Abstract
Several theories have been proposed regarding pain transmission mechanisms in tooth. However, the exact signaling mechanism from odontoblasts to pulp nerves remains to be clarified. Recently, ATP-associated pain transmission has been reported, but it is unclear whether ATP is involved in tooth pain transmission. In the present study, we focused on the vesicular nucleotide transporter (VNUT), a transporter of ATP into vesicles, and examined whether VNUT was involved in ATP release from odontoblasts. We examined the expression of VNUT in rat pulp by RT-PCR and immunostaining. ATP release from cultured odontoblast-like cells with heat stimulation was evaluated using ATP luciferase methods. VNUT was expressed in pulp tissue, and the distribution of VNUT-immunopositive vesicles was confirmed in odontoblasts. In odontoblasts, some VNUT-immunopositive vesicles were colocalized with membrane fusion proteins. Additionally P2X3, an ATP receptor, immunopositive axons were distributed between odontoblasts. The ATP release by thermal stimulation from odontoblast-like cells was inhibited by the addition of siRNA for VNUT. These findings suggest that cytosolic ATP is transported by VNUT and that the ATP in the vesicles is then released from odontoblasts to ATP receptors on axons. ATP vesicle transport in odontoblasts seems to be a key mechanism for signal transduction from odontoblasts to axons in the pulp.
Keywords: VNUT, ATP, pain, odontoblast, pain transmission
I. Introduction
In addition to the specific role of odontoblasts in dentin formation, odontoblasts located in the outermost cellular layer of dental pulp also function as sensory receptor cells in pain transmission in teeth [22]. Previously, several hypotheses have been proposed regarding the mechanism(s) of dental nociception [4, 18], such as the neural theory [30], hydrodynamic theory [2, 19], and odontoblast transducer theory. However, the signaling mechanism from odontoblasts to pulp nerves has remained unclear.
Recent studies have suggested that transient receptor potential (TRP) channels in odontoblasts or pulp nerve endings around odontoblasts are involved in the development of dental pain [6, 7, 31]. However, the exact nature of the signaling between odontoblasts and axons in the pulp near odontoblasts remains unknown.
Although adenosine triphosphate (ATP) was originally characterized as a molecule for intracellular energy transfer, more recently ATP has been found to play roles in intracellular and extracellular signaling, including as a neurotransmitter [3, 10, 32]. For extracellular signaling, ATP is released from cells through channels or gap junctions [8, 17, 33]. Furthermore, some reports have indicated the involvement of ATP in signaling from odontoblasts to pulpal nerves [5, 28]. However, the mechanism of ATP release from odontoblasts remains controversial.
Recently, vesicular nucleotide transporter (VNUT) was identified as an ATP transporter from the cytoplasm into vesicles for subsequent ATP secretion in purinergic signal transmission [27]. Since then, VNUT has been found in the taste cells of taste buds [14], in microglia [12], and in dorsal root ganglion neurons [24]. There is also evidence that P2X3 receptors, which are ATP receptors, are present in the nerve fibers of the odontoblast layer near dental pulp [1, 15, 26], and that NTPDase (an ATP-degrading enzyme) is expressed in dental nerve fibers around the odontoblast layer [20]. Thus, ATP signaling would be involved in pain transmission in teeth, but it is unclear whether or how ATP is released from odontoblasts.
In the present study, to investigate the involvement of VNUT in ATP signal transduction between odontoblasts and pulp nerves, we first examined the expression and the localization of VNUT in odontoblasts in vivo and in vitro. We then confirmed the involvement of VNUT in the release of ATP from odontoblast-like cells under heat stress. Our data contribute to understanding ATP signal transduction in pain transmission in teeth.
II. Materials and Methods
Animals
Male Sprague-Dawley (SD) rats weighing 150–200 g were used. Five male rats were habituated to the testing paradigms for 7 days before data collection. Animals had free access to food and water during experiments and were maintained in a temperature-controlled room (23°C) with a 12/12-hr light/dark cycle. The experimental protocol was reviewed and approved by the Kyushu Dental University Animal Care Committee.
Tissue preparation
Rats were anesthetized with diethylether and perfused transcardially with 4% paraformaldehyde in 0.2 M phosphate buffer (PFA, pH 7.4) containing 0.2% picric acid [9]. Maxillary bones, including upper molars, were then removed, post-fixed with PFA overnight, decalcified with EDTA for 2 weeks and then dehydrated through ascending graded sucrose solutions (10, 20 and 30%). Maxillary bones were rapidly frozen, cut into 6-μm-thick serial sections with a cryostat (Leica Instruments, Wetzlar, Germany), and mounted on gelatin/chrome alum-coated glass slides [13].
Cell culture
A rat clonal odontoblast-like cell line, KN-3, cloned by Noguchi et al. [25], was seeded in 35-mm culture dishes (3×105 cells/dish) and cultured with minimal essential medium, Eagle’s alpha modification (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS) (Cellgro; Mediatech Inc., Herndon, VA, USA), 100 mg/mL streptomycin (Sigma-Aldrich), and 100 U/mL penicillin (Sigma-Aldrich) in a humidified atmosphere of 5% CO2 at 37°C. Odontoblastic phenotype of the cells was confirmed by the expression of nestin, a marker of odontoblasts.
Immunohistochemistry
Antibody against rat VNUT was generated in rabbits using synthetic peptides corresponding to residues 5–19, RSSLMQPIPEETRKT. Specificity of the VNUT antibody in odontoblasts was confirmed by its preabsorption with the peptide (data not shown). Double labeling for VNUT with nestin (an odontoblast marker) or Snap25 (a marker of membrane fusion proteins), and double labeling for neurofilament 200 (NF200, a marker of nerve fibers) with P2X3 (an ATP receptor) were performed. First, the sections were pre-treated with 0.1% Triton X-100 for 10 min at room temperature and pre-incubated with Blocking One (1:20; Nacalai, Tesque Inc., Kyoto, Japan) in 0.1 M phosphate-buffered saline (PBS, pH 7.4) for 20 min at 37°C. The sections were then incubated with mouse monoclonal antibodies against nestin (1:100; Millipore, Billerica, MA, USA), rabbit polyclonal antibodies against Snap25 (1:500; Sigma-Aldrich), or rabbit polyclonal antibodies against NF200 (1:1000; Sigma-Aldrich) for 2 hr at 37°C. After rinsing with 0.1 M PBS, the sections were incubated with goat anti-mouse IgG 568 (1:400; Invitrogen, Carlsbad, CA, USA) or goat anti-rabbit IgG 488 (1:400; Invitrogen) for 2 hr at 37°C. After rinsing again with 0.1 M PBS, the sections were incubated with rabbit polyclonal antibodies against VNUT (1:100) or guinea pig polyclonal antibodies against P2X3 (1:250; Neuromics, Edina, MN, USA) for 2 hr at 37°C, washed in PBS, and incubated with goat anti-rabbit IgG 488 (1:400; Invitrogen) or goat anti-guinea pig IgG 546 (1:400; Invitrogen) for 2 hr at 37°C. After rinsing again with 0.1 M PBS, the sections were incubated with DAPI (Vector Laboratories Inc., Burlingame, CA, USA) for 5 min at room temperature. Negative control sections were incubated with secondary antibody in buffer alone. Finally, the sections were washed in 0.1 M PBS and covered with coverslips. Samples were examined under a fluorescence microscope (Keyence, Osaka, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
1. . Tissue preparation
Maxillary first molars and taste buds were obtained from the SD rats. The teeth were separated, and the pulps were isolated. Total RNA was extracted from freshly excised pulps and taste buds using RNAqueous (Ambion; Life Technologies, Austin, TX, USA) according to the manufacturer’s protocol. cDNA was synthesized from 2.0 μg of total RNA in 30 μL of reaction buffer composed of 500 μM dNTPs, 20 U ribonuclease inhibitor (Promega, Madison, WI, USA), and 200 U Superscript II reverse transcriptase (Invitrogen). Cycling conditions were 94°C for 60 sec, 60°C for 60 sec, and 72°C for 60 sec for 42 cycles. The primers used to amplify VNUT were 5'-AGAGTGCAGG AGAGCGAGAG-3' and 5'-GTGGTGTGACCCAGACAC AG-3', giving a PCR product of 563 bp. PCR products were subjected to 2% agarose gel electrophoresis with ethidium bromide staining and visualized under ultraviolet light illumination. The expression of Gapdh was used as an internal control.
2. . Cell culture and heat stress
KN-3 cells were seeded in 35-mm culture dishes (3×105 cells/dish) and cultured with minimal essential medium, Eagle’s alpha modification containing 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin in a humidified atmosphere of 5% CO2 at 37°C. After 24 hr, KN-3 cells were exposed to heat stress at 43°C for 45 min. For rapid heat stress, culture medium was changed to medium preheated at 43°C, and culture dishes were placed in an incubator preheated to 43°C, followed by incubation for 45 min at 43°C [23]. As a control, non-heat-treated KN-3 cells were cultured in medium containing 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin at 37°C. Total RNA was extracted from non-heat-treated and heat-stressed cells using RNAqueous at 1, 3, and 6 hr after heat stress. The following primers were used: for Hsp25 (Heat shock protein 25), 5'-GCAGGATGAACATGGCTA CATCTC-3' and 5'-TGGTGATCTCCGCTGATTGTG-3'; for VNUT, 5'-AGAGTGCAGGAGAGCGAGAG-3' and 5'-GTGGTGTGACCCAGACACAG-3', giving a PCR product of 279 bp (for Hsp25) and 563 bp (for VNUT). Each cycle consisted of a denaturation step at 94.0°C for 60 sec, an annealing step (Hsp25, 68°C for 60 sec for 38 cycles, VNUT, 60°C for 60 sec for 42 cycles), and an extension step at 72°C for 60 sec. The expression of Gapdh was used as an internal control.
siRNA and transfection
VNUT siRNA (ON-TARGET plus siRNA, GE Healthcare Japan, Tokyo, Japan) and negative control siRNA (GE Healthcare Japan) were used. At 24 hr before transfection, KN-3 cells were plated in 2.5 mL complete growth medium per well in a six-well plate. The density of KN-3 cells was 3.0×105 cells/well. After the cells were incubated overnight, siRNAs were transfected using the TransIT-X2 Dynamic Delivery System (Takara Bio, Shiga, Japan) according to the manufacturer’s protocol. At 24 hr after transfection, RT-PCR was then performed to assess the effects of the siRNA.
Measurement of ATP release
At 24 hr after transfection of siRNA, KN-3 cells were exposed to heat stress at 43°C for 45 min. Just after that 50 μL of the supernatant was collected. Similarly, 50 μL of the supernatant was collected with the medium of the control, non-heat-treated KN-3 cells. ATP concentrations in the culture medium samples were assayed with ATP assay reagent (Wako Pure Chemical Industries, Ltd., Osaka, Japan) according to the manufacturer’s protocol with an ATP standard curve (10 pM to 10 μM) [16]. Duplicate measurements were performed for each sample, and the average concentration was taken from five wells.
Statistical analysis
One-way analysis of variance (ANOVA) followed by individual post hoc comparisons (Scheffé) was used to assess the significance of differences.
III. Results
In vivo studies
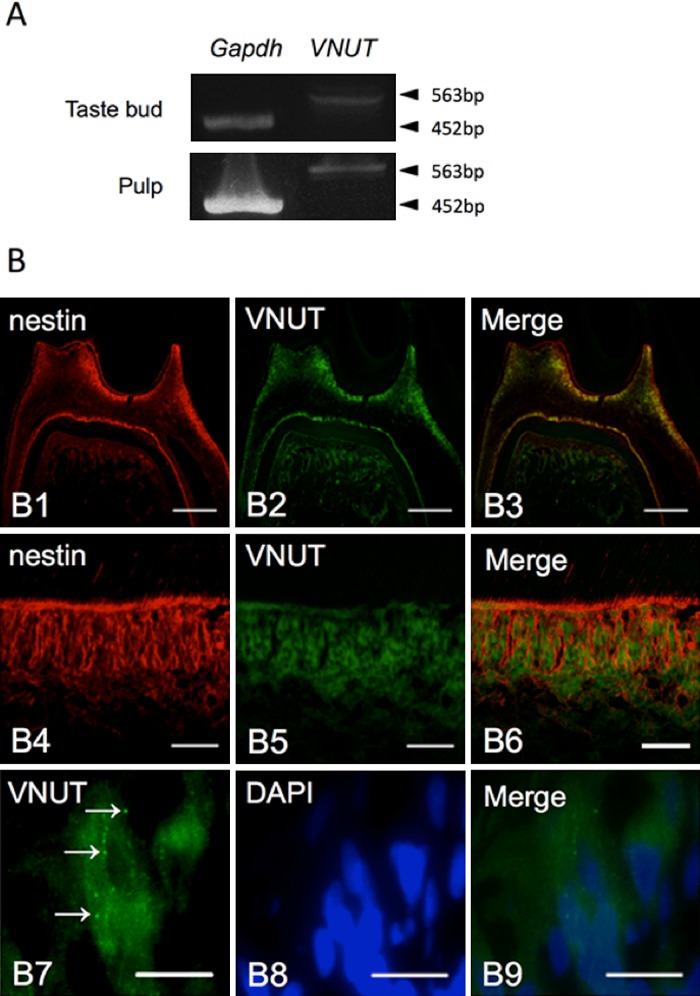
The expression of VNUT mRNA in rat pulp tissue, including odontoblasts, was examined initially by RT-PCR. The pulpal tissue including odontoblasts expressed VNUT mRNA of the same size as the taste buds (Fig. 1A). To investigate which cell type(s) expressed VNUT in pulpal tissue, we performed immunofluorescence staining using a rabbit polyclonal antibody against VNUT. As shown in Figure 1B (1–6), strong immunopositive reaction was found in odontoblasts that were immunopositive for nestin, an odontoblast marker. Additionally, the expression of vesicle-like VNUT was confirmed in an odontoblast at high magnification (Fig. 1B (7–9)). Sections that were incubated in the absence of primary antibody showed no specific staining (data not shown).
Fig. 1. .
VNUT expression in rat pulp. (A) VNUT mRNA expression in pulp and taste buds was confirmed by RT-PCR using primers specific to VNUT. (B) Immunofluorescence staining for VNUT (B2, B5, and B7) and nestin (B1 and B4) or DAPI (B8) in odontoblasts from rat tooth tissue. B3, B6, and B9 are merged images. Arrows indicate vesicle-like expression of VNUT (B7). Bars=200 μm (B1–3), 50 μm (B4–6), 20 μm (B7–9).
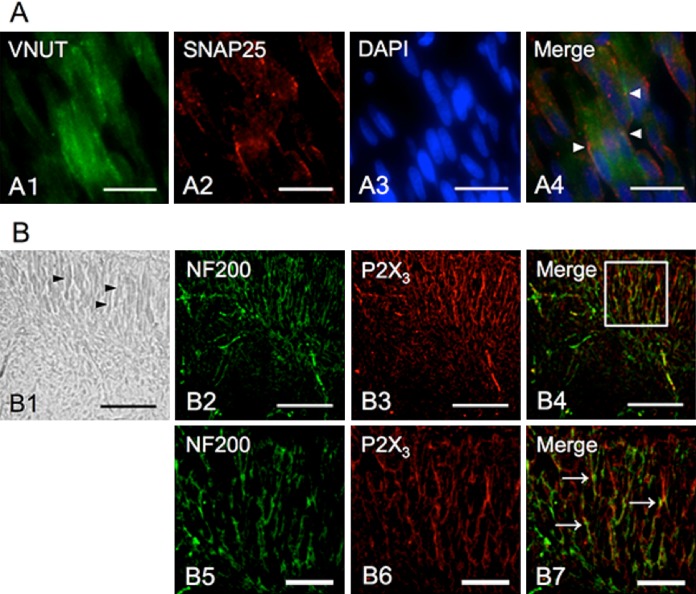
To confirm the involvement of VNUT in vesicle transport in odontoblasts, double immunofluorescence staining for VNUT and Snap25, a membrane fusion protein, was performed. Some immunopositive reactions for VNUT were colocalized with the immunopositive reactions for Snap25 at the periphery of odontoblasts (Fig. 2A (1–4)). Furthermore, to indicate the distribution of ATP receptors close to odontoblasts, we performed double staining for NF200, a neuron-specific neurofilament protein, and P2X3, a receptor for ATP. We found that most of the pulp nerves were immunoreactive with anti-NF200 antibodies, including many fine neurons running between odontoblastic cells. The immunopositive reactions for P2X3 receptors were distributed on the NF200 immunopositive axons between odontoblasts. These findings may possibly indicate that ATP vesicle transport had mediated the signal transduction from odontoblasts to axons in the pulp (Fig. 2B (1–7)).
Fig. 2. .
Immunohistochemical localization of Snap25 and P2X3 in rat odontoblasts and pulp nerve fibers. (A) Immunofluorescence staining of VNUT (A1), Snap25 (A2), and DAPI (A3) in rat odontoblasts. A4 is merged image. Arrowheads indicate the co-localization of VNUT and Snap25. (B) B1 is a bright-field image at low magnification of rat pulp, and the arrowheads indicate the location of odontoblasts. Immunofluorescence image for NF200 (B2 and B5) and P2X3 (B3 and B6) in odontoblasts from rat tooth tissue. B2–4 are immunofluorescence images in the same region as B1. B2–4 are high magnification images. P2X3 receptor expression on pulp nerve fibers was confirmed (arrows in B7). Bars=20 μm (A1–4), 100 μm (B1–4), 50 μm (B5–6).
In vitro studies
The odontoblastic phenotype in cultured KN-3 cells was confirmed by expression of the odontoblast marker nestin by immunofluorescence staining. The expression of VNUT in KN-3 cells was also confirmed (Fig. 3A). RT-PCR was performed to investigate the time-dependent changes in the expression of VNUT mRNA, compared with the expression of Hsp25 mRNA, in heat treatment in KN-3 odontoblastic cells. After KN-3 cells were exposed to heat stress at 43°C for 45 min, Hsp25 mRNA increased gradually from 1 to 3 hr after heat stress, whereas VNUT mRNA was expressed at the highest level at 1 hr after heat stress and then decreased (Fig. 3B).
Fig. 3. .
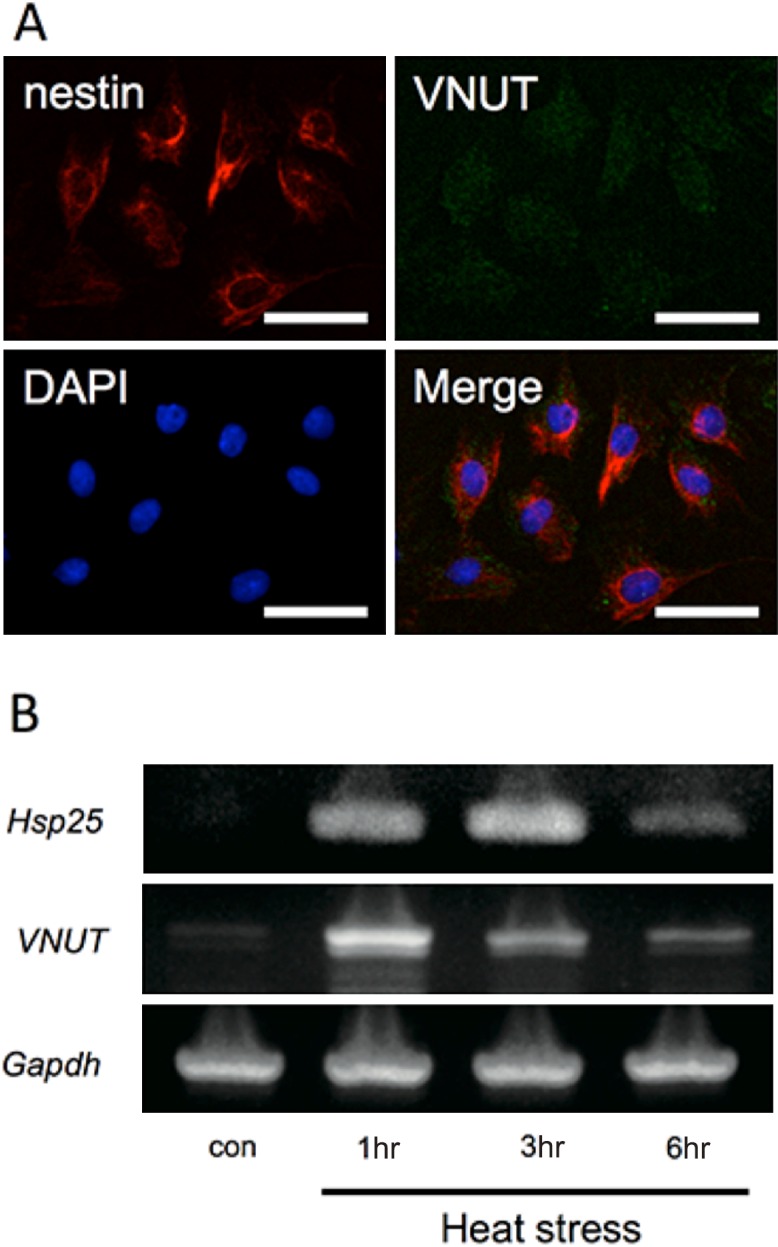
Immunohistochemical staining in KN-3 cells and Reverse-transcriptase PCR analysis for the expression of Hsp25 and VNUT in heat-treated KN-3 cells. (A) Immunofluorescence staining for VNUT and nestin and DAPI and merged in KN-3 cells. (B) Expression of Hsp25 was observed in KN-3 cells at 1, 3, and 6 hr after heat treatment. VNUT was most strongly expressed at 1 hr after heat treatment, subsequent to which the expression decreased gradually.
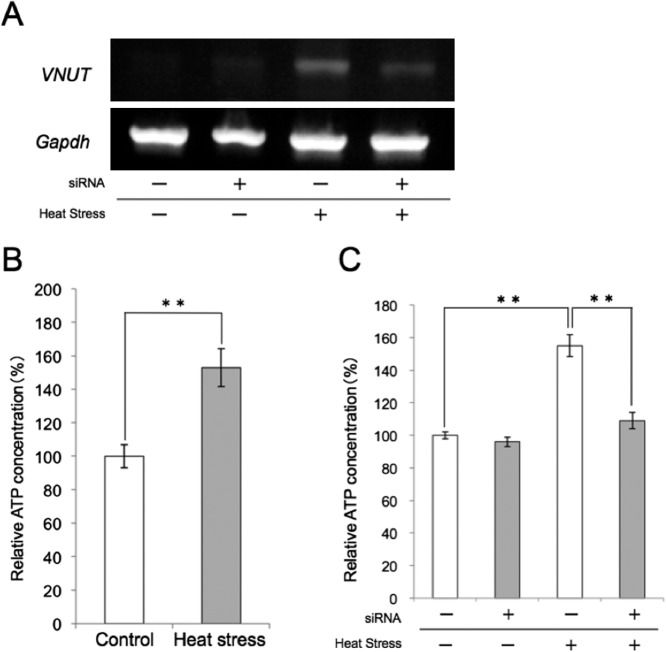
Finally, we examined the effects of VNUT siRNA interference on ATP release in KN-3 cells with and without heat stress. As indicated in Figure 4A, VNUT siRNA interference decreased VNUT mRNA expression after heat stress. RT-PCR was performed to confirm the effect after transfection. ATP release from KN-3 cells was then examined by an ATP luciferase assay. ATP release from KN-3 cells after heat treatment increased significantly compared with ATP release from non-heat-treated KN-3 cells (Fig. 4B). The increased ATP release after heat stress was decreased significantly by VNUT siRNA interference. There was no significant difference in ATP release between heat-stressed and non-heat-treated KN-3 cells after VNUT siRNA interference (Fig. 4C).
Fig. 4. .
ATP release by KN-3 cells after heat stress. (A) The expression of VNUT in non-heat-treated or heat-stressed KN-3 cells after siRNA or non-siRNA KN-3 cells. The expression of VNUT was strongest in heat-stressed KN-3 cells after non-siRNA treatment. (B) ATP release by heat-stressed KN-3 cells was significantly greater than the control (not heat treated). The values represent means±S.E. ** p<0.05. (C) The effect of heat stress under siRNA on ATP release by KN-3 cells. The increased ATP release after heat stress was decreased significantly by VNUT siRNA interference. The values represent means±S.E. **p<0.05.
IV. Discussion
Although the mechanism of pain transmission from odontoblasts to pulpal nerves remains to be clarified, in the present study we propose a possible mechanism for ATP signal transduction via VNUT in odontoblasts. Among the vesicle transporters, VNUT is a relatively recently discovered transporter that actively transports cytoplasmic ATP into vesicles [27]. If VNUT is present in odontoblasts, it may be involved in signal transduction from non-nerve cells to axons, as it is in taste buds [14]. In the present study, VNUT mRNA was detected in pulpal tissue, and then odontoblasts were shown to be immunopositive for VNUT. In a high-magnification view, ATP-immunopositive vesicle-like structures were detected in odontoblasts. This localization of VNUT in odontoblasts suggests the involvement of VNUT in ATP release from odontoblasts to pulpal axons. There are many reports that have shown the expression and function of TRP channels in odontoblasts [5, 6, 29, 31]. Taken together with these previous reports, this suggests that odontoblasts could receive stimulation by TRP channels on their membrane, activate VNUT, and release ATP to ATP receptors on axons in the pulp.
To confirm vesicle transport, we examined the colocalization of VNUT-positive vesicles with Snap25, a membrane fusion protein. The expression of Snap25 in dental pulp has been identified in previous studies [11]. In the present study, some VNUT-immunopositive vesicles colocalized with Snap25, close to the plasma membrane of odontoblasts. Because the colocalization of VNUT immunopositive vesicles and Snap25 suggested the direction of vesicle secretion, it was conjectured that ATP is being released in bilateral directions.
Furthermore, the P2X3 receptor, an ATP receptor, was demonstrated on the axons in the pulp mainly between odontoblasts (Fig. 2B). This is consistent with previous reports indicating the presence of ATP receptors in human dental pulp [1] and the expression of the ecto-ATPase NTPDase2 in dental pulp [20]. Not only the localization of VNUT but also the distribution of Snap25 in odontoblasts and the P2X3 receptor suggest an ATP-mediated signal transduction from odontoblasts to ATP receptors on axons in the pulp.
Next, we examined the functional role of VNUT for ATP release under thermal stimulation in odontoblast-like (KN-3) cells in vitro. After thermal stimulation, there was a rapid increase in VNUT mRNA expression, compared with the expression of Hsp25 mRNA. After KN-3 cells were exposed to heat stress at 43°C for 45 min, Hsp25 mRNA increased gradually from 1 to 3 hr after heat treatment, whereas VNUT mRNA was expressed at the highest level at 1 hr after heat treatment and then decreased. It was conjectured that the rapid increase in VNUT mRNA expression was due to its role in sensory signal transduction. Additionally, in VNUT siRNA cells, VNUT gene expression was barely increased compared with the case in cells that received the negative control siRNA. Odontoblasts have been suggested to contribute to thermal responses in the tooth via expression of TRP channels [5, 6, 31]. Although it remains to be determined which TRP channel(s) are associated with the activation or expression of VNUT in odontoblasts, our findings indicate that VNUT is involved in the thermal response of odontoblasts. Because there are some candidate molecules for ATP release in odontoblasts, we confirmed the role of VNUT in ATP release in odontoblasts using siRNA. Consequently, extracellular ATP release by thermal stimulation of odontoblast-like cells increased significantly compared with the control, and the increased ATP release by thermal stimulation was decreased significantly, to control levels, by adding siRNA. In a recent study, it is reported that pannexin 3 is involved in the release of ATP by external stimulus in the pulp [21]. However, our findings indicate that ATP release with thermal stimulation is related to the expression of VNUT. Further study is needed to clarify which TRP channel is involved in the expression of VNUT and how TRP channel signaling leads to the expression or activation of VNUT in odontoblasts.
In conclusion, the present study demonstrated that cytosolic ATP is transported by VNUT and that ATP in vesicles is released from odontoblasts to ATP receptors on axons. ATP vesicle transport in odontoblasts would seem to be a key mechanism for signal transduction from odontoblasts to axons in the pulp. From our findings, we propose a model of thermal pain signal transduction via a TRP-VNUT ATP release pathway, and suggest that VNUT may be a target molecule for the treatment of dentine hypersensitivity.
V. Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, to T. Goto (#26462791).
VI. References
- 1.Alavi A. M., Dubyak G. R. and Burnstock G. (2001) Immunohistochemical evidence for ATP receptors in human dental pulp. J. Dent. Res. 80; 476–483. [DOI] [PubMed] [Google Scholar]
- 2.Brannstrom M. and Johnson G. (1970) Movements of the dentine and pulp liquids on application of thermal stimuli. An in vitro study. Acta Odontol. Scand. 28; 59–70. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. and Wood J. N. (1996) Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr. Opin. Neurobiol. 6; 526–532. [DOI] [PubMed] [Google Scholar]
- 4.Chung G., Jung S. J. and Oh S. B. (2013) Cellular and molecular mechanisms of dental nociception. J. Dent. Res. 92; 948–955. [DOI] [PubMed] [Google Scholar]
- 5.Egbuniwe O., Grover S., Duggal A. K., Mavroudis A., Yazdi M., Renton T., Di Silvio L. and Grant A. D. (2014) TRPA1 and TRPV4 activation in human odontoblasts stimulates ATP release. J. Dent. Res. 93; 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Karim I. A., Linden G. J., Curtis T. M., About I., McGahon M. K., Irwin C. R. and Lundy F. T. (2011) Human odontoblasts express functional thermo-sensitive TRP channels: Implications for dentin sensitivity. Pain 152; 2211–2123. [DOI] [PubMed] [Google Scholar]
- 7.El Karim I. A., Linden G. J., Curtis T. M., About I., McGahon M. K., Irwin C. R., Killough S. A. and Lundy F. T. (2011) Human dental pulp fibroblasts express the “cold-sensing” transient receptor potential channels TRPA1 and TRPM8. J. Endod. 37; 473–478. [DOI] [PubMed] [Google Scholar]
- 8.Gomes P., Srinivas S. P., Van Driessche W., Vereecke J. and Himpens B. (2005) ATP release through connexin hemichannels in corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 46; 1208–1218. [DOI] [PubMed] [Google Scholar]
- 9.Gunjigake K. K., Goto T., Nakao K., Kobayashi S. and Yamaguchi K. (2009) Activation of satellite glial cells in rat trigeminal ganglion after upper molar extraction. Acta Histochem. Cytochem. 42; 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton S. G. (2002) ATP and pain. Pain Pract. 2; 289–294. [DOI] [PubMed] [Google Scholar]
- 11.Honma S., Taki K., Lei S., Niwa H. and Wakisaka S. (2010) Immunohistochemical localization of SNARE proteins in dental pulp and periodontal ligament of the rat incisor. Anat Rec (Hoboken). 293; 1070–1080. [DOI] [PubMed] [Google Scholar]
- 12.Imura Y., Morizawa Y., Komatsu R., Shibata K., Shinozaki Y., Kasai H., Moriishi K., Moriyama Y. and Koizumi S. (2013) Microglia release ATP by exocytosis. Glia 61; 1320–1330. [DOI] [PubMed] [Google Scholar]
- 13.Ishido M. and Kasuga N. (2015) Characteristics of the localization of connexin 43 in satellite cells during skeletal muscle regeneration in vivo. Acta Histochem. Cytochem. 48; 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwatsuki K., Ichikawa R., Hiasa M., Moriyama Y., Torii K. and Uneyama H. (2009) Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem. Biophys. Res. Commun. 388; 1–5. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J. and Gu J. (2002) Expression of adenosine triphosphate P2X3 receptors in rat molar pulp and trigeminal ganglia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 94; 622–626. [DOI] [PubMed] [Google Scholar]
- 16.Kim J., Kobayashi M., Fukuda M., Ogasawara D., Kobayashi N., Han S., Nakamura C., Inada M., Miyaura C., Ikebukuro K. and Sode K. (2010) Pyrroloquinoline quinone inhibits the fibrillation of amyloid proteins. Prion 4; 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarowski E. R. (2012) Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 8; 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M., Luo Z. Y., Bai B. F., Xu F. and Lu T. J. (2011) Fluid mechanics in dentinal microtubules provides mechanistic insights into the difference between hot and cold dental pain. PLoS One 6; e18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linden L. and Brannstrom M. (1967) Fluid movements in dentine and pulp. An in vitro study of flow produced by chemical solutions on exposed dentine. Odontol. Revy. 18; 227–236. [PubMed] [Google Scholar]
- 20.Liu X., Yu L., Wang Q., Pelletier J., Fausther M., Sévigny J., Malmström H. S., Dirksen R. T. and Ren Y. F. (2012) Expression of ecto-ATPase NTPDase2 in human dental pulp. J. Dent. Res. 91; 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Wang C., Fujita T., Malmstrom H. S., Nedergaard M., Ren Y. F. and Dirksen R. T. (2015) External dentin stimulation induces ATP release in human teeth. J. Dent. Res. 94; 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magloire, H., Couble, M. L., Thivichon-Prince, B., Maurin, J. C. and Bleicher, F. (2009) Odontoblast: a mechano-sensory cell. J. Exp. Zool. B Mol. Dev. Evol. 312B; 416–424. [DOI] [PubMed] [Google Scholar]
- 23.Morotomi T., Kitamura C., Toyono T., Okinaga T., Washio A., Saito N., Nishihara T., Terashita M. and Anan H. (2011) Effects of heat stress and starvation on clonal odontoblast-like cells. J. Endod. 37; 955–961. [DOI] [PubMed] [Google Scholar]
- 24.Nishida K., Nomura Y., Kawamori K., Moriyama Y. and Nagasawa K. (2014) Expression profile of vesicular nucleotide transporter (VNUT, SLC17A9) in subpopulations of rat dorsal root ganglion neurons. Neurosci Lett. 5; 579; 75–79. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi F., Kitamura C., Nagayoshi M., Chen K. K., Terashita M. and Nishihara T. (2009) Ozonated water improves lipopolysaccharide-induced responses of an odontoblast-like cell line. J. Endod. 35; 668–672. [DOI] [PubMed] [Google Scholar]
- 26.Renton T., Yiangou Y., Baecker P. A., Ford A. P. and Anand P. (2003) Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J. Orofac. Pain 17; 245–250. [PubMed] [Google Scholar]
- 27.Sawada K., Echigo N., Juge N., Miyaji T., Otsuka M., Omote H., Yamamoto A. and Moriyama Y. (2008) Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. U S A 105; 5683–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibukawa Y., Sato M., Kimura M., Sobhan U., Shimada M., Nishiyama A., Kawaguchi A., Soya M., Kuroda H., Katakura A., Ichinohe T. and Tazaki M. (2015) Odontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflugers Arch. 467; 843–863. [DOI] [PubMed] [Google Scholar]
- 29.Son A. R., Yang Y. M., Hong J. H., Lee S. I., Shibukawa Y. and Shin D. M. (2009) Odontoblast TRP channels and thermo/mechanical transmission. J. Dent. Res. 88; 1014–1019. [DOI] [PubMed] [Google Scholar]
- 30.Tarsa L., Bałkowiec-Iskra E., Kratochvil F. J. 3rd, Jenkins V. K., McLean A., Brown A. L., Smith J. A., Baumgartner J. C. and Balkowiec A. (2010) Tooth pulp inflammation increases brain-derived neurotrophic factor expression in rodent trigeminal ganglion neurons. Neuroscience 167; 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsumura M., Sobhan U., Sato M., Shimada M., Nishiyama A., Kawaguchi A., Soya M., Kuroda H., Tazaki M. and Shibukawa Y. (2013) Functional expression of TRPM8 and TRPA1 channels in rat odontoblasts. PLoS One 8; e82233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirkner K., Sperlagh B. and Illes P. (2007) P2X3 receptor involvement in pain states. Mol. Neurobiol. 36; 165–183. [DOI] [PubMed] [Google Scholar]
- 33.Zhao H. B., Yu N. and Fleming C. R. (2005) Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. U S A 102; 18724–18729. [DOI] [PMC free article] [PubMed] [Google Scholar]