and the circulating levels of related biochemicals (Figure 1). However, the combined effect of simultaneous insufficiency of these nutrients could be different due to the opposing effect of some of them. For example, lower protein intake results in higher rate of transmethylation of methionine while folate and B12 insufficiency causes a lower rate of methylation of homocysteine. The combined effect of these nutrient insufficiencies has not been examined in humans. The present data reports the net effect of the insufficiency of these nutrients on one carbon metabolism.
The concentrations of essential amino acids in the plasma were significantly lower in the Indian women in the fasting state. Since breakdown of proteins in the body, primarily skeletal muscle, is the major source of essential amino acids in the plasma, our data suggest a lower rate of protein breakdown or protein turnover in the Indian women. Although the present data cannot delineate the cause of lower rate of protein turnover in these subjects, it is likely to be related to lower dietary intake of proteins and consequent attempt at conservation of nitrogen. Dietary restriction of protein in healthy humans has been shown to cause a decrease in whole-body proteolysis as measured by the rate of appearance of leucine and a decrease in the rate of oxidation of leucine/protein27, 28 and cause a decrease in the rate of oxidation of leucine in the rat.29 These changes in essential amino acids were associated with small increase in the levels of glycine and serine in the plasma. The latter has been shown to increase in humans and in laboratory animals when dietary proteins are restricted.30–33 Tracer isotope studies have shown that increase in serine and glycine were the consequence of increased rates of de-novo synthesis of these amino acids 9,34. The higher levels of glycine and serine and their increased rates of synthesis may be related to the hepatic induction of PPARα as a result of low protein intake.9 Data in literature show that administration of PPARα agonist in mice results in increased levels and rate of turnover of glycine and serine in the plasma.35 The physiological significance of changes in glycine and serine metabolism during protein restriction, other than as source of methyl groups, has not been determined. It has been postulated that restriction of dietary protein results in higher methylation demand and a high rate transmethylation and consequently high rate of synthesis of serine and glycine.9 The negative correlation between plasma levels of folate and plasma levels of glycine and serine during fasting in the Indian women suggests that in addition to low protein, lower folate also may contribute to the higher levels of serine and glycine by attenuating the folate cycle.
A decrease in essential amino acids levels in the plasma was seen in all subjects following the administration of methionine mixed with orange juice and a low methionine breakfast, likely due to the expected suppression of whole body breakdown of proteins in response to carbohydrate (juice) and nutrients (breakfast) and associated increase in insulin.36,21 In contrast there was an increase in plasma concentration of glycine and serine in the nutritionally insufficient Indian women (Figure 3). These data suggest an active pathway for the synthesis of serine in the liver induced by low protein intake and rapid conversion of dietary carbohydrates into serine and glycine.37
The fasting plasma tHcy was markedly higher and glutathione was lower in the Indian women. There was a significant negative correlation between plasma levels of tHcy and pyridoxal phosphate levels (Figure 4), suggesting a dominant contribution of lower rate of transsulfuration to tHcy levels. The plasma levels of tHcy were not related to folate or B12 levels in this small group of women. None the less, the lower folate and B12 levels in the Indian women, by attenuating methylation of homocysteine, also would contribute to the increase in its plasma levels (Figure 1). The higher levels of formate in the plasma, in the Indian women, are consistent with an impaired rate of remethylation of homocysteine in these subjects.38 The cut off values at which a steep increase in plasma homocysteine concentration occurs in B12 insufficiency have been reported to be much higher (200–300pmol/l) than those seen here in the Indian women.4, 39 In addition lower protein intake by increasing the methionine cycle would also result in higher homocysteine.9 The individual contribution of inadequacies of these nutrients to the higher levels tHcy cannot be discerned from the present data. These observations underscore the importance of examining the impact of multinutrient deficiencies on metabolic biomarkers and the need for studies using isotopic tracers. The mechanism of lower concentration of glutathione in the plasma is unclear. It was probably not related to lower rate of transsulfuration due to lower B6 levels since the plasma levels of total cysteine were not different in the two groups. The lower levels could be the result of hormonally mediated decrease in glutathione synthesis as a result of altered nutritional state.40
We did the methionine load studies in order to (a) describe the net effect of multi-nutrient insufficiencies on one carbon metabolism and (b) to possibly reveal the contribution of transmethylation and transsulfuration of methionine to the observed changes in one carbon metabolism. As shown in figure 2 the plasma methionine response to oral load of 50mg/kg body weight of methionine was similar in Indian and American subjects, both in terms of plasma levels of methionine and of calculated incremental area under the curve. These data suggest that there was no difference in the two groups in relation to gastrointestinal absorption, the first pass metabolism and disposal of methionine. In contrast to the disposal of methionine, the incremental increase in plasma homocysteine concentrations and the area under the tHcy response curve were different in the two groups. However, it should be underscored that the changes in plasma homocysteine are a net effect of both transsulfuration and remethylation and therefore any differences in these processes cannot be evaluated from these data. Following a methionine load, there would be an increase in the intracellular concentration of s-adenosylmethionine (SAM). SAM is an allosteric inhibitor of methylenetetrahydrofolate reductase (MTHFR) and would result in a decrease in the remethylation of homocysteine via methionine synthase.41–43 SAM is also allosteric activator of cystathionine beta synthase and would cause an increase in transsulfuration pathway.40,41 Thus the higher plasma tHcy levels in the Indian women after methionine load, in the presence of similar levels of methionine, suggest a decrease in the disposal of homocysteine via the transsulfuration cascade. The significant correlation between plasma B6 levels and the plasma tHcy levels after methionine load support this inference. The mechanism of the observed changes in total glutathione in the plasma i.e. a decrease in the American women and increase in Indian women is not clear. Tracer isotope labelled methionine studies would be required to further interrogate the impact of multiple micro and macro-nutrient insufficiencies on components of methionine metabolism.
In summary, we have identified characteristic perturbations in one carbon metabolism and circulating levels of amino acids in response to multi-nutrient deficiency in the Indian women. A significant decrease in concentrations of essential amino acids in the plasma and an increase in serine and glycine suggest a lower isocaloric protein intake. Low B12 and B6 status resulted in higher homocysteine levels in the basal state and a higher homocysteine response to a methionine load. The net impact of these nutritional insufficiencies on transmethylation and transsulfuration of methionine will require careful tracer isotope studies.
Acknowledgments
Grant support: This work was supported in part by the National Institutes of Health grants, R21 HD073880 (to SCK), a Clinical and Translational Science Award RR024989 to Case Western Reserve University and Genomic and lifestyle predictors of foetal outcome relevant to diabetes and obesity and their relevance to prevention strategies in South Asian peoples (GIFTS), FP7-HEALTH-2011-two-stage/EC-GA no 278917
We are grateful to the study participants for taking part in this study. We thank Dr KJ Coyaji, Medical Director of the KEM Hospital and Dr VS Padbidri, Director Research for providing research facilities. We thank Mrs PC Yajnik for administrative support.
Footnotes
Conflict of interest: None of the authors had any financial or personal conflicts of interest associated with this manuscript.
References
- 1.Mason JB. Biomarkers of nutrient exposure and status in one-carbon (methyl) metabolism. J of Nutrition. 2003;133:941s–947s. doi: 10.1093/jn/133.3.941S. [DOI] [PubMed] [Google Scholar]
- 2.King WD, Ho V, Dodds L, Perkins SL, Casson RI, Massey TE. Relationships among biomarkers of one-carbon metabolism. Mol Biol Rep. 2012;39:7805–7812. doi: 10.1007/s11033-012-1623-y. [DOI] [PubMed] [Google Scholar]
- 3.Lamers Y. Indicators and methods for folate, vitamin B-12, and vitamin B-6 status assessment in humans. Current opinion in clinical nutrition and metabolic care. 2011;14:445–454. doi: 10.1097/MCO.0b013e328349f9a7. [DOI] [PubMed] [Google Scholar]
- 4.Selhub J, Jacques PF, Dallal G, Choumenkovitch S, Rogers G. The use of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B 12 status. Food and Nutrition bulletin. 2008;29:S67–S73. doi: 10.1177/15648265080292S110. [DOI] [PubMed] [Google Scholar]
- 5.Vogiatzoglou A, Oulhaj A, Smith AD, Nurk E, Drevon CA, Ueland PM, et al. Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B 12 status. Clinical Chemistry. 2009;55:2198–2206. doi: 10.1373/clinchem.2009.128678. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj A, Kumar D, Raina SK, Bansal P, Bhushan S, Chander V. Rapid Assessment for Coexistence of Vitamin B12 and Iron Deficiency Anemia among Adolescent Males and Females in Northern Himalayan State of India. Anemia. 2013:959605. doi: 10.1155/2013/959605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Nutrition Bulletin; Folate and vitamin B12 deficiencies: Proceedings of a WHO Technical Consultation; 18–21 October, 2005; Geneva, Switzerland. Jun, 2008. [PubMed] [Google Scholar]
- 8.Sukla KK, Nagar R, Raman R. Vitamin-B12 and folate deficiency, major contributing factors for anemia: A population based study. e-SPEN Journal. 2014;9:e45–e48. [Google Scholar]
- 9.Kalhan SC, Uppal SO, Moorman JL, Bennett C, Gruca LL, Parimi PS, et al. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J Biol Chem. 2011;286:5266–5277. doi: 10.1074/jbc.M110.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingenbleek Y, Hardilliera E, Jung L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrtion. 2002;18:40–46. doi: 10.1016/s0899-9007(01)00783-3. [DOI] [PubMed] [Google Scholar]
- 11.Ingenbleek Y, McCully KS. Vegetrainism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition. 2012;28:148–153. doi: 10.1016/j.nut.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Chambers JC, Ueland PM, Wright M, Dore CJ, Refsum H, Kooner JS. Investigation of relationship between reduced, oxidized, and protein-bound homocysteine and vascular endothelial function in healthy human subjects. Circ Res. 2001 Jul 20;89(2):187–92. doi: 10.1161/hh1401.093459. [DOI] [PubMed] [Google Scholar]
- 13.Chambers JC, Obeid OA, Kooner JS. Physiological increments in plasma homocysteine induce vascular endothelial dysfunction in normal human subjects. Arterioscler Thromb Vasc Biol. 1999 Dec;19(12):2922–7. doi: 10.1161/01.atv.19.12.2922. [DOI] [PubMed] [Google Scholar]
- 14.Sassi S, Cosmi B, Palareti G, Legnani C, Grossi G, Musolesi S, et al. Influence of age, sex and vitamin status on fasting and post-methionine load plasma homocysteine levels. Haematologica. 2002 Sep;87(9):957–64. [PubMed] [Google Scholar]
- 15.De JR, Griffioen PH, van ZB, Brouns RM, Visser W, Lindemans J. Evaluation of a shorter methionine loading test. Clin Chem Lab Med. 2004;42(9):1027–31. doi: 10.1515/CCLM.2004.207. [DOI] [PubMed] [Google Scholar]
- 16.Kalhan SC, Gruca LL, Parimi PS, O’Brien A, Dierker L, Burkett E. Serine metabolism in human pregnancy. Am J Physiol Endocrinol Metab. 2003;284:E733–E740. doi: 10.1152/ajpendo.00167.2002. [DOI] [PubMed] [Google Scholar]
- 17.Garcia AJ, Apitz-Castro R. Plasma total homocysteine quantification: an improvement of the classical high-performance liquid chromatographic method with fluorescence detection of the thiol-SBD derivatives. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;779:359–63. doi: 10.1016/s1570-0232(02)00401-4. [DOI] [PubMed] [Google Scholar]
- 18.Lammarre SG, MacMillan L, Morrow GP, Randell E, Pongnopparat T, Brosnan M, et al. An isotope-dilution, GC-MS assay for formate and its application to human and animal metabolism. Amino acids. 2014;46:1885–1891. doi: 10.1007/s00726-014-1738-7. [DOI] [PubMed] [Google Scholar]
- 19.Kelleher BP, Broin SD. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol. 1991;44:592–5. doi: 10.1136/jcp.44.7.592. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura T, Freeberg LE, Cornwell PE. Inhibition by EDTA of Growth of Lactobacillus casei in the folate microbiologic assay and its reversal by added manganese or Iron. Clin Chem. 1990;36:11. [PubMed] [Google Scholar]
- 21.Bergstrom J, Furst P, Vinnars E. Effect of a test meal, without and with protein, on muscle and plasma free amino acids. Clinical Science. 1990;79:331–337. doi: 10.1042/cs0790331. [DOI] [PubMed] [Google Scholar]
- 22.Rao S, Yajnik CS, Kanade, Fall CH, Margetts BM, Jackson AA, Shier R, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J Nutr. 2001;131:1217–24. doi: 10.1093/jn/131.4.1217. [DOI] [PubMed] [Google Scholar]
- 23.Hermann w, Schorr H, Obeid R, geisel J. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am J Clin Nutr. 2003;78:131–136. doi: 10.1093/ajcn/78.1.131. [DOI] [PubMed] [Google Scholar]
- 24.Antony AC. Vegetarianism and B-12 (cobalamin) deficiency. Am J Clin Nutr. 2003;78:3–6. doi: 10.1093/ajcn/78.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Pawlak R, lester SE, Babatunde T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr. 2014;68:541–548. doi: 10.1038/ejcn.2014.46. [DOI] [PubMed] [Google Scholar]
- 26.Quinlivan EP, Gregory JF. Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. 2003;77:221–225. doi: 10.1093/ajcn/77.1.221. [DOI] [PubMed] [Google Scholar]
- 27.Gaine PC, Pikosky MA, Martin WF, Bolster DR, maresh CM, rodriguez NR. Level of dietary protein impacts whole body protein turnover in trained males at rest. Metabolism. 2006;55:501–507. doi: 10.1016/j.metabol.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Lariviere F, Kupranycz DA, Chiasson J-L, Hoffer J. Plasma leucine kinetics and urinary nitrogen excretion in intensively treated diabetes mellitus. Am J Physiol. 1992;263:E173–E179. doi: 10.1152/ajpendo.1992.263.1.E173. [DOI] [PubMed] [Google Scholar]
- 29.Sketcher RD, James WP. Branched-chain amino acid oxidation in relation to catabolic enzyme activities in rats given a protein-free diet at different stages of development. British Journal of Nutrition. 1974;32:615–623. doi: 10.1079/bjn19740114. [DOI] [PubMed] [Google Scholar]
- 30.Adibi S. Influence of dietary deprivation on plasma concentration of free amino acids of man. Journal of Applied Physiol. 1968;25:52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- 31.Adibi, et al. Amino acid levels in plasma, liver, and skeletal muscle during protein deprivation. Am J Physiol. 1973;225:408–414. doi: 10.1152/ajplegacy.1973.225.2.408. [DOI] [PubMed] [Google Scholar]
- 32.Nagao, et al. Adaptational modification of serine and threonine metabolism in the liver to essential amino acid deficiency in rats. Amino acids. 2009;36:555–562. doi: 10.1007/s00726-008-0117-7. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi, et al. Characterization of dietary protein-dependent anibo acid metabolism by linking free amino acids with transcriptional profiles through analysis of correlation. Physiological Genomics. 2008;34:315–326. doi: 10.1152/physiolgenomics.00007.2008. [DOI] [PubMed] [Google Scholar]
- 34.Gibson, et al. Endogenous glycine and tyrosine production is maintained in adults consuming a marginal-protein diet. Am J Clin Nutr. 2002;75:511–518. doi: 10.1093/ajcn/75.3.511. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh K, Camejo G, Lanne B, Halvarsson T, Landergren MR, Oakes ND. Beyond lipids, pharmacological PPARa activation has important effects on amino acid metabolism as studied in the rat. Am J Physiol Endocrinol Metab. 2007;292:E1157–E1185. doi: 10.1152/ajpendo.00254.2006. [DOI] [PubMed] [Google Scholar]
- 36.Motil KJ, Matthews DE, Bier DM, Burke JF, Munro HN, Young VR. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol. 1981;240:E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- 37.Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino acid. Journal of Biological Chemistry. 2012;287:19786–19791. doi: 10.1074/jbc.R112.357194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamarre SG, maolloy AM, Reinke SN, Sykes BD, Brosnan ME, Brosnan JT. Formate can differentiate between hyperhomocysteinemia due to impaired remethylation and impaired transsulfuration. Am J Physiol Endocrinol Metab. 2012;302:E61–E67. doi: 10.1152/ajpendo.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–41. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 40.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 13:1169–1183. [PubMed] [Google Scholar]
- 41.Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Adaptation to methionine excess. Journal of Biological Chemistry. 1986;261:1582–1587. [PubMed] [Google Scholar]
- 42.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by s-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992;55:131–138. doi: 10.1093/ajcn/55.1.131. [DOI] [PubMed] [Google Scholar]
- 43.Grillo MA, Colombatto S. S-adenosylmethionine and its products. Amino acids. 2008;34:187–193. doi: 10.1007/s00726-007-0500-9. [DOI] [PubMed] [Google Scholar]