Abstract
Osteoporosis is a silent disease without any evidence of disease until a fracture occurs. Approximately 200 million people in the world are affected by osteoporosis and 8.9 million fractures occur each year worldwide. Fractures of the hip are a major public health burden, by means of both social cost and health condition of the elderly because these fractures are one of the main causes of morbidity, impairment, decreased quality of life and mortality in women and men. The aim of this review is to analyze the most important factors related to the enormous impact of osteoporotic fractures on population. Among the most common risk factors, low body mass index; history of fragility fracture, environmental risk, early menopause, smoking, lack of vitamin D, endocrine disorders (for example insulin-dependent diabetes mellitus), use of glucocorticoids, excessive alcohol intake, immobility and others represented the main clinical risk factors associated with augmented risk of fragility fracture. The increasing trend of osteoporosis is accompanied by an underutilization of the available preventive strategies and only a small number of patients at high fracture risk are recognized and successively referred for therapy. This report provides analytic evidences to assess the best practices in osteoporosis management and indications for the adoption of a correct healthcare strategy to significantly reduce the osteoporosis burden. Early diagnosis is the key to resize the impact of osteoporosis on healthcare system. In this context, attention must be focused on the identification of high fracture risk among osteoporotic patients. It is necessary to increase national awareness campaigns across countries in order to reduce the osteoporotic fractures incidence.
Keywords: Fracture prevention, Fracture risk, Fragility fracture, Osteoporosis, Hip fracture
Core tip: The osteoporosis burden is growing and 9 million fractures occur each year worldwide. Unfortunately, because of the underutilization of available preventive strategies, only a minority of women and men at high fracture risk are identified and successively referred for treatment. The aim of this review is to analyze the most important factors related to the enormous impact of osteoporotic fractures on population. Because early diagnosis is the key to reduce the impact of osteoporosis on healthcare system, attention must be focused on the identification of high fracture risk among osteoporotic patients.
INTRODUCTION
Osteoporosis has been defined as a systemic disease which affects the skeleton and is characterized by low bone mass, deterioration of microarchitecture of bone tissue and bone fragility increase with consequent susceptibility to fracture[1].
This bone pathology can be classified in primary or secondary forms. Primary osteoporosis is characterized by a progressive mineral bone lost as a function of people aging and it is influenced by changes of sex hormone. Instead, different pathologies as well as the use of specific medications, which affect skeletal health, can induce secondary osteoporosis. Primary form of osteoporosis comprises postmenopausal or senile disease (type I or type II respectively)[2]. Type I osteoporosis takes place in a subgroup of postmenopausal women, usually aged from 50 to 65 years, due to estrogen deficiency and consequent trabecular bone resorption. In this set of women fracture pattern mainly involves the spine and wrist. There is no evidence that postmenopausal bone loss itself causes any symptoms, and therefore, progressive bone loss has been called “the silent epidemic” or “silent thief”. The morbidity arises from the type of fracture sustained[3]. In senile osteoporosis, there is a balanced loss of both cortical and cancellous portions of bone tissue. Fractures of the hip, proximal humerus, tibia, and pelvis represent characteristic fractures of type II osteoporosis[4].
Although osteoporosis has long been considered a disease of women, an increase in age-related fractures has been observed also in men[5]. Nowadays, the number of males with osteoporosis is unknown, probably because of the infrequency of screening and controversies in bone mineral density (BMD) testing standards in men. Approximately the 50% of women and the 25% of men aged 50 and older will have an osteoporotic fracture in the lifetime[6]. Although many national and international organizations indicate to realize osteoporosis screening and treatment for men in their clinical guidelines, male osteoporosis remains recurrently not diagnosed and not treated[7,8].
The high socio-economic impact of osteoporosis is due to increased incidence of the disease, mortality and fracture-related costs. The occurrence of osteoporotic fractures is growing in several world areas as a consequence of the increased longevity of the population. Indeed, the number of hip fracture worldwide has reached 1.7 million by 1990[9,10]. In 2050, hip fractures could exceed 21 million[10,11]. In this context, attention must be focused on the identification of high fracture risk patients[12]. There is, therefore, the strong need to assess the best preventive methods and therapeutic approaches to contrast fracture widening accross populations. A large number of techniques can be used to assess the risk of fracture. In general, they fall into two major categories: Assessment of clinical risk factors (CRFs) and physical measurement of skeletal mass. Nowadays, the assessment of osteoporosis is based on bone density evaluation, and there are no satisfying clinical approaches, independent of BMD, for bone quality estimation[3].
The aim of this review is to analyze the main factors causing the huge impact of osteoporosis on the population, and to stress the importance of risk factors recognition and early identification of fracture risk in order to discriminate frail patients from non-frail patients. Currently, only a small number of high fracture risk patients are recognized and successively referred for therapy. In this context, the present report provides analytic evidences to assess the best practices in osteoporosis management as well as the indications for the adoption of a correct healthcare approach to decrease the socio-economic burden of osteoporosis.
FRACTURE RISK ASSESSMENT
Assessment of CRFs
A list of risk factors has been determined (Table 1) both for women and men. In several instances, exist a clear relationship between these risk factors and low bone density or other causes of osteoporotic fractures.
Table 1.
Clinical risk factors associated with increased risk of fragility fracture
| Risk factors associated with low BMD and fracture | Risk factors for falls |
| For woman as for men | |
| 1Increased age | Age |
| 1Low BMI | Environmental risk |
| 1Hystory of fragility fractures | Previous falls |
| 1Previous fragility fractures | Dehydratation |
| 1Parental hystory of fragility fractures | Depression |
| Recent falls | Poor vision |
| 1Premature menopause | Sarcopenia |
| 1Untrated hypogonadism | Urgent urinary incontinence |
| Poor neuromuscular function | Malnutrition |
| 1Prolonged immobilization and inactivity | Neurological risk factors |
| 1Alcohol intake | |
| 1Current smoking | |
| 1Glucocorticoids treatment (≥ 5 mg preadnisolone daily for 3 mo or more) | |
| 1Type I diabetes (and other endocrine disorders) | |
| Vitamin D insufficiency | |
| 1Rheumatoid arthritis (and other rheumatologic diseases) | |
| Aromatase inhibitor for breast cancer treatment | |
| Chemioterapy for breast cancer | |
| 1Thyroid disorders | |
| Cronic obstructive pulmonary disease | |
| Anorexia nervosa (and other hypogonadal states) | |
| Depressed mood | |
| Tricyclic antidepressant use | |
| Stroke | |
| IBD and other gastrointestinal disorders | |
| Organ transplantation | |
| Hematologic disorders | |
| Neurological and muscoloskeletal risks |
Adapted from Cosman et al[13] with modification.
CRFs for fracture risk assessment from tool FRAX®. BMD: Bone mineral density; CRFs: Clinical risk factors; IBD: Inflammatory bowel diseases; BMI: Body mass index.
In recent years, there have been a number of advances, principally in BMD measurement, osteoporosis diagnosis, fracture risk evaluation, the development of interventions that decrease risk of fractures and the creation of practice guidelines. Recently, a set of meta-analyses have been carried out to recognize CRFs to use in case finding strategies with or without the use of BMD. These are summarized below together with risk factors for falling because the majority of osteoporosis-related fractures derive from falls[13].
An important risk factor for hip fracture is low body mass index (BMI). Thus, the risk is nearly two-fold increased for individuals with a BMI of 25 kg/m2 vs 20 kg/m2[14-17]. Numerous studies show that a history of fragility fracture represent an important risk factor for further fracture independently of BMD[1,18,19]. It was shown that the risk of fracture is around doubled in the presence of a prior fracture[1,20].
Early menopause (before age 45 years), both natural and surgically induced, leads to an increased risk of mortality and fragility fractures[21] because these women are exposed to a hypogonadal state for a longer time[3].
Similarly, many abnormalities of menstrual function as well as late menarche and primary or secondary amenorrhea might also contribute to low BMD and therefore also increase the risk of osteoporosis[3,22,23]. Hypogonadism also occurs in a small proportion of men and might led to bone loss[24] and fractures[25]. Postmenopausal women are capable of producing adrenal steroids, of which androstenedione is converted to estrogens in adipose tissue. This might explain why thin women are at greater risk than their heavier counterparts, and possibly because smoking, which decreases appetite and body fat, is a risk factor[3]. There might be, however, additional factors related to smoking, and there is some evidence that smoking might accelerate the peripheral metabolism of exogenously administered estrogen[26]. Moreover, because female cigarette smokers are thinner than non-smokers, they have an earlier natural menopause[3].
On one hand crucial role for estrogen in bone loss is indicated by the increasing resorption of bone at menopause[5,27,28]; on the other hand, in men, although total serum testosterone and estrogen levels not vary with increasing age, the bioavailability fractions decrease progressively to 30%-50% of the young adults average after 80 years of age[5,29].
Lack of vitamin D is another risk factor. It is well known that vitamin D, calcium and protein insufficiency is highly frequent in the elderly. Vitamin D deficiency in adults can aggravate osteopenia and osteoporosis, and causes osteomalacia and muscle weakness, increasing the risk of fracture. Vitamin D can be obtained from diet or exposure to sunlight. Solar ultraviolet B radiation (wavelength, 290-315 nm) can convert 7-dehydrocholesterol to previtamin D3 and consequently to vitamin D3 by penetrating the skin. Vitamin D deficiency and bone fragility are also common in some countries such as Iran, where conservative cultural codes encourage body coverage and so limit sun exposure[30].
In addition to vitamin D insufficiency, hyperthyroidism and secondary hyperparathyroidism also take part in particular to age-related cortical bone loss in the elderly[31]. Other probable pathogenetic aspects comprise reduced serum levels of insulin-like growth factors[32]. Age-related bone loss and reduced bone strength (due to the imbalance between the activities of osteoblasts and the osteoclasts) are believed to start in both men and women from the beginning of the 5th decade until the end of life[33]. It is also possible that some early factors (i.e., perinatal nutrition) have affected the successive late-life risk of fractures in adults[8,34,35].
Bone loss is due to many disorders, such as insulin-dependent diabetes mellitus and Cushing’s disease. Myelomatosis might be present with osteoporotic crush. Rarer causes of osteoporosis include osteogenesis imperfecta, malabsorption, chronic renal failure and some drugs. However, all these disorders are comparatively rare and have relatively little impact upon any general screening strategy[3].
Other secondary causes of osteoporosis are linked with an increase in fracture risk (e.g., inflammatory bowel disease, endocrine disorders), but it is unclear if these are dependent on other risk factors. For example, the use of glucocorticoids is an important cause of osteoporosis and fractures according to the research groups of Kanis et al[1] and van Staa et al[36]. In contrast, rheumatoid arthritis determines a fracture risk independently of BMD and the use of glucocorticoids[37].
Immobility is also an important cause of bone loss. A woman immobilized for 1 mo can lose more bone than she would normally lose in 1 or 2 years of the osteoporotic process[3].
A family history of osteoporosis might be important and there is some evidence for a genetic component to peak bone mass[38]. Drugs such as corticosteroids and thyroid replacement treatments increase the risk of osteoporosis, as does excessive alcohol consumption[3]. These risk factors have a dose-dependent effect: The higher the exposure to these substances, the greater the risk (i.e., daily intake of about 3 units)[1,17].
Fracture risk assessment tool
All postmenopausal women and men 50 years of age and older should be assessed for osteoporosis risk in order to establish the need for BMD measurement and/or vertebral imaging.
Low BMD alone is a poor predictor of fracture in men and women, indicating the need for tools that predict fracture risk independent of, or in addition to, BMD. The use of risk assessment tools that include clinically relevant risk factors to predict fracture risk are being increasingly incorporated into osteoporosis screening and treatment guidelines.
The World Health Organization (WHO) and the International Osteoporosis Foundation advice that fracture risk should be expressed as a short-term absolute risk. The absolute risk of fracture is relative to age and life expectancy as well as the current relative risk, i.e., the probability over a 10-year interval[1,39]. The period of 10 years comprises the probable duration of treatment and the benefits that might persist once treatment is suspended.
Algorithm that combines the influence of CRFs on fracture risk, integrating or not data on BMD, is FRAX®[40,41] which takes into account the risk factors previously described in Table 1.
The FRAX tool (www.shef.ac.uk/FRAX) calculates alternatively the 10-year probability of hip or major osteoporotic fracture (hip, spine, hip, humerus or forearm fracture). Probabilities can be calculated for the different countries[40,41]. In all national treatment guidelines some case-finding approach is proposed for patient recognition. However, they differ on the basis of recognized risk factors, BMD and fracture risk assessment. Moreover, recommendations in national guidelines are not always implemented.
According to the Italian guidelines for osteoporosis treatment, postmenopausal women, men, and individuals taking glucocorticoids are included in the program of prevention, screening and diagnosis. Bone densitometry is suggested for all women aged 65 years and over, but, for men and younger postmenopausal women, only for those with CRFs. The guidelines recognize FRAX as a tool for estimating fracture probability and propose that pharmacological treatment should be indicated for people with “rather high” fracture risk but do not identify intervention thresholds[40].
Assessment of fracture risk: Available imaging methods
Osteoporosis causes loss of bone mass and deterioration of bone microarchitecture with a consequent reduction in bone stiffness and strength, thus resulting in an increased risk of fragility fractures.
Early diagnosis is essential for timely treatment and for identification of patients who are at a higher risk of fractures. Currently, osteoporosis diagnosis and fracture risk assessment are based on the quantitative BMD evaluation realized by the gold standard dual-energy X-ray absorptiometry (DXA). However, BMD assessment (which is a measure of bone mass) only partially provides information about bone strength. Indeed, on one hand BMD is a measure of bone mass, and on the other hand, bone fragility is dependent also on its microarchitecture quality which is determined by all the features (microarchitecture, microdamage and remodeling rates in bone) that influence a bone’s ability to resist fracture. The decay of trabecular bone microarchitecture has been acknowledged, among the features, as a major contributor to bone fragility[42].
Because DXA is a two-dimensional technique, it does have intrinsic limitations; it cannot aid in discriminating cortical from cancellous bone and cannot aid in distinguishing changes due to bone geometry from those due entirely to increased bone density.
There are several recently developed approaches that can provide complementary information for assessing fracture risks in addition to BMD. One of them is the quantitative computed tomography (QCT) which has been developed to assess bone loss[43]. In QCT trabecular bone can be examined separately from cortical portion of bone and a true value for mineral density is given, unlike other techniques[3,44].
In addition, currently, the microarchitecture of cancellous bone can be evaluated in vivo by high-resolution peripheral QCT techniques. However, such imaging techniques remain a high-end research tool rather than a diagnostic tool for clinical applications[42].
Other imaging techniques have been developed in order to improve the correct osteoporosis diagnosis, because the accurate diagnosis of osteoporosis leads to a better management in terms of prevention and appropriate pharmacologic or surgical treatment.
It has been demonstrated that BMD evaluations on reference anatomical sites, spine and proximal femur standardly evaluated by DXA examinations, are the most reliable available tool to predict the risk of osteoporotic fractures.
Unfortunately, DXA has precise limitations (i.e., bulky device, high cost, limited accessibility and use of ionizing radiation) that impede its application for population screenings and primary care diagnosis.
These limits have resulted in the development of an increasing number of radiation-free United States-based technologies as screening tools for early osteoporosis diagnosis and fracture prevention[45-48].
However, the actual clinical utility of United States devices for osteoporosis diagnosis is quite limited since they are referred only to peripheral sites (i.e., calcaneus, radius, tibia, etc.).
To overcome this limitation, a novel ultrasound approach has recently been developed to evaluate bone status and fragility fracture risk[49,50]. In this context, this new ultrasonic method is the first tool for bone characterization and microarchitecture assessment that enables the scanning of central axial reference sites (lumbar vertebrae and proximal femur) through an innovative approach without the use of ionizing radiations.
Unfortunately, in many countries, even patients at high risk of fractures might not be able to obtain therapy because effectual medicines are not reimbursed by government health insurance plans[10]. Moreover, worldwide osteoporosis is under-diagnosed, under-recognized and undertreated, and only a small number of patients with fractures receives proper investigation and therapy.
In 1994, a statement on the evaluation of fracture risk and its usage in screening for postmenopausal osteoporosis has been published by the WHO. In this report diagnostic criteria for BMD measurement have been provided and osteoporosis has been described as a recognized and well-defined disease that affected more than 75 million people in the world[3]. Based on WHO recommendations, national guidelines have been developed for osteoporosis management focusing mainly on the prevention of fractures in postmenopausal women.
In recent years, integrated programs to improve the management of osteoporosis are under development in some countries. Among the various aspects considered in these programs, those of particular relevance are related to education, improved screening and treatment efficacy monitoring. Indeed, many studies show that these programs reduce the risk of hip fractures compared to standard management[40]. Then, osteoporotic fractures are preventable by implementation of programs to assess and treat high risk individuals.
Geographical factors
Substantial difference has been shown in hip fracture incidence rates around the world due to environmental factors and lifestyle or cultural differences[8,51]. Generally the incidence of hip fracture increases with age. Moreover, the incidence rate differs across different regions or ethnic groups[30]. In Scandinavia and in North America, age-adjusted rates seem to be highest with almost seven-fold lower rates in Southern Europe[51,52]. The incidence of hip fracture is also lower in Asia, Latin America and Africa[53,54], particularly in rural areas[55-57].
In fact, bone mass is lower in Norwegian and Swedish with respect to other European people[58]. Whereas, Chinese, Japanese, South Koreans and black Africans show significantly lower bone mineral content (BMC) with respect to Western Caucasian populations[59-61]. One probable reason for this evidence is the difference between studies leading to different levels of underreporting[30]. Furthermore, compared to Caucasian, African Americans have a higher BMC, but likewise a lower prevalence of osteoporosis[30]. The lower hip fracture incidence rates among Asians and blacks can be due to also to their shorter hip axis length[62]. The genetic background of populations in the studied regions is an important factor to explain global variations in hip fractures. For example, the hip fracture incidence rates in Ontario are similar to those in the United Kingdom because older cohorts in Ontario are of English ancestry[63], Likewise, incidence rates are similar in Mexico and Spain; in fact, this two population group share the same genetic background[64]. Rates in Argentina are close to those from other predominantly populations because the ethnic background in Argentina is largely Caucasian. Furthermore, many Scandinavian cities where immigration is increasing have lower hip fracture rates than those with uniformly Scandinavian populations[65].
The worldwide risk of hip fracture is variable more in women than in men. Women have a higher osteoporosis risk; in the United States the lifetime risk of a hip fracture from age 50 years onward has been estimated at 17% and only 6% for Caucasian women and men, respectively. Among Asian, black, and Hispanic population, women and men were about 50% and 40% less susceptible to fracture with respect to white women and white men, respectively[66]. The rates in men and women are similar in low risk populations, particularly those of Asian or African heritage[30].
The incidence rate of hip fractures depends also on the country’s development. Where life expectancy at birth is low and the population is very young, as well as in African countries, the hip fracture incidence rates are the lowest[67,68]. However, the low incidence might be an artifact due to incomplete case ascertainment or national health database unavailability in developing countries.
The improvement of health care and the augmented life expectancy due to industrialization and urbanization have led to an increased incidence of hip fracture[30]. In fact, the rapid modernization and the consequent decline in routine load of physical activity induced an increased fracture incidence of the hip in Hong Kong[69] and Beijing[70]. Moreover urban settings have higher incidence rates than rural ones (i.e., Oslo, Norway )[56,71,72].
PREVALENCE OF OSTEOPOROTIC FRACTURE
Between 1990 and 2000, osteoporosis caused a 25% worldwide increment in hip fractures. The peak for hip or other fracture types occurs for both women and men aged 75-79 years and 50-59 years, respectively[73]. The annual number of hip fractures will grow significantly with the sustained ageing of the people. It is estimated that this demographic trend could induce a global increment of hip fractures from about 2 million in 1990 to a projected 6 million in 2050[9,10]. However, the future worldwide load of age-related fractures (hip and others) should be predicted by analyzing the variations in fracture incidence rates adjusted for demographic changes in the global population. Assuming a 1% annual rise in incidence adjusted for age, hip fractures in 2050 could exceed 8 million; if rates stabilized in Europe and North America but rose by 3% per year in the rest of the world, the total could account for more than 21 million[10,11] (Figure 1).
Figure 1.

Hip fractures expected impact. Incidence of hip fractures worldwide adjusted for demographic changes.
By 2050, the global incidence of hip fracture is expected to increase more than 300% and 240% in men and in women, respectively[11]. In women aged 45 years or more, the number of days spent in the hospital due to osteoporosis are greater than those due to other pathologies, such as breast cancer, diabetes, and myocardial infarction[74]. In Switzerland hospital bed days related to osteoporotic fracture are higher than those related to stroke and other cardiac disease[75]. In England, a fifth of all orthopedic beds are dedicated to hip fractures[10] and the cost related to osteoporotic fractures treatment in postmenopausal women has been estimated to reach about 2 billion dollars or more by 2020[76]. In Spain, there are around 2 million osteoporotic women (about 30% of them are aged 50 years or more): Each year 25000 fractures arise and cause, consequently, direct costs of about €120 million and indirect costs of about €400 million[77]. In Italy, approximately 4 million of women and 800 thousand men are thought to be affected by osteoporosis. In 1998, the European Commission estimated an incidence rise of hip fractures from 117000 to 240000 in the year 2000 in Germany[78]. However, the highest risk of hip fractures are shown in Northern Europe and the United States[79]. In Swedish male population the number of hospital bed days related to osteoporotic fractures are higher than those related to prostate cancer[80]. In Denmark, in population group aged 50 years or more, about 40% of women and 20% of men are osteoporotic[81]. In Finland, hip fractures total number augmented by 70% within a 10-year period (1992-2002)[82].
In the United States, among people aged 50 years or more, there were approximately 12 million cases of osteoporosis in 2010; this data are estimated to increase up to 14 million cases of disease by 2020[83], inducing the number of hip fractures to triple by 2040[84]. In Canada, osteoporosis affects 1.4 million postmenopausal women and the elderly. Almost 30000 hip fractures occur each year, and approximately 80% of these fractures are related to osteoporosis[85]. By the year 2030, the cases of hip fractures is estimated to quadruple[86].
In Australia, osteoporosis affects 2.2 million people (approximately 11% of men and 27% of women aged 60 years or more), causing 20000 hip fractures per year (growing by 40% every ten years), with total disease-related costs of $7.4 billion per year ($1.9 billion of direct costs)[87].
It is expected that approximately half of all osteoporotic hip fractures will take place in Asia by the year 2050[11]. Osteoporosis affects almost 70 million Chinese aged 50 years or more causing 687000 hip fractures each year[88]. In Japan, hip fractures total number was 153000 in 2010 and is projected to be 238000 in 2030[89].
Economic burden
In Europe, the total osteoporosis economic burden was estimated at €30.7 billion in 2010. The increment of direct costs is expected to be due to changes in demography to 76.7 billion in 2050[73]. In the United States, the medical cost of osteoporosis and related fractures is estimated at $20 billion per year[10], and can be predicted to be at $50 in 2050 due to the annual increase in incidence of osteoporotic fractures adjusted for age. China spent approximately $1.5 billion treating hip fracture in 2006. It is projected that this will grow to $12.5 billion in 2020 and to more than $264.7 billion by 2050[90] (Figure 2).
Figure 2.
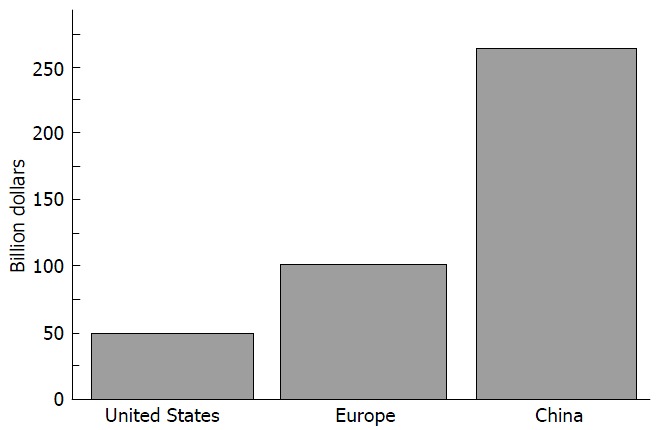
Economic burden. Expected costs in 2050 based on changes in demography.
The WHO considers osteoporosis to be second only to cardiovascular diseases as a crucial health problem[74]. The disability caused by osteoporosis is comparable or even greater than that produced by cancers and by different chronic non-transmissible pathologies, as reported by Johnell and Kanis[73] in 2006. Furthermore, the total costs per year of osteoporosis exceeds those for a variety of brain disorders[91] (Figure 3).
Figure 3.
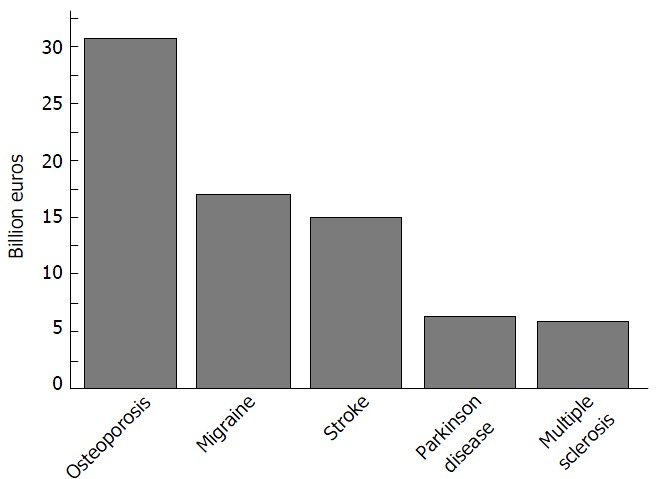
Total cost of osteoporosis vs brain disorders.
Currently in Europe, the annual expenditure for osteoporosis corresponds about to 3.5% of the total spent on health care[40]. However, osteoporosis total cost in a country is difficult to estimate because it depends on various factors, such as fracture risk related to age, size of population, acute hospital care, cost per fracture, long-term care at home, needs of nursing home care after hip fracture occurrence, medications, rehabilitation, treatment and loss of working days. Sometimes estimated costs are based on many assumptions that are difficult to test. Moreover, not all costs related to fracture come from a country’s healthcare budget (e.g., long-term care, community care).
Generally, a greater part of costs is related to incident fractures, whereas pharmacological treatment only represents less than 5% of total costs. The monetary burden depends mainly on the fracture risk; in fact, the cost per fracture increases with age (the 70% of the total costs are related to people aged more than 70 years). Also, fractures occurred in women represent the main part of the total cost[40].
Hip fractures account for more than half of the cost, whereas that of vertebral fractures is underestimated because of the difficulties of studying them. Only few people with clinical vertebral fractures become hospitalized[92] and, therefore, these cases are more complex to include in observational studies[93].
Outcome of osteoporotic fracture
Osteoporosis load is referred not only to fractures, costs, mortality, morbidity, but also to quality-adjusted life years (QALYs) lost.
Generally osteoporotic fractures caused more deaths and morbidity than cancer (except lung cancer). In particular, fracture of the hip is responsible for more deaths than suicide and transportation accidents[40].
The fractures effect on survival is related to the fracture type[94]. Hip fractures are the most dangerous, since approximately 10%-20% women with hip fractures die than expected for age within the first year. Moreover, the mortality is greater for men, and the death risk is greatest immediately after the fracture and decreases over time[10], even if it seems that mortality rates after fracture of hip have remained constant over the past 20 years[95]. Very often osteoporosis related fractures cause loss of physical functioning including loss of mobility and self-care. Approximately 7% of women become dependent on others to assist with the basic activities of daily living, and an additional 8% require nursing home care. The main long-term damage is in the capability to walk; half of patients able to walk before fracture cannot do so autonomously afterwards. Furthermore, up to a third of individuals who have a fracture of the hip can become totally dependent[96].
The principal vertebral fractures consequences are height loss, kyphosis, and back pain. Compression fractures cause acute symptoms[97] but many fractures seem to occur without pain. Women with vertebral deformities are substantially more likely to have chronic back pain as well as future fractures. Vertebral fractures, however, affect not only physical function but also physical aspect, and humor[10].
Osteoporosis load can also be quantified by loss of quality of life (QoL). Loss of QoL reflects the disutility or loss in utility due to both the pathology and increased mortality. The utility loss caused by fracture depend on the site of fracture; in fact, fractures of the axial anatomic sites (hip and vertebrae) induce more disutility with respect to forearm fractures. The loss of utility is similar for the both sexes[98]. During the first year after fracture of hip, vertebrae and wrist a person’s utility (relative to the age-specific utility) has been estimated to be 0.70, 0.59 and 0.96, respectively. On the other hand, in the subsequent years quality of life was assumed to be 80% of that of a healthy individual[99].
Combining mortality and loss of QoL, it is possible to evaluate the annual number of lost QALYs due to fractures. It is estimated that Germany has the highest number of lost QALYs due to its high fracture incidence and its great population. The estimation of QALYs lost due to fracture-related deaths was done considering an averaged interval time of four months between fracture and death[82]. Mortality during the first year after fractures represents approximately 1% and 3% of the total QALY-loss in women and men, respectively. A great part of the QALYs lost derives from the long-term disability after fractures due to osteoporosis. This component is larger in women, because men have a higher absolute mortality after fracture.
CONCLUSION
Osteoporosis incidence is rising in many countries. Osteoporotic fractures are a crucial public health concern and represent one of the main and frequent cause of disability and medical costs worldwide. Therefore, early diagnosis of patients with high risk of osteoporotic fractures is essential. Fortunately osteoporotic fractures are preventable. The comprehension of the main factors causing this “silent disease” could help the prediction of fractures in high-risk individuals worldwide. Early diagnosis of a larger range of the population is the key to resizing the impact of osteoporosis on the healthcare system. With this, it is necessary to encourage the widespread use of quick, cheap, non-invasive screening techniques and to increase national awareness campaigns promoting a healthy lifestyle across countries.
Footnotes
Supported by FESR P.O. Apulia Region 2007-2013 - Action 1.2.4, No. 3Q5AX31.
Conflict-of-interest statement: No potential conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 24, 2015
First decision: September 22, 2015
Article in press: December 8, 2015
P- Reviewer: Cheung WH, Lee YK, Vulcano E S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albright F. Annals of internal medicine, Volume 27, 1947: Osteoporosis. Nutr Rev. 1989;47:85–86. doi: 10.1111/j.1753-4887.1989.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 4.Anil G, Guglielmi G, Peh WC. Radiology of osteoporosis. Radiol Clin North Am. 2010;48:497–518. doi: 10.1016/j.rcl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmi G, Muscarella S, Leone A, Peh WC. Imaging of metabolic bone diseases. Radiol Clin North Am. 2008;46:735–754, vi. doi: 10.1016/j.rcl.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Guglielmi G NOF. Fast facts. 2008. Available from: http: //www.nof.org/aboutosteoporosis. [Google Scholar]
- 7.Willson T, Nelson SD, Newbold J, Nelson RE, LaFleur J. The clinical epidemiology of male osteoporosis: a review of the recent literature. Clin Epidemiol. 2015;7:65–76. doi: 10.2147/CLEP.S40966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giusti A, Bianchi G. Male osteoporosis. Reumatismo. 2014;66:136–143. doi: 10.4081/reumatismo.2014.786. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 11.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, Delmas P, Eisman J, Johnell O, Jonsson B, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002;13:527–536. doi: 10.1007/s001980200069. [DOI] [PubMed] [Google Scholar]
- 13.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riggs BL, Melton LJ. Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 15.Mazess RB, Barden HS. Bone densitometry for diagnosis and monitoring osteoporosis. Proc Soc Exp Biol Med. 1989;191:261–271. doi: 10.3181/00379727-191-42918. [DOI] [PubMed] [Google Scholar]
- 16.De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 17.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 18.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 19.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Eisman JA, McCloskey EV, Mellstrom D, Melton LJ, Pols HA, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029–1037. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Svejme O, Ahlborg HG, Nilsson JÅ, Karlsson MK. Low BMD is an independent predictor of fracture and early menopause of mortality in post-menopausal women--a 34-year prospective study. Maturitas. 2013;74:341–345. doi: 10.1016/j.maturitas.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Davies MC, Hall ML, Jacobs HS. Bone mineral loss in young women with amenorrhoea. BMJ. 1990;301:790–793. doi: 10.1136/bmj.301.6755.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prior JC, Vigna YM, Schechter MT, Burgess AE. Spinal bone loss and ovulatory disturbances. N Engl J Med. 1990;323:1221–1227. doi: 10.1056/NEJM199011013231801. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein JS, Klibanski A, Neer RM, Greenspan SL, Rosenthal DI, Crowley WF. Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med. 1987;106:354–361. doi: 10.7326/0003-4819-106-3-. [DOI] [PubMed] [Google Scholar]
- 25.Seeman E, Melton LJ, O’Fallon WM, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med. 1983;75:977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- 26.Baron JA. Smoking and estrogen-related disease. Am J Epidemiol. 1984;119:9–22. doi: 10.1093/oxfordjournals.aje.a113730. [DOI] [PubMed] [Google Scholar]
- 27.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 28.Saika M, Inoue D, Kido S, Matsumoto T. 17beta-estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-alpha. Endocrinology. 2001;142:2205–2212. doi: 10.1210/endo.142.6.8220. [DOI] [PubMed] [Google Scholar]
- 29.Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 30.Cheng SY, Levy AR, Lefaivre KA, Guy P, Kuramoto L, Sobolev B. Geographic trends in incidence of hip fractures: a comprehensive literature review. Osteoporos Int. 2011;22:2575–2586. doi: 10.1007/s00198-011-1596-z. [DOI] [PubMed] [Google Scholar]
- 31.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 32.Compston J. Clinical and therapeutic aspects of osteoporosis. Eur J Radiol. 2009;71:388–391. doi: 10.1016/j.ejrad.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 33.Guglielmi G, Muscarella S, Bazzocchi A. Integrated imaging approach to osteoporosis: state-of-the-art review and update. Radiographics. 2011;31:1343–1364. doi: 10.1148/rg.315105712. [DOI] [PubMed] [Google Scholar]
- 34.Evans JG, Seagroatt V, Goldacre MJ. Secular trends in proximal femoral fracture, Oxford record linkage study area and England 1968-86. J Epidemiol Community Health. 1997;51:424–429. doi: 10.1136/jech.51.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper C, Eriksson JG, Forsén T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int. 2001;12:623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 36.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 37.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton III LJ, Tenenhouse A, Reeve J, Silman AJ, Pols HA, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–899. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 38.Slemenda CW, Christian JC, Williams CJ, Norton JA, Johnston CC. Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991;6:561–567. doi: 10.1002/jbmr.5650060606. [DOI] [PubMed] [Google Scholar]
- 39.Kanis JA, Johnell O, Oden A, De Laet C, Jonsson B, Dawson A. Ten-year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone. 2002;30:251–258. doi: 10.1016/s8756-3282(01)00653-6. [DOI] [PubMed] [Google Scholar]
- 40.Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2011;6:59–155. doi: 10.1007/s11657-011-0060-1. [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 42.Dong X, Wang X. Assessment of bone fragility with clinical imaging modalities. Hard Tissue. 2013;2:7. doi: 10.13172/2050-2303-2-1-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams JE. Radiogrammetry and radiographic absorptiometry. Radiol Clin North Am. 2010;48:531–540. doi: 10.1016/j.rcl.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Guglielmi G, Lang TF. Quantitative computed tomography. Semin Musculoskelet Radiol. 2002;6:219–227. doi: 10.1055/s-2002-36719. [DOI] [PubMed] [Google Scholar]
- 45.Pais R, Campean R, Simon S, Bolosiu CR, Muntean L, Bolosiu HD. Accuracy of Quantitative Ultrasound Parameters in the Diagnosis of Osteoporosis. Cent Eur J Med. 2010;5:e478–e485. [Google Scholar]
- 46.Laugier P. Quantitative ultrasound of bone: looking ahead. Joint Bone Spine. 2006;73:125–128. doi: 10.1016/j.jbspin.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Holi MS, Radhakrishnan S, Swaranamani S, Ayavelan NA. Quantitative ultrasound technique for the assessment of osteoporosis and prediction of fracture risk. J Pure Appl Ultrason. 2005;27:e55–e60. [Google Scholar]
- 48.Khaw KT, Reeve J, Luben R, Bingham S, Welch A, Wareham N, Oakes S, Day N. Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: EPIC-Norfolk prospective population study. Lancet. 2004;363:197–202. doi: 10.1016/S0140-6736(03)15325-1. [DOI] [PubMed] [Google Scholar]
- 49.Conversano F, Franchini R, Greco A, Soloperto G, Chiriacò F, Casciaro E, Aventaggiato M, Renna MD, Pisani P, Di Paola M, et al. A novel ultrasound methodology for estimating spine mineral density. Ultrasound Med Biol. 2015;41:281–300. doi: 10.1016/j.ultrasmedbio.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Pisani P, Greco A, Renna MD, Conversano F, Casciaro E, Quarta L, Costanza D, Muratore M, Casciaro S. An Innovative Ultrasound-Based Method for The Identification of Patients at High Fracture Risk. Proceedings of the 3rd Imeko TC13 Symposium on Measurements in Biology and Medicine. New Frontiers in Biomedical Measurements, 2014: e50-e53 [Google Scholar]
- 51.Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, Melton LJ, Cummings SR, Kanis JA. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22:1277–1288. doi: 10.1007/s00198-011-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnell O, Gullberg B, Allander E, Kanis JA. The apparent incidence of hip fracture in Europe: a study of national register sources. Osteoporos Int. 1992;2:298–302. doi: 10.1007/BF01623186. [DOI] [PubMed] [Google Scholar]
- 53.Yan L, Zhou B, Prentice A, Wang X, Golden MH. Epidemiological study of hip fracture in Shenyang, People’s Republic of China. Bone. 1999;24:151–155. doi: 10.1016/s8756-3282(98)00168-9. [DOI] [PubMed] [Google Scholar]
- 54.Morales-Torres J, Gutiérrez-Ureña S. The burden of osteoporosis in Latin America. Osteoporos Int. 2004;15:625–632. doi: 10.1007/s00198-004-1596-3. [DOI] [PubMed] [Google Scholar]
- 55.Kaastad TS, Meyer HE, Falch JA. Incidence of hip fracture in Oslo, Norway: differences within the city. Bone. 1998;22:175–178. doi: 10.1016/s8756-3282(97)00247-0. [DOI] [PubMed] [Google Scholar]
- 56.Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, Kotowicz MA. Fracture rates lower in rural than urban communities: the Geelong Osteoporosis Study. J Epidemiol Community Health. 2002;56:466–470. doi: 10.1136/jech.56.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cooper C. Epidemiology of Osteoporosis. Osteoporos Int. 1999;2:eS2–eS8. doi: 10.1007/pl00004156. [DOI] [PubMed] [Google Scholar]
- 58.Lunt M, Felsenberg D, Adams J, Benevolenskaya L, Cannata J, Dequeker J, Dodenhof C, Falch JA, Johnell O, Khaw KT, et al. Population-based geographic variations in DXA bone density in Europe: the EVOS Study. European Vertebral Osteoporosis. Osteoporos Int. 1997;7:175–189. doi: 10.1007/BF01622286. [DOI] [PubMed] [Google Scholar]
- 59.Cundy T, Cornish J, Evans MC, Gamble G, Stapleton J, Reid IR. Sources of interracial variation in bone mineral density. J Bone Miner Res. 1995;10:368–373. doi: 10.1002/jbmr.5650100306. [DOI] [PubMed] [Google Scholar]
- 60.Russell-Aulet M, Wang J, Thornton JC, Colt EW, Pierson RN. Bone mineral density and mass in a cross-sectional study of white and Asian women. J Bone Miner Res. 1993;8:575–582. doi: 10.1002/jbmr.5650080508. [DOI] [PubMed] [Google Scholar]
- 61.Aspray TJ, Prentice A, Cole TJ, Sawo Y, Reeve J, Francis RM. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J Bone Miner Res. 1996;11:1019–1025. doi: 10.1002/jbmr.5650110720. [DOI] [PubMed] [Google Scholar]
- 62.Cummings SR, Cauley JA, Palermo L, Ross PD, Wasnich RD, Black D, Faulkner KG. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4:226–229. doi: 10.1007/BF01623243. [DOI] [PubMed] [Google Scholar]
- 63.Jaglal SB, Sherry PG, Schatzker J. The impact and consequences of hip fracture in Ontario. Can J Surg. 1996;39:105–111. [PMC free article] [PubMed] [Google Scholar]
- 64.Clark P, Lavielle P, Franco-Marina F, Ramírez E, Salmerón J, Kanis JA, Cummings SR. Incidence rates and life-time risk of hip fractures in Mexicans over 50 years of age: a population-based study. Osteoporos Int. 2005;16:2025–2030. doi: 10.1007/s00198-005-1991-4. [DOI] [PubMed] [Google Scholar]
- 65.Rogmark C, Sernbo I, Johnell O, Nilsson JA. Incidence of hip fractures in Malmö, Sweden, 1992-1995. A trend-break. Acta Orthop Scand. 1999;70:19–22. doi: 10.3109/17453679909000950. [DOI] [PubMed] [Google Scholar]
- 66.Tosteson AN, Melton LJ, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, Lindsay RL. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19:437–447. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Maghraoui A, Koumba BA, Jroundi I, Achemlal L, Bezza A, Tazi MA. Epidemiology of hip fractures in 2002 in Rabat, Morocco. Osteoporos Int. 2005;16:597–602. doi: 10.1007/s00198-004-1729-8. [DOI] [PubMed] [Google Scholar]
- 68.Zebaze RM, Seeman E. Epidemiology of hip and wrist fractures in Cameroon, Africa. Osteoporos Int. 2003;14:301–305. doi: 10.1007/s00198-002-1356-1. [DOI] [PubMed] [Google Scholar]
- 69.Lau EM, Cooper C. The epidemiology of osteoporosis. The oriental perspective in a world context. Clin Orthop Relat Res. 1996;(323):65–74. [PubMed] [Google Scholar]
- 70.Xu L, Haworth IS, Kulkarni AA, Bolger MB, Davies DL. Mutagenesis and cysteine scanning of transmembrane domain 10 of the human dipeptide transporter. Pharm Res. 2009;26:2358–2366. doi: 10.1007/s11095-009-9952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chevalley T, Herrmann FR, Delmi M, Stern R, Hoffmeyer P, Rapin CH, Rizzoli R. Evaluation of the age-adjusted incidence of hip fractures between urban and rural areas: the difference is not related to the prevalence of institutions for the elderly. Osteoporos Int. 2002;13:113–118. doi: 10.1007/s001980200002. [DOI] [PubMed] [Google Scholar]
- 72.Bjørgul K, Reikerås O. Incidence of hip fracture in southeastern Norway: a study of 1,730 cervical and trochanteric fractures. Int Orthop. 2007;31:665–669. doi: 10.1007/s00264-006-0251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 74.Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int. 1997;7:390–406. doi: 10.1007/BF01623782. [DOI] [PubMed] [Google Scholar]
- 75.Lippuner K, von Overbeck J, Perrelet R, Bosshard H, Jaeger P. Incidence and direct medical costs of hospitalizations due to osteoporotic fractures in Switzerland. Osteoporos Int. 1997;7:414–425. doi: 10.1007/pl00004149. [DOI] [PubMed] [Google Scholar]
- 76.Burge RT. The cost of osteoporotic fractures in the UK: Projections for 2000-2020. J Med Econ. 2001;(4):e51–e62. [Google Scholar]
- 77.Díaz Curiel M, García JJ, Carrasco JL, Honorato J, Pérez Cano R, Rapado A, Alvarez Sanz C. [Prevalence of osteoporosis assessed by densitometry in the Spanish female population] Med Clin (Barc) 2001;116:86–88. doi: 10.1016/s0025-7753(01)71732-0. [DOI] [PubMed] [Google Scholar]
- 78.Häussler B, Gothe H, Göl D, Glaeske G, Pientka L, Felsenberg D. Epidemiology, treatment and costs of osteoporosis in Germany--the BoneEVA Study. Osteoporos Int. 2007;18:77–84. doi: 10.1007/s00198-006-0206-y. [DOI] [PubMed] [Google Scholar]
- 79.Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK. International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res. 2002;17:1237–1244. doi: 10.1359/jbmr.2002.17.7.1237. [DOI] [PubMed] [Google Scholar]
- 80.Kanis JA, Johnell O, Oden A, De Laet C, Mellstrom D. Epidemiology of osteoporosis and fracture in men. Calcif Tissue Int. 2004;75:90–99. doi: 10.1007/s00223-004-0287-6. [DOI] [PubMed] [Google Scholar]
- 81.Vestergaard P, Rejnmark L, Mosekilde L. Osteoporosis is markedly underdiagnosed: a nationwide study from Denmark. Osteoporos Int. 2005;16:134–141. doi: 10.1007/s00198-004-1680-8. [DOI] [PubMed] [Google Scholar]
- 82.Borgström F, Sobocki P, Ström O, Jönsson B. The societal burden of osteoporosis in Sweden. Bone. 2007;40:1602–1609. doi: 10.1016/j.bone.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 83.National Osteoporosis Foundation. America’s bone health: the state of osteoporosis and low bone mass in our nation. Washington, DC: National Osteoporosis Foundation; 2002. [Google Scholar]
- 84.Schneider EL, Guralnik JM. The aging of America. Impact on health care costs. JAMA. 1990;263:2335–2340. [PubMed] [Google Scholar]
- 85.Melton LJ, Thamer M, Ray NF, Chan JK, Chesnut CH, Einhorn TA, Johnston CC, Raisz LG, Silverman SL, Siris ES. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:16–23. doi: 10.1359/jbmr.1997.12.1.16. [DOI] [PubMed] [Google Scholar]
- 86.Jackson SA, Tenenhouse A, Robertson L. Vertebral fracture definition from population-based data: preliminary results from the Canadian Multicenter Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:680–687. doi: 10.1007/s001980070066. [DOI] [PubMed] [Google Scholar]
- 87.Sambrook PN, Seeman E, Phillips SR, Ebeling PR. Preventing osteoporosis: outcomes of the Australian Fracture Prevention Summit. Med J Aust. 2002;176 Suppl:S1–16. doi: 10.5694/j.1326-5377.2002.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 88.China Health Promotion Foundation. Osteoporosis a Summary Statement of China. White Paper China. Washington, DC: National Osteoporosis Foundation; 2008. [Google Scholar]
- 89.Hagino H, Katagiri H, Okano T, Yamamoto K, Teshima R. Increasing incidence of hip fracture in Tottori Prefecture, Japan: trend from 1986 to 2001. Osteoporos Int. 2005;16:1963–1968. doi: 10.1007/s00198-005-1974-5. [DOI] [PubMed] [Google Scholar]
- 90.Luo L, Xu L. Analysis of direct economic burden of osteoporotic hip fracture and its influence factors. Chin J Epidemiol. 2005;9:9. [PubMed] [Google Scholar]
- 91.Ettinger B, Black DM, Nevitt MC, Rundle AC, Cauley JA, Cummings SR, Genant HK. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1992;7:449–456. doi: 10.1002/jbmr.5650070413. [DOI] [PubMed] [Google Scholar]
- 92.Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 93.Seeley DG, Browner WS, Nevitt MC, Genant HK, Scott JC, Cummings SR. Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1991;115:837–842. doi: 10.7326/0003-4819-115-11-837. [DOI] [PubMed] [Google Scholar]
- 94.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 95.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 96.Melton LJ. Who has osteoporosis? A conflict between clinical and public health perspectives. J Bone Miner Res. 2000;15:2309–2314. doi: 10.1359/jbmr.2000.15.12.2309. [DOI] [PubMed] [Google Scholar]
- 97.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pain and disability associated with new vertebral fractures and other spinal conditions. J Clin Epidemiol. 1994;47:231–239. doi: 10.1016/0895-4356(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 98.Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, Abdon P, Ornstein E, Lunsjö K, Thorngren KG, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006;17:637–650. doi: 10.1007/s00198-005-0015-8. [DOI] [PubMed] [Google Scholar]
- 99.Peasgood T, Herrmann K, Kanis JA, Brazier JE. An updated systematic review of Health State Utility Values for osteoporosis related conditions. Osteoporos Int. 2009;20:853–868. doi: 10.1007/s00198-009-0844-y. [DOI] [PubMed] [Google Scholar]


