Abstract
AIM: To study the interleukin-1 (IL-1) pathway as a therapeutic target for liver fibrosis in vitro and in vivo using the ATP-binding cassette transporter b4-/- (Abcb4-/-) mouse model.
METHODS: Female and male Abcb4-/- mice from 6 to 13 mo of age were analysed for the degree of cholestasis (liver serum tests), extent of liver fibrosis (hydroxyproline content and Sirius red staining) and tissue-specific activation of signalling pathways such as the IL-1 pathway [quantitative polymerase chain reaction (qPCR)]. For in vivo experiments, murine hepatic stellate cells (HSCs) were isolated via pronase-collagenase perfusion followed by density gradient centrifugation using female mice. Murine HSCs were stimulated with up to 1 ng/mL IL-1β with or without 2.5 μg/mL Anakinra, an IL-1 receptor antagonist, respectively. The proliferation of murine HSCs was assessed via the BrdU assay. The toxicity of Anakinra was evaluated via the fluorescein diacetate hydrolysis (FDH) assay. In vivo 8-wk-old Abcb4-/- mice with an already fully established hepatic phenotype were treated with Anakinra (1 mg/kg body-weight daily intraperitoneally) or vehicle and liver injury and liver fibrosis were evaluated via serum tests, qPCR, hydroxyproline content and Sirius red staining.
RESULTS: Liver fibrosis was less pronounced in males than in female Abcb4-/- animals as defined by a lower hydroxyproline content (274 ± 64 μg/g vs 436 ± 80 μg/g liver, respectively; n = 13-15; P < 0.001; Mann-Whitney U-test) and lower mRNA expression of the profibrogenic tissue inhibitor of metalloproteinase-1 (TIMP) (1 ± 0.41 vs 0.66 ± 0.33 fold, respectively; n = 13-15; P < 0.05; Mann-Whitney U-test). Reduced liver fibrosis was associated with significantly lower levels of F4/80 mRNA expression (1 ± 0.28 vs 0.71 ± 0.41 fold, respectively; n = 12-15; P < 0.05; Mann-Whitney U-test) and significantly lower IL-1β mRNA expression levels (1 ± 0.38 vs 0.44 ± 0.26 fold, respectively; n = 13-15; P < 0.001; Mann-Whitney U-test). No gender differences in the serum liver parameters [bilirubin; alanine aminotransferase (ALT); aspartate aminotransferase and alkaline phosphatase (AP)] were found. In vitro, the administration of IL-1β resulted in a significant increase in HSC proliferation [0.94 ± 0.72 arbitrary units (A.U.) in untreated controls, 1.12 ± 0.80 A.U. at an IL-1β concentration of 0.1 ng/mL and 1.18 ± 0.73 A.U. at an IL-1β concentration of 1 ng/mL in samples from n = 6 donor animals; P < 0.001; analyses of variance (ANOVA)]. Proliferation was reduced significantly by the addition of 2.5 μg/mL Anakinra (0.81 ± 0.60 A.U. in untreated controls, 0.92 ± 0.68 A.U. at an IL-1β concentration of 0.1 ng/mL, and 0.91 ± 0.69 A.U. at an IL-1β concentration of 1 ng/mL; in samples from n = 6 donor animals; P < 0.001; ANOVA) suggesting an anti-proliferative effect of this clinically approved IL-1 receptor antagonist. The FDH assay showed this dose to be non-toxic in HSCs. In vivo, Anakinra had no effect on the hepatic hydroxyproline content, liver serum tests (ALT and AP) and pro-fibrotic (collagen 1α1, collagen 1α2, transforming growth factor-β, and TIMP-1) and anti-fibrotic [matrix metalloproteinase 2 (MMP2), MMP9 and MMP13] gene expression after 4 wk of treatment. Furthermore, the hepatic IL-1β and F4/80 mRNA expression levels were unaffected by Anakinra treatment.
CONCLUSION: IL-1β expression is associated with the degree of liver fibrosis in Abcb4-/- mice and promotes HSC proliferation. IL-1 antagonism shows antifibrotic effects in vitro but not in Abcb4-/- mice.
Keywords: Cholestasis, Primary sclerosing cholangitis, The ATP-binding cassette transporter b4, Liver fibrosis, Interleukin-1
Core tip: Interleukin-1 (IL-1) critically participates in hepatic stellate cells (HSCs) pathophysiology and in the progression of liver injury to fibrosis. We found that fibrosis was more pronounced in female than in male ATP-binding cassette transporter b4-/- animals. This fibrosis was associated with higher IL-1β mRNA expression levels. We showed that IL-1β promoted the proliferation of murine HSCs and described an antifibrotic effect of the clinically approved IL-1 receptor antagonist Anakinra in vitro. Despite the promising antifibrotic effects in vitro, Anakinra failed to improve liver fibrosis in this preclinical primary sclerosing cholangitis model. Its potency in other models of liver injury and fibrosis remains to be determined.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease that is characterised by obliterative strictures of the intra- and extra-hepatic bile ducts. Because these strictures are associated with inflammation and fibrosis, PSC is supposed to be an immune-mediated liver disease. PSC primarily occurs in young adults and leads to liver cirrhosis with hepatic decompensation and portal hypertension and is frequently associated with malignancy of the biliary tract[1,2]. To date, there is no effective therapeutic option to halt disease progression. Thus, liver transplantation in end-stage liver disease is often required. The estimated median time for transplant-free survival may be as short as 12 to 13 years at tertiary referral centres[1,3]. Therefore, new therapeutic targets need to be identified to establish an urgently required effective therapy for PSC.
PSC mainly affects the large bile ducts and causes periductal fibrosis[4]. This leads to obliteration of the bile ducts, resulting in impaired bile flow and cholestasis. During chronic cholestatic disease, toxic bile acids accumulate[5] and induce cholangio- and hepatocellular apoptosis by specific signalling pathways[6-8]. The clinical significance of cholangiocellular and hepatocellular apoptosis in patients with chronic cholestatic disease was recently supported by the detection of serum markers of liver cell apoptosis[9].
Hepatocellular apoptosis is considered a trigger of liver fibrosis via the activation of hepatic stellate cells (HSCs)[10,11]. This results in the progression to liver fibrosis and cirrhosis in PSC.
HSCs are the major source of hepatic extracellular matrix deposition and crucially participate in the pathogenesis of liver fibrosis[12-14]. In addition to other factors, enhanced proliferation of this cell type is thought to be an important profibrotic mechanism[14]. In this regard, a recent study found that interleukin-1β (IL-1β) induces the proliferation of rat HSCs[15]. Furthermore, IL-1 was identified as a factor in the progression of liver injury to fibrosis[16]. Therefore, we hypothesised that blocking the IL-1 pathway during liver fibrosis might be a therapeutic approach in chronic liver disease.
Anakinra is a clinically approved IL-1 receptor antagonist that is listed for the treatment of rheumatoid arthritis by the European Medicines Agency and the United States Food and Drug Administration. Its implication for the treatment of chronic liver disease and fibrosis has not been investigated thus far.
The ATP-binding cassette transporter b4-/- (Abcb4-/-) mouse is an established preclinical model for biliary fibrosis and PSC[17-19]. Therefore, this model is widely used to study therapeutic strategies for biliary fibrosis and PSC in vivo[20].
Here, we tested the proliferative and pro-fibrotic properties of the IL-1 pathway in vitro, analysed its contribution to liver fibrosis in the Abcb4-/- model in vivo and tested the potential protective effects of the clinically approved IL-1 receptor antagonist Anakinra on the development of liver injury and fibrosis in this animal model.
MATERIALS AND METHODS
Animals
Animals were obtained from the Jackson Laboratory (United States) and Charles River (Germany). The animal protocol was designed to minimise pain or discomfort to the animals. The animals were housed at a 12/12 h light/dark cycle and were fed ad libitum. The animals were kept according to local regulations. The experiments were approved by local authorities. All ethical, institutional and national guidelines for the care and use of laboratory animals were followed.
Isolation and culture of primary murine HSCs
The isolation of primary murine HSC from female C57BL/6N wild-type animals was performed by pronase-collagenase perfusion followed by density gradient centrifugation in 13.2% Nycodenz® (Axis-Shield PoC, Norway)[21]. The cells were plated at a density of 25000 cells/cm2. The cells were kept in DMEM containing 10% fetal bovine serum and antibiotics (Sigma, Germany) in a humidified atmosphere with 5% CO2 and 21% O2 at 37 °C.
Toxicity assay
To investigate the potential toxic effects of Anakinra on murine HSCs, we performed the fluorescein diacetate hydrolysis (FDH) assay according to the method of Jones et al[22] after stimulation of murine HSCs for 24 h with 25 pg/mL to 2.5 μg/mL Anakinra.
Proliferation assay
The cells were stimulated with vehicle, 0.1 and 1 ng/mL IL-1β for 24 h at day three after isolation. Where indicated, the cells were co-incubated with 2.5 μg/mL Anakinra. The proliferation of primary murine HSCs was measured using a BrdU-assay kit (Roche, Germany) according to the manufacturer’s instructions.
In vivo experiments
Available material from female and male Abcb4-/- mice was kindly provided by the Research Unit for Experimental and Molecular Hepatology, Graz, Austria (Fickert P and Trauner M). The material from 6- to 13-mo-old animals was studied.
Eight-week old female Abcb4-/- (FVB) mice received daily intraperitoneally injections of Anakinra (1 mg/kg body-weight, a dosage used previously for animal studies, corresponding to the recommended dose for the treatment of rheumatoid arthritis[23] or NaCl 0.9% as a control for 4 wk, a time period previously shown to be sufficient to identify the modulation of liver fibrosis in this model[20]. After narcotisation with isoflurane (Abbott GmbH, Germany), the animals were sacrificed by cervical dislocation.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction was performed using a Sybr® green system. Glyceraldehyde-3-phosphate dehydrogenase or 18 s were used as housekeeping genes and were normalised against the means of the controls.
Serum biochemistry
The levels of bilirubin, alkaline phosphatase (AP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were analysed using the Cobas Integra 800 analyser (Roche Diagnostics, Germany) or the Hitachi 917 analyser (Boehringer Mannheim, Germany).
Quantification of hydroxyproline
The hydroxyproline content was determined according to the method of Edwards et al[24].
Sirius-red staining
Liver samples were fixed using 4% formaldehyde. After embedding in paraffin, 4-μm sections were stained with Sirius red.
Immunohistochemistry
Ki-67 immunohistochemistry was performed using the polyclonal rabbit anti-Ki-67 antibody (Novocastra, Germany). The ABC system with AEC as a substrate was used for the detection of antibody antigen binding. The number of positively stained cells was counted per mouse in 20 randomly chosen fields at 40-fold magnification.
Statistical analysis
Statistical calculations were performed using the SPSS 23 software package (IBM, United States). Where appropriate, the differences between groups were verified by the Mann-Whitney U-test.
Where appropriate, the analyses of variance (ANOVA) were calculated with the procedure UNIANOVA. The normality and homogeneity of the variances of the residuals were assessed by inspection of residual plots from the UNIANOVA procedure and P-P plots. Furthermore, Levene’s test was used to assess the equality of error variances (for details, see also supplemental material). The results are reported as the means ± SD. P-values less than 0.05 were considered to be statistically significant. The statistical analysis of this study was supported and reviewed by Christoph Glasmacher (Christoph Glasmacher - Biometrics and SAS-Programming for Clinical Research).
RESULTS
Gender differences in the liver disease of Abcb4-/- mice
Hepatic injury and liver fibrosis were evaluated in male and female Abcb4-/- animals from 6 to 13 mo of age. We found that the hepatic hydroxyproline levels were 37% lower in male animals than in female animals (Figure 1A; 274 ± 64 μg/g vs 436 ± 80 μg/g liver, respectively; n = 13-15; P < 0.001; Mann-Whitney U-test). Sirius red staining illustrated reduced collagen deposition in male animals (Figure 1B). In accordance with these findings, the livers of male animals showed 34% lower tissue inhibitor of metalloproteinase (TIMP-1) mRNA expression (Figure 1C; 1 ± 0.41 vs 0.66 ± 0.33 fold; n = 13-15; P < 0.05; Mann-Whitney U-test).
Figure 1.
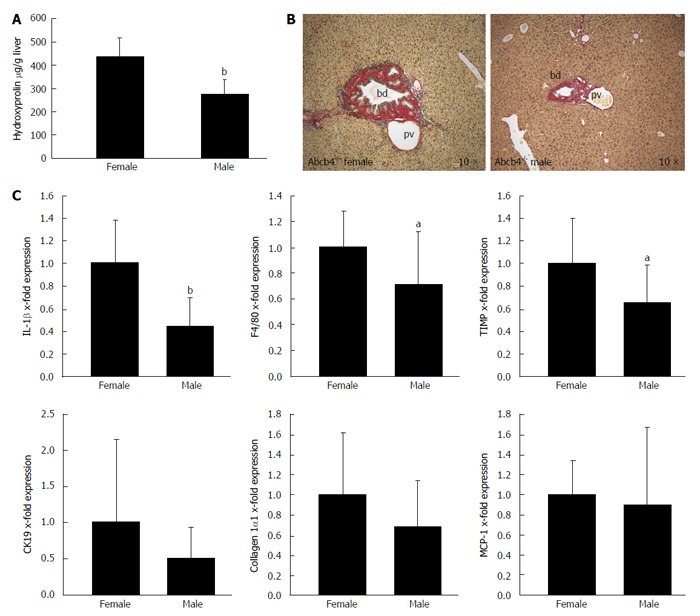
Gender differences in liver disease in ATP-binding cassette transporter b4-/- mice. Gender differences in liver fibrosis and liver inflammation were assessed with hydroxyproline measurement, Sirius red staining and quantitative real-time PCR in male and female Abcb4-/- mice between 6 and 13 mo of age. A: The hepatic hydroxyproline content of liver homogenates (μg/g liver) was lower in male animals than in female animals ( = 13-15; bP < 0.001; Mann-Whitney U-test); B: Sirius-red staining illustrates the gender differences regarding fibrosis in Abcb4-/- mice. Images were taken from 9- to 10-mo-old animals (original magnification, 10 ×; bd: bile duct; pv: portal vein); C: Gene expression was assessed via quantitative real-time PCR and was normalised for 18 s as a housekeeping gene. Expressions were normalised against the means of female mice. The hepatic IL-1β and F4/80 mRNA expression levels as markers for hepatic inflammation were significantly lower in male Abcb4-/- mice than in female animals (n = 12-15; bP < 0.001; aP < 0.05; Mann-Whitney U-test). The mRNA expression levels of the profibrotic gene TIMP-1 were also significantly lower in male Abcb4-/- mice than in female Abcb4-/- mice (n = 13-15; aP < 0.05; Mann-Whitney U-test). PCR: Polymerase chain reaction; TIMP-1: Tissue inhibitor of metalloproteinase-1; Abcb4-/-: ATP-binding cassette transporter b4-/-; IL-1: Interleukin-1; CK19: Cytokeratin 19; MCP-1: Monocyte chemotactic protein-1.
The mRNA expression of IL-1β and F4/80 as markers of hepatic-inflammation was 56% (Figure 1C; 1 ± 0.38 vs 0.44 ± 0.26 fold; n = 13-15; P < 0.001; Mann-Whitney U-test) and 29% lower (Figure 1C; 1 ± 0.28 vs 0.71 ± 0.41 fold; n = 12-15; P < 0.05; Mann-Whitney U-test) in the liver tissue of male animals than in the liver tissue of female animals.
No gender differences were observed regarding the hepatic mRNA expression of collagen 1α1, cytokeratin 19 and monocyte chemotactic protein-1; n = 13-15; Mann-Whitney U-test).
Additionally, no gender differences in serum biochemistry were observed regarding the bilirubin, ALT, AST, and AP levels (data not shown; n = 17-21; Mann-Whitney U-test). Furthermore, there were no sex differences regarding hepatic mitotic activity in Ki-67 immunohistochemistry (data not shown; n = 15; Mann-Whitney U-test).
In summary, we identified a lower grade of liver fibrosis in male than in female animals as illustrated by lower hydroxyproline levels and lower hepatic mRNA expression levels of the profibrogenic gene TIMP-1. Furthermore, lower fibrosis in male animals was associated with the reduced mRNA expression of the pro-inflammatory genes IL-1β and F4/80. These findings may support a potential role of the IL-1 pathway in the progression of liver injury to liver fibrosis.
IL-1β exerts proliferative effects in murine HSCs, while proliferation is reduced by the IL-1 receptor antagonist Anakinra in vitro
The importance of IL-1β in stellate cell proliferation was reported previously[15]. This work implicates inhibition of the IL-1 pathway as a potential target in the treatment of liver fibrosis. However, blockade of this target by the clinically available IL-1 receptor antagonist Anakinra has not been tested yet regarding HSC pathophysiology and liver fibrosis.
We addressed our in vitro experiments to investigate whether Anakinra could inhibit IL-1β-induced HSC proliferation.
In a first step, we evaluated the possible toxicity of Anakinra in primary murine HSCs using the FDH assay. This revealed no toxic effects of Anakinra at the tested doses, including the chosen dose of 2.5 μg/mL (data not shown, n = 3).
Furthermore, we observed that IL-1β increased the proliferation of murine HSCs [Figure 2A; 0.94 ± 0.72 (arbitrary units) A.U. in untreated controls, 1.12 ± 0.80 A.U. at an IL-1β concentration of 0.1 ng/mL and 1.18 ± 0.73 A.U. at an IL-1β concentration of 1 ng/mL in samples from n = 6 donor animals; general effect of IL-1β stimulation P < 0.001; control vs 0.1 ng/mL IL-1β P < 0.01; control vs 1 ng/mL IL-1β P < 0.001; ANOVA]. Due to the sufficient induction of HSC proliferation by IL-1β, the toxicity assay was not required for IL-1β.
Figure 2.
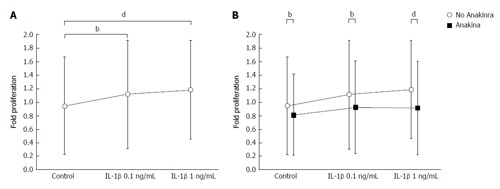
Interleukin-1β exerts proliferative effects in primary murine hepatic stellate cells, while Anakinra reduces hepatic stellate cell proliferation in vitro. The effects of IL-1β ± the IL-1 receptor antagonist Anakinra on the proliferation of murine HSCs were tested in vitro. A and B: The effects on HSC proliferation were examined using the BrdU assay after stimulation of cells with vehicle, and IL-1β at 0.1 and 1 ng/mL in the presence and absence of Anakinra (2.5 μg/mL), respectively (samples from n = 6 donor animals, bP < 0.01; dP < 0.001; ANOVA). HSC: Hepatic stellate cells; IL-1: Interleukin-1; ANOVA: Analyses of variance.
Proliferation of murine HSCs was reduced significantly by treatment with 2.5 μg/mL Anakinra (Figure 2B; 0.81 ± 0.60 A.U. in untreated controls, 0.92 ± 0.68 A.U. at an IL-1β concentration of 0.1 ng/mL, and 0.91 ± 0.69 A.U. at an IL-1β concentration of 1 ng/mL; in sample from n = 6 donor animals; general effect of Anakinra: P < 0.001; effect of Anakinra without IL-1β stimulation: P < 0.01; effect of Anakinra with 0.1 ng/mL IL-1β stimulation: P < 0.01; effect of Anakinra with 1.0 ng/mL IL-1β stimulation: P < 0.001; ANOVA), indicating a therapeutic effect of Anakinra on the proliferation of murine HSCs and, thereby, on liver fibrosis.
Taken together, Anakinra revealed promising anti-fibrotic effects in vitro.
Anakinra has no therapeutic effects in Abcb4-/- mice
The association of the degree of liver fibrosis and IL-1β expression in Abcb4-/- mice together with our in vitro data suggest a therapeutic effect of IL-1 antagonism on liver fibrosis in vivo. Therefore, we tested the therapeutic effects of Anakinra, a clinically approved IL-1 receptor antagonist, on biliary fibrosis and hepatic injury in female Abcb4-/- mice.
The hepatic hydroxyproline levels as a marker of liver fibrosis were lower in untreated Wt animals than in untreated Abcb4-/- mice (Figure 3A; n = 11; 82 ± 30 μg/g vs 227 ± 55 μg/g liver; P < 0.01; Mann-Whitney U-test) but were unaffected by Anakinra treatment (Figure 3A; n = 10-11; Mann-Whitney U-test). In accordance with this, we found differences regarding the serum parameters ALT (Figure 3A; 89 ± 87 U/L vs 454 ± 133 U/L; n = 8-10; P < 0.01; Mann-Whitney U-test) and AP (Figure 3A; 92 ± 17 U/L vs 530 ± 133 U/L; n = 8-10; P < 0.01; Mann-Whitney U-test) between the untreated Wt animals and Abcb4-/- mice but no effects of the treatment with Anakinra were observed (Figure 3A; n = 10-11; Mann-Whitney U-test).
Figure 3.
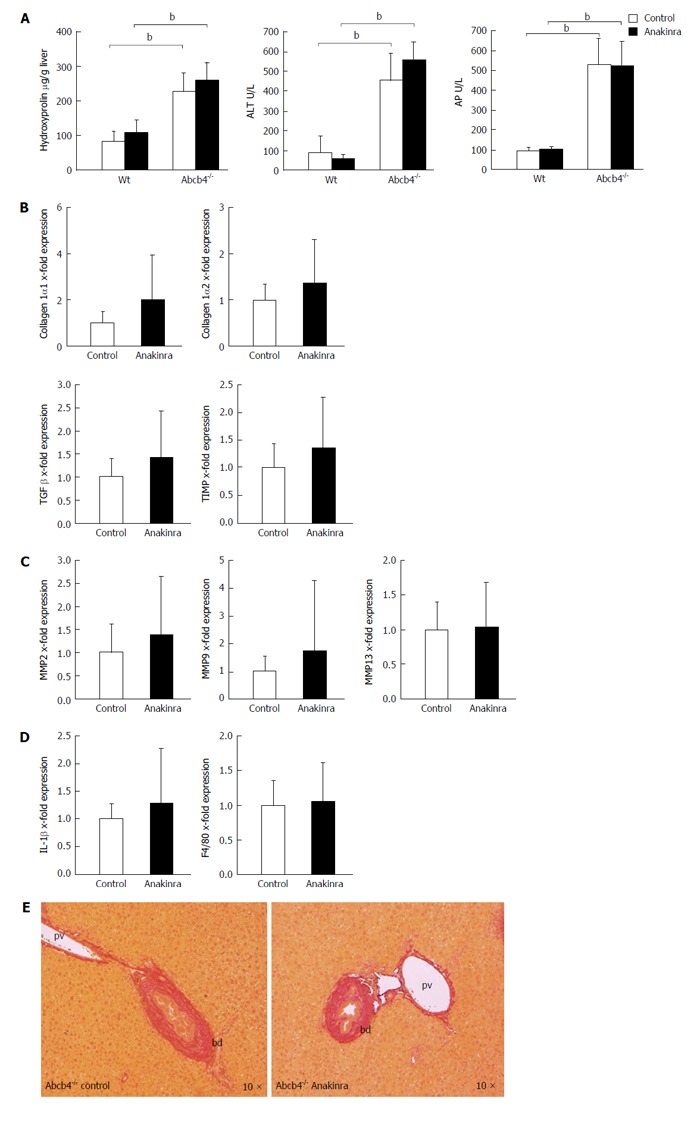
Anakinra does not reveal therapeutic effects on liver injury in ATP-binding cassette transporter b4-/- mice. Eight-week-old female Abcb4-/- animals were treated with daily intraperitoneal injections of saline (control) as vehicle or Anakinra (1 mg/kg body-weight) for 4 wk. A: The levels of hepatic hydroxyproline, alanine aminotransferase (ALT), and alkaline phosphatase (AP) were determined as described previously (n = 8-11, bP < 0.01; Mann-Whitney U-test). Gene expression was assessed via quantitative real-time PCR using glyceraldehyde-3-phosphate dehydrogenase as the housekeeping gene. Data were normalised against the means of the controls (saline); B: No alterations in the profibrotic genes were found [n = 10-11; not significant (n.s.); Mann-Whitney U-test]; C: No alterations in the antifibrotic genes were found (n = 10-11; n.s.; Mann-Whitney U-test); D: Anakinra treatment did not result in a significant change in the mRNA expression of the pro-inflammatory gene IL-1β or F4/80 (n = 10-11, n.s., Mann-Whitney U-test); E: The Sirius-red staining illustrates the absence of antifibrotic effects (original magnification 10 ×). bd: Bile duct; pv: Portal-vein; PCR: Polymerase chain reaction; TIMP: Tissue inhibitor of metalloproteinase; Abcb4-/-: ATP-binding cassette transporter b4-/-; IL: Interleukin; MMP: Matrix metalloproteinase; TGF: Transforming growth factor.
In a next step, we performed genetic profiling of fibrosis-related genes. Here, we found no influence of Anakinra on the profile of the profibrotic genes collagen 1α1, collagen 1α2, tissue growth factor-β, and TIMP-1 (Figure 3B; n = 10-11; Mann-Whitney U-test) between the groups.
In line with this finding, Anakinra treatment did not influence the expression of genes encoding the matrix-degrading enzymes matrix metalloproteinases 2, 9, and 13 (Figure 3C; n = 10-11; Mann-Whitney U-test).
The mRNA expression levels of IL-1β and F4/80 as indicators of hepatic inflammation were also unaffected by Anakinra treatment (Figure 3D; n = 10-11; Mann-Whitney U-test).
These missing antifibrotic effects were illustrated by Sirius red staining revealing no differences regarding periportal fibrosis (Figure 3E).
In summary, the in vivo administration of Anakinra showed no therapeutic effects on established fibrosis and hepatic injury in Abcb4-/- mice.
DISCUSSION
Few treatment options are available to prevent disease progression to liver fibrosis and cirrhosis in chronic cholestatic liver disease such as PSC. In PSC, the frequently applied bile acid ursodeoxycholic acid (UDCA) failed to demonstrate a positive effect on the clinical outcome[25], despite the beneficial effects of UDCA on serum biochemistry. Consequently, the American Association for the Study of Liver Disease no longer recommends the use of UDCA in PSC[2]. The loss of this therapeutic hope aggravates the need for an effective drug to treat PSC.
In the present study, we identified differences in the levels of hepatic fibrosis between female and male Abcb4-/- mice. Hepatic fibrosis was more aggravated in female mice as assessed by the determination of the liver hydroxyproline content, a finding that is in line with that of previous studies, which identified a more severe histological phenotype in female animals[26]. Here, the pronounced phenotype in females was accompanied by higher mRNA expression levels of IL-1β, F4/80, and TIMP-1.
TIMP is a well-known profibrotic player in the regulation of matrix degradation during fibrogenesis and resolution via the inhibition of matrix metalloproteinases[27] but also via its anti-apoptotic effects on hepatic myofibroblasts[28,29].
Therefore, the different TIMP levels might be a crucial factor for the observed gender differences in the degree of fibrosis. Hepatic inflammation, the role of chemokines, cytokines[30] and macrophages in the context of liver fibrosis are broadly discussed[31]. Macrophages may produce a wide spectrum of cytokines, including IL-1β, which are relevant for the pro-fibrogenic nature of HSCs[31-33]. Therefore, we hypothesised that the higher degree of fibrosis in female Abcb4-/- mice may be related to a more pronounced IL-1-driven hepatic inflammation and that the observed TIMP-1 alterations could reflect a consequence of inflammation. Thus, the IL-1 pathway might be a potential therapeutic target in PSC and fibrosis.
Supporting our hypothesis, we found that IL-1β stimulation exerts proliferative effects also on murine HSCs. These findings are in accordance with previously published data in rat HSCs[15]. Subsequently, we showed that the HSC proliferation was reduced by applying the clinically approved IL-1 receptor antagonist Anakinra. Because the proliferation of HSCs is a trigger of hepatic fibrosis[14], these effects might be of consequence for disease progression in vivo.
In light of recently published data showing that IL-1 receptor antagonism ameliorates inflammasome-dependent alcoholic steatohepatitis in mice[34], our in vitro findings led us to test the efficacy of medical IL-1 receptor antagonism to prevent liver damage and fibrosis in the Abcb4-/- model.
We applied Anakinra at an established dose (1 mg/kg body-weight)[23] for 4 wk. This time span has been established to be sufficient to detect the therapeutic effects in the Abcb4-/- model[20,35].
However, in the chosen setting, we found no effects on hepatic injury and fibrosis in this study. The serum markers of liver damage and cholestasis, as well as the analysis of liver tissue for fibrotic reaction, were unchanged.
This might be due to the distinctive pathophysiology of fibrosis in the Abcb4-/- model. The absence of phospholipids in Abcb4-/- mice results in a “toxic bile” constitution that leads to hepatic injury with scarring and obstruction of the biliary tree, finally causing cholestasis[36]. It is thought that portal myofibroblasts are primarily affected by the regurgitation of bile in this model[18]. These cells seem to rapidly acquire an activated phenotype in the early stages of biliary fibrosis[37] and might be the primary effectors of periportal fibrosis, which is the main type of fibrosis in the Abcb4-/- mouse[17,18]. This is also reflected by the previously observed increasing number in α-SMA-positive cells per portal field of Abcb4-/- over time[18]. Our in vitro studies revealed the effects on HSCs, possibly might have different characteristics than portal myofibroblasts[37]. This could explain the missing transferability of the in vitro effects in HSCs on the in vivo situation in the Abcb4-/- model. Thus, it seems likely that the IL-1-dependent effects may be of pathophysiologic relevance in other types of hepatic fibrosis that are primarily caused by the activation and proliferation of perisinusoidal HSCs, a situation that might be different from cholestatic fibrosis. One might also suspect that our results suggest that multiple pathways need to be considered for the therapeutic intervention of different types of liver fibrosis.
The observation that conventional immunosuppressive treatment has no therapeutic effect on PSC[2] is in accordance with the missing effects of our selective and putatively well-tolerable immunosuppressive approach by inhibition of the IL-1 pathway.
Taken together, this study demonstrated sex differences regarding hepatic inflammation and hepatic fibrosis in the Abcb4-/- model. Our in vitro experiments suggest the relevance of the IL-1 pathway in HSC proliferation and implicate the therapeutic potential of IL-1 antagonism. However, the treatment with the clinically approved IL-1 receptor antagonist Anakinra did not result in the amelioration of the hepatic phenotype in the Abcb4-/- model after 4 wk of treatment. Further studies might identify a therapeutic impact of this pathway in other types of liver fibrosis.
ACKNOWLEDGMENTS
Parts of this work were presented at the annual meeting of the Bavarian Society for Gastroenterology 2015 in Garmisch-Partenkirchen. The statistical methods of this study were reviewed by Christoph Glasmacher, an external biostatistician (Christoph Glasmacher - Biometrics and SAS-Programming for Clinical Research).
COMMENTS
Background
Hepatic stellate cells (HSCs) are the major source of hepatic extracellular matrix deposition and crucially participate in the pathogenesis of liver fibrosis. In addition to other factors, enhanced proliferation of this cell type is thought to be an important profibrotic mechanism. In this regard, a recent study found that interleukin-1β (IL-1)β induces the proliferation of rat HSCs. Furthermore, IL-1 was identified as a factor in the progression of liver injury to fibrosis. Gender differences regarding hepatic injury in ATP-binding cassette transporter b4-/- (Abcb4-/-) mice were reported previously. However, differences in liver fibrosis were not evaluated in detail so far. In this study, the authors describe gender differences regard liver fibrosis in Abcb4-/- animals. Interestingly these findings are associated with coherent alterations of the IL-1β mRNA expression levels. Therefore, they hypothesised that the clinically approved IL-1 receptor antagonist Anakinra could reduce the proliferative effects of IL-1β in HSCs and improve fibrosis in Abcb4-/- mice. They also hypothesised that blocking the IL-1 pathway during liver fibrosis might be a therapeutic approach in this chronic cholestatic liver disease.
Research frontiers
The proliferative effects of IL-1β on HSCs were described in former studies. However, a therapeutic approach via the clinically approved IL-1 receptor antagonist Anakinra was not investigated thus far regarding its efficacy in liver fibrosis.
Innovations and breakthroughs
This is the first study to evaluate the efficacy of the clinically approved IL-1 receptor antagonist Anakinra on HSC proliferation in vitro. They report on the antiproliferative effects of this agent on HSC proliferation and identified a potential therapeutic approach. Furthermore, this study reported on gender differences regarding liver fibrosis in the Abcb4-/- mouse model.
Applications
They in vitro findings support the importance of the IL-1 pathway in HSC proliferation. The effects of Anakinra on HSC proliferation suggest a therapeutic approach in liver fibrosis.
Terminology
HSCs are the major source of hepatic extracellular matrix deposition and crucially participate in the pathogenesis of liver fibrosis. The Abcb4-/- mouse model is a preclinical model for primary sclerosing cholangitis and biliary liver fibrosis.
Peer-review
This paper shows that the IL-1 signaling antagonist Anakinra can influence mouse HSC in vitro but not fibrosis in the Abcb4 mouse. Additional novel data is that female mice in this model are more affected than male mice.
Footnotes
Supported by The Münchener Medizinische Wochenschrift (MMW); B. Braun-Stiftung (to Reiter FP); the Deutsche Forschungsgemeinschaft (HO 4460/2-1 to Hohenester S and RU 742/6-1 to Rust C).
Institutional review board statement: This study includes no data or material from patients. We confirm that all of the required permissions for this study were obtained from our local authorities as mentioned in the Institutional animal care and use committee statement.
Institutional animal care and use committee statement: We confirm that the animals for our studies were kept according to the local regulations. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Regierung von Oberbayern; protocol number No.: 55.2.1.54-2532-104-11. All of the institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict-of-interest statement: All of the authors declare that they have no conflict of interest.
Data sharing statement: The technical appendix, statistical code, and dataset are available from the corresponding author at florian.reiter@med.uni-muenchen.de.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 11, 2015
First decision: September 18, 2015
Article in press: March 9, 2016
P- Reviewer: Fan XM, Gorrell MD, Penkova-Radicheva MP, Xu J S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
References
- 1.Farrant JM, Hayllar KM, Wilkinson ML, Karani J, Portmann BC, Westaby D, Williams R. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710–1717. doi: 10.1016/0016-5085(91)90673-9. [DOI] [PubMed] [Google Scholar]
- 2.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–2055. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 4.Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol. 2007;20 Suppl 1:S15–S30. doi: 10.1038/modpathol.3800684. [DOI] [PubMed] [Google Scholar]
- 5.Dilger K, Hohenester S, Winkler-Budenhofer U, Bastiaansen BA, Schaap FG, Rust C, Beuers U. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133–140. doi: 10.1016/j.jhep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Hohenester S, Gates A, Wimmer R, Beuers U, Anwer MS, Rust C, Webster CR. Phosphatidylinositol-3-kinase p110γ contributes to bile salt-induced apoptosis in primary rat hepatocytes and human hepatoma cells. J Hepatol. 2010;53:918–926. doi: 10.1016/j.jhep.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, Beuers U. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 8.Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem. 2009;284:2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
- 9.Denk G, Omary AJ, Reiter FP, Hohenester S, Wimmer R, Holdenrieder S, Rust C. Soluble intracellular adhesion molecule, M30 and M65 as serum markers of disease activity and prognosis in cholestatic liver diseases. Hepatol Res. 2014;44:1286–1298. doi: 10.1111/hepr.12304. [DOI] [PubMed] [Google Scholar]
- 10.Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387–392. doi: 10.1016/s1590-8658(02)80033-0. [DOI] [PubMed] [Google Scholar]
- 11.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 12.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 13.Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 14.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaping Z, Ying W, Luqin D, Ning T, Xuemei A, Xixian Y. Mechanism of interleukin-1β-induced proliferation in rat hepatic stellate cells from different levels of signal transduction. APMIS. 2014;122:392–398. doi: 10.1111/apm.12155. [DOI] [PubMed] [Google Scholar]
- 16.Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1324–G1331. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 20.Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161:207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- 22.Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- 23.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 24.Edwards CA, O’Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 25.Ali AH, Carey EJ, Lindor KD. Current research on the treatment of primary sclerosing cholangitis. Intractable Rare Dis Res. 2015;4:1–6. doi: 10.5582/irdr.2014.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Nieuwerk CM, Groen AK, Ottenhoff R, van Wijland M, van den Bergh Weerman MA, Tytgat GN, Offerhaus JJ, Oude Elferink RP. The role of bile salt composition in liver pathology of mdr2 (-/-) mice: differences between males and females. J Hepatol. 1997;26:138–145. doi: 10.1016/s0168-8278(97)80020-7. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran P, Iredale JP. Liver fibrosis: a bidirectional model of fibrogenesis and resolution. QJM. 2012;105:813–817. doi: 10.1093/qjmed/hcs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–939. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 29.Ismail MH, Pinzani M. Reversal of hepatic fibrosis: pathophysiological basis of antifibrotic therapies. Hepat Med. 2011;3:69–80. doi: 10.2147/HMER.S9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Affò S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, Millán C, Coll M, Caviglia JM, Arroyo V, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782–1792. doi: 10.1136/gutjnl-2013-306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15–S24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 34.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiter FP, Hohenester S, Nagel JM, Wimmer R, Artmann R, Wottke L, Makeschin MC, Mayr D, Rust C, Trauner M, et al. 1,25-(OH)2-vitamin D3 prevents activation of hepatic stellate cells in vitro and ameliorates inflammatory liver damage but not fibrosis in the Abcb4(-/-) model. Biochem Biophys Res Commun. 2015;459:227–233. doi: 10.1016/j.bbrc.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 36.Trauner M, Fickert P, Halilbasic E, Moustafa T. Lessons from the toxic bile concept for the pathogenesis and treatment of cholestatic liver diseases. Wien Med Wochenschr. 2008;158:542–548. doi: 10.1007/s10354-008-0592-1. [DOI] [PubMed] [Google Scholar]
- 37.Ramadori G, Saile B. Portal tract fibrogenesis in the liver. Lab Invest. 2004;84:153–159. doi: 10.1038/labinvest.3700030. [DOI] [PubMed] [Google Scholar]


