Abstract
This study investigated the alleviating effects of hydrogen sulfide (H2S), derived from sodium hydrosulfide (NaHS), on inflammation induced by dextran sulfate sodium (DSS) in both in vivo and in vitro models. We found that NaHS injection markedly decreased rectal bleeding, diarrhea, and histological injury in DSS-challenged mice. NaHS (20 μmol/L) reversed DSS-induced inhibition in cell viability in Caco-2 cells and alleviated pro-inflammation cytokine expression in vivo and in vitro, indicating an anti-inflammatory function for H2S. It was also found that H2S may regulate cytokine expression by inhibiting the nuclear factor-κB (NF-κB) signaling pathway. In conclusion, our results demonstrated that H2S alleviated DSS-induced inflammation in vivo and in vitro and that the signal mechanism might be associated with the NF-κB signaling pathway.
Keywords: Hydrogen sulfide (H2S), Inflammation, Nuclear factor-κB (NF-κB), Dextran sulfate sodium (DSS)
1. Introduction
Inflammatory bowel disease (IBD) patients suffer from chronic inflammation with the most common symptoms being weight loss, abdominal pain, (bloody) diarrhea, fatigue, and frequently extra-intestinal symptoms such as joint pain or skin and eye inflammation (Dubeau et al., 2013; Liu et al., 2014; Mileva et al., 2014; Malago et al., 2015; Xu et al., 2016). Kaplan (2015) reported that the incidence of IBD in the world is continuing to rise, with increasing prevalence in both industrialized and developing countries. While the exact etiology of IBD remains obscure, inflammation has been identified as a factor contributing to disease progression (Hirai and Matsui, 2015; Shimshoni et al., 2015).
The nuclear factor-κB (NF-κB) signaling pathway has been found to be involved in differentiation, immune response, proliferation, cell adhesion, angiogenesis, oxidative stress, and apoptosis (Watanabe et al., 2015). Compelling evidence indicates that NF-κB is associated with various inflammatory diseases, including ulcerative colitis and Crohn’s disease (Sun and Zhang, 2007). TLR4/Myd88, an upstream signal of NF-κB, can be activated in response to various inflammatory and infectious diseases. After activation, TLR4/Myd88 mediates the inflammatory response by activating NF-κB (Cao et al., 2014; Wang et al., 2015). Inhibitors of the NF-κB signaling pathway have been widely used to alleviate IBD (Sunil et al., 2010; McCann et al., 2015).
Hydrogen sulfide (H2S) is a gaseous molecule with various physiological functions, including neuromodulation, oxidative stress, regulation of blood pressure and cardiac function, inflammatory response, cellular energetics and apoptosis (Kabil et al., 2014). The beneficial role of H2S in various inflammatory responses has been validated (Gemici et al., 2015; Howell et al., 2015; Zhang et al., 2015), but there is little reference to the effects of H2S, or its mechanisms of action, in IBD. In this study we therefore evaluated the pharmacological effects of H2S from a sodium hydrosulfide (NaHS) source on inflammation and the NF-κB signal in dextran sulfate sodium (DSS)-induced inflammation in both in vivo and in vitro models of IBD.
2. Materials and methods
2.1. Animal model and groups
Thirty-two male ICR mice weighing 22–24 g were used in the experiment. Mice were divided into three groups each containing 10 animals: a control group (Cont), a DSS group (DSS), and a NaHS+DSS group (NaHS). In the control group, mice were allowed free access to tap water for drinking. Mice in the other two groups were allowed free access to a 5% (0.05 g/ml) DSS solution supplied as drinking water for 7 d to induce colonic inflammation. Mice from the NaHS group received freshly prepared NaHS solution (14 μmol/kg; Sigma-Aldrich) via intraperitoneal injection twice a day. Mice in the control and DSS groups received the same volume of sterile saline alone. The NaHS dosage was according to a previous report (Benetti et al., 2013). All mice were housed in polycarbonate cages at room temperature (25±3) °C, humidity (50±5)%, and a 12-h cycle of light and dark. During the experimental period, all mice were allowed free access to laboratory strip chows.
Afterwards, each mouse was weighed to calculate the average weight gain and then sacrificed. Colonic length and weight were measured. In addition, colonic samples from each mouse were collected and immediately frozen in liquid nitrogen and stored at −70 °C for further analyses.
2.2. Clinical evaluation of DSS colitis
Rectal bleeding and diarrhea from each mouse were recorded daily. The rectal bleeding was determined using Haemoccult kits (Beckman Coulter, Inc., CA, USA). The score of rectal bleeding was classified as follows: 0 for no blood (normal); 2 for positive haemoccult; and 4 for gross bleeding. The diarrhea score was classified as follows: 0 for well-formed pellets; 2 for pasty and semiformed stools; and 4 for liquid stools (Vlantis et al., 2015).
2.3. Histomorphometry determination
Haematoxylin and eosin (HE) staining (Yin et al., 2015b) was used for morphological evaluation after DSS treatment. Briefly, colon samples (0.5 cm) were kept in 4% neutral buffered 10% formalin, processed using routine histological methods and mounted in paraffin blocks. Then 6-μm-thick sections were cut and stained with HE. All specimens were examined under a light microscope (Nikon, Japan).
The histological examination was performed in a blinded fashion using a scoring system previously validated and described: severity of inflammation (0–3: none, slight, moderate, severe), depth of injury (0–3: none, mucosal, mucosal and submucosal, transmural), crypt damage (0–4: none, basal 1/3 damaged, basal 2/3 damaged, only surface epithelium intact, entire crypt and epithelium lost), and percentage of the involved area (0–4: 0%, 1%–10%, 10%–25%, 25%–50%, 50%–100%). Total scores, including the individual parameters added together, could range from 0 to 14.
2.4. Serum immunoglobulins
Orbital blood was collected and centrifuged at 3000 r/min for 10 min after 4 h clotting at 4 °C. Serum was separated and stored for further analyses. Assay kits for the analysis of serum immunoglobulins were obtained from Nanjing Jiancheng (China). Serum immunoglobulin A (IgA), IgG, and IgM were determined using an Automatic Biochemistry Radiometer system (Au640, Olympus).
2.5. Cell culture and treatment
Human colorectal adenocarcinoma-derived intestinal epithelial cells (Caco-2) (ATCC, Manassas, VA, USA) were grown in DMEM/F12 supplemented with 1 mmol/L sodium pyruvate, 20% fetal bovine serum, and 50 U/ml penicillin-streptomycin. Cells were treated with 2% (0.02 g/ml) DSS for 4 d to induce inflammation (Nighot et al., 2013). Cell viability was determined by the CKK-8 assay (Sigma-Aldrich, MO, USA) according to the manufacturer’s instructions. Briefly, 8×103 cells were seeded in 96-well plates. In the following day, cells were incubated with 1, 5, 10, 20, 50, and 100 μmol/L NaHS for 2 d and then assayed.
2.6. NF-κB activity
Cellular NF-κB activity after DSS and NaHS treatment was measured via an ELISA kit (Cell Biolabs, USA).
2.7. Complementary DNA (cDNA) synthesis and quantification of mRNA by real-time PCR analysis
RNA was isolated from colon and cell tissues with TRIZOL reagent according to the manufacturer’s instructions. Synthesis of the first strand (cDNA) was conducted using oligo (dT) 20 and Superscript II reverse transcriptase (Invitrogen, USA).
Primers were designed with Primer 5.0 according to the gene sequence of mouse; the sequences are shown in Table 1. Real-time PCR analysis was conducted according to previous studies (Yin et al., 2013a; 2014). The relative expression of different genes was normalized and presented as a ratio to their expression in the control group.
Table 1.
PCR primer sequences: the forward (F) primers and the reverse (R) primers
| Gene | Nucleotide sequences of primers (5'–3') |
| β-Actin | F: GTCCACCTTCCAGCAGATGT R: GAAAGGGTGTAAAACGCAGC |
| IL-1β | F: CTGTGACTCGTGGGATGATG R: GGGATTTTGTCGTTGCTTGT |
| IL-6 | F: TGCAAGAGACTTCCATCCAGT R: GTGAAGTAGGGAAGGCCG |
| IL-10 | F: ACAGCCGGGAAGACAATAAC R: CAGCTGGTCCTTTGTTTGAAAG |
| IL-17 | F: TACCTCAACCGTTCCACGTC R: TTTCCCTCCGCATTGACAC |
| IFN-γ | F: ATGAACGCTACACACTGCATCTTGGCTT R: CCTCAAACTTGGCAATACTCATGAATGC |
| TNF-α | F: AGGCACTCCCCCAAAAGAT R: TGAGGGTCTGGGCCATAGAA |
| TLR4 | F: TTCAGAACTTCAGTGGCTGGATT R: CCATGCCTTGTCTTCAATTGTTT |
| Myd88 | F: GCATGGTGGTGGTTGTTTCTG R: GAATCAGTCGCTTCTGTTGG |
2.8. Nuclear protein extraction and Western blot analysis
Nuclear proteins were extracted using nuclear and cytoplasmic extraction reagents in accordance with the manufacturer’s instructions (Thermo Fisher Scientific Inc., USA). Western blot was performed (Yin et al., 2015a) and NF-κBp65 (Abcam, Inc., USA) was used as the primary antibody. Rabbit proliferating cell nuclear antigen (PCNA) antibody (Sigma) was used as the nuclear protein loading control. The expression ratio of NF-κB was normalized against PCNA.
2.9. Statistical analysis
All statistical analyses were performed using SPSS 17.0 software. Group comparisons were performed using analysis of variance (ANOVA) and followed with Tukey’s multiple comparison test.
3. Results
3.1. Effects of NaHS on clinical indices in DSS-induced colitis
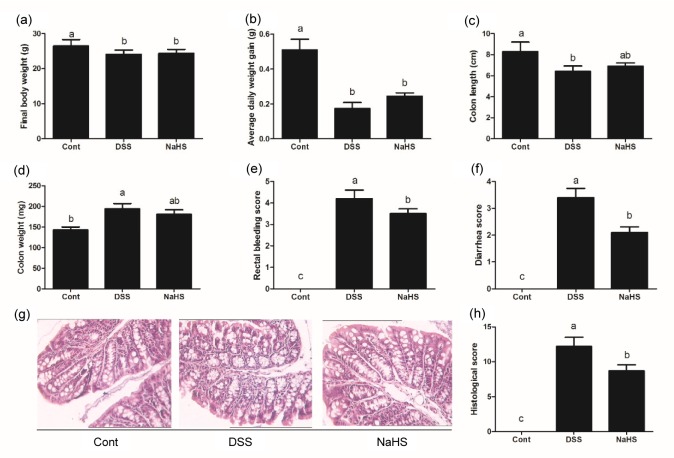
DSS treatment significantly reduced final body weight, daily weight gain, and colonic length, and increased colonic weight, rectal bleeding score, and diarrhea score (P<0.05; Fig. 1). Although NaHS administration failed to alleviate DSS-dysregulated body weight, colonic length, and colonic weight (P>0.05), it markedly decreased scores for rectal bleeding and diarrhea (P<0.05). HE staining results revealed that DSS caused colonic histological injury which was mitigated by NaHS (P<0.05).
Fig. 1.
Effects of NaHS on clinical parameters in DSS-induced colitis in mice
(a) Final body weight; (b) Average daily weight gain; (c) Colon length; (d) Colon weight; (e) Rectal bleeding score; (f) Diarrhea score; (g) HE staining; (h) Histological score. Data are expressed as mean±SD (n=10). Different letters above the columns are significant (P<0.05)
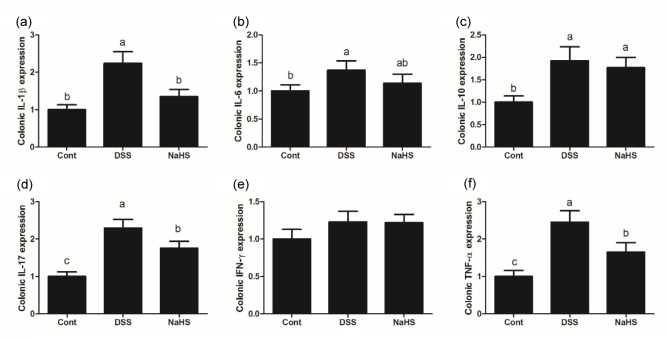
3.2. Effects of NaHS on inflammatory cytokines in DSS-induced colitis
Colonic interleukin-1β (IL-1β), IL-6, IL-10, IL-17, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) mRNA were measured by reverse transcription (RT)-PCR to evaluate the inflammatory response after DSS treatment in mice (Fig. 2). The results showed that adding 5% DSS to drinking water induced colonic inflammation in mice evidenced by the upregulation of IL-1β, IL-6, IL-10, IL-17, and TNF-α expression (P<0.05). Compared with the DSS group, NaHS administration significantly down-regulated colonic IL-1β, IL-17, and TNF-α expression (P<0.05), which indicated an anti-inflammatory function for NaHS.
Fig. 2.
Effects of NaHS on pro-inflammation cytokine expression in DSS-challenged mice
Expression of colonic IL-1β (a), IL-6 (b), IL-10 (c), IL-17 (d), IFN-γ (e), and TNF-α (f). Data are expressed as mean±SD (n=10). Different letters above the columns are significant (P<0.05)
3.3. Effects of NaHS on serum immunoglobulins in DSS-induced colitis
As shown in the Table 2, DSS treatment significantly reduced serum IgG and IgA (P<0.05). Although NaHS injection tended to alleviate DSS-induced inhibition of IgG and IgA levels, the difference was insignificant (P>0.05).
Table 2.
Serum immunoglobulins after DSS exposure
| Group | IgG (g/L) | IgM (g/L) | IgA (g/L) |
| Cont | 12.93±1.71a | 12.15±2.07 | 8.16±1.30a |
| DSS | 8.70±1.56b | 10.67±1.29 | 5.12±0.36b |
| NaHS | 9.15±2.04ab | 11.17±1.13 | 6.29±0.50ab |
Data are expressed as mean±standard deviation (SD) (n=10). Values in the same column with different superscripts are significant (P<0.05)
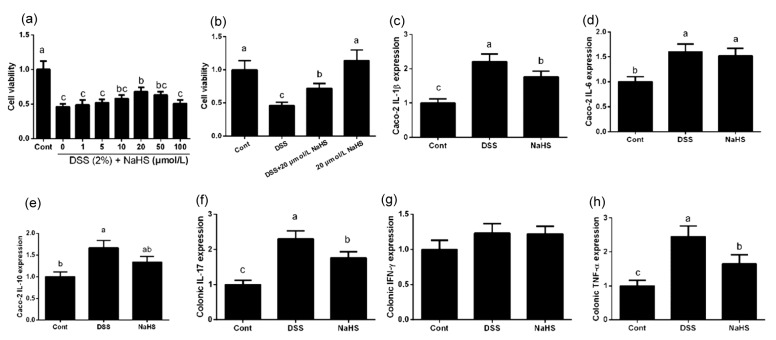
3.4. Effects of NaHS on inflammatory cytokines in DSS-challenged Caco-2 cells
We examined the role of NaHS in DSS-induced inflammatory response in a cell culture model. Cell viability was measured after treatment with different concentrations of NaHS (1, 5, 10, 20, 50, and 100 μmol/L). The 2% DSS inhibited cell viability (P<0.05; Fig. 3), whereas 20 μmol/L NaHS markedly reversed this inhibition in Caco-2 cells (P<0.05). Therefore, 20 μmol/L was used as the experimental dose for other tests.
Fig. 3.
Effects of NaHS on pro-inflammation cytokine expression in DSS-challenged Caco-2 cells
(a, b) Cell viability; (c) IL-1β expression; (d) IL-6 expression; (e) IL-10 expression; (f) IL-17 expression; (g) IFN-γ expression; (h) TNF-α expression. Data are expressed as mean±SD (n=10). Different letters above the columns are significant (P<0.05)
DSS significantly enhanced IL-1β, IL-6, IL-10, IL-17, and TNF-α mRNA abundances in Caco-2 cells (P<0.05), whereas NaHS alleviated DSS-induced inflammation by downregulating IL-1β, IL-17, and TNF-α expression (P<0.05). The in vitro results further validated the anti-inflammatory effect of NaHS.
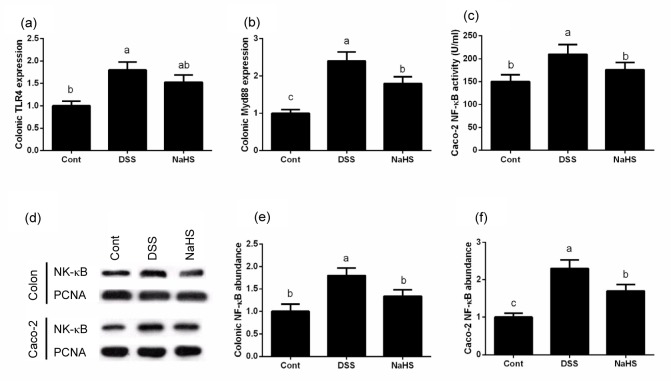
3.5. Effects of NaHS on the NF-κB signal in DSS-challenged in vivo and in vitro models
In the mouse model, DSS significantly activated the TLR4/Myd88 signal compared with the control group (P<0.05; Fig. 4). Although NaHS failed to down-regulate TLR4 expression, the Myd88 mRNA result demonstrated that DSS markedly enhanced the nuclear translocation of NF-κB and NaHS inhibited level in the NaHS group was significantly lower than that in the DSS group (P<0.05). The Western blotting result demonstrated that DSS markedly enhanced nuclear translocation of NF-κB and NaHS inhibited NF-κB activation (P<0.05). The ELISA and Western blotting results in Caco-2 cells further revealed that NaHS alleviated DSS-induced inflammation by inhibiting the NF-κB signal in vivo and in vitro.
Fig. 4.
Effects of NaHS on the NF-κB signal in DSS-induced inflammation in in vivo and in vitro models
(a) TLR4 expression; (b) Myd88 expression; (c) NF-κB activity; (d) Western blot result; (e) NF-κB abundance in mice; (f) NF-κB abundance in Caco-2 cells. Data are expressed as mean±SD (n=10). Different letters above the columns are significant (P<0.05)
4. Discussion
Accumulating evidence suggests that H2S may serve as an important biological gasotransmitter. H2S at physiological concentrations has been shown to protect cells in retinal neurons and act as a potential therapeutic target for retinal degeneration (Mikami et al., 2011; Eastep and Chen, 2015). H2S also regulates cellular Ca2+ homeostasis in microglial cells (Lee et al., 2006) and protects neurons from oxidative stress injury (Kimura and Kimura, 2004). In immune and inflammatory responses, H2S has been demonstrated to exhibit anti-inflammatory effects in various pathological situations (Bhatia, 2015; Pozsgai et al., 2015). However, some reports suggest that sulfur-containing compounds, including H2S released from the bacterial metabolism of non-absorbed sulfate, may be the injurious agents in the development of colitis, while Furne et al. (2000) confirmed that blocking fecal release of H2S via bismuth subsalicylate failed to alleviate intestinal inflammation. Although other sources of H2S have been reported in various types of inflammation, this study focused on the effect of NaHS-H2S on the DSS-induced colonic inflammatory response in in vivo and in vitro models to estimate the beneficial function of H2S.
We found that H2S, using NaHS as its source, had a clinically protective effect against DSS-induced colonic injury in mice. H2S markedly decreased rectal bleeding and diarrhea, and alleviated colonic histological injury. A previous study had shown that a marked increase in H2S generation contributes to ulcer healing and inflammation resolution in experimental colitis (Flannigan et al., 2014). Inhibition of endogenous H2S generation after cystathionine γ-lyase inhibitor treatment, a primary synthetase of H2S in the gastrointestinal tract, significantly exacerbated DSS-induced colitis (Hirata et al., 2011; Flannigan et al., 2014). These results indicate that H2S serves a beneficial role in DSS-induced colitis.
Compelling evidence in human and animal models has demonstrated that the generation of inflammatory cytokines and the inflammatory response in the gastrointestinal tract are involved in the progression of IBD (Beloqui et al., 2013; Sánchez-Fidalgo et al., 2013; Scharl et al., 2013; McCann et al., 2015). In this study, we found that DSS exposure significantly up-regulated IL-1β, IL-6, IL-10, IL-17, and TNF-α expression both in vivo and in vitro, whereas NaHS treatment alleviated this dysregulation; DSS treatment also decreased serum IgG and IgA, which was not alleviated by NaHS administration in our experiments. This may be because circulating immunoglobulins play an important role in the immune response: changes in immunoglobulins have been observed during the inflammatory response, suggesting their use as a potential therapy (Novokmet et al., 2014). Intransplantation-induced lung injury, NaHS injection has been demonstrated to inhibit the production of IL-1β and improve pulmonary function (Wu et al., 2013). Xu et al. (2015) reported that pre-treatment with NaHS ameliorates high glucose-induced inflammation in H9c2 cardiac cells, evidenced by the inhibition of IL-1β, IL-6, and TNF-α expression. Furthermore, treatment with an H2S donor in a rat model of non-erosive esophagitis markedly alleviated the inflammatory response and regulated serum IL-17 concentration (Zayachkivska et al., 2014). Thus, we speculate that H2S may serve as an anti-inflammatory agent in DSS-induced inflammation in vivo and in vitro.
NF-κB has been considered to be a key pro-inflammatory transcription factor involved in the expression of various genes, including cytokines (Shori and Baba, 2015; Yin et al., 2015b). Under normal conditions, NF-κB is sequestered in the cytoplasm via its inhibitory proteins, IκBs (Yin et al., 2013b). Various reports have revealed the relationship between IκBs and inflammation (Shin et al., 2012). Phosphorylation of IκBs is associated with its degradation and NF-κB activation (Yan and Polk, 1999). Compelling evidence suggests that NF-κB is activated in IBD and other inflammatory diseases (Cheon et al., 2006; Vinod and Guruvayoorappan, 2014; Rashti and Koohsari, 2015). Thus, inhibition of the NF-κB signaling pathway has been considered to be a potential target for IBD therapy. In this study, both in vivo and in vitro data showed that DSS exposure activated the NF-κB signal and NaHS treatment significantly inhibited this activation. Similarly, Zhou et al. (2014) reported that H2S exerts anti-inflammatory effects by inhibiting NF-κB signaling in high glucose-induced inflammation (McCann et al., 2015). As an upstream signal of NF-κB, TLR4/Myd88 is also activated by DSS exposure and down-regulated by NaHS treatment, further demonstrating the anti-inflammatory effect of H2S.
In conclusion, the present study provides in vivo and in vitro evidence that H2S derived from NaHS ameliorates the negative effects of DSS exposure in mice and Caco-2 cells and that this beneficial role may be associated with inhibition of the NF-κB signaling pathway.
Footnotes
Compliance with ethics guidelines: Xi CHEN and Xi-shuang LIU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Beloqui A, Coco R, Alhouayek M, et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int J Pharm. 2013;454(2):775–783. doi: 10.1016/j.ijpharm.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Benetti LR, Campos D, Gurgueira SA, et al. Hydrogen sulfide inhibits oxidative stress in lungs from allergic mice in vivo. Eur J Pharmacol. 2013;698(1-3):463–469. doi: 10.1016/j.ejphar.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M. H2S and inflammation: an overview. In: Moore PK, Whiteman M, editors. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide. Switzerland: Springer International Publishing; 2015. pp. 165–180. [Google Scholar]
- 4.Cao AT, Yao S, Stefka AT, et al. TLR4 regulates IFN-γ and IL-17 production by both thymic and induced Foxp3+ Tregs during intestinal inflammation. J Leukoc Biol. 2014;96(5):895–905. doi: 10.1189/jlb.3A0114-056RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheon JH, Kim JS, Kim JM, et al. Plant sterol guggulsterone inhibits nuclear factor-κB signaling in intestinal epithelial cells by blocking IκB kinase and ameliorates acute murine colitis. Inflamm Bowel Dis. 2006;12(12):1152–1161. doi: 10.1097/01.mib.0000235830.94057.c6. [DOI] [PubMed] [Google Scholar]
- 6.Dubeau MF, Iacucci M, Beck PL, et al. Drug-induced inflammatory bowel disease and IBD-like conditions. Inflamm Bowel Dis. 2013;19(2):445–456. doi: 10.1002/ibd.22990. [DOI] [PubMed] [Google Scholar]
- 7.Eastep J, Chen G. The relationships of high-fat diet and metabolism of lipophilic vitamins. Integr Food Nutr Metab. 2015;2(3):174–179. [Google Scholar]
- 8.Flannigan KL, Agbor TA, Blackler RW, et al. Impaired hydrogen sulfide synthesis and IL-10 signaling underlie hyperhomocysteinemia-associated exacerbation of colitis. PNAS. 2014;111(37):13559–13564. doi: 10.1073/pnas.1413390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furne JK, Suarez FL, Ewing SL, et al. Binding of hydrogen sulfide by bismuth does not prevent dextran sulfate-induced colitis in rats. Digest Dis Sci. 2000;45(7):1439–1443. doi: 10.1023/a:1005580709390. [DOI] [PubMed] [Google Scholar]
- 10.Gemici B, Elsheikh W, Feitosa KB, et al. H2S-releasing drugs: anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide. 2015;46:25–31. doi: 10.1016/j.niox.2014.11.010. (Available from: http://dx.doi.org/10.1016/j.niox.2014.11.010) [DOI] [PubMed] [Google Scholar]
- 11.Hirai F, Matsui T. Status of food intake and elemental nutrition in patients with Crohn’s disease. Integr Food Nutr Metab. 2015;2(2):148–150. [Google Scholar]
- 12.Hirata I, Naito Y, Takagi T, et al. Endogenous hydrogen sulfide is an anti-inflammatory molecule in dextran sodium sulfate-induced colitis in mice. Digest Dis Sci. 2011;56(5):1379–1386. doi: 10.1007/s10620-010-1461-5. [DOI] [PubMed] [Google Scholar]
- 13.Howell K, Yan F, Tokich A, et al. Iron sequestration is not the main mechanism in the inhibition of Staphylococcus aureus growth by cranberry phytochemicals. Integr Food Nutr Metab. 2015;2(3):184–188. [Google Scholar]
- 14.Kabil O, Motl N, Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta. 2014;1844(8):1355–1366. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. (Available from: http://dx.doi.org/10.1038/nrgastro.2015.150) [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18(10):1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 17.Lee SW, Hu YS, Hu LF, et al. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia. 2006;54(2):116–124. doi: 10.1002/glia.20362. (Available from: http://dx.doi.org/10.1002/glia.20362) [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Xiao QF, Zhang YL, et al. A cross-sectional study of irritable bowel syndrome in nurses in China: prevalence and associated psychological and lifestyle factors. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2014;15(6):590–597. doi: 10.1631/jzus.B1300159. (Available from: http://dx.doi.org/10.1631/jzus.B1300159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malago JJ, Sangu CL. Intraperitoneal administration of butyrate prevents the severity of acetic acid colitis in rats. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(3):224–234. doi: 10.1631/jzus.B1400191. (Available from: http://dx.doi.org/10.1631/jzus.B1400191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCann MJ, Dalziel JE, Bibiloni R, et al. An integrated approach to assessing the bio-activity of nutrients in vitro: the anti-oxidant effects of catechin and chlorogenic acid as an example. Integr Food Nutr Metab. 2015;2(3):197–204. (Available from: http://dx.doi.org/10.15761/IFNM.1000130) [Google Scholar]
- 21.Mikami Y, Shibuya N, Kimura Y, et al. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem. 2011;286(45):39379–39386. doi: 10.1074/jbc.M111.298208. (Available from: http://dx.doi.org/10.1074/jbc.M111.298208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mileva S, Galunska B, Gospodinova M, et al. Vitamin D3 status in children with acute diarrhea. Integr Food Nutr Metab. 2014;1(2):1–6. [Google Scholar]
- 23.Nighot P, Young K, Nighot M, et al. Chloride channel ClC-2 is a key factor in the development of DSS-induced murine colitis. Inflamm Bowel Dis. 2013;19(13):2867–2877. doi: 10.1097/MIB.0b013e3182a82ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novokmet M, Lukic E, Vuckovic F, et al. Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Sci Rep. 2014;4:4347. doi: 10.1038/srep04347. (Available from: http://dx.doi.org/10.1038/srep04347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozsgai G, Benko R, Bartho L, et al. Thermal spring water drinking attenuates dextran-sulfate-sodium-induced colitis in mice. Inflammopharmacology. 2015;23(1):57–64. doi: 10.1007/s10787-014-0227-7. [DOI] [PubMed] [Google Scholar]
- 26.Rashti Z, Koohsari H. Antibacterial effects of supernatant of lactic acid bacteria isolated from different Dough’s in Gorgan city in north of Iran. Integr Food Nutr Metab. 2015;2(3):193–196. [Google Scholar]
- 27.Sánchez-Fidalgo S, Cardeno A, Sanchez-Hidalgo M, et al. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24(7):1401–1413. doi: 10.1016/j.jnutbio.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Scharl M, Vavricka SR, Rogler G. Review: new anti-cytokines for IBD: what is in the pipeline? Curr Drug Targets. 2013;14(12):1405–1420. doi: 10.2174/13894501113149990159. [DOI] [PubMed] [Google Scholar]
- 29.Shimshoni E, Yablecovitch D, Baram L, et al. ECM remodelling in IBD: innocent bystander or partner in crime The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut. 2015;64(3):367–372. doi: 10.1136/gutjnl-2014-308048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin HS, Kang SI, Yoon SA, et al. Sinensetin attenuates LPS-induced inflammation by regulating the protein level of IκB-α. Biosci Biotechnol Biochem. 2012;76(4):847–849. doi: 10.1271/bbb.110908. [DOI] [PubMed] [Google Scholar]
- 31.Shori AB, Baba AS. Fermented milk derives bioactive peptides with antihypertensive effects. Integr Food Nutr Metab. 2015;2(3):178–181. [Google Scholar]
- 32.Sun XF, Zhang H. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol Histopathol. 2007;22(12):1387–1398. doi: 10.14670/HH-22.1387. [DOI] [PubMed] [Google Scholar]
- 33.Sunil Y, Ramadori G, Raddatzc D. Influence of NFκB inhibitors on IL-1β-induced chemokine CXCL8 and -10 expression levels in intestinal epithelial cell lines: glucocorticoid ineffectiveness and paradoxical effect of PDTC. Int J Colorectal Dis. 2010;25(3):323–333. doi: 10.1007/s00384-009-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinod VP, Guruvayoorappan C. Protective effect of marine mangrove Rhizophora apiculata on acetic acid induced experimental colitis by regulating anti-oxidant enzymes, inflammatory mediators and nuclear factor-κB subunits. Int Immunopharmacol. 2014;18(1):124–134. doi: 10.1016/j.intimp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Vlantis K, Polykratis A, Welz PS, et al. TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice. Gut, online. 2015 doi: 10.1136/gutjnl-2014-308323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Xia T, Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res. 2015;64(6):423–431. doi: 10.1007/s00011-015-0822-0. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe T, Sato T, Miyazaki M, et al. Effect of body composition intake with nano-lactic acid in rats. Integr Food Nutr Metab. 2015;2(2):156–158. [Google Scholar]
- 38.Wu J, Wei J, You X, et al. Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J Surg Res. 2013;182(1):e25–e33. doi: 10.1016/j.jss.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 39.Xu W, Chen J, Lin J, et al. Exogenous H2S protects H9c2 cardiac cells against high glucose-induced injury and inflammation by inhibiting the activation of the NF-κB and IL-1β pathways. Int J Mol Med. 2015;35(1):177–186. doi: 10.3892/ijmm.2014.2007. [DOI] [PubMed] [Google Scholar]
- 40.Yan F, Polk DB. Aminosalicylic acid inhibits IκB kinase α phosphorylation of IκBα in mouse intestinal epithelial cells. J Biol Chem. 1999;274(51):36631–36636. doi: 10.1074/jbc.274.51.36631. (Available from: http://dx.doi.org/10.1074/jbc.274.51.36631) [DOI] [PubMed] [Google Scholar]
- 41.Yin J, Ren W, Liu G, et al. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res. 2013;47(12):1027–1035. doi: 10.3109/10715762.2013.848277. [DOI] [PubMed] [Google Scholar]
- 42.Yin J, Ren WK, Wu XS, et al. Oxidative stress-mediated signaling pathways: a review. J Food Agric Environ. 2013;11(2):132–139. [Google Scholar]
- 43.Yin J, Wu MM, Xiao H, et al. Development of an antioxidant system after early weaning in piglets. J Anim Sci. 2014;92(2):612–619. doi: 10.2527/jas.2013-6986. [DOI] [PubMed] [Google Scholar]
- 44.Yin J, Liu M, Ren W, et al. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS ONE. 2015;10(4):e0122893. doi: 10.1371/journal.pone.0122893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin J, Duan J, Cui Z, et al. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 2015;5(20):15479–15486. (Available from: http://dx.doi.org/10.1039/C4RA13557A) [Google Scholar]
- 46.Xu XJ, Liu L, Yao SK. Nerve growth factor and diarrhea-predominant irritable bowel syndrome (IBS-D): a potential therapeutic target? J Zhejiang Univ.-Sci. B (Biomed. & Biotechnol) 2016;17(1):1–9. doi: 10.1631/jzus.B1500181. (Available from: http://dx.doi.org/10.1631/jzus.B1500181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zayachkivska O, Havryluk O, Hrycevych N, et al. Cytoprotective effects of hydrogen sulfide in novel rat models of non-erosive esophagitis. PLoS ONE. 2014;9(10):e110688. doi: 10.1371/journal.pone.0110688. (Available from: http://dx.doi.org/10.1371/journal.pone.0110688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang P, Li F, Wiegman CH, et al. Inhibitory effect of hydrogen sulfide on ozone-induced airway inflammation, oxidative stress, and bronchial hyperresponsiveness. Am J Resp Cell Mol Biol. 2015;52(1):129–137. doi: 10.1165/rcmb.2013-0415OC. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X, Feng Y, Zhan Z, et al. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem. 2014;289(42):8827–8834. doi: 10.1074/jbc.M114.596593. [DOI] [PMC free article] [PubMed] [Google Scholar]