Abstract
HIV targets the gut mucosa early in infection, causing immune and epithelial barrier dysfunction and disease progression. However, gut mucosal sensing and innate immune signaling through mucosal pattern recognition receptors (PRR) during HIV infection and disease progression are not well defined. Using the simian immunodeficiency virus (SIV)-infected rhesus macaque model of AIDS, we found a robust increase in PRR and inflammatory cytokine gene expression during the acute SIV infection in both peripheral blood and gut mucosa, coinciding with viral replication. PRR expression remained elevated in peripheral blood following the transition to chronic SIV infection. In contrast, massive dampening of PRR expression was detected in the gut mucosa, despite the presence of detectable viral loads. Exceptionally, expression of TLR4 and 8 was down modulated and diverged from expression patterns for most other TLRs in the gut. Decreased mucosal PRR expression was associated with increased abundance of several pathogenic bacterial taxa, including Pasteurellaceae members, Aggregatibacter and Actinobacillus, and Mycoplasmataceae family. Early anti-retroviral therapy led to viral suppression, but only partial maintenance of gut PRR and cytokine gene expression. In summary, SIV infection dampens mucosal innate immunity through PRR dysregulation and may promote immune activation, gut microbiota changes and ineffective viral clearance.
Introduction
The gut-associated lymphoid tissue (GALT) is an important early site of HIV replication and severe mucosal CD4+ T cell depletion1–4. A stable viral reservoir is established in the gut very early in infection that is not eradicated even during long-term suppressive anti-retroviral therapy (ART)2,5. The loss of mucosal CD4+ T cells, altered T cell homeostasis, and epithelial barrier disruption are linked to microbial translocation, chronic immune activation and disease progression2,6,7. However, changes in the gut microenvironment during early HIV infection are not adequately reflected in the peripheral blood, highlighting the importance of the gut mucosal assessments to investigate pathogenic mechanisms.
Chronic HIV infection is characterized by persistent viral replication, unresolved immune activation, and progressive decline of immune function2,5,6,8. Despite effective suppression of viral replication during long-term ART, it fails to eradicate viral reservoirs and fully resolve chronic inflammation2,6,9,10. Increased inflammatory cytokine production and altered gut microbiota are reflective of pathogenic effects of chronic HIV infection11–13. Signaling through pattern recognition receptors (PRR) in the GI tract drives the sensing of pathogens and initiation of innate inflammatory immune responses while maintaining immune tolerance to resident gut microbiota. Therefore, it is possible that PRR expression and signaling may be exploited by pathogens to invade the gut mucosa. Conversely, the host may modulate PRR expression to limit the pathogen-driven inflammation and damage to the gut microenvironment. Current understanding is limited on whether HIV infection impairs PRR expression and innate immune response at the gut mucosal surfaces.
The human gut microbiota plays an essential role in maintaining immune homeostasis. In addition to providing a physical barrier against outgrowth of pathogenic bacteria, components of the gut microbiota aid in digestion of nutrients, produce important factors that support epithelial growth and barrier function and guide the development of the immune system14. Changes in the composition and diversity of gut microbiota have been linked to inflammation and disease15. Disruption of the gut microbiota involving increased microbial diversity and increased presence of potentially pathogenic bacterial families is reported in HIV-infected patients and in SIV-infected non-human primates11–13,16–19. These studies primarily focused on the lower GI tract and oral cavity. However, shifts in microbiota inhabiting the small intestine engaged in nutrient digestion and absorption have been under-investigated. Moreover, it is not known whether SIV/HIV infection modulates changes in the mucosal expression of receptors that sense microbial products, including PRRs, and whether infection-associated changes in PRR expression influence aberrant production of inflammatory cytokines as well as gut microbiota composition.
Using the SIV model of AIDS, we found a robust increase in the expression of multiple PRRs and associated cytokines in the gut mucosa during early SIV infection that was followed by a remarkable dampening of PRR and cytokine gene expression during therapy-naive chronic viral infection, despite the persistence of high viral loads. In contrast, increased PRR expression was maintained in peripheral blood at all stages of infection. The exception was for TLRs 4 and 8 whose expression was down modulated or unchanged during SIV infection. Altered PRR expression in SIV infection was associated with changes in microbiome diversity and increased immune activation. An early initiation of ART resulted in elevated levels of PRR expression and enhanced immune recovery. Our findings suggest that modulation of PRR signaling during viral infection may be linked to incomplete viral clearance, increased immune activation and decreased microbiome diversity. Therefore, PRR signaling and/or microbiota modulation in the gut may be beneficial therapeutic targets to reverse the pathological consequences of HIV infection.
Results
Viral replication and CD4+ T cell depletion in peripheral blood and gut mucosa during SIV infection
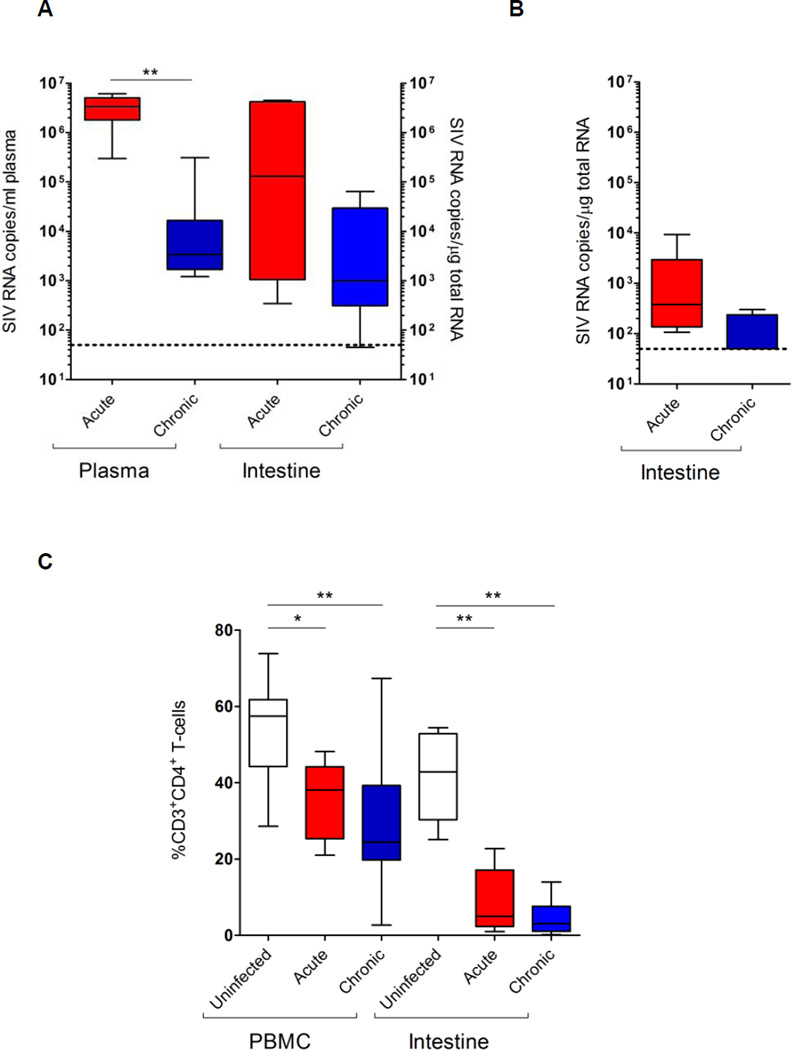
Viral RNA loads were detected in both peripheral blood and jejunum samples during acute and chronic stages of pathogenic SIVmac251 infection (Supplemental Table 1 and Figure 1A). Viral loads averaged 2.8 × 106 copies/ml plasma and 1.5 × 105 copies/Jg total RNA from jejunum tissue during acute SIV infection. Average viral loads were reduced to 4.4 × 104 copies/ml in plasma and to 104 copies/µg total RNA in jejunum tissues during chronic SIV infection (Figure 1A, p<0.01 compared with acute infection). Severe CD4+ T cell loss was consistently detected in the gut mucosa (9.1 ± 8.4% CD3+ CD4+ cells) during acute SIV infection that persisted (4.3 ± 4.1%) through chronic viral infection (Figure 1C). CD4+ T cell loss in peripheral blood was progressive in acute (35.8 ± 10.0%) and in chronic infection (31.8 ± 19.0%), compared to uninfected controls (52.2%). As previously reported20, early initiation of ART (1 week post-SIV infection) resulted in a remarkable decrease in viral loads in both plasma and jejunum samples and >80% of CD4+ T cell restoration (Supplemental Table 1). Despite having plasma viral loads below detection limits, residual viral replication was detected in the gut.
Figure 1.
Viral loads and CD4+ T cell depletion in peripheral blood and gut mucosal compartments during SIV infection. Viral RNA loads were measured in the plasma and gut tissue during acute and chronic stages of pathogenic SIV mac251 (A) and nonpathogenic SIVmac1A11 (B) infection by RT-qPCR. Percentages of CD3+CD4+ T cells were determined by flow cytometry (C). Statistical analysis was performed by comparison with uninfected controls. *, p<0.05; **, p<0.01.
SIVmac1A11, a non-pathogenic viral strain, successfully establishes infection in the host but does not cause immunodeficiency21,22. Experimental SIVmac1A11 infection led to low levels of viral replication (average 1.9 × 103 copies/Jg total RNA) in the jejunum during acute viral infection (Figure 1B). They are significantly lower (~3 log difference) compared to those with pathogenic SIVmac251 infection (p<0.05) (Figure 1A). In chronic SIVmac1A11 infection, all but one animal had viral loads below the limit of detection.
Increased expression of PRRs in peripheral blood during acute SIV infection that persists through chronic infection
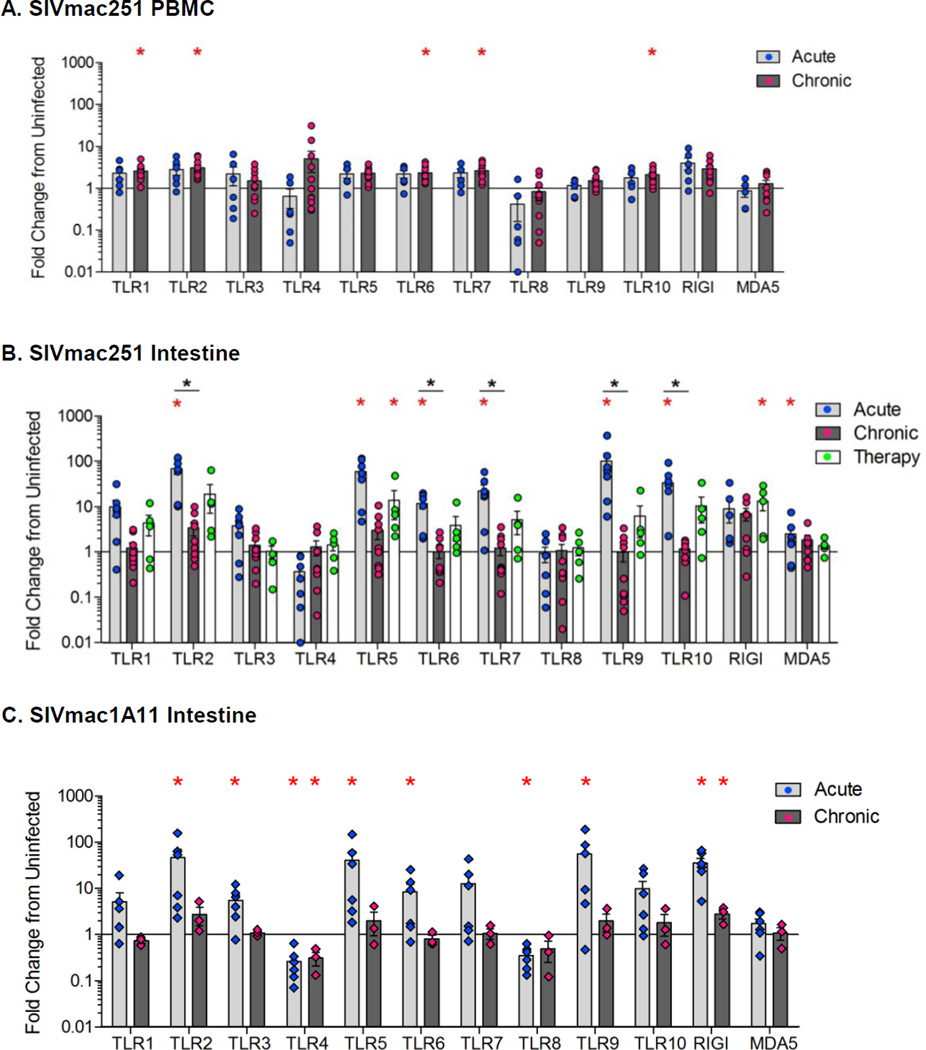
We sought to determine whether PRR expression was altered in the peripheral blood during the acute and chronic stages of SIV infection. A significant increase in the expression of toll-like receptor (TLR) 1, 2, 6, 7 and 10 (p<0.05) was seen during chronic SIV infection, as compared to uninfected controls (Figure 2A). Expression of cytosolic sensors, RIG-I and MDA5 was not significantly altered (Figure 2A). Thus, chronic SIV infection resulted increased TLR expression in PBMC.
Figure 2.
Expression profiles of pattern recognition receptors during acute and chronic stages of SIV. The PRR gene expression was determined by qRT-PCR in the PBMC (A) and gut tissue (B) during acute or chronic (therapy-naive or with ART) stages of SIVmac251 infection compared to SIV-negative healthy controls. The PRR expression was measured in gut samples during the acute and chronic stages of non-pathogenic SIVmac1A11 infection (C). Data are shown as the average fold changes compared to uninfected controls. *p<0.05 (red asterisk), compared with uninfected control animals; *p<0.05 (black asterisk), compared with chronically infected animals.
Marked increase in PRR expression in the gut during acute SIV infection followed by massive dampening during chronic infection
We investigated changes in the expression of PRRs in the gut mucosa during SIV infection. A robust increase in the expression of MDA-5 and TLRs 2, 5, 6, 7, 9, and 10 (p<0.05) was detected in intestinal tissue during acute SIV infection (Figure 2B). These changes mirrored the increased TLR expression observed in peripheral blood samples (Figure 2A). However, the expression of these PRRs was substantially higher in the gut than in peripheral blood (Figure 2A, 2B). Further, robust expression of TLRs 5 and 9 was seen only in the gut mucosal tissues, but not in PBMCs of SIV-infected animals compared to uninfected controls.
During chronic SIV infection, a remarkable dampening of PRRs expression (P<0.05; TLRs 2, 6, 7, 9, 10) was observed in gut mucosal tissues, when compared to those in acute SIV infection (Figure 2B). The dampening of PRR expression occurred despite the persistence of viral loads in the gut (Figure 1C, 2B). Among TLRs, endosomal TLR3, TLR7, and TLR9 are known to detect HIV and SIV nucleic acids. Expression levels of TLR7 and 9 genes were markedly increased in the gut during acute infection but decreased to baseline levels during chronic infection. In summary, there is discordance between peripheral blood and gut mucosal compartments for PRR expression, which was most pronounced during chronic SIV infection.
Although majority of the TLRs had a striking increase in their expression levels in the gut and peripheral blood, TLR4 and TLR8 had a divergent expression pattern. No increase in TLR4 or TLR8 expression in the gut was observed during acute as well as chronic stages of SIV infection (Figure 2B). This is surprising considering the presence of viral RNA and antigens, as well as, active viral replication in the gut. There was a trend of decreased TLR4 expression during the acute SIV infection although the values did not reach statistical significance. Decreased expression was also observed for TLR4 and TLR8 during the acute SIV infection in peripheral blood (Figure 2A).
Preservation of PRR expression in the gut following the early initiation of ART
We found that early initiation of ART during HIV and SIV infection can suppress viral replication and restore CD4+ T cells in the gut mucosa6,20,23. In order to determine the effects of therapeutic intervention on the restoration of gut innate immune homeostasis, we measured the PRR gene expression in jejunum samples from animals that received ART during acute SIV infection. As previously reported, the early initiation of ART (at one week post-SIV infection) led to suppression of viral RNA loads to very low to undetectable levels and resulted in >80% restoration of CD4+ T-cells in the gut, and nearly to pre-infection levels in the peripheral blood compared to animals initiating ART during chronic SIV infection and therapy-naive SIV infected controls20. Early ART partially prevented the dampening of the PRR expression in gut tissues of these animals (Figure 2B). The PRR expression profile during therapy was similar to that observed during acute SIV infection. These data suggest that early ART was able to partially restore the mucosal sensing and response through PRR expression, and this coincided with the suppression of viral loads and restoration of mucosal CD4+ T cells.
Preservation of PRR expression in animals with non-pathogenic SIV1A11 infection
We sought to determine whether dampening of the PRR expression in the gut during chronic SIV infection was related to viral loads and pathogenicity of the virus. We determined PRR expression in animals infected with non-pathogenic SIVmac1A1121. An increase in the PRR expression was detected during acute SIVmac1A11 infection and the trend was similar to that during acute pathogenic SIV infection. A significant increase in the expression of TLRs 2, 3, 4, 5, 9, and RIG-I was detected in SIVmac1A11 infected animals, compared to uninfected controls (p<0.05, Figure 2C). During chronic SIVmac1A11 infection, PRR expression decreased to the levels comparable to those seen in SIV-negative controls (Figure 2C). This decline coincided with an effective suppression of viral replication in the gut mucosa, suggesting that increased TLR expression during acute viral infection was in response to viral loads. These findings are in contrast to our findings from pathogenic chronic SIVmac251 infection that showed decreased gut mucosal PRR expression in spite of persistent viral replication and detectable viral loads, further validating the dysregulation of mucosal TLR expression during pathogenic SIV infection (Figure 2B and 2C).
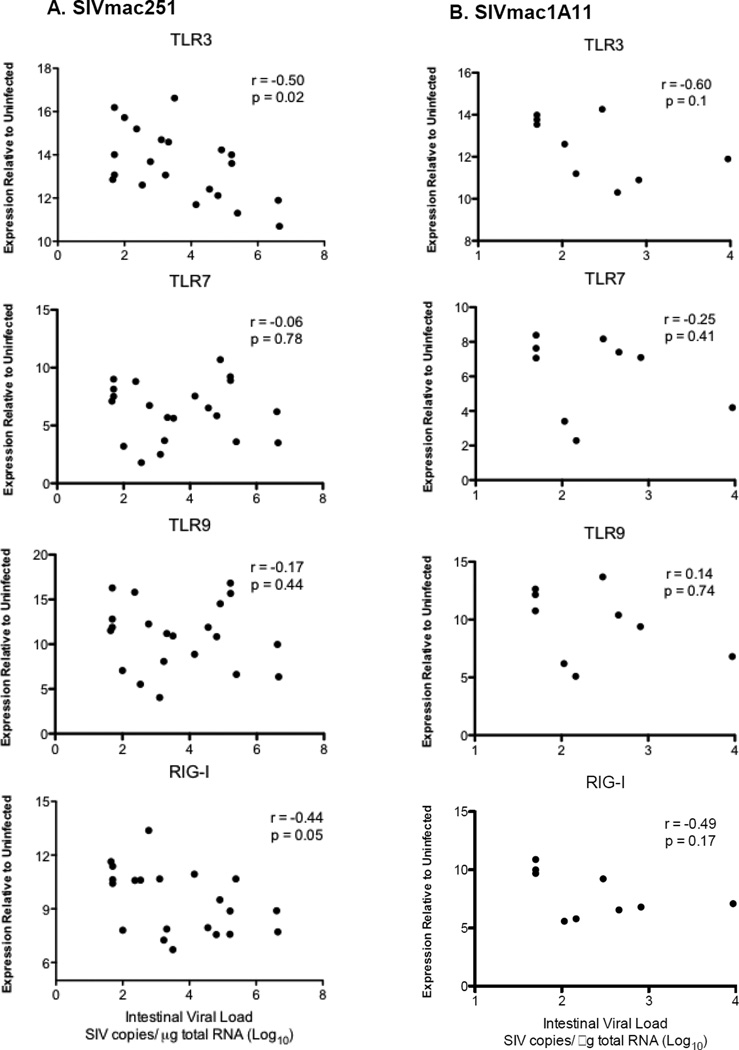
PRR expression of RIG-I or TLR3 negatively correlates with SIV replication
To further investigate whether expression of PRR plays a role in the suppression of viral replication, we performed correlation analyses. Among these PRRs, only expression of RIG-I or TLR3 displayed a significant negative correlation with SIVmac251 loads (Figure 3A). Both viral nucleic acid sensors RIG-I and TLR3 were previously shown to inhibit HIV or SIV replication24,25. Rapid induction of RIG-I and TLR3 during acute SIV infection suggests that the virus was detected by these PRRs. In contrast, there was no correlation of any PRRs, including RIG-I or TLR3, with SIVmac1A11 replication (Figure 3B), indicating that induction of RIG-I or TLR3 during acute SIVmac1A11 infection might have partly contributed to effective suppression of SIVmac1A11 replication.
Figure 3.
A correlation of pattern recognition receptor expression with viral replication during acute SIV infection. Spearman rank correlations were performed between expression levels of TLR3, TLR7, TLR9 and RIG-I and intestinal viral loads from SIVmac251 (A) and SIVmac1A11 (B) infected animals.
Inflammatory cytokine expression reflects changes in PRR expression during SIV infection
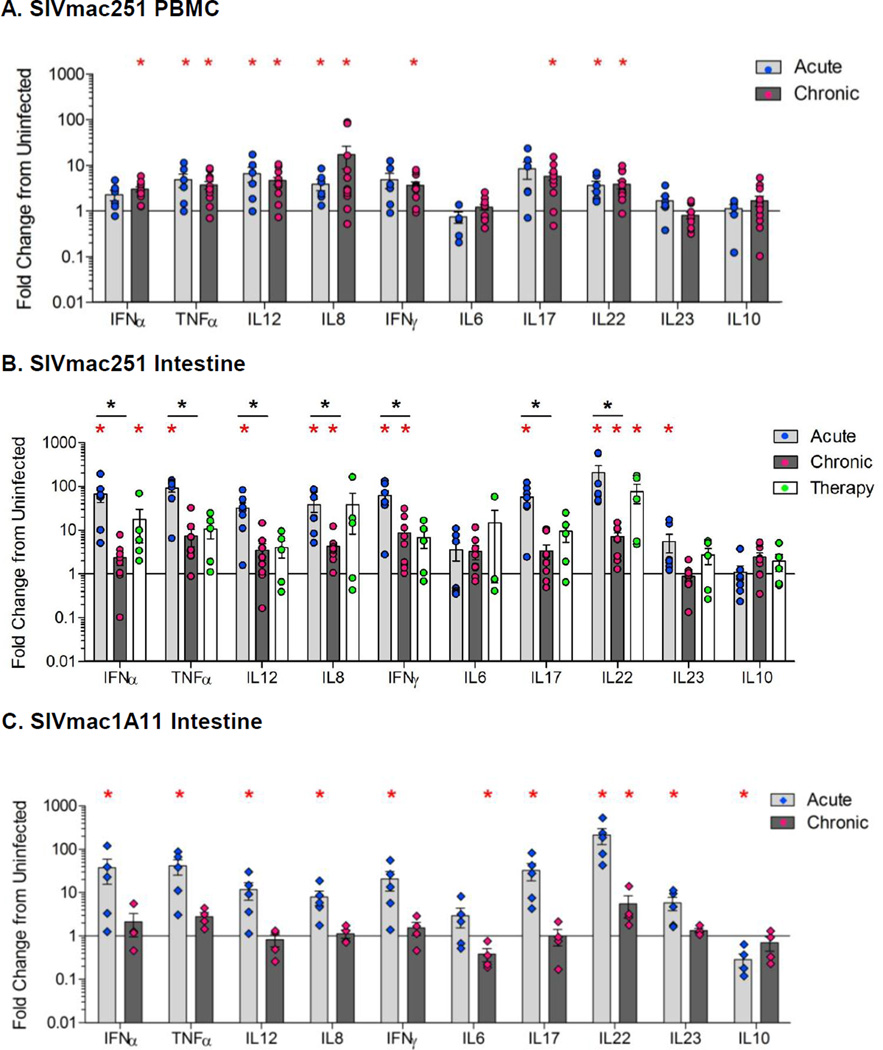
To determine whether changes in PRR expression during SIV infection were also accompanied by associated mucosal responses, we measured the expression of inflammatory and immune-modulatory cytokines. There was a significant increase (p<0.05) in the expression of the pro-inflammatory cytokines TNFα, IL-12, IL-8, and IL-22 in the peripheral blood (Figure 4A) and IFNα, IFNγ, TNFα, IL-12, IL-8, IL-17, IL-22, and IL-23 in the jejunum compartments (Figure 4B) of animals with acute SIV infection, as compared to uninfected controls. The magnitude of the induction of inflammatory cytokines in the gut mucosa was substantially greater than that seen in peripheral blood.
Figure 4.
Changes in the cytokine expression during acute and chronic stages of SIV infection. Cytokine gene expression was analyzed in the PBMC (A) and gut tissue (B) during acute and chronic (therapy-naive or with ART) stages of pathogenic SIV infection compared to SIV-negative controls using qRT-PCR. Cytokine gene expression was measured in gut tissue during acute and chronic stages of non-pathogenic SIVmac1A11 infection (C). The data are shown as an average fold change compared to SIV-negative controls. *p<0.05 (red asterisk), compared with uninfected control animals; *p<0.05 (black asterisk), compared with chronically infected animals.
Cytokines induced during acute SIV infection (IFNγ, IFNα, TNFα, IL-17, IL-12, IL-8, and IL-22) were significantly down-modulated in the gut during the transition from acute to chronic SIV infection. Nevertheless, IL-8, IFNγ, and IL-22 levels remained elevated compared to SIV-negative controls (Figure 4B). In contrast to the gut, the expression of cytokines remained elevated in the peripheral blood during both acute and chronic SIV infection (Figure 4A). Initiation of ART during early SIV infection resulted in the control of viral replication and an elevated level of several cytokines involved in immune response.
Expression of pro-inflammatory cytokines was also increased in non-pathogenic SIVmac1A11-infected animals during acute infection and was similar to the profile seen during SIVmac251 infection (Figure 4B, 4C). However, cytokine expression was reduced to basal levels during chronic SIV1A11 infection that was comparable to uninfected controls. No demonstrable change in the expression of anti-inflammatory cytokine IL-10, was seen during acute or chronic SIVmac251 infection while IL-10 expression was elevated during chronic SIV1A11 infection. Collectively, these data indicate that the elevated PPR expression induced during SIV infection was associated with inflammatory cytokine expression, and that early ART did not decrease levels of both PRR and inflammatory cytokines to the basal control levels in the gut mucosa, possibly due to the residual viral replication.
SIV infection leads to changes in the gut microbiota
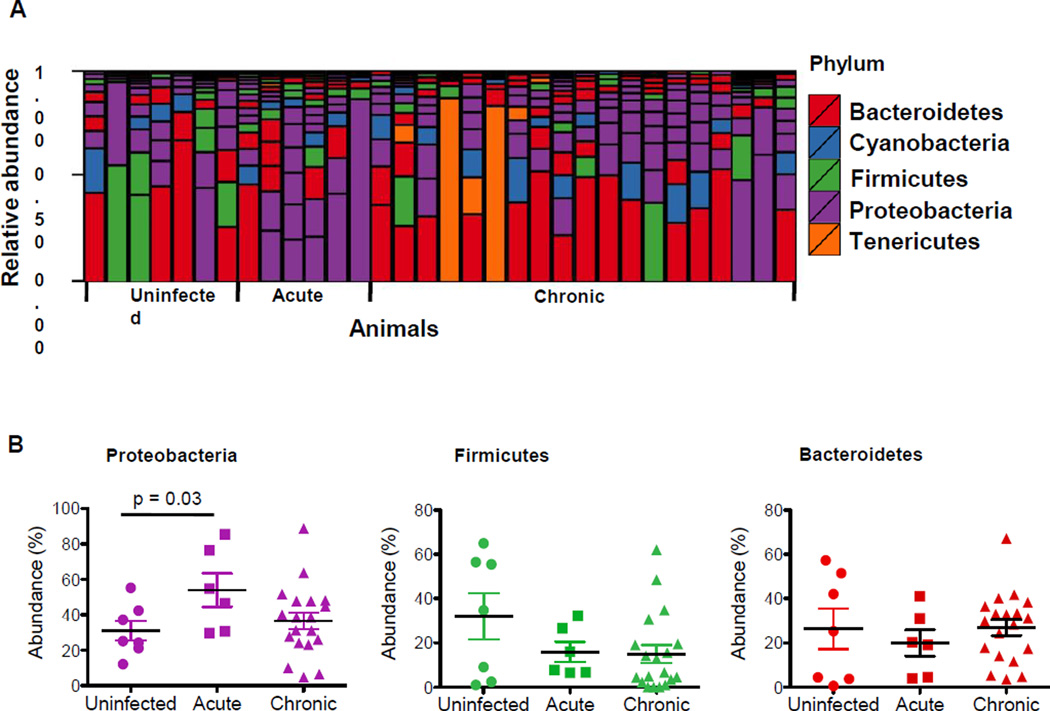
Recent studies have reported alterations in the composition and complexity of gut microbial communities during chronic HIV and SIV infections11–13,16–18. Based on the differential kinetics of PRR and cytokine expression in intestinal mucosa during SIV infection, we sought to investigate whether the pro-inflammatory environment during SIV infection would cause alterations in the gut microbiota from the same sites of PRR and cytokine analyses. In contrast, most of the previous studies of gut microbiota during HIV and SIV infections evaluated fecal samples. To determine the changes occurring in the gut microbiota during SIV infection, we constructed and sequenced 16S amplicon libraries from gut tissue of SIV-infected animals and healthy SIV-negative controls. Gut microbial communities differed from one animal to another (Figure 5A). Microbiota from jejunum samples of all the animals predominantly consisted of three phyla: Firmicutes, Bacteroidetes, and Proteobacteria (Figure 5A). The most notable change during acute SIV infection was a significant increase in the levels of Proteobacteria (Figure 5B). A decrease in Firmicutes levels was also detected during both acute and chronic SIV infection; although not statistically significant (Figure 5B). The abundance of Bacteroidetes was comparable between SIV-negative controls and SIV infected animals, and there was no detectable difference in the remaining phyla.
Figure 5.
SIV infection induced changes in gut microbiota. The gut microbiota from jejunum tissue samples of SIV infected macaques were sequenced for 16S amplicons. Data from animals during the acute (n=6) and chronic SIV infection (n=19) were compared to those from SIV-negative healthy controls (n=7). (A) Bar charts represent the group average percentage of the total filtered reads that aligned to the indicated phylum. (B) Relative abundance of Proteobacteria, Bacteroidetes, and Firmicutes in SIV-negative and SIV-infected macaques during acute and chronic stages of infection.
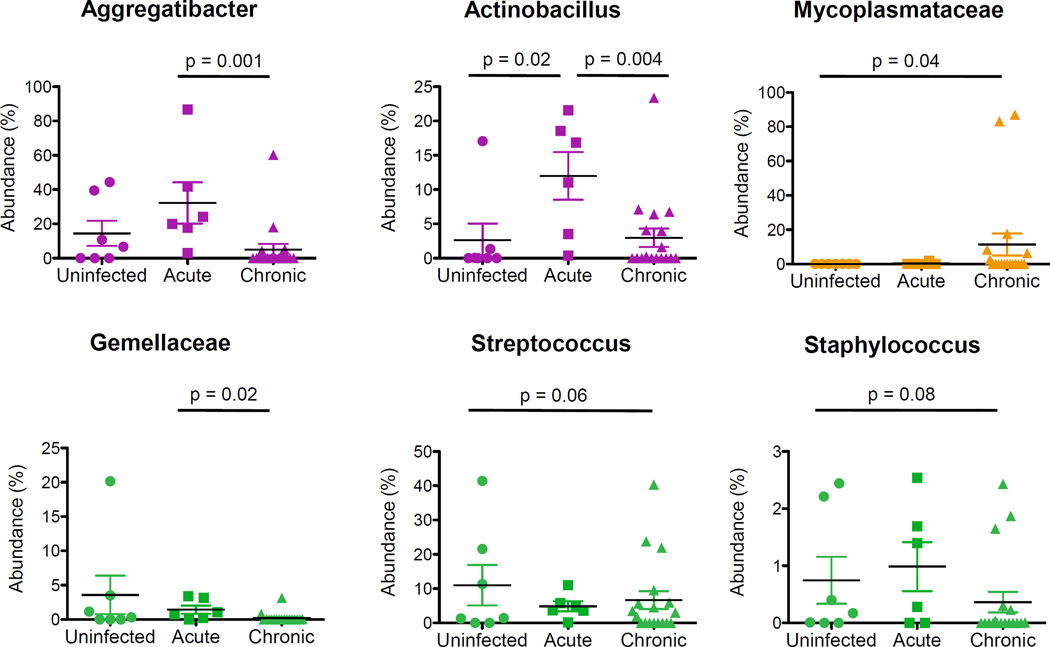
Few changes were observed at lower bacterial taxonomic levels. Within the Proteobacteria phylum, Pasteurellaceae family members, including Aggregatibacter and Actinobacillus, became more abundant during acute SIV infection and then significantly less abundant during the chronic viral infection (Figure 6). A significant increase in the abundance of Mycoplasmataceae, a family within the Tenericutes phylum, was also detected during chronic infection compared with uninfected animals (Figure 6). No significant changes were observed within other phyla. However, there was a decreased trend (p =0.08) for Streptococcus abundance during acute and chronic infection, and for increased abundance of Staphylococcus abundance only in the chronic SIV infection that approached significance (Figure 6).
Figure 6.
An increased abundance of potentially pathogenic microbial taxa during SIV infection. Dot plots show relative abundance of Aggregatibacter, Actinobacillus, Mycoplasmataceae, Gemellaceae, Streptococcus, and Staphyclococcus in SIV-negative and SIV-infected animals. Significant differences between groups were determined using non-parametric Mann-Whitney U tests (p-value ≤ 0.05).
Alterations in gut microbiota correlate with aberrant cytokine and PRR expression
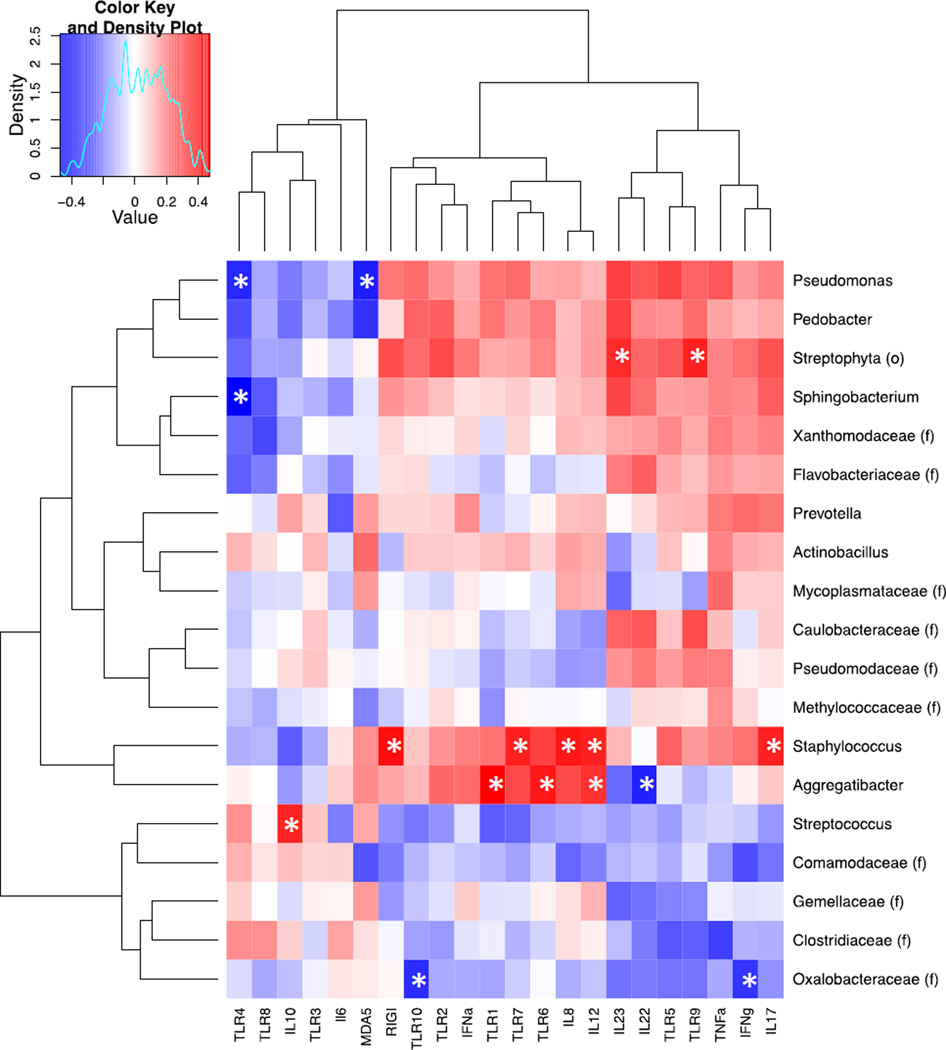
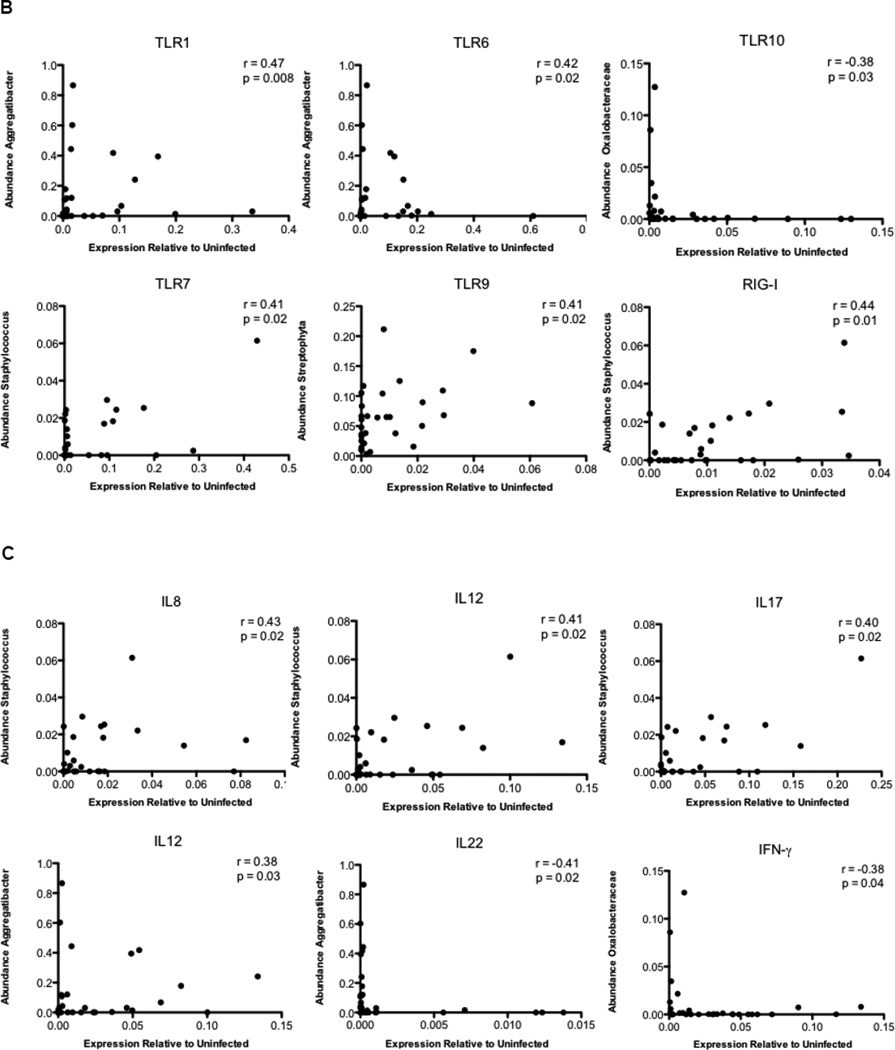
To investigate whether changes in the microbiome of SIV-infected animals could be directly associated with expression of specific PRRs or cytokines, we examined correlations between the most abundant OTUs (>0.5% in all samples) and expression of PRR and cytokine genes during SIV infection (Figure 7A). We found that Aggregatibacter abundance was positively correlated with the expression of TLR1, TLR6, and IL-12, but negatively correlated with IL-22 expression. Members of this genus are known to possess virulence factors that inhibit activation and function of immune cells26. Similarly, the abundance of organisms belonging to the genus Staphylococcus positively correlated with inflammatory cytokines and PRRs, including IL-8, IL-12, IL-17, TLR7, and RIG-I, and the abundance of Oxalobacteraceae negatively correlated with the expression of IFNγ and TLR10 (Figures 7B–C). Our data suggest that shifts in bacterial composition and abundance may influence changes in the expression of PRRs and cytokines in the gut during SIV infection.
Figure 7.
Correlation of the expression of PRRs and cytokines with changes in the gut microbiota during SIV infection. (A) A heatmap of Spearman correlation coefficients was generated for abundance of microbial families and relative expression of pattern recognition receptors and cytokines in the gut during SIV infection. The red color indicates a positive correlation coefficient and the blue color represents a negative coefficient. Significant correlations (unadjusted p-value≤ 0.05) were indicated with an asterisk. Spearman rank correlations between TLR (B) and Cytokines (C) expression and abundance of the most abundant OTUs.
Discussion
A complex interplay exists among immune cells, intestinal epithelium, and microbial communities in the maintenance of gastrointestinal homeostasis. Previous studies have demonstrated that disruption of the gut epithelial barrier during chronic HIV and SIV infections may contribute to microbial translocation, increased T cell turnover and systemic immune activation7,2,4,8,11,27,28. These pathogenic changes may be initiated early in infection and be driven by severe loss of mucosal CD4+ T cells1,29,30. Although majority of HIV infections occur through mucosal routes, a significant number of new HIV infections occur following an intravenous exposure of the virus3,5. Rhesus macaques infected with SIV through either mucosal route or intravenous exposure progress to acute and chronic stages of infection and demonstrate similar levels of severe CD4+ T cell depletion, altered T cell homeostasis and increased inflammatory cytokines in the gut29–32. Our study highlights the role of the early innate sensing and response through TLR signaling in SIV infection and potential implications in viral pathogenesis. Our findings suggest that SIV infection causes a rapid up-regulation of PRR and cytokine gene expression in the gut mucosa during acute SIV infection. However, these increases are markedly diminished during the chronic viral infection. The PRR and cytokine gene expression were associated with changes in the gut microbiota. Thus, we identified a potential link between SIV infection associated PRR-mediated innate immune modulation and microbial dysbiosis. One of the striking observations in our study was the discordance between the peripheral blood and gut mucosal compartments regarding the induction of PRR and cytokine expression during SIV infection. Cells from the peripheral blood are known to be functionally and phenotypically different from the cells that reside in the gut, and this may affect the differential PRR expression and cytokine responses in the peripheral blood versus the gut mucosal compartment.
Robust changes in PRR gene expression in the gut during early viral infection can originate from direct engagement of the receptors with PRR ligands, increased pro-inflammatory cytokines, or through altered distribution of mucosal cell populations33. Our findings show induction of massive innate immune response in the gut mucosa during acute SIV infection that was reflected by a robust increase in the expression of TLR7, TLR9, MDA5, and RIG-I, and by relatively modest increase in TLR3 expression. In agreement with previously reported studies regarding these PRRs inhibiting HIV or SIV replication25,34, we also found that in vivo expression of RIG-I and TLR3 negatively correlated with the replication of pathogenic SIVmac251, but not with non-pathogenic SIVmac1A11. While the induction of RIG-I or TLR3 expression could dampen SIV replication, the virus may disarm the innate immune signaling during chronic SIV infection. Modest changes in TLR3 levels in our study could be possibly due to the whole tissue analysis which may mask increased TLR expression localized to a small proportion of responding mucosal cells. Although TLR7 agonist could possibly reactivate latent SIV reservoirs (James Whitney et al. Abstract #108, CROI, 2015), we did not find a correlation of TLR7 expression with SIV replication. Engagement of TLR7 was shown to elicit an anergic state in human CD4+ T cells, which contributes to CD4+ T cells hyporesponsiveness35. These observations highlight the need for further investigations prior to applying TLR agonists in HIV cure study. We recently reported an induction of TLR4, TLR7 and TLR8 expression in the gut mucosa during very early SIV infection (at 2.5 days following SIV infection)36. This coincided with an increase in IL-1β expression and disruption of epithelial barrier36. Collectively, our data suggest that SIV induces robust TLR expression in the gut immediately following the viral exposure. Thus, early mucosal response to the viral infection is driven by the robust TLR expression and may set the stage for the immune dysfunction.
Expression of TLRs 4 and 7 in the gut mucosa was decreased during acute SIV infection compared to uninfected animals, and remained unchanged during chronic viral infection. Since TLR4 is involved in activating innate immunity and HIV specific cytotoxic T lymphocyte activity, impaired TLR4 expression may adversely affect virus-specific immunity. This is noteworthy since the decreased TLR4 expression in the gut mucosa suggests that it may lead to ineffective anti-viral immune response. Impairment of TLR4 expression could be a direct effect of viral antigens. HIV tat is shown to cause decreased TLR4 expression37. A widespread suppression of genes regulating innate immunity, including LPS receptors, CD14 and TLR4 was shown in PBMC of cynomolgus monkeys during acute SHIV 89.6p infection38. We also found that TLR8 expression in the gut mucosa was not changed during acute and chronic SIV infection despite the presence of viral loads. TLR8 is known to sense viral single stranded RNA and induce innate immune response. Decreased TLR8 expression was correlated with increased HIV replication in dendritic cells in vitro39. Interestingly, the modulation of TLR8 signaling is mediated through the complement system and has resulted in enhanced HIV infection, suggesting this as an important viral mechanism of immune evasion40. Thus, the virus may highjack innate immunity in the gut mucosa through suppression of TLR4 and TLR8. Our findings suggest that agonists of these TLRs should be explored for enhancing anti-viral immunity.
We found a remarkable increase in TLR2 and TLR5 expression during acute SIV infection in the gut. These data suggest an early encroachment of bacteria into lamina propria, and consequent microbial stimulation of intestinal immune cells. Substantial dampening of TLR2 and TLR5 expression during chronic infection was surprising, because acute SIV infection is associated with disruption of gut epithelial barriers and increased microbial translocation, indicating a possible negative feedback regulation of TLR2/5 signaling and/or changes of gut microbiota diversity2,3.
The increased TLR expression in the gut during acute SIV infection coincided with up-regulation of several inflammatory cytokines, including TNFα and IFNγ, which may contribute to viral control either by killing infected cells or by modulating other immune cells. Following the transition to chronic SIV infection, there was a precipitous decline in the expression of PRRs and cytokines in the gut mucosa. In contrast, the levels of PRRs and cytokines in the peripheral blood remained elevated during chronic SIV infection. Changes in the expression of inflammatory cytokines in the intestinal mucosa during chronic HIV infection have been previously reported41. We found that antigen-presenting cells from peripheral blood of individuals with chronic HIV infection showed aberrant inflammatory cytokine responses to the commensal bacteria, which was in part due to elevated expression of TLR2 on these cells42. It is not fully understood why the gut mucosa experiences precipitous dampening of the PRR and cytokine expression during chronic SIV infection despite the ongoing viral replication. First, it is possible that the severe depletion of CD4+ T cells in the gut and altered T cell homeostasis may cause changes in the PRR expression. Disruption of the gut epithelium and its renewal during SIV and HIV infections may also impact on the TLR expression. Secondly, SIV or HIV antigens could inhibit the innate immune response that would enable viral replication and persistence43. Third, during chronic viral infection, changes in the gut microbiota composition may down-modulate expression of PRRs (Figure 7) and the associated inflammatory cytokines. Reduced PRR expression during chronic SIV infection may compromise the ability of gut immune cells to sense and respond to the virus itself as well as to other pathogens, while dampening of PRR and cytokine expression in the gut may limit the gut tissue damage caused by chronic inflammation.
We found that the initiation of ART during early SIV infection resulted in preservation of higher PRR and cytokine expression in the gut (Figures 2B and 4B), and led to effective suppression of viral replication, high recovery of CD4+ T cells and induction of mucosal repair/renewal associated gene expression in the gut as compared to those initiating ART during chronic infection20,23. However, early ART failed to completely suppress viral replication or eradicate the virus. Lack of total recovery of the gut mucosal immune compartment and PRR expression may be reflective of residual viral replication and immune activation as well as microbial translocation in the mucosa. Previous report indicates that very early ART in the acute SIV/HIV infection has limited effect on eradication of viral reservoirs, which may be associated with dampened PRR-cytokine signaling44.
The non-pathogenic SIVmac1A11 successfully establishes infection in the host but does not cause immunodeficiency21,22. The acute SIV1A11 infection elicited an increase in PRR and cytokine gene expression levels in the gut that was similar to the increase observed during acute SIVmac251 infection. However, PRR and cytokine expression levels decreased during chronic SIVmac1A11 infection, which were similar to those in uninfected controls. Previously, we found that dissemination and tissue localization of pathogenic SIVmac251 was very different from non-pathogenic SIVmac1A11, which may be associated with increased migration of inflammatory cells into the intestinal mucosa45. Together, these findings suggest that, unlike pathogenic SIVmac251, infection with non-pathogenic SIVmac1A11 might not cause epithelial damage and microbiota translocation. Alternatively, SIVmac1A11-induced gut epithelial damage might be repaired during chronic viral infection.
We found that gut microbiota from SIV-infected macaques showed decreased abundance of Firmicutes and increased abundance of Proteobacteria. Similar findings have been previously reported in HIV-infected patients11–13,16. Administration of sevelamer, which binds to microbial LPS, reduced immune activation in SIV infected macaques46. However, sevelamer failed to decrease circulating LPS or soluble CD14 levels in ART-naive HIV infected patients47. These findings suggest that blocking of LPS in circulation alone was not sufficient to completely resolve immune activation. Thus, further investigations are needed to develop strategies to reverse changes in the gut microbiota during pathogenic infections.
To date, most studies have examined colon contents and fecal samples for assessing changes in gut microbiota. However, recent studies have clearly showed that composition and diversity of microbiota in various parts of the gastrointestinal tract (including small and large intestine) are somewhat different. These differences may be driven by the local microenvironment that may differ in its pH, oxygen, carbon, ions and minerals and microbial exposures. Our study focused on the small intestine where the majority of the terminal nutrient digestion and absorption occurs and HIV and SIV infections cause gut epithelial barrier disruption, nutrient malabsorption and severe CD4+ T cell depletion1,4,27,29,30,32. We examined changes in the gut microbiota from the small intestine in order to investigate correlations of the mucosal TLR expression and inflammation with gut microbiota changes at the same local site in the gut microenvironment.
Altered microbiota composition could be responsible for changes in PRR expression in the gut, leading to increased recognition of the commensal microbiota, resulting in progressive inflammation and dysbiosis. Alternatively, the increased expression of PRR and inflammatory cytokines could mediate changes of the microbiota composition, also resulting in progressive inflammation and dysbiosis. Consistent with this possibility, an increased prevalence of Proteobacteria is usually observed in intestinal inflammation because the inflamed gut provides new sources of nutrients for bacterial species within this phylum48. Furthermore, PRR signaling could be positively or negatively selected for specific taxa49. In this regard, we observed several associations between gut microbiota and PRR and cytokine expression. For example, Aggregatibacter, which has been previously shown to induce TLR2 expression, was positively associated with TLR1, TLR6 and IL-12 expression (Figures 7B–C). It is known that TLR2 signaling occurs via heterodimerization with either TLR1 or TLR6 and that heterodimers can sense a wide range of molecules mainly from Gram-positive, but also from Gram-negative bacteria, generating an inflammatory milieu33. The increased abundance of Aggregratibacter coupled with increased TLR1/2/6 expression suggests a link between local inflammation and dysbiosis during acute SIV infection. Moreover, the abundance of Staphylococcus correlates with several cytokines and PRR. These results are also consistent with previous reports that highlight the ability of members of this genus to induce the production of inflammatory cytokines50.
Our study captures the in vivo gut mucosal response to SIV infection and highlights changes in PRR and cytokine gene expression, as well as their potential effects on the resident gut microbiota. Early ART partially restored PRR and cytokine expression in the gut. Future studies on the functionality of individual cell subsets such as macrophages, dendritic cells, or intestinal epithelial cells will help define their role in the innate gut immune response during early infection. A better understanding of HIV-induced PRR signaling and its impact on the innate immune responses is critical to the development of improved therapeutic strategies for viral suppression and enhanced immune recovery.
Methods
Animal subjects, infection, and sample collection
The study involved a cross-sectional analysis of samples of jejunum and peripheral blood mononuclear cells from rhesus macaques infected intravenously with either pathogenic SIVmac251 or non-pathogenic SIVmac1A11 (Supplemental Table 1). Tissue samples were collected at necropsy, and immediately cryopreserved −80 °C, during acute (at 1 and 2 weeks following SIVmac251 or SIVmac1A11 infection) or chronic stages (at 10 to 34 weeks post-SIVmac251 infection and at 8 to 23 weeks post-SIV1A11 infection) of SIV infection as well as from SIV-negative healthy controls. Tissue samples were also obtained from rhesus macaques that started receiving combination anti-retroviral therapy (PMPA and FTC) at 1 week post-SIVmac251 infection and continued for 30 weeks thereafter as previously reported20.
Cell isolation and flow cytometry
Peripheral blood mononuclear cells (PBMC) from peripheral blood and lamina propria lymphocytes (LPL) from gut tissue samples were isolated as previously described31,36. Flow cytometric analysis was performed to assess the prevalence of CD3+CD4+ T cells using T cell phenotype markers CD3 (SP34-2, BD Bioscience) and CD4 (OKT4, eBioscience) as previously described36. Isolated PBMC and gut tissue samples were flash frozen in liquid nitrogen and cryopreserved for further analysis.
RNA isolation and gene expression analysis
50 mg of frozen tissues and pelleted cryopreserved PBMC were used for RNA extraction using RNeasy kit (Qiagen, Valencia, CA). Quantitative real-time PCR (qRT-PCR) was performed to measure RNA levels of TLR and cytokine genes as previously reported28. Levels of gene transcription were normalized to GAPDH or β-actin, and data were expressed as a relative fold change from uninfected controls (Supplementary Information). SIV RNA loads in plasma and gut tissue samples were determined by real-time PCR assay as described previously31,36.
Gut microbiota analysis
DNA was isolated from jejunal tissue samples using PowerSoil kit protocol (MoBio), amplified using a nested PCR protocol and barcoded primer specific for V4 region of 16S rRNA and quantified. Sequences were generated (MiSeq Sequencer, Illumina), filtered for quality and analyzed using QIIME software. Details are provided in the Supplementary information.
Statistical analysis
Statistical differences between groups were analyzed using ANOVA with Tukey post-test on normalized Ct values. Spearman correlation coefficients were determined by calculating using normalized Ct values. Differences in microbial abundance were analyzed using non-parametric Mann-Whitney U tests. Correlation analysis and heatmaps were performed with the R Statistical Software.
Supplementary Material
Acknowledgements
We thank Drs. Charles Bevins and Patricia Castillo for their design of the nested PCR protocol. This work was supported by the UC Davis RISE grant and NIH grants AI43274 and DK61297 to SD, and by a postdoctoral fellowship from the Brazilian funding agency CNPq to CSR. We thank Beau Parry for help in manuscript preparation.
Footnotes
Author Contributions
Conceived and designed the experiments: TWG and SD. Performed the experiments: TWG, CAG, CSR, SSW, LAH, and MR. Analyzed the data: TWG, CSR, CAG, LRG, AJB, GJ and SD. Contributed to the writing of the manuscript: TWG, GJ, CSR, CAG, LAH and SD.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. Journal of virology. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annual review of pathology. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 3.Dandekar S, George MD, Baumler AJ. Th17 cells, HIV and the gut mucosal barrier. Current opinion in HIV and AIDS. 2010;5:173–178. doi: 10.1097/COH.0b013e328335eda3. [DOI] [PubMed] [Google Scholar]
- 4.Sankaran S, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. Journal of virology. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- 6.Guadalupe M, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. Journal of virology. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends in microbiology. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 9.Keedy KS, Margolis DM. Therapy for persistent HIV. Trends in pharmacological sciences. 2010;31:206–211. doi: 10.1016/j.tips.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macal M, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal immunology. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 11.Vujkovic-Cvijin I, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Science translational medicine. 2013;5:193ra191. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutlu EA, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS pathogens. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozupone CA, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell host & microbe. 2013;14:329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nature immunology. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nature reviews. Gastroenterology & hepatology. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 16.Dillon SM, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal immunology. 2014;7:983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klase Z, et al. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal immunology. 2015 doi: 10.1038/mi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ocon S, et al. Transcription profiling reveals potential mechanisms of dysbiosis in the oral microbiome of rhesus macaques with chronic untreated SIV infection. PloS one. 2013;8:e80863. doi: 10.1371/journal.pone.0080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moeller AH, et al. SIV-induced instability of the chimpanzee gut microbiome. Cell host & microbe. 2013;14:340–345. doi: 10.1016/j.chom.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhoeven D, Sankaran S, Silvey M, Dandekar S. Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. Journal of virology. 2008;82:4016–4027. doi: 10.1128/JVI.02164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marthas ML, et al. Rhesus macaques inoculated with molecularly cloned simian immunodeficiency virus. Journal of medical primatology. 1989;18:311–319. [PubMed] [Google Scholar]
- 22.Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. The American journal of pathology. 1994;145:428–439. [PMC free article] [PubMed] [Google Scholar]
- 23.George MD, Reay E, Sankaran S, Dandekar S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. Journal of virology. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Wang X, Li J, Zhou Y, Ho W. RIG-I activation inhibits HIV replication in macrophages. Journal of leukocyte biology. 2013;94:337–341. doi: 10.1189/jlb.0313158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buitendijk M, Eszterhas SK, Howell AL. Toll-like receptor agonists are potent inhibitors of human immunodeficiency virus-type 1 replication in peripheral blood mononuclear cells. AIDS research and human retroviruses. 2014;30:457–467. doi: 10.1089/AID.2013.0199. [DOI] [PubMed] [Google Scholar]
- 26.Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology 2000. 1999;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 27.Sankaran S, et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9860–9865. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffatellu M, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nature medicine. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veazey RS, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 30.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. Journal of virology. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhoeven D, et al. Enhanced innate antiviral gene expression, IFN-alpha, and cytolytic responses are predictive of mucosal immune recovery during simian immunodeficiency virus infection. Journal of immunology. 2014;192:3308–3318. doi: 10.4049/jimmunol.1302415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heise C, Vogel P, Miller CJ, Halsted CH, Dandekar S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. The American journal of pathology. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 33.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annual review of immunology. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sang M, Liu JB, Dai M, Wu JG, Ho WZ. Toll-like receptor 3 signaling inhibits simian immunodeficiency virus replication in macrophages from rhesus macaques. Antiviral research. 2014;112:103–112. doi: 10.1016/j.antiviral.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominguez-Villar M, Gautron AS, de Marcken M, Keller MJ, Hafler DA. TLR7 induces anergy in human CD4(+) T cells. Nature immunology. 2015;16:118–128. doi: 10.1038/ni.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirao LA, et al. Early mucosal sensing of SIV infection by paneth cells induces IL-1beta production and initiates gut epithelial disruption. PLoS pathogens. 2014;10:e1004311. doi: 10.1371/journal.ppat.1004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben Haij N, et al. HIV-1 Tat Protein Induces Production of Proinflammatory Cytokines by Human Dendritic Cells and Monocytes/Macrophages through Engagement of TLR4-MD2-CD14 Complex and Activation of NF-kappaB Pathway. PloS one. 2015;10:e0129425. doi: 10.1371/journal.pone.0129425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. The Journal of clinical investigation. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gringhuis SI, et al. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nature immunology. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- 40.Ellegard R, et al. Complement opsonization of HIV-1 results in decreased antiviral and inflammatory responses in immature dendritic cells via CR3. Journal of immunology. 2014;193:4590–4601. doi: 10.4049/jimmunol.1401781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGowan I, Radford-Smith G, Jewell DP. Cytokine gene expression in HIV-infected intestinal mucosa. Aids. 1994;8:1569–1575. doi: 10.1097/00002030-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Nagy LH, et al. Chronic HIV infection enhances the responsiveness of antigen presenting cells to commensal Lactobacillus. PloS one. 2013;8:e72789. doi: 10.1371/journal.pone.0072789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:54. doi: 10.1186/1742-4690-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitney JB, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stone JD, Heise CC, Canfield DR, Elices MJ, Dandekar S. Differences in Viral Distribution and Cell-Adhesion Molecule Expression in the Intestinal-Tract of Rhesus Macaques Infected with Pathogenic and Nonpathogenic Siv. Journal of medical primatology. 1995;24:132–140. doi: 10.1111/j.1600-0684.1995.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 46.Kristoff J, et al. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. The Journal of clinical investigation. 2014;124:2802–2806. doi: 10.1172/JCI75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandler NG, et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. The Journal of infectious diseases. 2014;210:1549–1554. doi: 10.1093/infdis/jiu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winter SE, Lopez CA, Baumler AJ. The dynamics of gut-associated microbial communities during inflammation. EMBO reports. 2013;14:319–327. doi: 10.1038/embor.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Round JL, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards LA, et al. Enterotoxin-producing staphylococci cause intestinal inflammation by a combination of direct epithelial cytopathy and superantigen-mediated T-cell activation. Inflammatory bowel diseases. 2012;18:624–640. doi: 10.1002/ibd.21852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.