Abstract
Background & Objective:
The results of epidemiologic studies on the relationship between the coffee consumption and pancreatic cancer risk were inconsistent. Thus, we performed an update meta-analysis of cohort studies to quantitatively summarize the association between coffee consumption and pancreatic cancer risk.
Methods:
We searched CBM (China Biology Medicine disc) and MEDLINE for studies of coffee consumption and pancreatic cancer risk up to June 2015. A total of 20 cohort studies were identified in this meta-analysis, and we analyzed these studies using random effects model. The dose-response analysis was conducted too.
Results:
The overall relative risk (RR) for highest coffee consumption versus lowest coffee consumption was 0.75 (95% Confidence Interval (CI), 0.63-0.86). Statistic significant heterogeneity was found among these studies (I2 =37.8%, P for heterogeneity =0.045). The pooled RR for increment of 1 cup/day of coffee consumption was 0.99 (95%CI, 0.96-1.03) for the nine studies, without statistically significant.
Conclusions:
High coffee consumption is associated with a reduced pancreatic cancer risk. However, the result should be accepted with caution, due to the potential confounder and bias could not be excluded. Further well designed studies are needed to confirm the finding.
KEY WORDS: Coffee, Pancreatic cancer, Meta-analysis
INTRODUCTION
Pancreatic cancer is the eighth most common cause of death from cancer worldwide.1 About 46 420 new pancreatic cancer cases were diagnosed and 39 590 people died from this cancer in the United States in 2014.2 Although the diagnosis and treatment of pancreatic cancer has been evident improvement, the five-year survival rate for the disease is still no more than 5%.3 Thus, it is very important to detect modifiable risk factors that may develop into the primary prevention for this cancer.
Coffee is one of the most popular beverages over the world. Since the early 1980s, epidemiologic studies on the relationship between the coffee consumption and pancreatic cancer risk have been conducted in different countries,4-21 and two meta-analysis studies have been performed on this topic too.22,23 However, the results of the two meta- analysis studies were contrary to each other. Furthermore, several new prospective cohort studies on this topic had been published recently, and the results of these new cohort studies are inconsistent too.24,25 Therefore, it is necessary to perform an update meta-analysis to quantitatively summarize the association between coffee consumption and pancreatic cancer risk. Moreover in order to reduce the bias, we recruited prospective cohort studies only.
METHODS
Search strategy
We searched CBM (China Biology Medicine disc) and MEDLINE for studies of coffee consumption and pancreatic cancer risk up to June 2015. Key words searched were as follows: (pancreatic OR pancreas) AND (cancer OR tumor OR carcinoma) AND (coffee OR caffeine OR drinking OR beverages OR diet OR lifestyle). Moreover, we have scan reference lists of retrieved articles to search for additional studies. The language of the studies was limited to English or Chinese.
Study selection
The inclusion criteria for the present meta-analysis were: (1) prospective cohort study design; (2) presented the consumption of coffee; and (3) provided the relative risk (RRs) (or odds rations or hazard ration) with their confidence intervals (CIs) (or data to calculate them). The exclusion criteria were: (1) case-control design; (2) data about coffee consumption was insufficient; (3) duplicate reports; (4) if multiple articles were from the same study population, only the one with largest sample or most information was included.
Data extraction
Two authors (Ran and Wang) independently extracted all data and tabulated them, discrepancies were resolved by discussion. The following data from each eligible study was extracted: first author’s last name, year of publication, country, period of follow-up, number of participants, RRs of pancreatic cancer with corresponding 95% CIs for each level of coffee consumption, and variables adjusted for the statistical analysis.
Statistical analysis
For all the included cohort studies, we computed overall RRs with 95% CIs for the highest versus lowest level of coffee consumption. Then subgroup analysis to evaluate the influence of geographic areas was performed too.
Statistical heterogeneity was investigated by Q test and I2 statistic. For the Q test, P < 0.10 was considered present heterogeneity. If the heterogeneity was statistically significant, a random effects model was conducted. Otherwise, a fixed effects model was used.
For the dose-response analysis of coffee consumption, the method proposed by Greenland et al was used to evaluate linear trends (study-specific slopes) from the correlated natural logs of the RRs through categories of coffee consumption.26 We only recruited those studies that showed the number of cases and person-years and RRs with variance estimates for at least three quantitative exposure categories. For each study, we assigned the midpoint of each exposure category as the dose corresponding, and the open-ended upper category was assumed to have the same amplitude as the previous category.
Finally, publication bias was evaluated by the Begg’s and Egger’s tests. Statistical analyses were performed with Stata (version 12.0; StataCorp, College Station, TX, USA).
RESULTS
Study characteristics
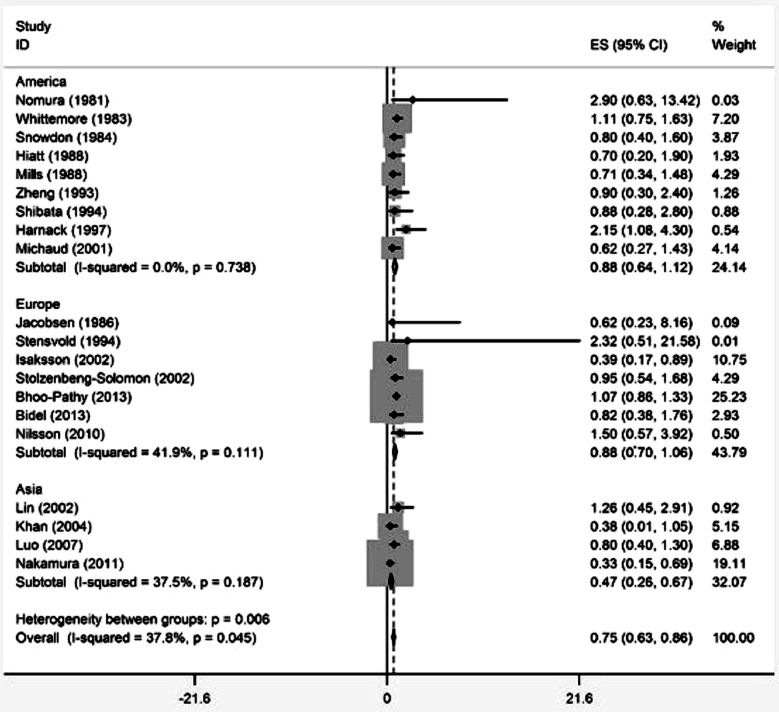
A total of 20 cohort studies were identified in this meta-analysis, including 1341876 participants and 2872 cases of pancreatic cancer.4-21,24,25 The process of selecting studies is shown in Fig.1. Of the all eligible studies, nine studies were conducted in America (the United States),4-10,12-14 four studies in Asia (Japan),16,18,19,21 and seven studies in Europe (two each in Norway, Sweden, Finland, and one study was conducted in ten European countries by the European Prospective Investigation into Nutrition and Cancer cohort).7,11,15,17,20,24,25 The sample size varied from 412 to 477 312, and the number of pancreatic cancer cases ranged from 21 to 865 (Table-I).
Fig.1.
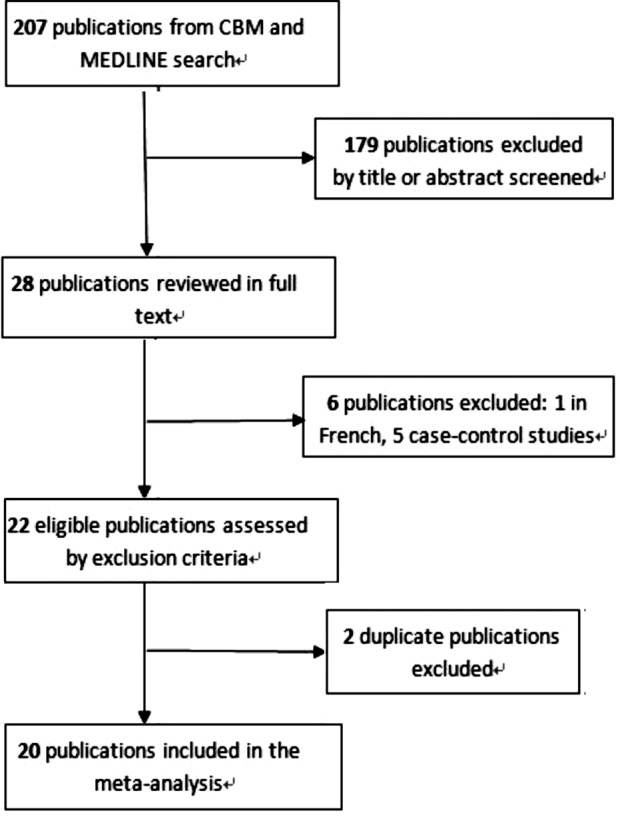
Flow diagram of selection of relevant publications.
Table-I.
Characteristics of Studies of Coffee Consumption and Pancreatic cancer risk.
| Study | Country | Study period | Cases/Subjects | Consumption categories | Relative risk (95% ci) | Adjustments |
|---|---|---|---|---|---|---|
| Nomura 1981 [4] | America | 1968-1981 | 28/8032 | nondrinkers | 1.00(reference) | age, smoking |
| 1-2 cup/d | 2.74(0.61-12.36) | |||||
| 3-4 cup/d | 1.80(0.36-8.89) | |||||
| >5 cup/d | 2.90(0.63-13.42) | |||||
| Whittemore 1983 [5] | America | 1966-1983 | 84/412 | nondrinkers | 1.00(reference) | age, college, class year |
| drinkers | 1.11(0.75-1.63) | |||||
| Snowdon 1984 [6] | America | 1960-1980 | 71/23912 | <1 cup/d | 1.00(reference) | age, sex |
| 1cup/d | 1.7(0.9-3.3) | |||||
| >2 cup/d | 0.8(0.4-1.6) | |||||
| Jacobsen 1986 [7] | Norway | 1967-1978 | 63/16555 | ≤2 cup/d | 1.00(reference) | age, smoking, residence |
| 3-4 cup/d | 1.22(0.23-2.20) | |||||
| 5-6 cup/d | 0.53(0.24-1.19) | |||||
| ≥7 cup/d | 0.62(0.23-1.68) | |||||
| Hiatt 1988 [8] | America | 1978-1984 | 48/122894 | nondrinkers | 1.00(reference) | age, smoking, tea, alcohol, ethic, blood glucose |
| <1 cup/d | 0.8(0.3-2.6) | |||||
| 1-3 cup/d | 0.9(0.4-2.1) | |||||
| >4 cup/d | 0.7(0.2-1.9) | |||||
| Mills 1988 [9] | America | 1976-1983 | 40/34000 | Never | 1.00(reference) | age, sex |
| < Daily | 0.65(0.22-1.89) | |||||
| ≥ Daily | 0.71(0.34-1.48) | |||||
| Zheng 1993 [10] | America | 1966-1986 | 56/17633 | <3 cup/d | 1.00(reference) | age, smoking, alcohol |
| 3-4 cup/d | 0.6(0.3-1.2) | |||||
| 5-6 cup/d | 0.7(0.4-1.6) | |||||
| >7 cup/d | 0.9(0.3-2.4) | |||||
| Stensvold 1994[11] | Norway | 1977-1988 | 41/42973 | ≤2 cup/d | 1.00(reference) | age, smoking, country of residence |
| 3-4 cup/d | 2.58(0.58-23.44) | |||||
| 5-6 cup/d | 2.80(0.65-25.27) | |||||
| ≥7 cup/d | 2.32(0.51-21.58) | |||||
| Shibata 1994 [12] | America | 1981-1990 | 63/13979 | <1 cup/d | 1.00(reference) | age, sex, smoking |
| 1cup/d | 1.82(0.75-4.43) | |||||
| 2-3 cup/d | 1.67(0.74-3.77) | |||||
| ≥4 cup/d | 0.88(0.28-2.80) | |||||
| Harnack 1997 [13] | America | 1986-1994 | 66/33976 | ≤7cup/week | 1.00(reference) | age, smoking |
| 8-17.5cup/week | 1.91(0.92-4.00) | |||||
| ≥17.5cup/week | 2.15(1.08-4.30) | |||||
| Michaud 2001[14] | America | 1980-1998 | 288/136593 | nondrinkers | 1.00(reference) | age, smoking, body mass index, diabetes mellitus, history of cholecystectomy |
| <1 cup/d | 0.94(0.65-1.36) | |||||
| 1 cup/d | 0.60(0.38-0.94) | |||||
| 2-3 cup/d | 0.88(0.65-1.21) | |||||
| >3 cup/d | 0.62(0.27-1.43) | |||||
| Isaksson 2002 [15] | Sweden | 1961-1997 | 131/21884 | 0-2 cup/d | 1.00(reference) | age, sex, smoking |
| 3-6 cup/d | 0.91(0.60-1.38) | |||||
| >7 cup/d | 0.39(0.17-0.89) | |||||
| Lin 2002 [16] | Japan | 1988-1997 | 225/99527 | nondrinkers | 1.00(reference) | age, smoking |
| 1-2 cup/m | 0.78(0.46-1.26) | |||||
| 1-4 cup/w | 0.55(0.34-0.86) | |||||
| 1 cup/d | 0.55(0.30-0.95) | |||||
| 2-3 cup/d | 0.39(0.20-0.71) | |||||
| ≥4 cup/d | 1.26(0.45-2.91) | |||||
| Stolzenbeng-Solomon 2002 [17] | Filand | 1985-1997 | 163/27111 | reference category | 1.00(reference) | age, smoking |
| low | 1.48(0.89-2.46) | |||||
| moderately low | 1.12(0.61-2.03) | |||||
| moderately high | 1.72(1.01-2.86) | |||||
| high | 0.95(0.54-1.68) | |||||
| Khan 2004 [18] | Japan | 1984-2002 | 25/3155 | nondrinkers | 1.00(reference) | age, smoking, education |
| drinkers | 0.38(0.01-1.05) | |||||
| Luo 2007 [19] | Japan | 1990-2003 | 233/102137 | rarely | 1.00(reference) | age, sex, smoking, body mass index, tea, alcohol, diabetes mellitus |
| 1-2 cup/w | 1.0(0.7-1.4) | |||||
| 3-4 cup/w | 1.1(0.7-1.7) | |||||
| 1-2 cup/d | 0.9(0.6-1.3) | |||||
| ≥3 cup/d | 0.8(0.4-1.3) | |||||
| Nilsson 2010 [20] | Sweden | 1992-2007 | 74/61569 | <1 cup/d | 1.00(reference) | age, sex, education, smoking, body mass index, physical activity |
| 1-3 cup/d | 1.18(0.47-3.02) | |||||
| ≥4 cup/d | 1.50(0.57-3.92) | |||||
| Nakamura 2011 [21] | Japan | 1992-1999 | 52/30826 | nondrinkers | 1.00(reference) | |
| low | 0.44(0.21-0.82) | |||||
| high | 0.33(0.15-0.69) | |||||
| Bhoo-Pathy 2013 [24] | Europe | 1992-2000 | 865/477312 | low | 1.00(reference) | age, sex, high, weight, education, smoking, body mass index, diabetes mellitus, physical activity |
| nondrinkers | 1.09(0.80-1.50) | |||||
| moderately low | 1.11(0.92-1.31) | |||||
| moderately high | 0.99(0.81-1.21) | |||||
| high | 1.07(0.86-1.33) | |||||
| Bidel 2013 [25] | Finland | 1972-2006 | 235/60041 | nondrinkers | 1.00(reference) | age, sex, alcohol, tea, study year, education, smoking, body mass index, diabetes mellitus, physical activity |
| 1-2 cup/d | 0.86(0.42-1.74) | |||||
| 3-4 cup/w | 0.86(0.45-1.64) | |||||
| 5-6 cup/w | 0.78(0.41-1.47) | |||||
| 7-9 cup/w | 0.92(0.46-1.83) | |||||
| ≥10 cup/d | 0.82(0.38-1.76) |
Highest versus lowest drinking category
As various measurement units for coffee consumption were used in the included studies, we just considered to compare the highest coffee consumption category with lowest coffee consumption category, and take the latter as the reference category.
The overall RR for highest coffee consumption versus lowest coffee consumption was 0.75 (95%CI, 0.63-0.86). Statistic significant heterogeneity was found among these studies (I2 =37.8%, P for heterogeneity =0.045) (Fig.2). Neither Egger’s test (P for bias =0.436) nor Begg’ test (P for bias =0.078) indicated a significant publication bias (Fig.3).
Fig.2.
Forest plot (random-effects model) of coffee consumption (highest versus lowest category) and pancreatic cancer risk.
Fig.3.
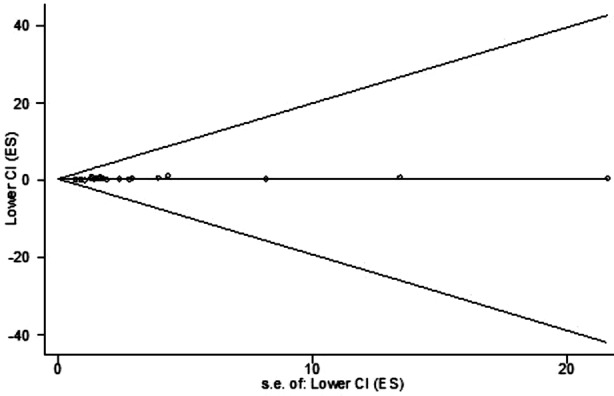
Begg’s funnel plot for publication bias.
Subgroup analysis on geographic areas
Nine studies had been conducted in the America, analysis of the nine studies showed that there was no association between coffee consumption and pancreatic cancer risk (RR, 0.88; 95%CI, 0.64-1.12; P for heterogeneity =0.738). And similar result was found in the seven studies in Europe (RR, 0.88; 95%CI, 0.70-1.06; P for heterogeneity =0.111). In contrast, when the four studies in Asia were pooled, coffee consumption was associated with a reduced pancreatic cancer risk (RR, 0.88; 95%CI, 0.70-1.06; P for heterogeneity =0.187) (Fig.2).
Dose-response meta-analysis
Nine studies were included in the dose-response analysis for the relationship between coffee consumption and pancreatic cancer risk.10-14,16,19,20,25 The pooled RR for an increment of one cup/day of coffee consumption was 0.99 (95%CI, 0.96-1.03), without statistically significant. The Goodness-of-fit indicated no significant heterogeneity among these studies (Q = 35.29; p = 0.23).
DISCUSSION
Up to now, more and more evidences, which is provided by epidemiological studies have demonstrated the inverse association between coffee consumption and some cancer risk, such as breast cancer, prostate cancer, liver cancer, and colorectal cancer.27-31 However the relationship between coffee consumption and pancreatic cancer risk had a series of inconsistent result. In 2011, a meta-analysis based on fourteen cohort studies showed a significant association between coffee consumption and reduced pancreatic cancer risk (RR, 0.68; 95%CI, 0.51-0.84).22 After that, Turati et al performed another meta-analysis with 37 case-control studies and 17 cohort studies, the result suggested non-significant association between coffee consumption and the risk of pancreatic cancer (RR, 1.13; 95%CI, 0.99-1.29).23 Specially, our update meta-analysis, which is based on 20 cohort studies, supported the protective effect of high coffee consumption for pancreatic cancer risk (RR, 0.75; 95%CI, 0.63-0.86).
In our meta-analysis, when the 20 studies were pooled, high coffee consumption was associated with a reduced pancreatic cancer risk, but the results of subgroup, which stratify by geographic area, were diverse. Studies in America and Europe showed a non-significant association between coffee consumption for pancreatic cancer risk. In contrast, studies in Asia revealed a significant inverse association between coffee consumption and pancreatic cancer risk. The diverse results among subgroup analysis may be owing to different race and environment.
Coffee interfere the cancerous process with different stage. The molecular mechanisms for anticancer effects of coffee compounds are as follows: (1) The antioxidant of coffee may reduce reactive oxygen species (ROS), that can induce DNA damage provoked.32 (2) Coffee can enhance endogenous defense systems by inducing a complex of nuclear clear factor erythroid-2-like 2 factor (Nrf2), and the cafestol of the coffee can increase the endogenous antioxidant too.33,34 (3) Coffee’s chemopreventive effect can induce DNA repair capacity. In vitro experiment suggested that cafestol and kahweol decreased 50% genotoxicity of human-derived hepatoma cells. 35 (4) Coffee consumption can decreased inflammation marker, such as the level of IL-18, c-reactive protein and E- selectin. Several compounds of coffee can also inhibit the activation of nuclear factor kappa B (NF-κB), that is the key transcription factor of inflammatory process.36,37 (5) Experiment showed that coffee component cafestol, kahweol, and caffeine can induce apoptosis. 38 These molecular mechanisms could well explain our findings in our meta-analysis that high coffee consumption with a decreased pancreatic cancer risk.
Several limitations of our meta-analysis should be discussed. First, we could not obtain enough information to calculate the adjusted RR in some studies, so we just combined unadjusted RRs. This could have influence on the quality of the meta-analysis. Second, as all cohort studies, the potential bias could not be completely avoided. Third, different classification coffee consumption among studies may contribute to the heterogeneity when pooled analysis. Finally, the different measurement units, brewing method, and coffee type may be the cause of heterogeneity too.
In summary, the present meta-analysis suggested that high coffee consumption is associated with a reduced pancreatic cancer risk. However, the result should be accepted with caution, due to the potential confounder and bias could not be completely excluded. Further well designed studies are needed to confirm the finding.
Footnotes
Source of funding: None.
Declaration of interest: None.
Authors’ Contribution
RHQ and WJZ proposed the study. RHQ and SCQ performed research and wrote the first draft.
RHQ and WJZ collected and analyzed the data.
RHQ is the guarantor.
All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. doi:10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma JM, Zou ZH, Jemal A. Global cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. doi:10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Ries L, Melbert D, Krapcho M, Marios M. Risk Determination for Pancreatic Cancer. JOP. 2014;15(4):289–291. doi: 10.6092/1590-8577/2591. [DOI] [PubMed] [Google Scholar]
- 4.Nomura A, Heilbrun LK, Stemmermann GN. Coffee and pancreatic cancer. Lancet. 1981;22:415. doi: 10.1016/s0140-6736(81)90849-7. doi:10.1016/S0140-6736(81)90849-7. [DOI] [PubMed] [Google Scholar]
- 5.Whittemore AS, Paffenbarger RS, Anderson K, Halpern J. Early precursors of pancreatic cancer in college men. J Chronic Dis. 1983;36(3):251–256. doi: 10.1016/0021-9681(83)90059-0. doi:10.1016/0021-9681(83)90059-0. [DOI] [PubMed] [Google Scholar]
- 6.Snowdon DA, Phillips RL. Coffee consumption and risk of fatal cancers. Am J Pub Health. 1984;74(8):820–823. doi: 10.2105/ajph.74.8.820. doi:10.2105/AJPH.74.8.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen BK, Bjelke E, Kvåle G, Heuch I. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J National Cancer Institute. 1986;76(5):823–831. [PubMed] [Google Scholar]
- 8.Hiatt RA, Klatsky AL, Armstrong MA. Pancreatic cancer, blood glucose and beverage consumption. Int J Can. 1988;41(6):794–797. doi: 10.1002/ijc.2910410603. [DOI] [PubMed] [Google Scholar]
- 9.Mills PK, Beeson WL, Abbey DE, Fraser GE, Phillips RL. Dietary habits and past medical history as related to fatal pancreas cancer risk among Adventists. Cancer. 1988;61(12):2578–2585. doi: 10.1002/1097-0142(19880615)61:12<2578::aid-cncr2820611232>3.0.co;2-0. doi:10.1002/1097-0142(19880615)61:12%3C2578:: AID-CNCR2820611232%3E3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Zheng W, McLaughlin JK, Gridley G, Bjelke E, Schuman LM, Silverman DT, et al. A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States) Cancer Causes Control. 1993;4(5):477–482. doi: 10.1007/BF00050867. doi:10.1007/BF00050867. [DOI] [PubMed] [Google Scholar]
- 11.Stensvold MI, Jacobsen BK. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control. 1994;5(5):401–408. doi: 10.1007/BF01694753. doi:10.1007/BF01694753. [DOI] [PubMed] [Google Scholar]
- 12.Shibata A, Mack TM, Paganini-Hill A, Ross RK, Henderson BE. A prospective study of pancreatic cancer in the elderly. Int J Can. 1994;58(1):46–49. doi: 10.1002/ijc.2910580109. doi:10.1002/ijc.2910580109. [DOI] [PubMed] [Google Scholar]
- 13.Harnack LJ, Anderson KE, Zheng W, Folsom AR, Sellers TA, Kushi LH. Smoking, alcohol, coffee, and tea intake and incidence of cancer of the exocrine pancreas: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 1997;6(12):1081–1086. [PubMed] [Google Scholar]
- 14.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol Biomarkers Prev. 2001;10(5):429–437. [PubMed] [Google Scholar]
- 15.Isaksson B, Jonsson F, Pedersen NL, Larsson J, Feychting M, Permert J. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin Registry. Int J Can. 2002;98(3):480–482. doi: 10.1002/ijc.10256. doi:10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Tamakoshi A, Kawamura T, Inaba Y, Kikuchi S, Motohashi Y, et al. Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. Int J Can. 2002;99(5):742–746. doi: 10.1002/ijc.10402. doi:10.1002/ijc.10402. [DOI] [PubMed] [Google Scholar]
- 17.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;55(9):783–792. doi: 10.1093/aje/155.9.783. doi:10.1093/aje/155.9.783. [DOI] [PubMed] [Google Scholar]
- 18.Khan MMH, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, et al. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer sites and sex. Asian Pacific J Can Prev. 2004;1:58–65. [PubMed] [Google Scholar]
- 19.Luo J, Inoue M, Iwasaki M, Sasazuki S, Otani T, Ye W, et al. Green tea and coffee intake and risk of pancreatic cancer in a large-scale, population-based cohort study in Japan (JPHC study) Euro J Can Prev. 2007;16(6):542–548. doi: 10.1097/CEJ.0b013e32809b4d30. doi:10.1097/CEJ.0b013e32809b4d30. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson LM, Johansson I, Lenner P, Lindahl B, Van Guelpen. Consumption of filtered and boiled coffee and the risk of incident cancer: a prospective cohort study. Cancer Causes Control. 2010;21(10):1533–1544. doi: 10.1007/s10552-010-9582-x. doi:10.1007/s10552-010-9582-x. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Nagata C, Wada K, Tamai Y, Tsuji M, Takatsuka N, et al. Cigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in Japan. Jap J Clin Oncol. 2011;41(2):225–231. doi: 10.1093/jjco/hyq185. doi:10.1093/jjco/hyq185. [DOI] [PubMed] [Google Scholar]
- 22.Dong J, Zou J, Yu XF. Coffee drinking and pancreatic cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2011;17(9):1204–1210. doi: 10.3748/wjg.v17.i9.1204. doi:10.3748/wjg.v17.i9.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turati F, Galeone C, Edefonti V, Ferraroni M, Lagiou P, La Vecchia C, et al. A meta-analysis of coffee consumption and pancreatic cancer. Ann Oncol. 2012;23(2):331–318. doi: 10.1093/annonc/mdr331. doi:10.1093/annonc/mdr331. [DOI] [PubMed] [Google Scholar]
- 24.Bhoo–Pathy N, Uiterwaal CSPM, Dik VK, Jeurnink SM, Bech BH, Overvad K, et al. Intake of coffee, decaffeinated coffee, or tea does not affect risk for pancreatic cancer: results from the European prospective investigation into nutrition and cancer study. Clin Gastroenterol Hepatol. 2013;11(11):1486–1492. doi: 10.1016/j.cgh.2013.05.029. doi:10.1016/j.cgh.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Bidel S, Hu G, Jousilahti P, Pukkala E, Hakulinen T, Tuomilehto J. Coffee consumption and risk of gastric and pancreatic cancer - A prospective cohort study. Int J Can. 2013;132(7):1651–1659. doi: 10.1002/ijc.27773. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 27.Lowcock EC, Cotterchio M, Boucher BA, El-Sohemy A. High coffee intake, but not caffeine, is associated with reduced estrogen receptor negative and postmenopausal breast cancer risk with no effect modification by CYP1A2 genotype. Nutr Can. 2013;65(3):398–409. doi: 10.1080/01635581.2013.768348. doi:10.1080/01635581.2013.768348. [DOI] [PubMed] [Google Scholar]
- 28.Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, Stampfer MJ, et al. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. J National Cancer Institute. 2011;103(11):876–884. doi: 10.1093/jnci/djr151. doi:10.1093/jnci/djr306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sang LX, Chang B, Li XH, Jiang M. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterol. 2013;13(1):13–34. doi: 10.1186/1471-230X-13-34. doi:10.1186/1471-230X-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian C, Wang W, Hong Z, Zhang X. Coffee consumption and risk of colorectal cancer: a dose–response analysis of observational studies. Cancer Causes Control. 2013;24(6):1265–1268. doi: 10.1007/s10552-013-0200-6. doi:10.1007/s10552-013-0200-6. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama K, Kuriyama S, Akhter M, Kakizaki M, Nakaya N, Ohmori-Matsuda K, et al. Coffee consumption and mortality due to all causes, cardiovascular disease, and cancer in Japanese women. J Nutr. 2010;104:1007–1013. doi: 10.3945/jn.109.109314. doi:10.3945/jn.109.109314. [DOI] [PubMed] [Google Scholar]
- 32.Bøhn SK, Blomhoff R, Paur I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Molecular Nutr Food Res. 2014;58(5):915–930. doi: 10.1002/mnfr.201300526. [DOI] [PubMed] [Google Scholar]
- 33.Volz N, Boettler U, Winkler S, Teller N, Schwarz C, Bakuradze T, et al. Effect of coffee combining green coffee bean constituents with typical roasting products on the Nrf2/ARE pathway in vitro and in vivo. J Agricultural Food Chem. 2012;60(38):9631–9641. doi: 10.1021/jf302258u. doi:10.1021/jf302258u. [DOI] [PubMed] [Google Scholar]
- 34.Boettler U, Volz N, Teller N, Haupt LM, Bakuradze T, Eisenbrand G, et al. Induction of antioxidative Nrf2 gene transcription by coffee in humans: depending on genotype? Molecular Biology Reports. 2012;39(6):7155–7162. doi: 10.1007/s11033-012-1547-6. doi:10.1007/s11033-012-1547-6. [DOI] [PubMed] [Google Scholar]
- 35.Majer BJ, Hofer E, Cavin C, Lhoste E, Uhl M, Glatt HR, et al. Coffee diterpenes prevent the genotoxic effects of 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) and N-nitrosodimethylamine in a human derived liver cell line (HepG2) Food Chem Toxicol. 2005;43(3):433–441. doi: 10.1016/j.fct.2004.11.009. doi:10.1016/j.fct.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84(4):888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 37.Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Jaakko T. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91(4):950–957. doi: 10.3945/ajcn.2009.28548. doi:10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 38.Hwang YP, Jeong HG. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Letters. 2008;582(17):2655–2662. doi: 10.1016/j.febslet.2008.06.045. doi:10.1016/j.febslet.2008.06.045. [DOI] [PubMed] [Google Scholar]