Abstract
Use of the dietary supplement quercetin is on the rise. Because previous studies imply an inhibitory effect of quercetin on male fertility, we explored the effects of this flavonoid on fertility in female mice. Birth outcomes, and ovarian morphology in 4-week-old offspring, were assessed in mice receiving dietary quercetin (5 mg kg−1 day−1) for 9 months during two breeding periods: from 2 to 6 months (prime reproductive age) and 8 to11 months of age. Quercetin increased birth spacing, leading to a 60% reduction in the number of litters, but enhanced folliculogenesis in ovaries of female offspring. While in young females quercetin caused an almost 70% increase in litter size, in older animals this effect was reversed. Consistent with the inhibitory activity of quercetin on the enzyme transglutaminase 2 (TG2), genetic ablation of TG2 in mice mirrors the effects of quercetin on birth outcomes and follicular development. Further, TG2-null mice lack responsiveness to quercetin ingestion. Our study shows for the first time that dietary quercetin can cause reduced reproductive potential in female mice and implies that TG2 may regulate ovarian ageing.
Additional keywords: fecundity, folliculogenesis, litter size, offspring, ovary
Introduction
Dietary bioflavonoids represent a large class of polyphenolic compounds found in most plants. Many flavonoids, including quercetin (3,3′,4′,5,7-pentahydroxyflavone), are reported to possess strong antioxidant properties and to have beneficial health effects. Accumulating evidence that dietary bioflavonoids are beneficial for health and longevity (Wojcik et al. 2010) combined with the recent classification of quercetin as generally recognised as safe (GRAS) has led to a widening use of quercetin as a food supplement in the general population including people of reproductive age. However, the impact of quercetin on fertility and reproduction requires further investigation.
The effects of quercetin on male fertility as studied in vitro and in vivo are controversial, ranging from a demonstrated impairment of male fertility both in humans and animal models (Aravindakshan et al. 1985; Khanduja et al. 2001; Ranawat et al. 2013), to the use of quercetin as an alternative drug for the treatment of male infertility (Taepongsorat et al. 2008). The ability of this flavonoid to stimulate intense redox activity in human spermatozoa in vitro was proposed to underlie the aetiology of male infertility induced by quercetin (Bennetts et al. 2008). In contrast to the multiple investigations on the effects of quercetin on male fertility, its effects on female reproduction are less studied.
Among the pleiotropic effects of quercetin is its ability to inhibit the activity of enzyme transglutaminase 2 (TG2; Beazley et al. 2013a). TG2 is a ubiquitous calcium-dependent enzyme that catalyses protein cross-linking, polyamination or deamidation (Lorand and Graham 2003).Women with coeliac disease, an autoimmune disorder targeting TG2 (Özgör and Selimoglu 2010), experience a variety of reproductive disorders contributing to unexplained infertility; however, the mechanisms contributing to this remain unclear. TG2 is present in the endometrial epithelium where it participates in adhesion and migratory events during embryo implantation (Fujimoto et al. 1996; Kabir-Salmani et al. 2005). In addition, TG2 regulates various signalling cascades, including the canonical β-catenin pathway (Beazley et al. 2012) that has been implicated in regulating ovulation (Fan et al. 2010; Usongo et al. 2012).
The goal of this study was to explore the effects of dietary quercetin supplementation on female fecundity and fertility in mice, both during their prime reproductive age (2–6 months old) and as they near reproductive cessation (8–11 months old). Moreover, accounting for the ability of quercetin to inhibit TG2 as well as TG2-dependent signalling (Beazley et al. 2012; Beazley et al. 2013a), we also investigated the role of TG2 in quercetin effects on female reproduction. Our data suggest that consumption of quercetin has unwanted mild effects on female fertility, mediated at least in part by TG2, raising awareness about the long-term use of this dietary supplement in females of reproductive age.
Materials and methods
Animal maintenance and breeding
C57BL/6 mice or transglutaminase 2 (TG2)-null mice (a kind gift from Robert Graham, Victor Chang Cardiovascular Institute, Darlinghurst, NSW, Australia) were maintained under controlled lighting (12 : 12 h light : dark) and temperature (22°C) with ad libitum access to food and water. All animal experiments were approved by the Animal Care and Use Committee at the University of Maryland Medical School and were conducted in accordance with the National Institute of Health (NIH) guidelines for the care and use of laboratory animals.
For breeding experiments, 2-month-old nulliparous female C57BL/6 mice or TG2-null mice were housed 2 : 1 with proven males (four females and two males in total of each genotype for each condition tested; power = 0.87). To determine the effects of quercetin on female reproduction and fertility, breeding cages were randomly assigned to receive either quercetin (Quercegen Pharma, Boston, MA, USA) or an equal volume of dimethylsulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA) vehicle via drinking water. This dose of quercetin is sufficient to block TG2 activity in vitro (Beazley et al. 2012) and to prevent β-catenin activation in vivo (Beazley et al. 2013a). Quercetin was prepared as a stock solution at 80 mgmL−1 in DMSO. Dosage was determined by monitoring water consumption over a 3-week period (Table 1) and using the calculation 80 mgmL−1 quercetin × average volume water consumed per animal per day (mL)/average weight per animal (kg) to determine the amount of quercetin stock to add to drinking water. Based on these data, animals received either 5mg kg−1 day−1 quercetin or DMSO vehicle 0.05% v/v in drinking water. For each dam, the number of litters and litter sizes were recorded. From these data, the time between successful pregnancies (birth spacing) was calculated. Resulting offspring were weighed weekly until 4 weeks of age at which time animals were killed and ovaries were harvested from female offspring for morphometric and histologic analyses.
Table 1.
Average weight and water consumption over a 3-week period by vehicle-treated (control) and quercetin-treated (quercetin) wild-type or TG2-knockout mice
| Parameter | Wild-type | TG2-knockout | ||
|---|---|---|---|---|
| Control | Quercetin | Control | Quercetin | |
| Bodyweight (g) | 23.6 ± 1.2 | 24.1 ± 0.6 | 23.8 ± 0.1 | 22.6 ± 1.2 |
| Water consumption (mL day−1) | 3.2 ± 0.2 | 2.9 ± 0.3 | 3.1 ± 0.2 | 3.2 ± 0.1 |
| Quercetin dose (mg kg−1 day−1) | – | 5.2 ± 0.3 | – | 5.7 ± 0.3 |
n = 3, all groups
Ovarian histology and follicle counting
To determine the effect of quercetin treatment on folliculogenesis, ovaries were harvested from one 4-week-old female offspring per litter born to either untreated control dams or quercetin-treated dams, both wild-type and TG2-null genotypes. These female offspring were receiving quercetin during the first 3 weeks of life either via drinking water or mother’s milk (De Feo et al. 2006) and then for 1 additional week after weaning in drinking water. Collected ovaries were weighted and then fixed in 4% paraformaldehyde, embedded in Optimum Cutting Temperature (OCT; Electron Microscopy Sciences, Hatfield, PA, USA) freezing medium and serially sectioned at 10-νm thickness. Haematoxylin and eosin staining (H&E) was performed according to standard protocols. Ovarian follicles were analysed on every 10th serial section through the ovary using a Leica DMIL microscope with attached SPOT-RT camera (Diagnostic Instruments, Sterling Heights, MI, USA). Follicles were counted and classified as primordial (Po), primary (Pr), secondary (Se) or antral (An) based on the following characteristics: primordial follicles were defined as oocytes surrounded by a single layer of flattened granulosa cells; primary follicles were defined as oocytes surrounded by a single layer of cuboidal granulosa cells; secondary follicles were defined as oocytes surrounded by two or more layers of cuboidal granulosa cells and antral follicles were defined by the presence of an antrum. The abundance of each type of follicle in quercetin and control ovaries was normalised to total ovarian area and reported as a percentage of control (control = 100%).
Statistical analysis
Power analysis to determine sample size was calculated using G*Power software V.3.1 (Faul et al. 2007). Statistical analyses were performed using Student t-test for two groups (SigmaStat, V.2.03; SPSS, Chicago, IL, USA) comparing the quercetin treatment groups to controls. Data were tested for normality as well as equal variance. For groups of three or more, one-way analysis of variance (ANOVA) was used. Data are presented as mean ± s.e.m.
Results
Birth outcomes in quercetin-treated mice
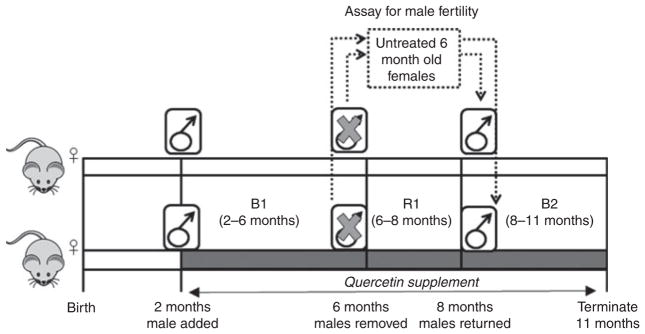
To determine the effects of quercetin on female reproduction and fertility, breeding cages were randomly assigned to receive 5 mg kg−1 day−1 quercetin in DMSO or an equal volume of DMSO vehicle via drinking water (Fig. 1). Control and quercetin-treated animals were allowed to breed during two breeding periods; B1 when females were 2–6 months old followed by B2 when females were 8–11 months old. During a 2-month resting period (R1) between these breeding periods, males were removed from the cages and allowed to breed with untreated females to test whether a 4-month-long quercetin exposure affected male fertility (Fig. 1, dashed box). Birth outcomes including the dates of birth and numbers of pups per litter born to each female were recorded during each breeding period.
Fig. 1.
Experimental breeding scheme for wild-type or TG2-knockout mice treated with 5mg kg−1 day−1 quercetin (dark grey) or vehicle control (light grey). Breeding was monitored in the same females during two breeding periods, when the females were 2–6 months old (B1) and 8–11 months old (B2). During the resting period (R1) females continued to receive quercetin or vehicle while males were removed and bred with untreated 6-month-old untreated females (dashed box). n = 4 females and 2 males for each of the following groups: quercetin-treated males × quercetin-treated females, vehicle-treated males × vehicle-treated females and quercetin- or vehicle-treated males × untreated females.
Quercetin had no effect on maternal bodyweight after the first 2 weeks of treatment (Fig. 2) or after 4 months of treatment (Fig. 2), during the resting period between breeding periods. However, we did observe a significant decrease in the number of litters born to both young and aged quercetin-treated females compared with age-matched control dams (Fig. 3a). Females in the B2 period may represent those at the onset of reproductive senescence as the number of litters born to them, normalised to the duration of breeding, was reduced by ~80% compared with young females in both control (grey bars) and quercetin-treated females (black bars; Fu et al. 2013). These data indicate that quercetin does not alter the onset of reproductive senescence. The significant 58.3% quercetin-dependent decrease in litter number in young females during the first breeding period (Fig. 3a) was accompanied by a similar 61.9% increase in the spacing between successful births (from 3.6 ± 0.2 weeks in control females to 5.8 ± 0.8 weeks in quercetin-treated females, P<0.001; Fig. 3b), accounting for the observed reduction in litters born to quercetin-treated females and suggesting reduced reproductive potential in quercetin-treated female mice regardless of age.
Fig. 2.
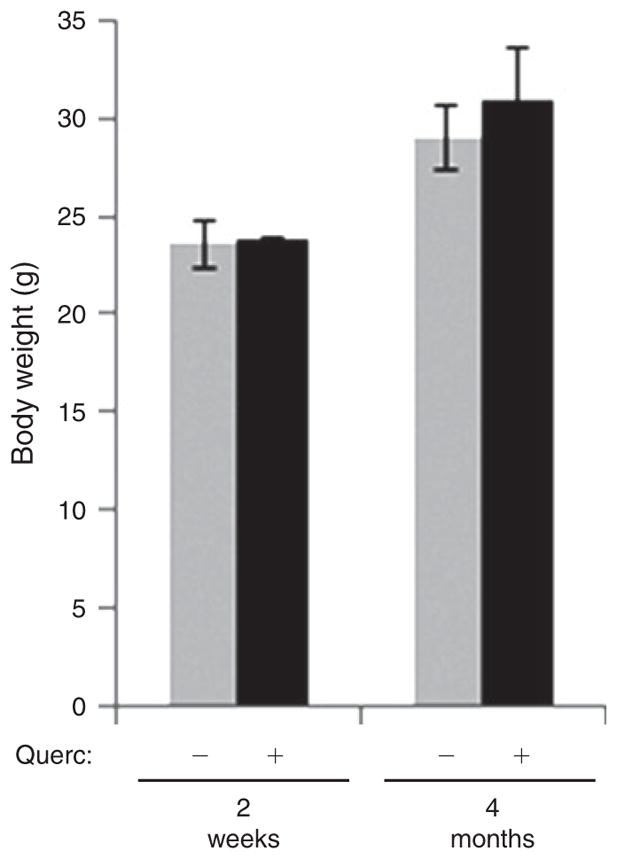
Quercetin treatment does not affect maternal bodyweight. Female dams receiving quercetin (5 mg kg−1 day−1; + Querc) or vehicle (− Querc) continuously via their drinking water were weighed 2 weeks after the start of quercetin treatment and again 4 months later between breeding periods. n = 4.
Fig. 3.
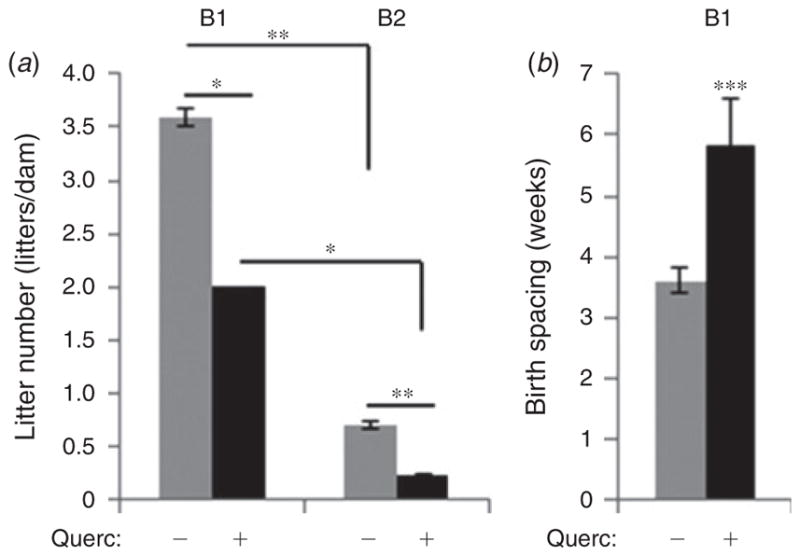
Dietary quercetin reduces fertility in female mice. (a) Number of litters born per dam, normalised to duration of breeding, during both early (B1) and late (B2) breeding periods and (b) time between the births of the litters during the early (B1) breeding period was recorded in female mice receiving quercetin (5 mg kg−1 day−1; + Querc) or vehicle (− Querc) continuously via their drinking water. n = 4. *P<0.05, **P<0.01, ***P<0.001.
Quercetin promotes increased fecundity
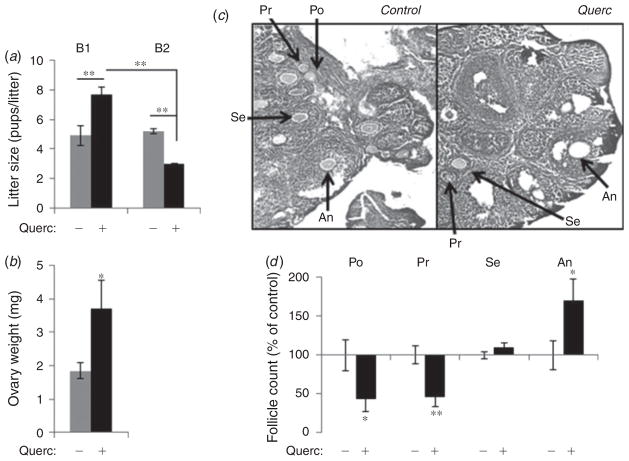
To determine whether quercetin treatment also impacts fecundity, we next examined the average litter size in quercetin-treated and control untreated females. In the control female mice the average number of ~5 pups per litter remained constant throughout both breeding periods, regardless of the age of the females (Fig. 4a, grey bars). In contrast, in quercetin-treated females we observed a significant increase in pups per litter (from 4.9 ± 0.7 to 7.7 ± 0.5, P<0.05) in young females (Fig. 4a, B1) and a significant decrease (from 5.2 ± 0.2 to 3.0 ± 0.1, P<0.01) in aged females (Fig. 4a, B2). This dual response in young and old females suggests that females receiving quercetin may be producing more mature follicles early but fewer follicles as they age.
Fig. 4.
Quercetin enhances follicle development. (a) Litter size was recorded in female mice receiving quercetin (5 mg kg−1 day−1; + Querc) or vehicle control (− Querc) continuously via their drinking water. n = 4. **P<0.01. (b) Ovary weight is increased in quercetin-treated female mice (n = 6) compared with control mice (n = 8). (c–d ) Follicle counts in serial sections of ovaries from control (n = 5) and quercetin-treated (+ Querc; n =5) female mice. (c) Representative sections of ovaries. (d) Quantification of the stages of folliculogenesis in serial sections through each ovary. Follicles were classified according to histomorphology as: primordial (Po), primary (Pr), secondary (Se) or antral (An). *P<0.05, **P<0.01.
The increase in litter size observed in young quercetin-treated females suggests enhanced follicular development. However, it was not feasible to examine both birth outcomes and ovarian histology in the breeding dams, as the dams were near cessation of oestrus when we concluded the breeding studies (see Fig. 3a). Therefore, we took an indirect measure to determine the potential for quercetin treatment to affect folliculogenesis by examining ovarian histology in 4-week-old female offspring born to quercetin-treated mothers and treated with quercetin until ovaries were collected.
The birthweight and growth of the offspring during post-natal development up to 4 weeks of age were not affected by quercetin (Table 2). However, analysis of the offspring revealed a significant increase in ovary weight (Fig. 4b) in quercetin-treated mice compared with the control, although no visual gross abnormalities were noted. Next, we examined the stages of follicular development in female offspring using H&E-stained serial sections of ovaries. Gross observations showed abundant antral (An) follicles in ovaries from quercetin-treated animals compared with control animals (Fig. 4c). Quantitative analysis of the number of follicles at different stages from at least 10 serial sections spaced 100 νm apart within each ovary revealed a significant increase in the number of antral (An) follicles in quercetin-treated offspring, while both primordial (Po) and primary (Pr) follicles were similarly decreased (P<0.01, Fig. 4d). These data suggest that quercetin stimulates more rapid maturation of follicles at the expense of reducing the numbers of the earlier stage (primordial and primary) follicles. Further, the 70.2 ± 27.4% (P<0.05) increase in antral follicles closely mirrors the 69.9 ± 10.2% (P<0.01) increase in litter size in quercetin-treated females, indicating a potential relationship between increased folliculogenesis and the increase in litter size and supporting an overall increase in female fecundity in response to dietary quercetin.
Table 2.
Bodyweight of vehicle-treated (control) and quercetin-treated (quercetin) female offspring from birth through sexual maturity (4 weeks old)
| Age (weeks) | Bodyweight (g) | ||
|---|---|---|---|
| Control | Quercetin | P value | |
| 0.5 | 2.4 ± 0.3 | 2.8 ± 0.3 | 0.107 |
| 1 | 5.7 ± 0.4 | 5.6 ± 0.3 | 0.416 |
| 2 | 7.6 ± 0.9 | 6.6 ± 0.6 | 0.074 |
| 3 | 8.4 ± 0.8 | 9.1 ± 0.6 | 0.118 |
| 4 | 13.5 ± 0.1 | 13.2 ± 0.3 | 0.254 |
n = 8, control; n = 6, quercetin
No effects of quercetin on male potency at 5mg kg−1 day−1
Previous studies reported a decrease in male fertility in mice caused by higher doses of quercetin (20–400 mg kg−1 day−1; Aravindakshan et al. 1985; Khanduja et al. 2001; Ranawat et al. 2013). In the studies described here, both male and female mice in quercetin-treated breeding cages were exposed to quercetin. Therefore, to determine whether the reduced litter production in response to quercetin might be due to a decrease in male fertility, the males from the B1 breeding cages (both exposed to 5mg kg−1 day−1 quercetin for 4 months and control untreated) were mated for 2 months with additional untreated 6-month-old female mice (Fig. 1, dashed box). In these breedings, litter size and the spacing between successful pregnancies (birth spacing) were similar in cages with quercetin-treated and control male mice (Table 3), suggesting that the 4-month-long exposure to this low dose of quercetin did not affect male fertility.
Table 3.
Test of effect of quercetin on male fertility; birth outcomes in untreated female mice bred with male mice that were treated with quercetin (5 mg kg−1 day−1) or vehicle control
| Birth outcome | Control | Quercetin | P value |
|---|---|---|---|
| Litter size (pups per litter) | 5.8 ± 0.9 | 5.7 ± 1.2 | 0.421 |
| Birth spacing (weeks) | 3.5 ± 0.3 | 3.6 ± 0.6 | 0.389 |
n = 4
Transglutaminase 2 mediates the effects of quercetin on birth outcomes
Previously, we showed that quercetin inhibits the enzyme TG2 and its downstream intracellular signalling including the β-catenin pathway (Beazley et al. 2012, 2013a), both of which have been implicated in fertility (Fujimoto et al. 1996; Tulac et al. 2003; Kabir-Salmani et al. 2005; Fan et al. 2010; Liu et al. 2010; Usongo et al. 2012; Wetendorf and DeMayo 2012). We therefore reasoned that if quercetin effects involved the inhibition of TG2 then quercetin supplement would not affect fertility in TG2-null female mice. To test this hypothesis, male and female TG2-knockout mice were treated with quercetin and bred according to the same scheme as wild-type mice (see Fig. 1). In contrast to what we observed in wild-type mice, no difference in litter size, number of litters or time between successful pregnancies was observed in TG2-null animals during the early breeding period, in females that were 2 to 6 months old, and bodyweight at both 2 and 6 months old was not affected by quercetin treatment (Table 4). Similarly, detailed examination of ovarian histology revealed no difference in follicular development between quercetin-treated and control TG2-deficient females. However, in comparison to wild-type mice, genetic ablation of TG2 alone (in the presence or absence of quercetin) promoted a significant increase in the number of antral (An) follicles (Table 5), mirroring the effects of quercetin on wild-type females.
Table 4.
Birth outcomes and bodyweight of female TG2-knockout mice receiving quercetin (5 mg kg−1 day−1) or vehicle (control) continuously via their drinking water
| Parameter | Control | Quercetin | P value |
|---|---|---|---|
| Litter size (pups per litter) | 7.5 ± 0.4 | 7.8 ± 0.6 | 0.383 |
| Litter number (litters per dam) | 4.0 ± 0.7 | 4.3 ± 0.5 | 0.288 |
| Time between litters (weeks) | 3.7 ± 0.3 | 3.4 ± 0.1 | 0.116 |
| Bodyweight (g) | |||
| 2 months old | 24.1 ± 0.6 | 22.8 ± 1.2 | 0.162 |
| 6 months old | 30.3 ± 1.8 | 32.0 ± 2.5 | 0.368 |
Bodyweight of control or quercetin-treated TG2-null dams was recorded at the beginning and end of the 4-month breeding period. n = 4
Table 5.
Effects of quercetin on follicle development in wild-type and TG2-knockout animals
| Stage of follicular development | Wild-type | TG2-knockout | ||
|---|---|---|---|---|
| Control n = 8 | Quercetin n = 6 | Control n = 5 | Quercetin n = 4 | |
| Primordial | 83.96 ± 16.59 | 36.27 ± 13.72* | 35.33 ± 4.28** | 32.50 ± 4.77** |
| Primary | 95.98 ± 10.77 | 43.14 ± 11.23* | 54.34 ± 7.14* | 50.00 ± 5.27* |
| Secondary | 212.53 ± 8.47 | 235.44 ± 6.00 | 226.00 ± 16.98 | 200.83 ± 23.28 |
| Antral | 70.08 ± 12.98 | 122.86 ± 23.82* | 124.33 ± 9.86* | 121.67 ± 10.96* |
Follicle counts in serial sections spaced 100 νm apart through a 1-mm area (10 total sections counted) of ovaries from control and quercetin-treated female mice. Follicles were classified according to histomorphology as: primordial, primary, secondary or antral. Results are presented as mean number of follicles per animal at each stage in ovaries from control or quercetin-treated wild-type or TG2-null females.
P<0.05 and
P<0.01 for each group compared with untreated wild-type animals
Discussion
Quercetin is a major bioflavonoid in the human diet. In the United States, average daily intake of quercetin from plant food sources ranges from 25 mg to 210 mg (or 3 mg kg−1 day−1; Harwood et al. 2007; Corega et al. 2014). Quercetin has been characterised as an anti-oxidant and anti-inflammatory agent and preliminary research implies its beneficial effects on cancer, metabolic syndrome, the cardiovascular system and oral health (Huxley and Neil 2003; Edwards et al. 2007; Egert et al. 2009; Beazley et al. 2013a, 2013b; Corega et al. 2014; Sak 2014). In recent years, research on quercetin has evolved from animal experiments to human clinical studies and trials, and its popularity as a daily supplement is growing among the general population. The common daily dose of quercetin as a nutritional supplement is 10 mg kg−1 day−1 but can be even higher (Harwood et al. 2007). Despite accumulating reports on the beneficial effects of dietary quercetin there are also indications that in some conditions quercetin supplements should be used with caution. For example, quercetin regulates the activity of several enzymes involved in the metabolism of xenobiotics in the body and may therefore be contraindicated with some antibiotics. In addition, quercetin is a xenoestrogen (Kamel 2013) and may also affect oestrogen-dependent processes involved in reproduction.
Here we show that oral quercetin intake (5 mg kg−1 day−1) increases fecundity in young female mice, evidenced by an increase in litter size in females of prime reproductive age and increased folliculogenesis in female offspring. In contrast to younger females, in aging mice quercetin causes a decline in litter size, suggesting that increased fecundity in young females occurs at the expense of depleting ovarian reserves earlier. This is supported by the observation in female quercetin-treated offspring that a significantly increased number of antral follicles is associated with a significant decrease in primordial follicles. This change is a hallmark of ovarian reserve depletion leading to premature ovarian failure (Santoro 2003) and reproductive senescence.
In addition, we observed that the increase in litter size in young mice is associated with a reduction in the number of litters born and increased spacing between litters, suggesting a seemingly paradoxical effect of quercetin on female fecundity and fertility. In agreement with our findings, similar effects were also reported in female offspring of Wistar rats treated with 10 mg kg−1 day−1 quercetin, in which increased gestation length, a 10% reduction in pregnancy success rate and an increase in litter size (Johnson et al. 2009) were observed, although this study in rats did not report any statistical significance of the results. Nevertheless, an impairment of female fertility by low-dose quercetin (5–10 mg kg−1 day−1), which is relevant to dietary consumption by humans, has been observed in both species.
The effects of dietary quercetin on female fertility and fecundity are similar to the effects induced by genetic ablation of the quercetin-sensitive enzyme TG2. Thus, we observed a similar increase in the number of antral follicles in TG2-null ovaries (71.2 ± 10.8%, P<0.05) and in in quercetin-treated wild-type ovaries (70.2% ± 27.4%, P<0.05) compared with untreated control females. Further, loss of TG2 attenuates quercetin effects on female reproduction observed in wild-type mice, in agreement with quercetin acting via inhibition of TG2. A plausible mechanism for the involvement of TG2 relates to its ability to activate the β-catenin signalling pathway (Beazley et al. 2013a), which in the normal ovary is associated with non-ovulatory follicles (Usongo et al. 2012) and can impair ovulation (Fan et al. 2010). Therefore, it can be proposed that blocking TG2-mediated β-catenin signalling with quercetin (Beazley et al. 2013a) may allow for enhanced ovulation; however, further studies will be necessary to directly establish the link between quercetin, TG2 and β-catenin in this process. Nonetheless, our findings potentially open an entirely new avenue of research as it is the first evidence of a role for TG2 in ovulation.
Of note, an autoimmune disorder targeting TG2, such as coeliac disease, is associated with a variety of reproductive disorders including unexplained early-onset of menopause (Özgör and Selimoglu 2010), although the pathogenesis of the TG2-associated reproductive disorders remains unclear. Consistent with a role for TG2 as a target of quercetin in ovulation, TG2-deficient female mice also showed a reduction in primordial follicles similar to quercetin-treated wild-type females, and aging TG2-null females lacked a sufficient number of litters for statistical analysis of litter size. Together these data suggest a potential mechanism for the association of TG2 with reproductive senescence.
In contrast to female fertility, we did not observe any effects of the 5mg kg−1 day−1 quercetin treatment on male reproduction and quercetin-treated males produced the same progeny as control males when mated with untreated females. However, higher doses of quercetin were shown to be risky and reduced male fertility in mice (Aravindakshan et al. 1985; Taepongsorat et al. 2008; Ranawat et al. 2013). A possible explanation for this discrepancy is in the chosen delivery method, with previous in vivo studies on male fertility employing intraperitoneal (Aravindakshan et al. 1985; Taepongsorat et al. 2008) or subcutaneous (Ranawat et al. 2013) injections of quercetin. In our studies, quercetin was delivered via oral consumption to mimic human dietary intake. Indeed, of the ingested dose of quercetin~53 percent is absorbed in humans (Walle et al. 2001) and close to 60 percent is absorbed in rats (Chen et al. 2005). Accordingly, total plasma concentrations of quercetin are ~0.2 νmol L−1 following oral intake of 1 mg kg−1 quercetin in both rodents and humans, with reported plasma concentrations in rats peaking at 51 νmol L−1 after 225 mg kg−1 oral dose (Manach et al. 1997) and in humans peaking at 0.3 νmol L−1 after ~1.5 mg kg−1 oral dose (Hollman et al. 1997). Therefore, our findings on the effects of oral 5mg kg−1 day−1 quercetin on fertility in mice may be relevant to the effects of this bioflavonoid in humans.
In conclusion, our data support the proposition that some activities of quercetin and its metabolites may overshadow the beneficial effects of its supplementation in females of reproductive age, calling for further analysis of the clinical data to relate our findings in mice to humans.
Acknowledgments
We thank Dr Candace Kerr for constructive discussion of this study and Mr. Steven Reckard and Ms. Shweta Das for technical assistance. This study was supported by the NIH grant HL093305 (to M. N.) and postdoctoral fellowship training grant AR007592 (to K. B.).
References
- Aravindakshan M, Chauhan PS, Sundaram K. Studies on germinal effects of quercetin, a naturally occurring flavonoid. Mutat Res. 1985;144:99–106. doi: 10.1016/0165-7992(85)90010-7. [DOI] [PubMed] [Google Scholar]
- Beazley KE, Deasey S, Lima F, Nurminskaya MV. Transglutaminase 2-mediated activation of beta-catenin signalling has a critical role in warfarin-induced vascular calcification. Arterioscler Thromb Vasc Biol. 2012;32:123–130. doi: 10.1161/ATVBAHA.111.237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazley KE, Banyard D, Lima F, Deasey SC, Nurminsky DI, Konoplyannikov M, Nurminskaya MV. Transglutaminase inhibitors attenuate vascular calcification in a preclinical model. Arterioscler Thromb Vasc Biol. 2013a;33:43–51. doi: 10.1161/ATVBAHA.112.300260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazley KE, Reckard S, Nurminsky D, Lima F, Nurminskaya M. Two sides of MGP-null arterial disease: chondrogenic lesions dependent on transglutaminase 2 and elastin fragmentation associated with induction of adipsin. J Biol Chem. 2013b;288:31 400–31 408. doi: 10.1074/JBC.M113.495556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetts LE, De Iuliis GN, Nixon B, Kime M, Zelski K, McVicar CM, Lewis SE, Aitken RJ. Impact of oestrogenic compounds on DNA integrity in human spermatozoa: evidence for cross-linking and redox cycling activities. Mutat Res. 2008;641:1–11. doi: 10.1016/J.MRFMMM.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Chen X, Yin OQ, Zuo Z, Chow MS. Pharmacokinetics and modelling of quercetin and metabolites. Pharm Res. 2005;22:892–901. doi: 10.1007/S11095-005-4584-1. [DOI] [PubMed] [Google Scholar]
- Corega C, Vaida L, Festila DG, Rigoni G, Albanese M, D’Agostino A, De SD, Pardo A, Nocini PF, Bertossi D. The benefits of quercetin for dentistry and maxillofacial surgery: a systematic review. Minerva Stomatol. 2014 EPUB ahead of print. [PubMed] [Google Scholar]
- De Feo V, Quaranta E, Fedele V, Claps S, Rubino R, Pizza C. Flavonoids and terpenoids in goat milk in relation to forage intake. Ital J Food Sci. 2006;18:85–92. [Google Scholar]
- Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, Wolffram S, Muller MJ. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- Fan HY, O’Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinisation. Mol Endocrinol. 2010;24:1529–1542. doi: 10.1210/ME.2010-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioural and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Fu X, Cheng J, Hou Y, Zhu S. The association between the oocyte pool and aneuploidy: a comparative study of the reproductive potential of young and aged mice. J Assist Reprod Genet. 2013;31:323–331. doi: 10.1007/S10815-013-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Kanzaki H, Nakayama H, Higuchi T, Hatayama H, Iwai M, Kaneko Y, Mori T, Fujita J. Requirement for transglutaminase in progesterone-induced decidualisation of human endometrial stromal cells. Endocrinology. 1996;137:1096–1101. doi: 10.1210/endo.137.3.8603579. [DOI] [PubMed] [Google Scholar]
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/J.FCT.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/S0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–908. doi: 10.1038/SJ.EJCN.1601624. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Makaji E, Ho S, Boya X, Crankshaw DJ, Holloway AC. Effect of maternal raspberry leaf consumption in rats on pregnancy outcome and the fertility of the female offspring. Reprod Sci. 2009;16:605–609. doi: 10.1177/1933719109332823. [DOI] [PubMed] [Google Scholar]
- Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Sakai K, Iwashita M. Tissue transglutaminase at embryo–maternal interface. J Clin Endocrinol Metab. 2005;90:4694–4702. doi: 10.1210/JC.2005-0240. [DOI] [PubMed] [Google Scholar]
- Kamel HH. Role of phyto-oestrogens in ovulation induction in women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;168:60–63. doi: 10.1016/J.EJOGRB.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Khanduja KL, Verma A, Bhardwaj A. Impairment of human sperm motility and viability by quercetin is independent of lipid peroxidation. Andrologia. 2001;33:277–281. doi: 10.1046/J.1439-0272.2001.00432.X. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EH, Yeung WS, Ho PC, Lee KF. Excessive ovarian stimulation upregulates the Wnt-signalling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod. 2010;25:479–490. doi: 10.1093/HUMREP/DEP429. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/NRM1014. [DOI] [PubMed] [Google Scholar]
- Manach C, Morand C, Demigne C, Texier O, Regerat F, Remesy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409:12–16. doi: 10.1016/S0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- Özgör B, Selimoglu MA. Coeliac disease and reproductive disorders. Scand J Gastroenterol. 2010;45:395–402. doi: 10.3109/00365520903508902. [DOI] [PubMed] [Google Scholar]
- Ranawat P, Kaushik G, Saikia UN, Pathak CM, Khanduja KL. Quercetin impairs the reproductive potential of male mice. Andrologia. 2013;45:56–65. doi: 10.1111/J.1439-0272.2012.01311.X. [DOI] [PubMed] [Google Scholar]
- Sak K. Site-specific anti-cancer effects of dietary flavonoid quercetin. Nutr Cancer. 2014;66:177–193. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol (Paris) 2003;64:87–92. [PubMed] [Google Scholar]
- Taepongsorat L, Tangpraprutgul P, Kitana N, Malaivijitnond S. Stimulating effects of quercetin on sperm quality and reproductive organs in adult male rats. Asian J Androl. 2008;10:249–258. doi: 10.1111/J.1745-7262.2008.00306.X. [DOI] [PubMed] [Google Scholar]
- Tulac S, Nayak NR, Kao LC, Van WM, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC. Identification, characterisation and regulation of the canonical Wnt signalling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–3866. doi: 10.1210/JC.2003-030494. [DOI] [PubMed] [Google Scholar]
- Usongo M, Rizk A, Farookhi R. Beta-catenin/Tcf signalling in murine oocytes identifies non-ovulatory follicles. Reproduction. 2012;144:669–676. doi: 10.1530/REP-12-0291. [DOI] [PubMed] [Google Scholar]
- Walle T, Walle UK, Halushka PV. Carbon dioxide is the major metabolite of quercetin in humans. J Nutr. 2001;131:2648–2652. doi: 10.1093/jn/131.10.2648. [DOI] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualisation and glandular development via a complex paracrine signalling network. Mol Cell Endocrinol. 2012;357:108–118. doi: 10.1016/J.MCE.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik M, Burzynska-Pedziwiatr I, Wozniak LA. A review of natural and synthetic antioxidants important for health and longevity. Curr Med Chem. 2010;17:3262–3288. doi: 10.2174/092986710792231950. [DOI] [PubMed] [Google Scholar]