Abstract
Entrapment of middle cluneal nerves induces low back pain and leg symptoms. The middle cluneal nerves can become spontaneously entrapped where this nerve pass under the long posterior sacroiliac ligament. A case of severe low back pain, which was completely treated by release of the middle cluneal nerve, was presented. Entrapment of middle cluneal nerves is possibly underdiagnosed cause of low-back and/or leg symptoms. Spinal surgeons should be aware of this clinical entity and avoid unnecessary spinal surgeries and sacroiliac fusion. This paper is to draw attention by pain clinicians in this unrecognized etiology.
Keywords: Entrapment neuropathy, Superior cluneal nerve, Middle cluneal nerve, Sacroiliac joint, Low back pain, Neuropathic pain
Core tip: A case of severe low back pain, which was completely treated by release of the middle cluneal nerve, was presented. Clunealgia is underdiagnosed cause of low back pain and leg pain. The middle cluneal nerve may be entrapped where this nerve pass under or through the long posterior sacroiliac ligament.
INTRODUCTION
Recently, clunealgia has become known as an under-diagnosed cause for chronic low back pain (LBP) or leg pain[1-5]. Trescot[2] stated that cluneal neuralgia is more commonly the result of an entrapped nerve rather than a nerve injury resulting from iliac crest bone harvest. Kuniya et al[3] reported that patients with superior cluneal nerve (SCN) entrapment occurs where pierce fascial attachment at posterior iliac crest. SCN disorders comprised 12% of all patients presenting with a chief complaint of LBP and/or leg symptoms in their clinic and approximately 50% of SCN disorder patients had leg pain and/or tingling.
The concept of a relationship between the cluneal nerve and LBP is not new. A relationship between the cluneal nerve and LBP was sporadically reported several decades ago[6,7]. The first detailed description was made by Strong et al[7] in 1957. Deafferentation of the SCN and/or middle cluneal nerve (MCN) was attempted in 30 patients because these nerves were considered to cause LBP with or without referred leg pain. Of 30 patients, five had referred pain in their leg in the S1 and/or S2 area and had deafferentation of the MCN with favorable outcomes.
Maigne et al[8] and Lu et al[4] performed cadaveric dissections to study anatomy of the SCN and concluded that that the medial branch of the SCN consistently passed through an osteofibrous tunnel and might be spontaneously entrapped in the tunnel. Following these anatomical studies, several surgeons reported successful surgical outcomes of SCN release[3,5,9]. However, surgical reports of release are limited to SCN entrapment. Until now, MCN entrapment has not yet been reported in English literature. This paper is to firstly present a case of MCN entrapment and to draw attention by pain clinicians in MCN entrapment.
CASE REPORT
In April 2013, a 48-year-old woman presented complaining of LBP and buttock pain radiating to both legs that had gradually developed over 10 years. L4-5 discectomy performed at another hospital two years before resulted in no improvement. The pain was continuous and severe even with long-term daily use of tramadol 225 mg/acetaminophen 1950 mg, pregabalin 50 mg and loxoprofen sodium 180 mg. The visual analog scale (VAS) score was 67 mm and the Roland-Morris Disability Questionnaire (RDQ) score was 18. A neurologic examination revealed no sensory or motor disturbance in her legs. Lumbar motion was greatly limited in all directions because of pain (Appendix, video 1). The finger floor distance in flexion was 50 cm. Palpation of the superior SCN tender point, located 7 cm laterally to the midline on the bilateral iliac crest[7], replicated the postero-lateral aspect of calf pain. She also had significant tender points approximately 1.5 cm caudal to the palpable margin of the bilateral posterior superior iliac spine, by the lateral sides of the long posterior sacroiliac ligament (LPSL). These loci were along the running course of the MCN as described in an anatomical report by Tubbs et al[10]. Palpation of MCN tender points provoked mid-posterior thigh pain. Repetitive infiltration of a local anesthetic, Lidocaine, into each tender point consistently resulted in clear improvement of symptoms for three hours.
The patient was informed that release was previously performed exclusively for SCN entrapment and had never been applied for MCN entrapment. She gave their informed consent to undergo surgical decompression. In May 2013, microscopic SCN and MCN releases were attempted. Surgeries were approved by the Institutional Ethics Committee of our institution. Surgery was performed bilaterally under general anesthesia with the patient in the prone position. An oblique 10 cm skin incision was made over the iliac crests. Being careful not to injure nerve branches passing through subcutaneous tissues, the superficial layer of the thoracolumbar fascia was opened. Two branches of the SCN were identified within 5 cm above the iliac crest and were seen to emerge from the lateral margin of the deep layer of the thoracolumbar fascia. These SCN branches were traced in a caudal direction until they passed over the iliac crest. In agreement with a recent anatomical study, the two medial branches of the SCN where they pierce the thoracolumbar fascia over the iliac crest were found to be entrapped in adhesions. A thin branch of the MCN perforating the thoracolumbar fascia was identified just medial to the posterior superior iliac spines. Although obvious entrapment was not observed, the perforating orifices were opened.
Within one week following surgery, the patient reported that her pain had completely disappeared around the upper iliac crests, but remained around the LPSL on both sides. Palpation on the LPSL consistently induced LBP and leg tingling radiating from the buttocks to the calves on both sides. Injections around the LPSL were repeated every month. Each time, the patient reported reappearance of leg tingling during the block procedure and, soon after, complete improvement in LBP and leg tingling that continued for three days. Consequently, blocks were repeated six times over six months without substantial permanent change in LBP. The VAS score was 50 mm at six months after surgery. Near-full range of flexion was obtained with no pain reappearance, but lumbar extension was still severely limited.
In an attempt to eliminate remaining pain, in December 2013, revision surgery was done. Previous operative incisions were reopened. After gluteal muscle splitting approach, the bilateral MCNs were explored where the nerves penetrate the LPSL (Figure 1). Proximally, the nerves possibly arose from the S2 foramen. The MCNs were decompressed by excising the LPSL where the nerve penetrates the ligament. After revision, pain dramatically improved, precluding need for any medication. The patient had no limitation in lumbar motion. The VAS score at eight months after revision was 0 mm and the RDQ score was 1.
Figure 1.
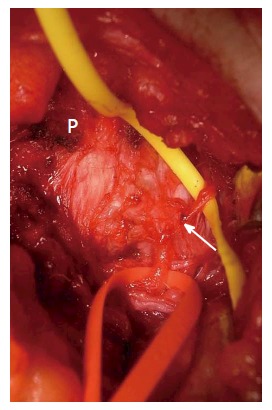
Photo taken during the surgical release of a left-side middle cluneal nerve. Medially to the posterior superior iliac crest (P), the MCN is identified passing under the superficial layer of the long posterior sacroiliac ligament. The nerve is seen to be entrapped under the deeper layer of the long posterior sacroiliac ligament where it penetrates the ligament (arrow). The yellow and red tapes have been used to lift the proximal and distal portions of MCN branch, respectively. MCN: Middle cluneal nerve.
DISCUSSION
The MCN is composed of sensory branches of the dorsal rami of S1 to S3 foramina and travels below the PSIS in an approximately horizontal course to supply the skin overlying the posteromedial area of the buttock[10-12]. The evidence that predominantly the S1 and S2 lateral branches may explain why MCN disorder could cause leg symptoms in posterior thigh to calf.
Tubbs et al[10] studied anatomy running course of MCN and concluded that the MCN would be less likely to become entrapped because MCN travels superficial to the LPSL. On the other hand, anatomical studies by Horwitz[13], Grob et al[12], and McGrath et al[14] showed that the primary and secondary loops of the posterior sacral nerve plexus passed through or underneath the LPSL. They suggested entrapment of the penetrating nerves within or under the ligament is a potential cause for LBP and peripartum pelvic pain.
A diagnosis of SCN/MCN entrapment was made by palpation of the iliac crest or LPSL resulting in marked tenderness and provocation of symptoms, and by pain relief after local anesthetic injection. The SCN tender point was on the posterior iliac crest approximately 70 mm from the midline and 45 mm from the PSIS[3]. The MCN tender point was on the LPSL within 40 mm caudal to the PSIS (Figure 2).
Figure 2.
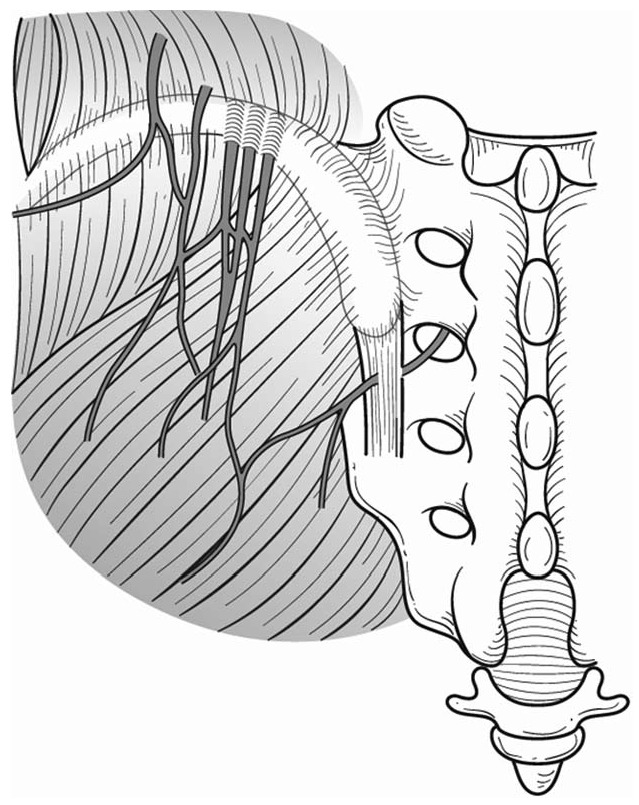
Schematic illustration of typical running courses and entrapment of superior and middle cluneal nerves. Multiple branches of the superior cluneal nerve may be entrapped where they pierce the thoracolumbar fascia over the iliac crest. Middle cluneal nerve may be entrapped where this nerve pass under or through the long posterior sacroiliac ligament.
Pain due to MCN entrapment may be treated as sacroiliac joint pain. Although sacroiliac joint pain remains a controversial subject, it is thought to cause 15% to 30% of LBP and is often associated with buttock to lower extremity symptoms[15]. There are no medical history or physical examination findings consistently capable of identifying sacroiliac joint pain[16]. The physical examination tests, such as Patrick’s test or Gaenslen’s test, have weak predictive value[15]. Radiological imaging contributes little to diagnosis[15]. The current gold standard for the diagnosis is fluoroscopically guided sacroiliac joint blocks[15]. Fortin et al[17] analyzed contrast extravasation patterns during 76 sacroiliac joint arthrograms by using computerized tomography and found dorsal leakage around LPSL in 18 cases (24%) and dorsal leakage into the S1 foramen in 6 (8%) The LPSL is a significant posterior ligamentous structure, resisting shearing of the sacroiliac joint and is a potential pain generator[14,18]. Murakami et al[19] compared the effect of injections into the intraarticular space and periarticular region around the LPSL in patients with sacroiliac joint pain. The injection around the LPSL was effective in all 25 patients, whereas the intraarticular injection was effective in only nine out of 25 patients (36%). Furthermore, all 16 patients without pain relief after the intraarticular injection reported almost complete pain relief after injection around the LPSL. Fortin et al[16] stated that sacroiliac joint patients could localize their pain with one finger and the area pointed to was immediately inferomedial to the posterior superior iliac spine within 1 cm. Murakami et al[20] observed a positive effect with periarticular sacroiliac joint block in 18 patients out of 25 patients who indicated the main site of pain within 2 cm of the posterior superior iliac spine. These results suggest that sacroiliac joint pain can originate from the LPSL[20].
LBP is one of the most common problems that most people suffer at some point in their life. There are many sources of LBP. In most LBP patients, the exact cause of LBP is not clear. Thus, one of the most difficult tasks with LBP is to identify the actual pain generator. Large epidemiological studies show that 20% to 37% of patients with back pain suffer from a neuropathic pain component[21]. So far, neuropathic pain is considered to be caused by lesions of nociceptive sprouts within the degenerated disc, mechanical compression of the nerve root, or by action of inflammatory mediators originating from the degenerative disc[22]. SCN/ MCN entrapment must not be forgotten as cause of neuropathic LBP.
CONCLUSION
MCN entrapment is underdiagnosed cause of low back pain and leg pain. For a structure to be considered as a potential source of pain, pain must be provoked by palpation or relieved by local anesthetic injection of the tender point around LPSL. Knowledge of this clinical entity would avoid unnecessary sacroiliac joint fusion and spinal surgeries.
Footnotes
Conflict-of-interest statement: The author has no conflict of interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 8, 2015
First decision: October 16, 2015
Article in press: December 21, 2015
P- Reviewer: Feher G, Peng BG S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Akbas M, Yegin A, Karsli B. Superior cluneal nerve entrapment eight years after decubitus surgery. Pain Pract. 2005;5:364–366. doi: 10.1111/j.1533-2500.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- 2.Trescot AM. Cryoanalgesia in interventional pain management. Pain Physician. 2003;6:345–360. [PubMed] [Google Scholar]
- 3.Kuniya H, Aota Y, Kawai T, Kaneko K, Konno T, Saito T. Prospective study of superior cluneal nerve disorder as a potential cause of low back pain and leg symptoms. J Orthop Surg Res. 2014;9:139. doi: 10.1186/s13018-014-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu J, Ebraheim NA, Huntoon M, Heck BE, Yeasting RA. Anatomic considerations of superior cluneal nerve at posterior iliac crest region. Clin Orthop Relat Res. 1998;(347):224–228. [PubMed] [Google Scholar]
- 5.Maigne JY, Doursounian L. Entrapment neuropathy of the medial superior cluneal nerve. Nineteen cases surgically treated, with a minimum of 2 years’ follow-up. Spine (Phila Pa 1976) 1997;22:1156–1159. doi: 10.1097/00007632-199705150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Drury BJ. Clinical evaluation of damage to the superior cluneal nerves. Am J Orthop Surg. 1968;10:102–106. [PubMed] [Google Scholar]
- 7.Strong EK, Davila JC. The cluneal nerve syndrome; a distinct type of low back pain. Ind Med Surg. 1957;26:417–429. [PubMed] [Google Scholar]
- 8.Maigne JY, Maigne R. Trigger point of the posterior iliac crest: painful iliolumbar ligament insertion or cutaneous dorsal ramus pain? An anatomic study. Arch Phys Med Rehabil. 1991;72:734–737. [PubMed] [Google Scholar]
- 9.Morimoto D, Isu T, Kim K, Imai T, Yamazaki K, Matsumoto R, Isobe M. Surgical treatment of superior cluneal nerve entrapment neuropathy. J Neurosurg Spine. 2013;19:71–75. doi: 10.3171/2013.3.SPINE12420. [DOI] [PubMed] [Google Scholar]
- 10.Tubbs RS, Levin MR, Loukas M, Potts EA, Cohen-Gadol AA. Anatomy and landmarks for the superior and middle cluneal nerves: application to posterior iliac crest harvest and entrapment syndromes. J Neurosurg Spine. 2010;13:356–359. doi: 10.3171/2010.3.SPINE09747. [DOI] [PubMed] [Google Scholar]
- 11.Sittitavornwong S, Falconer DS, Shah R, Brown N, Tubbs RS. Anatomic considerations for posterior iliac crest bone procurement. J Oral Maxillofac Surg. 2013;71:1777–1788. doi: 10.1016/j.joms.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Grob KR, Neuhuber WL, Kissling RO. [Innervation of the sacroiliac joint of the human] Z Rheumatol. 1995;54:117–122. [PubMed] [Google Scholar]
- 13.Horwitz TM. The anatomy of (a) teh lumbosacral nerve plexus - its relation to variations of vertebral segmentation, and (b) the posterior sacral nerve plexus. Anat Rec. 1939;74:91–107. [Google Scholar]
- 14.McGrath MC, Zhang M. Lateral branches of dorsal sacral nerve plexus and the long posterior sacroiliac ligament. Surg Radiol Anat. 2005;27:327–330. doi: 10.1007/s00276-005-0331-x. [DOI] [PubMed] [Google Scholar]
- 15.Vanelderen P, Szadek K, Cohen SP, De Witte J, Lataster A, Patijn J, Mekhail N, van Kleef M, Van Zundert J. 13. Sacroiliac joint pain. Pain Pract. 2010;10:470–478. doi: 10.1111/j.1533-2500.2010.00394.x. [DOI] [PubMed] [Google Scholar]
- 16.Fortin JD, Falco FJ. The Fortin finger test: an indicator of sacroiliac pain. Am J Orthop (Belle Mead NJ) 1997;26:477–480. [PubMed] [Google Scholar]
- 17.Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. AJNR Am J Neuroradiol. 1999;20:1429–1434. [PMC free article] [PubMed] [Google Scholar]
- 18.Vleeming A, Pool-Goudzwaard AL, Hammudoghlu D, Stoeckart R, Snijders CJ, Mens JM. The function of the long dorsal sacroiliac ligament: its implication for understanding low back pain. Spine (Phila Pa 1976) 1996;21:556–562. doi: 10.1097/00007632-199603010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12:274–280. doi: 10.1007/s00776-007-1126-1. [DOI] [PubMed] [Google Scholar]
- 20.Murakami E, Aizawa T, Noguchi K, Kanno H, Okuno H, Uozumi H. Diagram specific to sacroiliac joint pain site indicated by one-finger test. J Orthop Sci. 2008;13:492–497. doi: 10.1007/s00776-008-1280-0. [DOI] [PubMed] [Google Scholar]
- 21.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 22.Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185–190. doi: 10.1007/s11916-009-0032-y. [DOI] [PubMed] [Google Scholar]


