Abstract
Purpose/Objective(s)
We sought to identify swallowing muscle dose-response thresholds associated with chronic radiation-associated dysphagia (RAD) after IMRT for oropharyngeal cancer.
Materials/Methods
T1-4 N0-3 M0 oropharyngeal cancer patients who received definitive IMRT and systemic therapy were examined. Chronic RAD was coded as any of the following ≥ 12 months post-IMRT: videofluoroscopy/endoscopy detected aspiration or stricture, gastrostomy tube and/or aspiration pneumonia. DICOM-RT plan data were autosegmented using a custom region-of-interest (ROI) library and included inferior, middle and superior constrictors (IPC, MPC, and SPC), medial and lateral pterygoids (MPM, LPM), anterior and posterior digastrics (ADM, PDM), intrinsic tongue muscles (ITM), mylo/geniohyoid complex (MHM), genioglossus (GGM), ), masseter (MM), Buccinator (BM), palatoglossus (PGM), and cricopharyngeus (CPM), with ROI dose-volume histograms (DVHs) calculated. Recursive partitioning analysis (RPA) was used to identify dose-volume effects associated with chronic-RAD, for use in a multivariate (MV) model.
Results
Of 300 patients, 34 (11%) had chronic-RAD. RPA showed DVH-derived MHM V69 (i.e. the volume receiving ≥69Gy), GGM V35, ADM V60, MPC V49, and SPC V70 were associated with chronic-RAD. A model including age in addition to MHM V69 as continuous variables was optimal among tested MV models (AUC 0.835).
Conclusion
In addition to SPCs, dose to MHM should be monitored and constrained, especially in older patients (>62-years), when feasible.
Keywords: Dysphagia, Intensity-Modulated Radiation Therapy, Oropharyngeal Cancer, Mylohyoid, Geniohyoid, Dose-volume
Introduction
Dysphagia is a potentially devastating late toxicity of head and neck radiation therapy (RT)[1, 2]. Radiation-associated dysphagia (RAD) is often cited as a dose limiting toxicity in this population[3, 4]. Patients with severe RAD may require lifelong tube feeding[5], or suffer potentially life-threatening aspiration[3, 4]. Population level data suggest 3-fold elevated risk of aspiration pneumonia in head and neck cancer (HNC) patients treated with chemoradiotherapy (CRT) relative to non-cancer controls, and 42% excess mortality among cancer survivors who develop pneumonia[6]. Pooled analysis of Radiation Therapy Oncology Group (RTOG) trials of CRT for HNC reported unacceptably high rates of severe late toxicity (i.e., 43% of patients with adequate baseline function had grade 3–4 late laryngopharyngeal toxicity) suggesting that further dose intensification cannot be safely achieved without new technique(s) to protect against late effects[7]. The therapeutic benefits of aggressive RT for HNC are clear[8–10], but understanding the structure-specific doses predisposing to long-term toxicity is paramount to patient care[11–13].
Swallowing requires complex coordination of numerous structures, and the exact contribution of each is incompletely understood[14]. Intensity-modulated radiation therapy (IMRT), now standard for HNC, substantially reduces normal tissue dose[15]. However, with more beam paths, greater volumes of non-target normal tissue (which may not have been exposed in conventional RT treatments) receive bystander dose[16]. Various studies have concluded that sparing dysphagia-related structures likely improves outcomes[1, 17, 18]. The wide array of candidate dysphagia-associated structures implicated by our group and others in previous studies reflects the complicated nature of RAD and suggests that further insight into its mechanism could be helpful in preparing future treatment regimens. To this end, as part of an ongoing HNC toxicity reduction program[19–33], specific aims of our study include:
Identify dose-volume parameters of candidate swallowing-related muscular ROI related to chronic-RAD after IMRT.
Identify candidate single- and multiple-muscle ROI dose-volume response thresholds associated with chronic-RAD
Identify clinical and dosimetric parameters independently associated with risk of chronic-RAD.
Material and Methods
Study Design and Sampling Method
Patients treated with curative intent IMRT and systemic therapy for oropharyngeal cancer at The University of Texas, MD Anderson Cancer Center between 2002 and 2011 were retrospectively reviewed under an approved Institutional Review Board (IRB) protocol. Eligibility criteria were: Pathologically confirmed diagnosis of oropharyngeal squamous cell carcinoma (OPSCC), IMRT as a definitive treatment, available IMRT plan in the MDACC archive, and a minimum follow-up of ≥12 calendar months after end IMRT. Of 349 patients identified, 49 were excluded because radiotherapy treatment plans could not be restored to analyze DVHs, leaving a total of 300 patients for analysis.
IMRT
We have previously reported in detail our IMRT approach for oropharyngeal cancer[9]. In brief, IMRT was used to treat the primary tumor and upper neck nodes. IMRT was delivered using “split-field” technique with lower neck below the isocenter treated with an anterior beam, with a larynx midline block. While “whole-field” IMRT used only when tumor might be underdosed using the split-field approach. All patients were treated with definitive bilateral IMRT with systemic therapy.
Data Collection
Chronic-RAD was defined as any of the following criteria occurring ≥12 months post-IMRT: videofluoroscopy/endoscopy detected aspiration or stricture, gastrostomy tube and/or aspiration pneumonia. Gastrostomy tube dependence was coded at 1-year follow-up, 2-year follow-up, and last disease-free follow-up. While, videofluoroscopic studies were conducted for patients referred with post-radiation symptoms of dysphagia (106 patients, 69 of these were ≥12 months post-radiation).
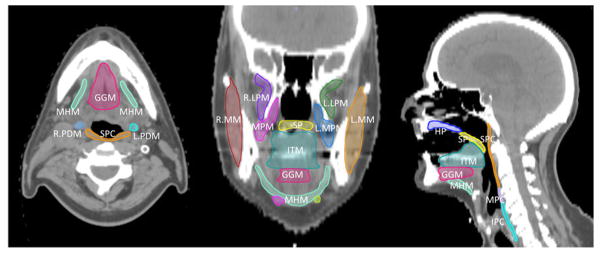
Clinical variables included age, sex, ethnicity, AJCC stage, TNM classification, tumor subsite (tonsil, base of tongue, or other) smoking history (never smoker, former/<10 pack-years, current/>10 pack-years), and chemotherapy regimen. Treatment plan and dosimetric data were restored using Pinnacle 9.6 software (Phillips Medical Systems, Andover, MA). Planning CT DICOM files were exported into a benchmarked [34] commercial deformable registration/segmentation software (Velocity AI 3.0.1, Velocity Medical Solutions, Atlanta, GA). For each patient, dysphagia-related musculature were software autosegmented using an existing atlas dataset [34] and subsequently reviewed by two radiation oncologists (ASR and CDF). DVHs were generated for the following muscle-specific regions of interest (ROIs): inferior, middle and superior constrictors (IPC, MPC, and SPC), medial and lateral pterygoids (MPM, LPM), anterior and posterior digastrics (ADM, PDM), intrinsic tongue muscles (ITM), mylo/geniohyoid complex (MHM), genioglossus (GGM), palatoglossus (PGM), masseter (MM), buccinator (BM), and cricopharyngeus (CPM). Exemplar ROIs are shown in Figure 1; indicative ROIs from 11 selected cases are included as DICOM-RT datasets at http://figshare.com/authors/Abdallah_Mohamed/551961. Prescription dose was as per standard practice, and is detailed in Table 1.
Figure 1. Exemplar swallow-related ROI.
Axial, coronal, and sagittal images of the contoured segments.
Abbreviations: GGM – Genioglossus Muscle; HP – Hard Palate; IPC – Inferior Pharyngeal Constrictor; ITM – Intrinsic Tongue Muscles; LPM – Lateral Pterygoid Muscle; MHM – Mylo/geniohyoid Complex; MM – Masseter Muscle; MPM – Medial Pterygoid Muscle; PDM – Posterior Dygastric Muscle; SP – Soft Palate; SPC – Superior Pharyngeal Constrictor, R.-right, L.-left.
Table 1.
Demographic and clinical data of study patient population.
| All patients (n=300) | Asymptomatic Swallowing (n=266) | Chronic-RAD (n=34) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| T-category | n. | Percent | n. | Percent | n. | Percent |
| 1 | 53 | 17.67% | 51 | 19.17% | 2 | 5.88% |
| 2 | 139 | 46.33% | 128 | 48.12% | 11 | 32.35% |
| 3 | 68 | 22.67% | 60 | 22.56% | 8 | 23.53% |
| 4 | 40 | 13.33% | 27 | 10.15% | 13 | 38.24% |
| N-category | ||||||
| 0 | 16 | 5.33% | 14 | 5.26% | 2 | 5.88% |
| 1 | 12 | 4.00% | 12 | 4.51% | 0 | 0.00% |
| 2a | 21 | 7.00% | 21 | 7.89% | 0 | 0.00% |
| 2b | 167 | 55.67% | 150 | 56.39% | 17 | 50.00% |
| 2c | 71 | 23.67% | 57 | 21.43% | 14 | 41.18% |
| 3 | 13 | 4.33% | 12 | 4.51% | 1 | 2.94% |
| Site | ||||||
| Base of tongue | 164 | 54.67% | 144 | 54.14% | 20 | 58.82% |
| Tonsil | 131 | 43.67% | 118 | 44.36% | 15 | 44.12% |
| Other | 5 | 1.67% | 4 | 1.50% | 1 | 2.94% |
| Smoking status | ||||||
| Never | 55 | 18.33% | 46 | 17.29% | 9 | 26.47% |
| <10 pack-years | 109 | 36.33% | 99 | 37.22% | 10 | 29.41% |
| >10 pack-years | 136 | 45.33% | 121 | 45.49% | 15 | 44.12% |
| Sex | ||||||
| Male | 272 | 90.67% | 238 | 89.47% | 34 | 100.00% |
| Female | 28 | 9.33% | 28 | 10.53% | 0 | 0.00% |
| Ethnicity | ||||||
| White | 283 | 94.33% | 251 | 94.36% | 32 | 94.12% |
| Black | 7 | 2.33% | 6 | 2.26% | 1 | 2.94% |
| Hispanic | 9 | 3.00% | 8 | 3.01% | 1 | 2.94% |
| Asian/Pacific Islander | 1 | 0.33% | 1 | 0.38% | 0 | 0.00% |
| Chemotherapy | ||||||
| Cisplatin | 195 | 65.00% | 99 | 37.22% | 6 | 17.65% |
| Cetuximab | 105 | 35.00% | 167 | 62.78% | 28 | 82.35% |
| IMRT technique | ||||||
| Split-field | 284 | 94.6% | 253 | 95% | 31 | 91% |
| Whole-field | 16 | 5.4% | 13 | 5% | 3 | 9% |
| IMRT fractionation | ||||||
| Once-daily | 262 | 87% | 233 | 87.5% | 29 | 85% |
| Accelerated | 38 | 13% | 33 | 12.5% | 5 | 15% |
| Mean±SD | Median (Range) | Mean±SD | Median (Range) | Mean±SD | Median (Range) | |
| Age (years) | 56±9 | 56 (28–81) | 56±8 | 55 (36–81) | 59±10 | 59 (28–81) |
| RT dose (Gy) | 69±2 | 70 (64–75) | 68±2 | 70 (64–75) | 70±1 | 70 (66–72) |
| RT fractions delivered | 33±2 | 33 (29–35) | 33±2 | 33 (29–35) | 33±2 | 33 (30–35) |
Statistical analysis
ROI summary parameters, including mean dose (Dmean) were first investigated non-parametrically. Bivariate plots of cumulative group dose volume histograms (DVH) were dichotomized by the presence or absence of chronic-RAD, with subsequent Wilcoxon rank sum test and p-values plotted via heat map analysis. Multivariate bootstrap resample recursive partitioning analysis (RPA)[35] was conducted to identify and test candidate dose-volume parameters associated with increased probability of chronic-RAD. RPA (also known as classification and regression trees) was selected over other parametric methodologies as it allows selection of candidate “thresholds” for continuous variables using a binary endpoint (e.g. chronic-RAD).
Sequential random forest/RPA analysis is especially robust when limited priors preclude knowledge-based selection of continuous candidate covariates, and is comparatively unaffected by multi-collinearity and/or potential hyper-dimensional interactions within/between candidate clinical and dosimetric covariates[36], in contrast to standard logistic regression models, and thus require no direct transformation.
Detailed statistical methods are provided in supplementary table S1. RPA and regression models were applied systematically in the following steps to:
identify candidate ROI dose-volume parameters using bootstrap resampled RPA for whole ROI Dmean, whole ROI Dmax, and ROI V1-V75 for all patients, with chronic-RAD status as a discriminant variable (step 1),
define dose-volume thresholds for chronic-RAD within “best” candidate parameters for each ROI using Receiver Operating Characteristic (ROC) and K-fold cross validation (step 2),
identify a predictive model for chronic-RAD by testing “best” dose-volume ROI candidates and clinical variables using stepwise nominal regression with Bayesian Information Criteria (BIC) minimization optimization for model selection and comparison (step 3), and
plot population level estimates of continuous toxicity-response profile probabilities using post hoc bootstrapped logistic probability models and subsequent unsupervised nonlinear curve fits similar to the methodology of Wedenberg [37] (step 4) as an alternative to NTCP curve assessment that mandates a 0% to 100% probability range for our chronic-RAD outcome of interest that is implausible at standard RT doses for OPSCC and given a non-zero baseline rate of age-/comorbidity-related dysphagia.
For this exploratory analysis and model construction, uncorrected p-values are presented, with a priori α=.05 considered for provisional statistical significance. Bonferroni correction(s), effect sizes, and LogWorth values (wherein Log Worth represents -log10[p-value], such that p=0.01 is equivalent to a LogWorth of 2.0, p=0.001 is denoted by LogWorth of 3.0, etc.) are detailed further for interpretative clarity. All statistical analysis was performed using commercial statistical analysis software (MatLab R2011a, Mathworks, Natick, MA; JMP v12Pro, SAS Institute, Cary, NC, USA; IBM SPSS 22.0, Chicago, IL).
Results
Patient and treatment characteristics
A total of 300 oropharyngeal cancer patient cases were accrued after eligibility screening. The median follow up was 48 months (range 12–110). The majority were male 91% with median age of 56 years. Median IMRT dose was 70 Gy (range 64–75) delivered using standard fractionation (87%) and split-field technique (95%) in the majority of patients. Detailed demographic, disease, and treatment data are shown in Table 1.
Chronic-RAD
According to the pre-specified criteria, a total of 34 patients (11%) had chronic-RAD (videofluoroscopy detected aspiration n=21 (7%), videofluoroscopy detected stricture n=10 (3%), gastrostomy tube at 12months n=18 (6%), at 24months n=10 (3%), at last disease free follow-up n=12 (4%), and/or aspiration pneumonia n=8 (2.6%)). Of these chronic-RAD patients, only 5 (14.7%) showed clinical evidence of dysphagia prior to radiation therapy (i.e. ≥ grade 2 according to the common terminology criteria of adverse events version 4.0), however, all but one had clear progression of the dysphagia grade following chemoradiation.
Univariate correlates
Age (p=0.0134), T-category (p=0.004), N-category (p=0.03), sex (p=0.008), radiotherapy prescription dose (p=0.003), number of fractions (p=0.0005), and cytotoxic chemotherapy (p=0.03) were significantly associated with differentials in chronic-RAD rates, while smoking status (p=0.4), subsite (p=0.7) and ethnicity (p=0.9) failed to demonstrate an association with chronic-RAD.
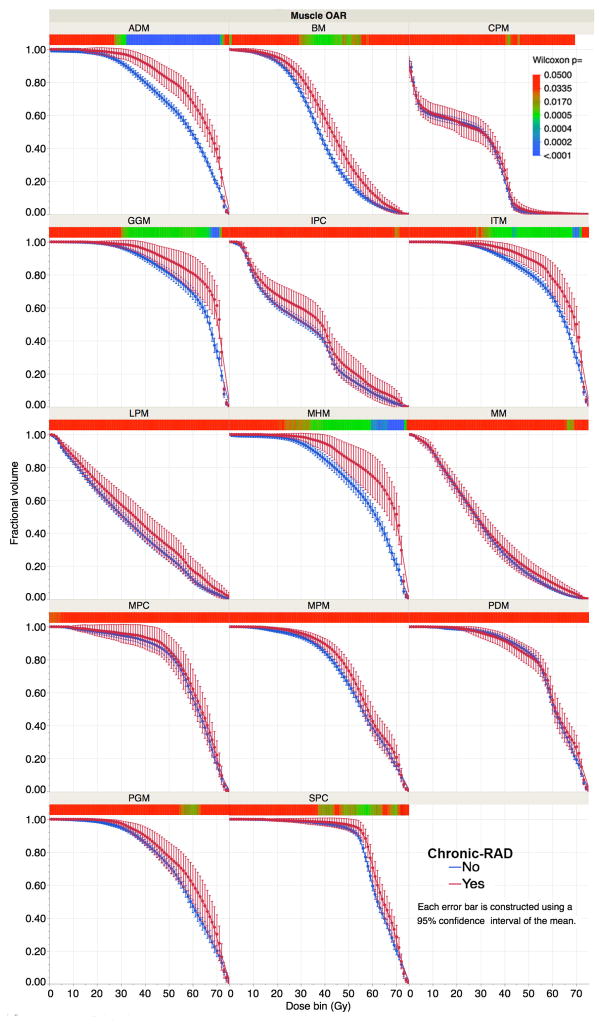
Graphical analysis of composite DVHs shows patients with chronic-RAD had numerically higher dose delivery across all DVHs than those without RAD, with some variability of magnitude across ROIs (see Figure 2). After bonferroni correction, significant pairwise dose-volume differences were observed for ADM, GGM, ITM, and MHM (denoted in blue in the heat map for each ROI).
Figure 2. Swallow-related ROI DVH stratified by chronic-RAD.
Note non-overlapping confidence intervals of dose in 1-Gy bins visually suggests a magnitude difference of p<0.05 using a parametric assessment (i.e. t-test). To account for multiple comparisons and avoid potential error from normal distribution assumptions while illustrating pairwise dose differentials between chronic-RAD and non-RAD subgroups, a heat map is displayed below each ROI DVH to quantify the magnitude of p-values for each 1-Gy bin (per nonparametric Wilcoxon rank sum test for each bin). To account for the comparison across 75 dose levels (0 to 75 Gy), for 14 OARs, a Bonferroni-corrected p=0.000048, denoting significance despite large-scale multiple comparison, is indicated on the heat map by blue shading while red shades denote failure to meet the significance threshold.
Post-hoc assessment of RPA-derived DVH and clinical parameters revealed that none demonstrated an absolute-value correlation of |r| >0.7 (wherein 1= perfect correlation and 0=no correlation), the canonical threshold (confirmed by Dormann et al.[37]) for data distortion, with maximum of |r| = 0.68 observed between ADM V60 and MHM V69. No clinical variables (age, sex, T-category, etc.) showed a collinearity with dosimetric parameters derived from RPA. Consequently, we feel that the resultant stepwise-regression model, while not impervious to collinearity considerations, is unlikely to be inaccurate as a function of ROI dose-parameter covariance.
Assessment of whole ROI Dmean is described in Supplementary Figure S1, with significant (p<0.05) mean dose differentials between chronic-RAD and no-RAD subgroups for ADM, GGM, MHM, ITM, and SPC ROIs. All but SPC remained significant after Bonferroni correction for multiple comparisons.
Dose-volume thresholds
ROI-specific dose-volume thresholds associated with chronic-RAD were next explored via RPA decision tree analysis, with training and validation ROC AUCs, Again ADM, GGM, ITM, MHM, and SPC (which showed whole ROI mean dose-response signal, vide supra) were statistically significant, as well as MPM dose-volume “cutpoints” (Table 2). The resultant statistically significant binary cutpoints were interrogated by confirmatory logistic regression to establish effect size, communicated as odds ratios and relative risk ratios, for ease of interpretation in Table 2. MHM and SPC dose-volume parameters (specifically MHM V69 and SPC V70) showed lower (superior) substantively BIC values than the other (“Very Strong” evidence grade, consistent with a >99% posterior probability of improved model performance); MHM V69 was only slightly more informative when compared to SPC V70.
Table 2.
Univariate RPA-derived ROI specific dose-volume thresholds. LogWorth represents the negative logarithm of the p-value.
| Recursive partitioning analysis | Confirmatory univariate nominal logistic regression | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle OAR | V-level | Percent-threshold | ROC AUC Cohort (test) | ROC AUC Holdback (verification) | LogWorth | P-value | SS | Odds Ratio (95% CI) | Relative risk (95% CI) | ΔBIC | BIC | Evidence Grade § |
| ADM | 60 | 79% | 0.68 | 0.60 | 5.95 | <.0001 | ** | 2.88 (1.32–6.12) | 2.48 (1.32–4.65) | 216.55 | 12.21 | Very Strong |
| BM | 35 | 65.8% | 0.65 | 0.57 | 1.09 | 0.0815 | n.s. | - | ||||
| CPM | 45 | 0.35% | 0.64 | 0.51 | 1.00 | 0.0998 | n.s. | - | ||||
| GGM | 35 | 98.9% | 0.70 | 0.55 | 2.74 | 0.0018 | ** | 3.65 (1.69–8.54) | 3.17 (1.53–6.57) | 212.08 | 7.73 | Strong |
| IPC | 70 | 98.2% | 0.60 | 0.51 | 1.08 | 0.0831 | n.s. | |||||
| ITM | 47 | 99.9% | 0.67 | 0.44 | 2.83 | 0.0015 | * | 2.66 (1.13–5.90) | 2.30 (1.18–4.48) | 218.48 | 14.14 | Very Strong |
| LPM | 66 | 13.1% | 0.53 | 0.35 | 1.07 | 0.0860 | n.s. | - | ||||
| LRX | 63 | 1% | 0.61 | 0.47 | 0.89 | 0.1274 | n.s. | - | ||||
| MHM | 69 | 17.5% | 0.74 | 0.64 | 6.77 | <.0001 | ** | 4.54 (2.14–10.33) | 3.81 (1.89–7.67) | 204.34 | 0.00 | BICminimum (reference) |
| MM | 66 | 4.4% | 0.61 | 0.53 | 0.88 | 0.1314 | n.s. | - | ||||
| MPC | 49 | 99.9% | 0.63 | 0.54 | 0.17 | 0.6825 | n.s. | - | ||||
| MPM | 70 | 1% | 0.59 | 0.45 | 3.31 | 0.0005 | * | 2.64 (1.27–5.72) | 2.37 (1.22–4.60) | 216.60 | 12.25 | Very Strong |
| PDM | 69 | 13.5% | 0.60 | 0.48 | 0.15 | 0.7070 | n.s. | - | ||||
| PGM | 65 | 68.9% | 0.62 | 0.49 | 0.24 | 0.5732 | n.s. | - | ||||
| SPC | 70 | 6.35% | 0.68 | 0.47 | 5.09 | <.0001 | ** | 10.60 (3.12–45.16) | 9.00 (2.20–36.83) | 205.14 | 0.80 | Weak |
statistically significant at P<0.05;
statistically significant after Bonferroni correction.
Multivariate model
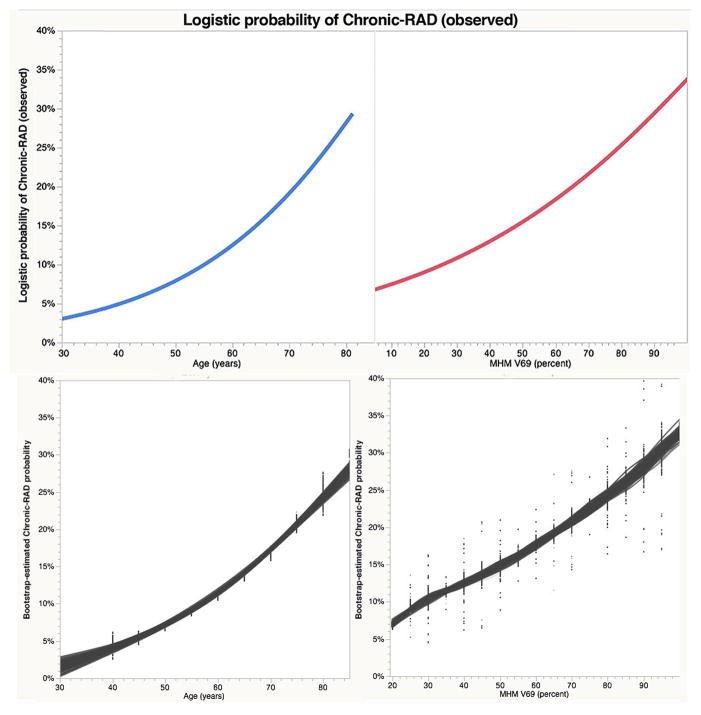
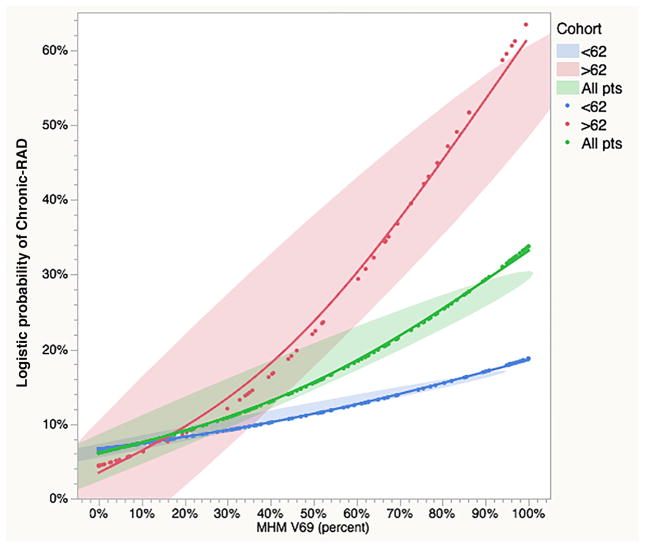
A BIC-minimizing forward stepwise regression model was constructed using the clinical parameters (T- and N-category, chemotherapy, sex, age) and the RPA-derived dose volume thresholds; the resultant model indicated MHM V69 and age as most predictive covariates (AUC=0.835). Post hoc RPA comparison of continuous versus binary MHM V69 and age model effects is summarized in Supplementary Table S2. While both models were sound in terms of goodness of fit, significance (i.e. p-value/LogWorth), and detectable effect size, BIC comparison found a continuous model of MHM V69 and age had superior performance (BIC difference 6.28, “Strong” by evidence grade) to a model using binary MHM V69 >79.5% and age of >62 years as model effects. Observed and bootstrapped predicted models for MHM V69 and age are plotted in Figure 3a–b and 3c–d, respectively. Finally, to illustrate interaction, a plot of the observed probability of chronic-RAD as a function of MHM dose, stratified by age over or under 62-years, is shown in Figure 4.
Figure 3. Observed and Predicted probabilities of chronic-RAD by age and MHMV69.
Observed nominal logistic continuous variable regressor-response plots for age (3a) and MHM V69 (3b). Bootstrap estimated population probability of Chronic-RAD plots for age (3c) and MHM V69 (3d); points are shown in 5 Gy/5-year “bins” with gray lines representing unsupervised fits across 104 resampled distribution, indicative of the expected range of uncertainty attributable to differentials between the observed sample and the parent oropharyngeal cancer patient true population.
Figure 4. Chronic RAD as a function MHM V69 by Age.
Composite plot of MHM V69 (as a continuous variable) and age cohort (green shading denotes the observed whole population; red identifies patients over 62 years of age; blue indicates patients less than 62 years old). Smoothed fits are shown with color-specific ellipses covering 95% of observed values for each cohort as a visual uncertainty estimator.
Discussion
Optimizing functional outcomes is a paramount goal in contemporary management of OPSCC. HPV-associated cancers now account for the majority of new OPSCC cases[38]. Clinically-distinct from tobacco related disease, HPV-associated OPSCC is diagnosed in younger patients who have favorable prognosis for long-term survival such that most survivors have potential to live years with effects of therapy. RAD is a priority issue for survivors[39], drives perception of QOL[40], and significantly predicts for aspiration pneumonia[41]. Even in modern practice, up to 60% of patients require feeding tube placement during IMRT[42]. More alarmingly, we previously reported a 7.6% chronic aspiration rate amongst head and neck (primarily oropharyngeal) squamous cell carcinoma patients undergoing chemoradiotherapy[33], and the Michigan group have reported that up to 20% of survivors develop chronic aspiration even with dysphagia-optimized IMRT planned specifically to minimize dose to non-target swallow critical structures including the constrictors and larynx[43–45].
Chronic radiation-associated dysphagia is an exquisitely complex and challenging toxicity. The state of the field is such that there is no effective treatment to reverse chronic-RAD in long-term survivors; intensive and costly therapies are required for incremental gains in functionality. The persistence of refractory RAD in modern practice motivates clinicians to refine preventive efforts through enhanced treatment paradigms. The complexity of swallowing function belies simple definition of dose response to clinical radiotherapy, as in dose-related xerostomia as a function of salivary gland dose. While several groups continue to actively define important benchmarks for RAD[43, 46–57], most investigators study heterogeneous therapy cohorts (e.g. post-operative and definitive cases) or combine a mélange of organ sites for which beam paths to non-target structures vary widely. Head and neck cancers, owing to anatomical complexities, are far from monolithic. Mixing laryngeal cases, where retropharyngeal (and thus SPC) dose coverage is frequently unnecessary for treatment of subclinical disease, with nasopharyngeal cases, where obligate SPC coverage is the norm, but laryngeal coverage is exempted, is useful given large aggregates of cases, but may obscure systematic dose-response relationships (and achievable constraints) in more homogenous cohorts. Even within oropharyngeal tumors, the obligate muscle coverage when treating base of tongue and tonsillar cancers is quite distinct. The use of a uniform case mix as presented herein overcomes many issues of anatomic heterogeneity, as does the fact that all included patients had comparatively uniform definitive curative-intent IMRT in this non-surgical OPSCC cohort.
Several studies have attempted to link dysphagia to the dose-volume received by specific structures. In particular, Levendag et al found a statistically important correlation between dose to the superior and middle constrictors and dysphagia[5]. Our group likewise found a significant correlation between mean superior pharyngeal constrictor dose and late-onset radiation-associated dysphagia (late-RAD) in a small case-control study[4]. Eisbruch et al. implicated the pharyngeal constrictors and, furthermore, correlated supraglottic and glottic larynx dosage with aspiration[58]. One recent study found that dose to the inferior pharyngeal constrictor best predicted the need for long- term gastrostomy tube[59].
Like others groups, our data points to SPC dose (especially SPC V70) as a strong associate of RAD, consistent with the host of well-documented series above[43, 46–56]. However, in our OPC chemoIMRT-only dataset, MHM dose was a more consistent classifier of chronic-RAD than SPC dose. To our knowledge, our data are the first to specifically characterize mylohyoid/geniohyoid dose-response at a volumetric level in multivariate models as a predictor of RAD. However, recent work by the John Hopkins group shows a similar trend[60], as did a previous work by our group suggesting a more nonspecific anterior oral cavity ROI predictive of long-term modified barium swallow-defined dysphagia[11]. These smaller OPC series which showed trends from floor of mouth muscle ROIs, were limited primarily by sample size (46 patients in the Hopkins series, 31 in our previous MDACC series), but, given the effect size seen in the current study, both pilot series appear to have detected meaningful trends. The correlation of videofluoroscopic kinematics with geniohyoid dose by the Hopkins team points to the importance of these muscle groups, and provides an evidentiary correlate of pathophysiology that may underlie chronic-RAD in our larger cohort.
In our dataset, we characterized the geniohyoid/mylohyoid muscles as a single structural ROI, which raises the necessary caveat in terms of OAR ROI definition for toxicity analyses[61] (vide infra). However, the physiologic function of these muscle groups for sensorimotor swallow initiation[62] as well both anterior and superior hyoid lift is well known[63], and serves as potential explanatory rationale for the large observed dose-dependent effect sizes (Supplementary Table S2). While PubMed search for “mylohyoid” and “radiotherapy” resulted in no relevant series, the fact that swallowing tasks have identifiable MRI-demonstrated mylohyoid-related recruitment, also lends credence to our findings despite their comparative novelty[64]. Suprahyoid muscles are implicated as the anterior sling for airway closure in morphometric analyses of normal swallows. These data point to a need for multi-site functional dose-toxicity validation, and suggest a move to consider dose constraint to the MHM muscles in IMRT planning for OPC primary tumors, in addition to the SPC, when clinically feasible given requisite tumor/nodal coverage.
As part of similar programmatic multi-OAR dose-response assessment/optimization efforts undertaken by the Groningen group[56, 65–69], a recent seminal LASSO-based analysis of a heterogeneous HNC cohort [56] investigated PEG-tube dependence. By way of comparison, we saw some similar associations (e.g. T-category, mean SPC dose), some divergence (IPC and CPM dose were not significant in our cohort), as well as several non-overlapping variables (weight alteration, age). This is likely a factor of demographics (a minority of OPC patients [28%] in the Groningen dataset compared to our exclusively OPSCC cohort) which inform therapy (e.g. with OPC cases we favor a midline block[70, 71], comparatively reducing IPC and CPM dose compared to laryngeal/hypopharyngeal cases where coverage of these structures is normative, which were the majority (53%) in Groningen). Like this series, Wopken et al. [56] used BIC-based classification with resampling, pointing to a growing acceptance of these techniques, for toxicity, and likewise permits assessment of toxicity correlates in cases where model assumptions for parametric methods (such as LKB NTCP models) are either inappropriate or as yet undefined[72].
The finding of age as a substantive correlate of chronic-RAD echoes findings by Beetz et al.[73, 74], showing a distinctive age-related functional recovery differential in the parotid glands of elderly patients. The rationale for these increased age-related radiosensitivity observations are unclear. Fundamentally, our understanding of the biological bases of radiation-associated dysphagia remain opaque, as it is unclear if direct muscle damage (such as late radiation fibrosis[75–77]) and/or denervation effects[78–80] are primary drivers of severe chronic-RAD. Furthermore, identification of mechanistic genomic processes[81–84] which are potentially altered by aging might provide insight into the observed age-dose-toxicity interaction. Since age is strong correlate of pneumonia in HNC chemoradiotherapy patients, as shown by Merlano et al.[85], it may be that preventing incipient dysphagia in the elderly might preclude secondary aspiration pneumonia events, and even reduce mortality in high-risk elderly populations.
Our study has several notable limitations, as with any DVH-based analysis, spatial data is lost in the transition from 3-dimensional dose distributions, precluding sub-ROI volumetric effects, as well as data regarding proximate voxels in distinct structures. Lack of 3D data also precludes incorporation of tumor/node spatial considerations (which themselves are the true driver of systematic OAR ROI dose), and which serves as a general confounder in almost all HNC dose-response models, which tacitly imply dose independence of ROIs from potentially proximate target volumes. Though we used a previously benchmarked OAR segmentation and atlas workflow[34], ROI segmentation variability can substantively alter normal tissue complication assessment and should always be noted as a dependency[61]. Given the longitudinal, retrospective nature of this study, the potential for underreporting is also of concern. However, it should be noted that median follow-up was 48 months, and 94% of patients were followed for more than two years and though videoflouroscopy was not used routinely creating the potential for missed “silent aspiration,” the 7% rate of chronic aspiration detected by videoflouroscopy in the current study is very close to the 7.6% aspiration rate we previously reported for a group of patients in a prospective institutional organ preservation trial using routine videoflouroscopic dysphagia assessment[33]. Finally, our choice of recursive partitioning analytic methods represents an inherently “greedy” model system, and classification and regression tree approaches often are sensitive to “noise” from random variation within the dataset. In addition to a use of test-training methodology with 20% “holdback” verification and 10-fold cross validation, as suggested by Lemon et al.[86], we used bootstrap resampling with traditional methods such as logistic regression for confirmation/validation whenever possible. The “oversensitivity” of RPA to intrinsic patterns in the extant data (as opposed to conceptual reliance on distributional attributes) provides potential limits to generalizability outside the current dataset, though we have attempted to address this via population risk estimation using bootstrap methods.
Despite inherent limitations, our data represent the largest OPSCC chemo-IMRT study using benchmarked/curated autosegmentation to investigate multivariate clinical and dosimetric correlates from a curated database of patients receiving direct dysphagia-specialist speech pathologist-rated objective swallowing dysfunction for chronic-RAD, and the resultant findings are potentially useful for clinical practice. Our data demonstrate that, in addition to SPC, other OAR ROIs (notably oral cavity/FOM ROIs ADM, GGM, ITM, and MHM) showed a substantive dose-response signal, as did specific clinical/demographic characteristics (T- and N-category, gender, age, and chemotherapy status, prescription dose and fractionation). Our data point to MHM as a strong associate of chronic-RAD in our OPC patients, and point to MHM V69 as a potential target constraint for clinical implementation. Further, the relationship of age confirms observations seen in other head and neck OARs[74] and points to potential risk stratification approach for older head and neck patients.
Moving forward, our goal is to develop multivariate 3D models which incorporate both spatially and hyper-dimensionally covariate data. Our goal is to develop a model that accounts for dose to multiple structures, and gives evidence-based decision tools for therapy, rehabilitation, or supportive care interventions, such as patient/physician/speech pathology for shared decision-making. The identification of high-risk subsets of patients (e.g. older patients with high MHM dose) could drive swallowing exercise, and thus serve as prophylaxis against dysphagia.
Conclusion
Swallowing muscles (SPC, ADM, GGM, ITM, MHM, and MPM) dose-volume parameters were associated with chronic-RAD. A model using age and MHM V69 was the preferred model to identify chronic-RAD. Our data suggest mylohyoid dose and age may be cofactors of interest for reducing or risk-stratifying for dysphagia in future oropharyngeal cancer populations.
Supplementary Material
Boxplot display of whole muscle ROI mean doses, stratified by the presence or absence of chronic-RAD; p-values are presented. *-indicates p<0.05; **-indicates Bonferroni-corrected p< 0.0036.
†Contributing authors
Timothy Dale, MBA1,5**, Katherine Hutcheson, PhD2, **, Abdallah S. R. Mohamed, MD, MSc1,6, Jan S. Lewin, PhD2, G. Brandon Gunn, MD1, Arvind U.K. Rao, PhD3, Jayashree Kalpathy-Cramer, PhD7, Steven J. Frank, MD1, Adam S. Garden, MD1, Jay A. Messer, B.S.1,9, Benjamin Warren, B.S.1,9, Stephen Y. Lai, MD, PhD2, Beth M. Beadle, MD, PhD1, William H. Morrison, MD,1 Jack Phan, MD, PhD1, Heath Skinner, MD, PhD1, Neil Gross, MD2, Renata Ferrarotto, MD4, Randal S. Weber, MD2, David I. Rosenthal, MD1, Clifton D. Fuller, MD, PhD1,8,*.
1Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
2Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
3Department of Bioinformatics and Computational Biology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
4Department of Thoracic & Head and Neck Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
5Baylor College of Medicine, Houston, TX, USA.
6Department of Clinical Oncology, University of Alexandria, Alexandria, Egypt.
7Athinoula A. Martinos Center for Biomedical Imaging/Massachusetts General Hospital/Massachusetts Institute of Technology, Charlestown, MA, USA.
8Medical Physics Program, The University of Texas Graduate School of Biomedical Sciences, Houston, TX, USA.
9The University of Texas Health Science Center at Houston Medical School, Houston, TX, USA.
**co-first author contributing equally
†- Co-author specific contributions:
All listed co-authors performed the following:
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work;
Drafting the work or revising it critically for important intellectual content;
Final approval of the version to be published;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Specific additional individual cooperative effort contributions to study/manuscript design/execution/interpretation, in addition to all criteria above are listed as follows:
TD-Drafted initial manuscript, undertook supervised analysis and interpretation of data. KH-Co-primary investigator; with CDF conceived project and interpreted study results, direct and final oversight of toxicity and clinical data collection; direct oversight of trainee personnel (TD).
ASRM-Undertook clinical and imaging data collection; executed and quality assured immobilization, direct oversight of all image registration/segmentation, and data collection workflow; direct oversight of trainee personnel (TD, JM, BW), and participated in data analysis, interpretation, and manuscript drafting and final editing . GBG,JSL,SJF,ASG, SYL, BMB, WHM,JP, HS, NG, RF, RW- Direct patient care provision, direct toxicity assessment and clinical data collection; interpretation and analytic support.
AR- Provided direct statistical support and data interpretation assistance.
JKC- Assisted with project inception; segmentation analysis quality assurance; provided statistical support and data interpretation assistance.
JM, BW- Segmentation workflow and imaging registration execution; supervised data analysis.
DIR- Responsible for data collection, project integrity, manuscript oversight and correspondence, programmatic oversight, toxicity assessment and clinical data collection; direct oversight of trainee personnel (ASRM, CDF).
CDF- Corresponding author; co-primary investigator; conceived, coordinated, and directed all study activities, responsible for data collection, project integrity, manuscript content and editorial oversight and correspondence; direct oversight of trainee personnel (TD, JM, BW, ASRM).
Conflict of Interest Statement/Funding sources and financial disclosures: Dr. Fuller received/receives grant and/or salary support from: the National Institutes of Health/National Cancer Institute’s Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant Program. Dr. Hutcheson receives grant support from the MD Anderson Institutional Research Grant Program and the National Cancer Institute (R03 CA188162). This work was supported in part infrastructure support by National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. These listed funders/supporters played no role in the study design, collection, analysis, interpretation of data, manuscript writing, or decision to submit the report for publication.
Footnotes
Portions of this dataset were presented at the 2015 American Society for Radiation Oncology Annual Meeting, San Antonio, TX, and the 2016 Multidisciplinary Head and Neck Cancer Symposium, Scottsdale, AZ.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–6. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 2.Bhide SA, Newbold KL, Harrington KJ, Nutting CM. Clinical evaluation of intensity-modulated radiotherapy for head and neck cancers. The British journal of radiology. 2012;85:487–94. doi: 10.1259/bjr/85942136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisbruch A, Lyden T, Bradford CR, Dawson LA, Haxer MJ, Miller AE, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. International journal of radiation oncology, biology, physics. 2002;53:23–8. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 4.Awan MJ, Mohamed AS, Lewin JS, Baron CA, Gunn GB, Rosenthal DI, et al. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral oncology. 2014;50:746–52. doi: 10.1016/j.oraloncology.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levendag PC, Teguh DN, Voet P, van der Est H, Noever I, de Kruijf WJ, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Boero IJ, Hwang L, Le QT, Moiseenko V, Sanghvi PR, et al. Aspiration pneumonia after concurrent chemoradiotherapy for head and neck cancer. Cancer. 2014 doi: 10.1002/cncr.29207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beadle BM, Liao KP, Elting LS, Buchholz TA, Ang KK, Garden AS, et al. Improved survival using intensity-modulated radiation therapy in head and neck cancers: a SEER-Medicare analysis. Cancer. 2014;120:702–10. doi: 10.1002/cncr.28372. [DOI] [PubMed] [Google Scholar]
- 9.Garden AS, Dong L, Morrison WH, Stugis EM, Glisson BS, Frank SJ, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. International journal of radiation oncology, biology, physics. 2013;85:941–7. doi: 10.1016/j.ijrobp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. The Lancet Oncology. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. International journal of radiation oncology, biology, physics. 2010;78:1356–65. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortensen HR, Jensen K, Aksglaede K, Behrens M, Grau C. Late dysphagia after IMRT for head and neck cancer and correlation with dose-volume parameters. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;107:288–94. doi: 10.1016/j.radonc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Langendijk JA, Doornaert P, Rietveld DH, Verdonck-de Leeuw IM, Leemans CR, Slotman BJ. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;90:189–95. doi: 10.1016/j.radonc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2007;85:74–82. doi: 10.1016/j.radonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CR, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;106:364–9. doi: 10.1016/j.radonc.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NC, Garcia J, Kies MS, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. International journal of radiation oncology, biology, physics. 2008;72:747–55. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peponi E, Glanzmann C, Willi B, Huber G, Studer G. Dysphagia in head and neck cancer patients following intensity modulated radiotherapy (IMRT) Radiation oncology. 2011;6:1. doi: 10.1186/1748-717X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paleri V, Roe JW, Strojan P, Corry J, Gregoire V, Hamoir M, et al. Strategies to reduce long-term postchemoradiation dysphagia in patients with head and neck cancer: an evidence-based review. Head & neck. 2014;36:431–43. doi: 10.1002/hed.23251. [DOI] [PubMed] [Google Scholar]
- 19.Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. 2014;89:292–302. doi: 10.1016/j.ijrobp.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Awan MJ, Mohamed ASR, Lewin JS, Baron CA, Gunn GB, Rosenthal DI, et al. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: A preliminary dosimetric comparison. Oral oncology. 2014 doi: 10.1016/j.oraloncology.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhayani MK, Hutcheson KA, Barringer DA, Roberts DB, Lewin JS, Lai SY. Gastrostomy tube placement in patients with hypopharyngeal cancer treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head & neck. 2013;35:1641–6. doi: 10.1002/hed.23199. [DOI] [PubMed] [Google Scholar]
- 22.Gunn GB, Mendoza TR, Fuller CD, Gning I, Frank SJ, Beadle BM, et al. High symptom burden prior to radiation therapy for head and neck cancer: A patient-reported outcomes study. Head & neck. 2012 doi: 10.1002/hed.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutcheson KA, Bhayani MK, Beadle BM, Gold KA, Shinn EH, Lai SY, et al. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngol Head Neck Surg. 2013;139:1127–34. doi: 10.1001/jamaoto.2013.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MWS, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–9. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheson KA, Yuk MM, Holsinger FC, Gunn GB, Lewin JS. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy in long-term oropharyngeal cancer survivors: Video case reports. Head & neck. 2014 doi: 10.1002/hed.23840. [DOI] [PubMed] [Google Scholar]
- 26.Kocak-Uzel E, Gunn GB, Colen RR, Kantor ME, Mohamed ASR, Schoultz-Henley S, et al. Beam path toxicity in candidate organs-at-risk: Assessment of radiation emetogenesis for patients receiving head and neck intensity modulated radiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014 doi: 10.1016/j.radonc.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson SK, Shinn EH, Basen-Engquist K, Demark-Wahnefried W, Prokhorov AV, Baru C, et al. Identifying early dehydration risk with home-based sensors during radiation treatment: a feasibility study on patients with head and neck cancer. J Natl Cancer Inst Monographs. 2013;2013:162–8. doi: 10.1093/jncimonographs/lgt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NCS, Garcia J, Kies MS, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. International journal of radiation oncology, biology, physics. 2008;72:747–55. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenthal DI, Mendoza TR, Fuller CD, Hutcheson KA, Wang XS, Hanna EY, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: A prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer. 2014 doi: 10.1002/cncr.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. International journal of radiation oncology, biology, physics. 2010;78:1356–65. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS. Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Qual Life Res. 2013;22:2331–9. doi: 10.1007/s11136-013-0380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai CJ, Hofstede TM, Sturgis EM, Garden AS, Lindberg ME, Wei Q, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. International journal of radiation oncology, biology, physics. 2013;85:415–20. doi: 10.1016/j.ijrobp.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 33.Hutcheson KA, Lewin JS, Holsinger FC, Steinhaus G, Lisec A, Barringer DA, et al. Long-term functional and survival outcomes after induction chemotherapy and risk-based definitive therapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2014;36:474–80. doi: 10.1002/hed.23330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed AS, Ruangskul MN, Awan MJ, Baron CA, Kalpathy-Cramer J, Castillo R, et al. Quality assurance assessment of diagnostic and radiation therapy-simulation CT image registration for head and neck radiation therapy: anatomic region of interest-based comparison of rigid and deformable algorithms. Radiology. 2015;274:752–63. doi: 10.1148/radiol.14132871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kocak-Uzel E, Gunn GB, Colen RR, Kantor ME, Mohamed AS, Schoultz-Henley S, et al. Beam path toxicity in candidate organs-at-risk: assessment of radiation emetogenesis for patients receiving head and neck intensity modulated radiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014;111:281–8. doi: 10.1016/j.radonc.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychological methods. 2009;14:323–48. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carre G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46. [Google Scholar]
- 38.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients’ perspectives. Otolaryngol Head Neck Surg. 2011;145:767–71. doi: 10.1177/0194599811414506. [DOI] [PubMed] [Google Scholar]
- 40.Khan MK, Koyfman SA, Hunter GK, Reddy CA, Saxton JP. Definitive radiotherapy for early (T1–T2) glottic squamous cell carcinoma: a 20 year Cleveland Clinic experience. Radiat Oncol. 2012;7:193. doi: 10.1186/1748-717X-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter KU, Lee OE, Lyden TH, Haxer MJ, Feng FY, Schipper M, et al. Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head & neck. 2013 doi: 10.1002/hed.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhayani MK, Hutcheson KA, Barringer DA, Lisec A, Alvarez CP, Roberts DB, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: Factors affecting placement and dependence. Head & neck. 2013;35:1634–40. doi: 10.1002/hed.23200. [DOI] [PubMed] [Google Scholar]
- 43.Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. International journal of radiation oncology, biology, physics. 2011;81:e93–9. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. International journal of radiation oncology, biology, physics. 2007;68:1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 45.Feng FY, Kim HM, Lyden TH, Haxer MJ, Worden FP, Feng M, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–8. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caglar HB, Tishler RB, Othus M, Burke E, Li Y, Goguen L, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. International journal of radiation oncology, biology, physics. 2008;72:1110–8. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 47.De Ruyck K, Duprez F, Werbrouck J, Sabbe N, Sofie de L, Boterberg T, et al. A predictive model for dysphagia following IMRT for head and neck cancer: introduction of the EMLasso technique. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;107:295–9. doi: 10.1016/j.radonc.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Deantonio L, Masini L, Brambilla M, Pia F, Krengli M. Dysphagia after definitive radiotherapy for head and neck cancer. Correlation of dose-volume parameters of the pharyngeal constrictor muscles. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2013;189:230–6. doi: 10.1007/s00066-012-0288-8. [DOI] [PubMed] [Google Scholar]
- 49.Dirix P, Abbeel S, Vanstraelen B, Hermans R, Nuyts S. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. International journal of radiation oncology, biology, physics. 2009;75:385–92. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 50.Duprez F, Madani I, De Potter B, Boterberg T, De Neve W. Systematic review of dose--volume correlates for structures related to late swallowing disturbances after radiotherapy for head and neck cancer. Dysphagia. 2013;28:337–49. doi: 10.1007/s00455-013-9452-2. [DOI] [PubMed] [Google Scholar]
- 51.Haderlein M, Semrau S, Ott O, Speer S, Bohr C, Fietkau R. Dose-dependent deterioration of swallowing function after induction chemotherapy and definitive chemoradiotherapy for laryngopharyngeal cancer. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2014;190:192–8. doi: 10.1007/s00066-013-0493-0. [DOI] [PubMed] [Google Scholar]
- 52.Mazzola R, Ricchetti F, Fiorentino A, Fersino S, Giaj Levra N, Naccarato S, et al. Dose-volume-related dysphagia after constrictor muscles definition in head and neck cancer intensity-modulated radiation treatment. The British journal of radiology. 2014;87:20140543. doi: 10.1259/bjr.20140543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanguineti G, Gunn GB, Parker BC, Endres EJ, Zeng J, Fiorino C. Weekly dose-volume parameters of mucosa and constrictor muscles predict the use of percutaneous endoscopic gastrostomy during exclusive intensity-modulated radiotherapy for oropharyngeal cancer. International journal of radiation oncology, biology, physics. 2011;79:52–9. doi: 10.1016/j.ijrobp.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 54.Sanguineti G, Rao N, Gunn B, Ricchetti F, Fiorino C. Predictors of PEG dependence after IMRT+/−chemotherapy for oropharyngeal cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;107:300–4. doi: 10.1016/j.radonc.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 55.Teguh DN, Levendag PC, Noever I, van Rooij P, Voet P, van der Est H, et al. Treatment techniques and site considerations regarding dysphagia-related quality of life in cancer of the oropharynx and nasopharynx. International journal of radiation oncology, biology, physics. 2008;72:1119–27. doi: 10.1016/j.ijrobp.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 56.Wopken K, Bijl HP, van der Schaaf A, van der Laan HP, Chouvalova O, Steenbakkers RJ, et al. Development of a multivariable normal tissue complication probability (NTCP) model for tube feeding dependence after curative radiotherapy/chemo-radiotherapy in head and neck cancer. Radiother Oncol. 2014;113:95–101. doi: 10.1016/j.radonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Salama JK, Stenson KM, List MA, Mell LK, Maccracken E, Cohen EE, et al. Characteristics associated with swallowing changes after concurrent chemotherapy and radiotherapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1060–5. doi: 10.1001/archotol.134.10.1060. [DOI] [PubMed] [Google Scholar]
- 58.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? International journal of radiation oncology, biology, physics. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 59.Vlacich G, Spratt DE, Diaz R, Phillips JG, Crass J, Li CI, et al. Dose to the inferior pharyngeal constrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014;110:435–40. doi: 10.1016/j.radonc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Kumar R, Madanikia S, Starmer H, Yang W, Murano E, Alcorn S, et al. Radiation dose to the floor of mouth muscles predicts swallowing complications following chemoradiation in oropharyngeal squamous cell carcinoma. Oral oncology. 2014;50:65–70. doi: 10.1016/j.oraloncology.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Brouwer CL, Steenbakkers RJ, Gort E, Kamphuis ME, van der Laan HP, Van’t Veld AA, et al. Differences in delineation guidelines for head and neck cancer result in inconsistent reported dose and corresponding NTCP. Radiother Oncol. 2014;111:148–52. doi: 10.1016/j.radonc.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 62.Paterson WG. Alteration of swallowing and oesophageal peristalsis by different initiators of deglutition. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 1999;11:63–7. doi: 10.1046/j.1365-2982.1999.00131.x. [DOI] [PubMed] [Google Scholar]
- 63.Pearson WG, Jr, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–51. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. International journal of radiation oncology, biology, physics. 2012;83:210–9. doi: 10.1016/j.ijrobp.2011.06.1954. [DOI] [PubMed] [Google Scholar]
- 65.Beetz I, Schilstra C, van Luijk P, Christianen ME, Doornaert P, Bijl HP, et al. External validation of three dimensional conformal radiotherapy based NTCP models for patient-rated xerostomia and sticky saliva among patients treated with intensity modulated radiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;105:94–100. doi: 10.1016/j.radonc.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Kierkels RG, Korevaar EW, Steenbakkers RJ, Janssen T, van’t Veld AA, Langendijk JA, et al. Direct use of multivariable normal tissue complication probability models in treatment plan optimisation for individualised head and neck cancer radiotherapy produces clinically acceptable treatment plans. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2014;112:430–6. doi: 10.1016/j.radonc.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 67.van der Laan HP, Gawryszuk A, Christianen ME, Steenbakkers RJ, Korevaar EW, Chouvalova O, et al. Swallowing-sparing intensity-modulated radiotherapy for head and neck cancer patients: treatment planning optimization and clinical introduction. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;107:282–7. doi: 10.1016/j.radonc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 68.van der Laan HP, Bijl HP, Steenbakkers RJ, van der Schaaf A, Chouvalova O, Vemer-van den Hoek JG, et al. Acute symptoms during the course of head and neck radiotherapy or chemoradiation are strong predictors of late dysphagia. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;115:56–62. doi: 10.1016/j.radonc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 69.Wopken K, Bijl HP, van der Schaaf A, Christianen ME, Chouvalova O, Oosting SF, et al. Development and validation of a prediction model for tube feeding dependence after curative (chemo-) radiation in head and neck cancer. PloS one. 2014;9:e94879. doi: 10.1371/journal.pone.0094879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dabaja B, Salehpour MR, Rosen I, Tung S, Morrison WH, Ang KK, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. International journal of radiation oncology, biology, physics. 2005;63:1000–5. doi: 10.1016/j.ijrobp.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 71.Lee N, Mechalakos J, Puri DR, Hunt M. Choosing an intensity-modulated radiation therapy technique in the treatment of head-and-neck cancer. International journal of radiation oncology, biology, physics. 2007;68:1299–309. doi: 10.1016/j.ijrobp.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Allen Li X, Alber M, Deasy JO, Jackson A, Ken Jee KW, Marks LB, et al. The use and QA of biologically related models for treatment planning: short report of the TG-166 of the therapy physics committee of the AAPM. Medical physics. 2012;39:1386–409. doi: 10.1118/1.3685447. [DOI] [PubMed] [Google Scholar]
- 73.Beetz I, Schilstra C, van der Schaaf A, van den Heuvel ER, Doornaert P, van Luijk P, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: the role of dosimetric and clinical factors. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;105:101–6. doi: 10.1016/j.radonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Beetz I, Steenbakkers RJ, Chouvalova O, Leemans CR, Doornaert P, van der Laan BF, et al. The QUANTEC criteria for parotid gland dose and their efficacy to prevent moderate to severe patient-rated xerostomia. Acta Oncol. 2014;53:597–604. doi: 10.3109/0284186X.2013.831186. [DOI] [PubMed] [Google Scholar]
- 75.Semiz Oysu A, Ayanoglu E, Kodalli N, Oysu C, Uneri C, Erzen C. Dynamic contrast-enhanced MRI in the differentiation of posttreatment fibrosis from recurrent carcinoma of the head and neck. Clin Imaging. 2005;29:307–12. doi: 10.1016/j.clinimag.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 76.Moloney EC, Brunner M, Alexander AJ, Clark J. Quantifying fibrosis in head and neck cancer treatment: An overview. Head & neck. 2015;37:1225–31. doi: 10.1002/hed.23722. [DOI] [PubMed] [Google Scholar]
- 77.Glazer HS, Lee JK, Levitt RG, Heiken JP, Ling D, Totty WG, et al. Radiation fibrosis: differentiation from recurrent tumor by MR imaging. Radiology. 1985;156:721–6. doi: 10.1148/radiology.156.3.4023233. [DOI] [PubMed] [Google Scholar]
- 78.Hutcheson KA, Lewin JS, Barringer DA, Lisec A, Gunn GB, Moore MW, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–9. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang AT, Song S, Dominguez LM, Nguyen J, Goldman RA, Reiter ER. Delayed lower cranial neuropathies following primary radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. 2013;123:1207–9. doi: 10.1002/lary.23938. [DOI] [PubMed] [Google Scholar]
- 80.Shin HY, Park HJ, Choi YC, Kim SM. Clinical and electromyographic features of radiation-induced lower cranial neuropathy. Clin Neurophysiol. 2013;124:598–602. doi: 10.1016/j.clinph.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Ghazali N, Shaw RJ, Rogers SN, Risk JM. Genomic determinants of normal tissue toxicity after radiotherapy for head and neck malignancy: a systematic review. Oral oncology. 2012;48:1090–100. doi: 10.1016/j.oraloncology.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 82.Rattay T, Talbot CJ. Finding the genetic determinants of adverse reactions to radiotherapy. Clin Oncol (R Coll Radiol) 2014;26:301–8. doi: 10.1016/j.clon.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Venkatesh GH, Manjunath VB, Mumbrekar KD, Negi H, Fernandes DJ, Sharan K, et al. Polymorphisms in radio-responsive genes and its association with acute toxicity among head and neck cancer patients. PloS one. 2014;9:e89079. doi: 10.1371/journal.pone.0089079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Popanda O, Marquardt JU, Chang-Claude J, Schmezer P. Genetic variation in normal tissue toxicity induced by ionizing radiation. Mutat Res. 2009;667:58–69. doi: 10.1016/j.mrfmmm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Merlano MC, Monteverde M, Colantonio I, Denaro N, Lo Nigra C, Natoli G, et al. Impact of age on acute toxicity induced by bio- or chemo-radiotherapy in patients with head and neck cancer. Oral oncology. 2012;48:1051–7. doi: 10.1016/j.oraloncology.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 86.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–81. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Boxplot display of whole muscle ROI mean doses, stratified by the presence or absence of chronic-RAD; p-values are presented. *-indicates p<0.05; **-indicates Bonferroni-corrected p< 0.0036.