Abstract
Gastrointestinal helminth infections are extremely prevalent in many human populations, and are associated with down-modulated immune responsiveness. In the experimental model system of Heligmosomoides polygyrus, a chronic infection establishes in mice, accompanied by a modulated Th2 response and increased regulatory T cell activity. To determine if DC populations in the lymph nodes draining the intestine are responsible for the regulatory effects of chronic infection, we first identified a population of CD11clo non-plasmacytoid DCs which expand following chronic H. polygyrus infection. The CD11clo DCs are under-represented in magnetic bead-sorted preparations, and spared from deletion in CD11c-DTR mice. Following infection, CD11clo DCs did not express CD8, CD103, PDCA, or Siglec H and were poorly responsive to TLR stimuli. In DC:T cell co-cultures, CD11clo DCs from naïve and H. polygyrus-infected mice could process and present protein antigen, but induced lower levels of antigen-specific CD4+ T cell proliferation and effector cytokine production, and generated higher percentages of Foxp3+ T cells in the presence of TGF-β. Treg generation was also dependent on retinoic acid receptor signaling. In vivo, depletion of CD11chi DCs further favored the dominance of the CD11clo DC phenotype. Following CD11chi DC depletion, effector responses were dramatically inhibited but the expansion in regulatory T cell numbers following H. polygyrus infection barely compromised, showing a significantly higher regulatory:effector CD4+ T cell ratio compared to CD11chi DC-intact animals. Thus, the pro-regulatory environment of chronic intestinal helminth infection is associated with the in vivo predominance of a newly defined phenotype of CD11clo tolerogenic DCs.
Introduction
Helminth parasites infect over two billion humans in the world today, the vast majority of these infections being with gastrointestinal nematodes (1). Each species of gut nematode is able to neutralize host immunity and survive for many months or years. Moreover, there is an inverse relationship between helminth infection and the incidence of immunopathological diseases targetting allergens (2-4) or autoantigens (5-7). Hence, it is thought that active diversion and suppression of the immune system occurs, rather than simple ignorance, and that these parasites can generate or sustain an immunoregulatory environment.
To model this important relationship, we have studied dendritic cell (DC) populations in gastrointestinal nematode infections. DCs are a phenotypically diverse set of cells which play a fundamental role in sensing the presence of pathogens and selecting the mode of immune response mounted by antigen-specific T cells (8-10). The crucial role of DCs, rather than other cell types such as basophils, in generating anti-helminth effector responses has very recently been confirmed (11-13). The ability of DCs to sense and discriminate between different pathogen types enables them to direct CD4+ and CD8+ T cell responses to varying pathogens in a highly effective manner.
It is now recognized that DCs also play an essential immunoregulatory role, maintaining immunological tolerance (14-16) or dampening effector responses through the induction of regulatory cytokines and T cells (17-19). Notably, a diverse range of DC subtypes are able to act in this fashion, including ex vivo mucosal CD103+ DCs (20), CD11cloCD45RBhi DCs (21), splenic CD8+CD205+ cells (22) as well as myeloid DCs treated in vitro with pro-regulatory agents such as IL-10, TGF-β and retinoic acid (20, 23, 24). Regulatory DCs may themselves produce IL-10 (25) or indoleamine 2,3-dioxygenase (IDO) (26), or act through surface ICOS-L (27), CTLA-4 and PD-1 (28). It is interesting to note reports of enhanced regulatory DC activity in a number of pathogen contexts such as Bordetella pertussis (29), mycobacteria (30), Candida albicans (31) and malaria (32).
Several alternative models exist to explain how DCs differ in their ability to positively or negatively influence immunity. First, it has been known for some time that immature DCs which have not been classically activated (for example by TLR ligation) are able to induce regulatory, IL-10-producing T cells (33-35). Secondly, DCs derived from mucosal tissues have a high intrinsic pro-regulatory capacity, including but not restricted to the CD103+ subset mentioned above (36-38). Finally, rather than activation status or location per se, it has been suggested that specialized subsets of DCs are dedicated to the maintenance of tolerance; in particular, plasmacytoid DCs (pDCs) are more closely associated with immunoregulatory outcomes than other, conventional, types of DCs (27, 39).
Helminth infection is closely associated with the induction of a Th2 immune response characterized by the production of IL4 and IL13 by CD4+ T cells, exemplified by the model nematode Nippostrongylus brasiliensis, which is expelled after a short-lived acute infection (40). In this and other helminth systems, in vitro-derived DCs pulsed with parasite antigens are able to induce strongly Th2-polarized immune responses on adoptive transfer to naïve hosts (41, 42). Certain parasites, such as Heligmosomoides polygyrus, can also elicit the expansion of regulatory T cells, which suppress effector T cell proliferation, attenuate pathology and may allow survival within the host for many months or years (43-46). Although little information is yet available on the in vivo phenotype and function of DCs over the course of gastrointestinal nematode infection, we hypothesised that a pro-regulatory subset of DC would become more prominent during chronic H. polygyrus infection. This hypothesis is consistent with a study showing that pooled splenic and MLN DCs from H. polygyrus-infected mice suppressed immunity in Citrobacter rodentium-infected recipients (47). We therefore investigated whether DC populations during both acute and chronic infection models displayed altered phenotypic and functional characteristics consistent with the stimulation of an immunoregulatory environment.
Our results, presented below, demonstrate significant changes in DC composition during infection, in particular highlighting a novel CD11clo subset. We have previously reported that, within the CD11chi population purified by magnetic bead sorting, CD8αint, CD103+ DCs diminish in frequency in the MLN, but that overall CD11chi DCs from infected mice remain immunogenic (48). We now show that infection also expands a population of MLN CD11clo DCs identified by flow cytometry but which is poorly represented in magnetic bead-sorted preparations, and escapes deletion in CD11c-DTR mice. Critically, these DCs are most efficient at de novo induction of CD4+Foxp3+ Tregs, and predominate during chronic infection. Because primarily CD11chi DCs are eliminated in DTx-mediated depletion in CD11c-DTR mice, we are also able to show that the CD11clo subset which preferentially survives DTx treatment, maintains the frequency of Treg cells in vivo.
Materials and Methods
Mice
BALB/c, C57BL/6 and DO11.10 mice were bred and maintained in a specific pathogen-free facility at the University of Edinburgh. CD11c.DOG mice on the C57BL/6 background expressing the human diphtheria toxin receptor and the ovalbumin fragment aa 140-386 under the control of the CD11c promoter were rederived into the same facility from the line previously described (49, 50).
H. polygyrus and N. brasiliensis infection and parasite antigen preparation
Mice were infected with 200 H. polygyrus bakeri L3 larvae using a gavage tube or injected subcutaneously with 250 N. brasiliensis L3 larvae and harvested 7 days post-infection. Excretory-secretory antigens from adult H. polygyrus (HES) and N. brasiliensis (NES) were prepared as previously described (51, 52).
Dendritic cell phenotyping
Mesenteric lymph nodes (MLNs) were removed into HBSS before digesting in 0.5 mg/ml Liberase CI (Roche) for 30 minutes at 37°C in a shaking incubator with the addition of 0.02 M EDTA (pH 7.3) for the last 5 minutes. MLNs were then washed and homogenized in HBSS, centrifuged at 400 g for 5 minutes before resuspending in FACS buffer (0.5 % BSA, 0.05% sodium azide, 1×PBS). DCs were phenotyped by labeling with 1/200 FITC-conjugated antibody to MHC class II (clone M5/114.15.2; BioLegend), F4/80 (MF48020; Caltag), NK1.1 (clone PK136), CD24 (clone M1/69; eBioscience); PE-conjugated anti-CD40 (clone 3/23), CD80 (clone 16-10A1), CD86 (clone GL1), 1/25 PDCA-1 (clone JF05-1C2.4.1; Miltenyi); PerCP-conjugated anti-B220 (clone RA3-6B2), APC-conjugated anti-CD11c (clone N418; Biolegend), Ly6C (clone AL-21); PE-Cy7-conjugated anti-CD8 (clone 53-6.7; eBioscience); APC-ef780-conjugated anti-B220 (clone RA3-6B2; eBioscience), CD11c (clone N418; ebioscience), a combination of biotin-conjugated anti-CD103 (clone 2E7, eBioscience), CD8α (clone 53-6.7, eBioscience), CD11b (clone M1/70; BioLegend), Siglec-H (clone 551.3D3; Miltenyi) and streptavidin-conjugated PerCP (BioLegend) or PerCP-Cy5.5 (Biolegend) or the relevant isotype controls before analysing by FACS. All fluorochrome-labeled antibodies were obtained from BD Pharmingen unless otherwise stated, and samples were analyzed by flow cytometry using Becton-Dickinson Canto or LSR-II flow cytometers.
Dendritic cell purification
Following liberase digestion as described above, MLN were resuspended in FACS buffer (0.5 % BSA, 0.05% sodium azide, 1×PBS). Following labeling of cells with 1/200 PE-conjugated anti-CD3 (clone 17A2; BD Pharmingen) at 4°C for 15 minutes, cells were incubated with anti-PE microbeads (Miltenyi Biotec) and the flow-through collected from an LS column. After centrifugation at 400 g for 5 mins, cells were resuspended at 106/ml FACS buffer and labeled with 1/200 APC-conjugated anti-CD11c (clone N418; BioLegend) for 20 minutes, before washing as previously. Cells were sorted on a FACS Aria based on high and low expression of CD11c (CD11chi and CD11clo). Live cells were >90% pure following sorting. For experiments where B and T cells were depleted, cells were labelled with 1/200 PE-conjugated anti-IgM (clone RMM-1; Biolegend) and anti-IgD (clone 11-26c.2a; BD pharmingen) as well as anti-CD3 before collecting the flow through. Cells were sorted for CD11c expression and the absence of PE and CD49b PeCy7 (clone DX5; eBiosciences).
Dendritic cell antigen presentation, regulatory T cell induction and T cell polarization
Sorted CD11chi and CD11clo dendritic cells were plated in triplicate at 10:1, 1:1 and 1:10 ratios with 2×104 CFSE-labeled CD4+CD25– DO11.10 T cells in the presence of 0.03 μg/ml OVA peptide or 4mg/ml filter sterilised grade V Ovalbumin protein (Sigma). Having determined the optimal proliferative responses in the T cell population for both antigen conditions, regulatory T cell induction experiments were performed with a 1:1 ratio DC:T cell, 0.03ug/ml OVA peptide + 2 ng/ml recombinant TGF-β, 0.1 μg/ml OVA peptide + 2 ng/ml recombinant TGF-β, 0.03 μg/ml OVA peptide + 2 ng/ml recombinant TGF-β + 1 μM of the retinoic acid receptor antagonist LE540 (Tocris Bioscience), or 0.03 μg/ml OVA peptide alone. Cells were fed at days 2 and 4 with 5 ng/ml recombinant IL-2 and harvested at day 5. Antigen presentation and DO11.10 proliferation was assessed by CFSE dilution within the CD4+KJ126+ T cell population. Regulatory T cell induction was assessed by Foxp3 expression using PE-conjugated anti-Foxp3 (clone FJK-16s; eBiosciences) within the cultured CD4+KJ126+ T cell population and comparing it to freshly sorted CD4+KJ126+ T cells (99.8% Foxp3–). Supernatants from the co-cultures were harvested to analyse for T cell polarization by IFN-γ, IL4, IL-6, IL-10 and IL-17 production using commercially available cytokine ELISA (BD pharmingen).
Isolation of naïve transgenic CD4+ T cells for in vitro regulatory T cell induction
The spleen and peripheral lymph nodes were removed from a DO11.10 mouse, homogenized and labeled with CD4 (L3T4) microbeads (Miltenyi Biotec) before positive selection using an LS column. Cells were then labeled with PerCP-conjugated anti-CD4 (clone GK1.5; Biolegend) and PE-conjugated anti-CD25 (clone PD61 5.3; Caltag) for 15 minutes at 4°C before sorting the CD4+CD25– population on a FACSAria. Cells were labeled with 5 μM CFSE before co-culture using standard protocols (53).
In vivo depletion of dendritic cells
CD11c.DOG mice were depleted of CD11chiMHCII+ dendritic cells by intraperitoneal injection of 8 ng/g diphtheria toxin daily from day −1 to 6 after infection (49, 50). The efficacy of this depletion was assessed by liberase digestion of the spleen and MLN followed by flow cytometry using a combination of the aforementioned fluorochrome-conjugated antibodies specific for surface CD11c, MHC class II and B220. Plasmacytoid (PDCA-1+) DCs were depleted by administration of 100μg of 120G8 ip on days 0, 2, 4, 5, 8 and 11 post-infection with H. polygyrus.
Ex vivo MLN analysis following DC depletion
The MLNs of infected and naïve mice were removed for intracellular cytokine staining, regulatory T cell profiling and antigen-specific restimulation. For intracellular staining, 6×106 cells/well were plated in a 24-well plate with 0.5 μg/ml PMA and 1 μg/ml ionomycin for 1 hour before the addition of 10 μg/ml Brefeldin A for a further 3 hours. Cells were then washed, and blocked by resuspension in FACS buffer containing FcR block for 15 mins. After washing, cells were incubated with 1/200 anti-CD8-FITC and anti-CD4-PerCP for 20 minutes, washed again then fixed for 20 minutes with 200 μl Fix/Perm buffer (BD Pharmingen). Fixation buffer was removed with two washes with permeabilization buffer (BD Pharmingen) and samples were split and subsequently stained for intracellular cytokines using 1/200 anti-IFN-gamma-APC, anti-IL-4-PE, anti-IL-9 PE, anti-IL-10-APC, anti-IL-13-APC, anti-IL-17-PE or the relevant isotype control for 20 minutes in Perm buffer. After another wash in Perm Buffer, samples were resuspended in FACS buffer and analysed by flow cytometry using a Becton-Dickinson Canto or LSR-II flow cytometer. For regulatory T cell profiling, 106 cells were blocked as previously, then surface stained for 20 minutes with 1/200 anti-CD4-PerCP, anti-CD25-PE and anti-CD103-biotin with a combination of Streptavidin-PerCP. After washing, these cells were fixed with eBioscience Fix/Perm buffer for between 45 minutes and 18 hours in accordance with the manufacturer’s instructions, before washing twice with Perm buffer and staining with 1/200 anti-Foxp3-APC in Perm Buffer for 30 minutes. Samples were then washed in Perm buffer and resuspended in FACS buffer before analysis by flow cytometry. For antigen-specific restimulation, 106 cells were plated in the presence of media or 2 μg/ml HES for 72 hours at 37°C for 5% CO2 before, centrifuging at 400 g for 5 minutes and freezing the supernatants at −20°C, which were then analysed for IFN-γ, IL-4, IL-5, IL-10, IL-13 and IL-17 by commercially available ELISA (BD Pharmingen).
Statistical analysis
For statistical analysis comparing two groups, data were assessed for normality and equal variances, log transforming if necessary to achieve normality and equal variance. If the data fulfilled these criteria an unpaired t test was used, while if this was not possible the non-parametric Mann-Whitney U test was used. Where more than three groups were being tested, data was assessed and if necessary transformed similarly for variance and normality and either a parametric 1 way ANOVA (followed by Tukey’s multiple comparison test) or a non-parametric Kruskal-Wallis test (followed by a Dunns multiple comparison) used.
Results
Predominance of CD11clo DC population in the MLN following chronic helminth infection
Gastrointestinal helminth infections stimulate powerful Th2-dominated immune responses both to parasite and bystander antigens (40), as well as in certain cases Foxp3+ Treg expansion (43, 44). Although we and others have previously shown that the Th2 response to helminths such as N. brasiliensis (42) and Schistosoma mansoni (41) can be recapitulated through antigen-pulsed DCs, it is not yet known whether helminth-stimulated DC populations can favor the expansion of regulatory T cell activity.
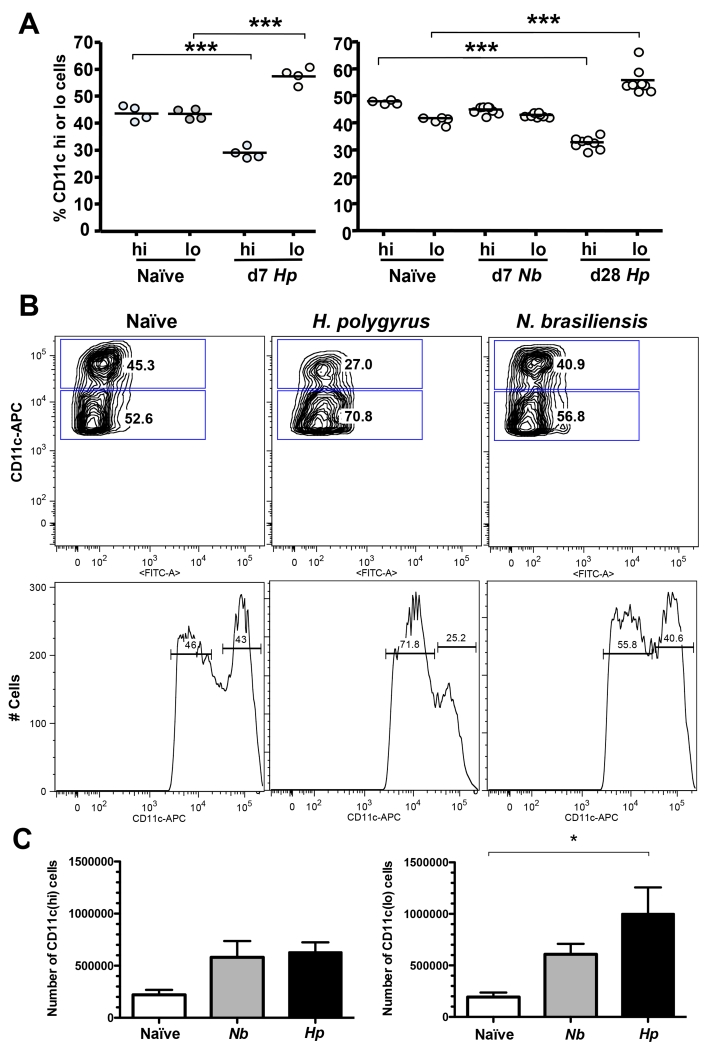
We therefore examined CD11c+ DC populations in the draining MLNs of mice infected with the nematode H. polygyrus, which establishes a chronic infection and drives Treg expansion, and N. brasiliensis which is expelled by a potent Th2 response after an acute infection of 6-8 days. MLNs were found to contain all the common subsets of DCs, conventional CD11chi (both CD11b+ and CD11b–), CD11chiCD103+ and plasmacytoid CD11cloB220+. However, 7 days following H. polygyrus infection, at a time point associated with magnified Treg numbers (43) the proportion of CD11clo to CD11chi DCs showed a striking increase (Fig 1 A), which remained in place at 28 days (Fig 1 A, B). Within the MLNs of H. polygyrus infected mice, conventional CD11chi represented only 30% of the DC population, with a downshift in peak expression levels. In contrast, no significant alteration occurred in mice mounting effective immunity to expel N. brasiliensis (Fig 1 A, B). This change reflected preferential expansion of the CD11clo subset in H. polygyrus infection, which stimulates a doubling or more of total lymph node cell numbers (Fig. 1 C). Moreover, we could confirm a previous report (49), that the CD11clo population is under-represented in preparations sorted on anti-CD11c beads, and found this was true for both PDCA+ and PDCA– populations. Thus, flow-sorted DCs were used for all subsequent functional analyses.
Figure 1. CD11chi and CD11clo DC Subsets in Naïve and Infected MLNC.
A. Proportions of CD11chi and CD11clo DC subsets in MLNs of groups of naïve and infected mice. Left-hand panel shows naïve and d7 H. polygyrus infections; right hand panel d28 H. polygyrus and d7 N. brasiliensis infections. Significant differences in the percentage of CD11chi and CD11clo cells are denoted by ***(p ≤ 0.001).
B. Representative CD11c expression analyzed by flow cytometry in MLNs from individual naïve, d28 H. polygyrus-infected and d7 N. brasiliensis-infected mice, showing gatings used and the proportions of CD11chi and CD11clo populations.
C. Total numbers of CD11chi and CD11clo DC subsets in MLN for naïve, H. polygyrus and N. brasiliensis-infected infected mice. Significant difference is denoted by * (p ≤ 0.05).
Expansion of non-plasmacytoid CD11clo DCs in infection
To determine which DC subsets are most altered during H. polygyrus infection, more extensive phenotypic data were collected. The CD11chi population included most CD11bhi (Fig. 2 A, B), CD8αhi and CD8αint (Fig. 2 C) cells. In contrast, the CD11clo subset was found to be largely CD11b-negative and CD8α-negative, consistent with our previous report that CD11c+CD8αint DCs are lost from the MLNC during these nematode infections (48).
Figure 2. Phenotypes of CD11chi and CD11clo DC subsets in MLNC.
A. Representative flow cytometry plots of CD11b expression by CD11chi and CD11clo DC populations within naïve and d28 H. polygyrus-infected MLNs.
B. Percentage of total MLN cells represented by CD11chi and CD11clo DCs of either CD11b+ or CD11b– phenotype within naïve and d28 H. polygyrus-infected mice. Significant differences are denoted by ** (p ≤ 0.01).
C. Representative plots of CD8α expression by DC populations of naïve and H. polygyrus-infected mice.
D-K. Overlay of histograms showing expression levels among CD11chi (Black) and CD11clo (dark gray) DCs of the markers of PDCA-1 (D), Siglec H (E), B220 (F), CD19 (G), Ly6C (H), F4/80 (I), NK1.1 (J) and Siglec F (K). Isotype controls for each antibody are shown as light gray lines.
Because plasmacytoid dendritic cells (pDCs) express lower levels of CD11c and have been strongly implicated to have tolerogenic or pro-regulatory activity (27, 39, 54), we next analyzed the CD11clo population for markers of this cell type, namely B220, PDCA-1 (55) and Siglec H (56). In naïve mice, the CD11clo gate included most of the B220+PDCA-1+Siglec H+ pDCs, but following infection expression of both PDCA-1 (Fig. 2 D) and Siglec H (Fig. 2 E) actually declined. In addition, while a proportion of the CD11clo cells expressed B220 (Fig 2 F) and CD19 (Fig. 2 G), and the monocyte marker Ly6C (Fig. 2 H) they did not express F4/80 (Fig. 2 I), NK1.1 (Fig. 2 J) or the eosinophil marker Siglec F (Fig. 2 K).
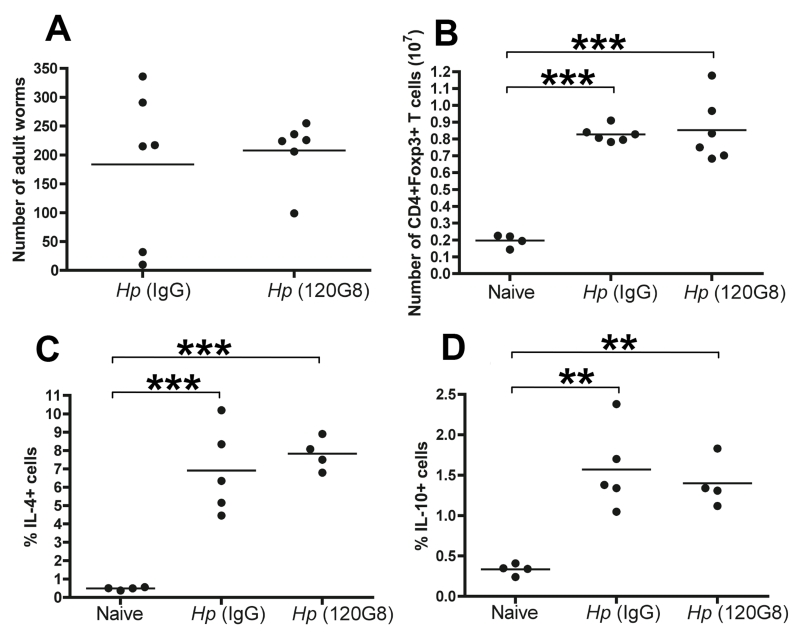
To further test whether the small number of pDCs within the CD11clo population play a tolerogenic role in H. polygyrus infection, we tested the effects of pDC depletion with the anti-PDCA-1 antibody 120G8 (55), prior to and during H. polygyrus infection. We continued the depletion protocol until day 11, when the adult H. polygyrus have emerged from the gut wall and mice were analysed at d28 to determine the effect of depletion on chronic worm burden and T cell responses. Depletion did not alter adult worm survival (Fig. 3 A) or the increase in regulatory T cell numbers seen with H. polygyrus infection at this time point (Fig. 3 B). Th2 responses, as measured by intracellular IL-4 (Fig. 3 C) and IL-10 (Fig 3 D), as well as IL-5 and IL-13 recall responses to HES antigen (data not shown) were undiminished in anti-120G8 treated mice.
Figure 3. Depletion of PDCA-1+ plasmacytoid DCs does not alter response to infection.
Plasmacytoid DCs were depleted in vivo by administration of 120.G8 antibody from d0-d11 of infection with H. polygyrus, and parasitological and immunological parameters measured at d28. Data are representative of two similar experiments and significant differences are denoted by ** (p ≤ 0.01) and ***(p ≤ 0.001). No significant differences were found between recipients of 120G.8 and control IgG antibodies.
A. Intestinal worm burden
B. Foxp3+ T cell numbers in MLN
C, D Intracellular staining for IL-4 and IL-10, gated on CD4+ T cells
CD11clo DCs do not express high levels of CD103
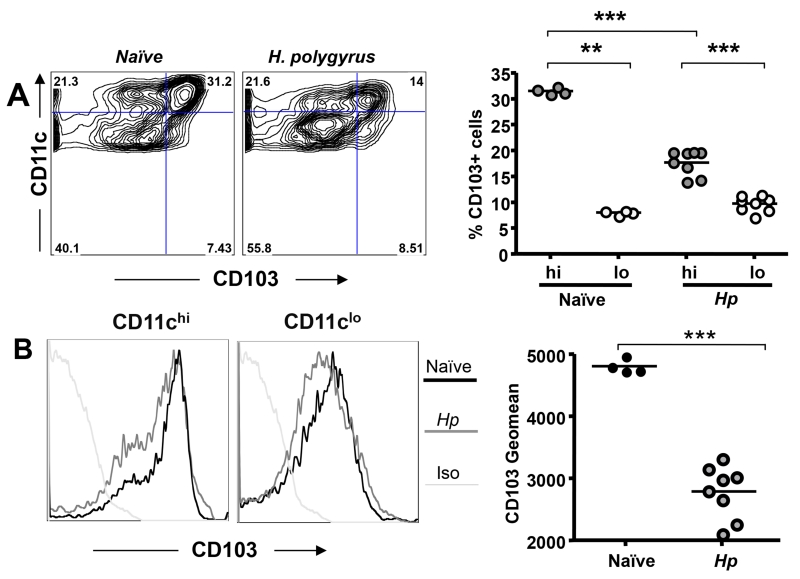
Expression of CD103, the αE chain of integrin αEβ7, has been reported to be an important attribute of mucosal DCs involved in immune regulation, as demonstrated with magnetic bead-purified (ie CD11chi) cells (20, 38, 57). We therefore measured levels of this marker in MLN DCs from naive and infected mice. Surprisingly, despite the overall pro-regulatory environment associated with H. polygyrus infection, we found that the proportion of CD103-expressing CD11c+ cells declined sharply. Few CD11clo DCs expressed CD103 either in the naïve or infected MLN, and by d28, the predominant DC phenotype (>50%) was CD11cloCD103– (Fig. 4 A). Notably, CD103 expression was restricted to the CD11chi population (Fig. 4 A), but even within the latter, CD103 intensity waned following infection (Fig. 4 B).
Figure 4. CD103 Expression on DCs during Gastrointestinal Nematode Infection.
A. Expression of CD103 within the CD11c+ DC population in naïve and d28 H. polygyrus-infected mice; left hand panel shows representative bivariant plots, right hand panel presents percentages of the indicated CD11c+ subset expressing CD103 in MLNs from experimental groups of 4-8 mice. Significant differences in the expression of CD103 are denoted by **(p ≤ 0.01) and ***(p ≤ 0.001).
B. Intensity of CD103 expression on CD11chi and CD11clo MLN DCs from naïve and day 28 H. polygyrus infected mice; isotype controls are shown in light gray. Representative histograms (left panels) and mean MFI (right panel) from experimental groups of 4-8 mice are presented.
Co-stimulatory phenotype of DC populations in the MLN following infection
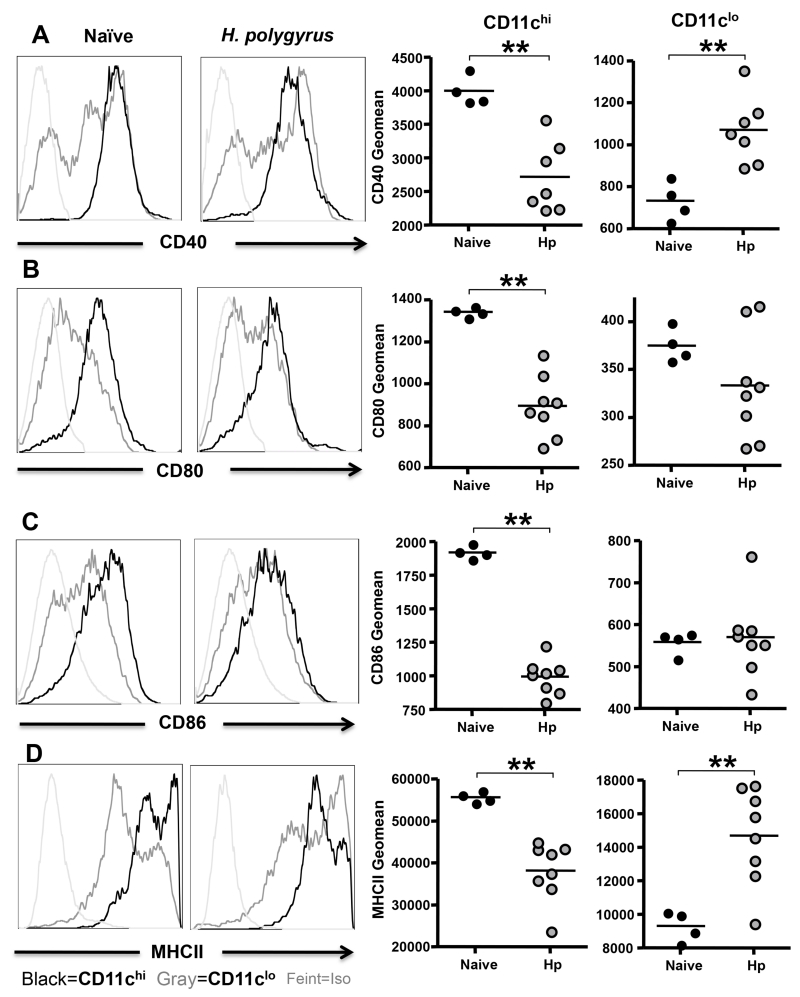
We then compared the expression of CD40, CD80, CD86 and MHCII among CD11c subsets following infection with H. polygyrus. In both naïve and infected mice, the CD11clo DC population expressed lower levels of CD40, CD80, CD86 and MHC class II compared to the CD11chi population, but the two subsets showed converse trends as a result of infection. Thus, each surface marker was downregulated in the CD11chi population of DCs from infected mice (Fig. 5 A-D), while CD40 and MHCII expression were significantly increased in the population of CD11clo DCs following infection (Fig. 5 A, D). Hence, the CD11chi population in infection presents with a lower level of maturation and activation markers than is found in naïve animals, while the CD11clo subset displays significant upregulation of MHCII and CD40.
Figure 5. Activation Phenotypes of DCs in Naïve and Infected MLNC.
A. Expression of the costimulatory molecule CD40 by CD11chi and CD11clo dendritic cells from the MLN of naïve and d28 H. polygyrus-infected mice. Left hand panels: histogram of CD40 expression in naïve mice and H. polygyrus infections; black line denotes CD11chi; gray line, CD11clo; isotype controls are shown in light gray. Right hand panels: geometric mean fluorescent intensity for CD40 among CD11chi and CD11clo dendritic cells; note different scales reflecting substantially lower expression levels on CD11clo DCs.
B-D. As above, expression of CD80 (B), CD86 (C) and MHCII (D).
Significant differences in costimulatory molecule expression are denoted by **(p ≤ 0.01)
Characterization of CD11clo DC function following H. polygyrus infection
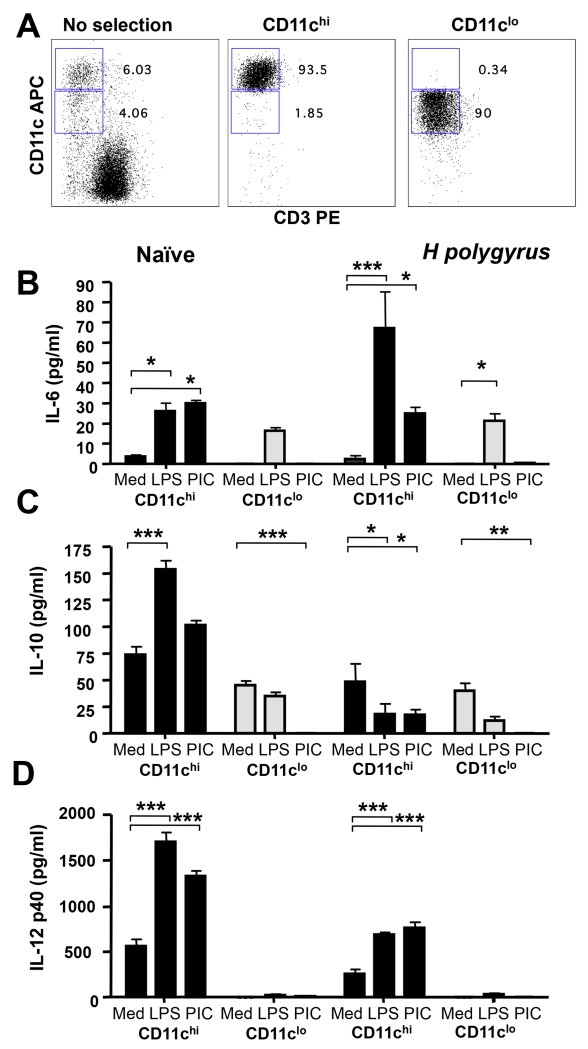
Having demonstrated significant increases in the ratio of CD11clo:CD11chi DC in the MLN of H. polygyrus infected mice, we compared the functional profile of these cells in terms of responsiveness to innate ligands and potential for antigen presentation to T cells. To isolate required numbers of each cell type, MLNs from naïve and H. polygyrus-infected mice were pooled, treated with liberase/EDTA, depleted of CD3+ cells, and flow sorted on CD11c expression. Purities of >90% were achievable, with <2% of CD11chi DCs within the CD11clo preparation, and vice versa (Fig. 6 A).
Figure 6. Responses of CD11chi and CD11clo DCs to TLR ligation.
A. Representative flow cytometry of unseparated MLNs (left-hand panel) and FACS-sorted CD11chi (center panel) and CD11clo DCs (right-hand panel).
B-D FACS-sorted CD11chi and CD11clo MLN cells from naïve and d7 H. polygyrus-infected mice were cultured with medium alone or the TLR ligands LPS or PolyIC. Concentrations of IL-6 (B), IL-10 (C) and IL-12p40 (D) in culture supernatants harvested after 3 days were measured by ELISA. No IL-12p70 or IFN-α was detected. Significant differences are denoted by * (p ≤ 0.05), ** (p ≤ 0.01) and ***(p ≤ 0.001).
The two subsets from naive and infected mice were first tested for responsiveness to TLR ligands LPS and poly-IC. Naive CD11chi DCs were highly responsive to stimulation, producing IL-6 (Fig. 6 B), IL-10 (Fig 6 C) and IL-12p40 (Fig. 6 D); however, production of the latter two cytokines was diminished in equivalent cells from H. polygyrus-infected mice. In contrast, CD11clo DCs failed to respond to TLR ligation with IL-10 and IL-12p40. Production of IL-6 by CD11clo DCs was in response to LPS alone and was unaffected by infection status. The restricted TLR responsiveness corresponded to the relatively low levels of TLR-2, -3, -4 and -9 expressed by the CD11clo population as determined by RT-PCR (data not shown).
T cell polarization
To test the capacity of these cells to stimulate an antigen-specific CD4+ effector T cell response, CD11chi and CD11clo cells from naïve and H. polygyrus-infected mice were pulsed with OVA peptide in co-cultures with CFSE-labelled CD4+CD25− DO11.10 T cells. While CD11chi DCs were able to drive a high level of T cell proliferation, this was not the case for the CD11clo subset from both naïve and H. polygyrus-exposed mice (Fig. 7 A). In similar in vitro cultures, cytokine production in response to presentation by each subtype was measured. Naive CD11chi DCs strongly stimulated IFN-γ, IL-4, IL-6, IL-10 and IL-17 (Fig. 7 B-F); following infection, responses elicited by this subset were similar, but with higher IL-4 showed a skew towards Th2. CD11clo DCs were consistently poorer at generating T cell cytokine responses; following infection, this subset showed enhanced stimulation of IL-10 responses (Fig. 7 E), but it was notable that negligible IL-6 or IL-17 production was elicited by CD11clo DCs (Fig. 7 D, F).
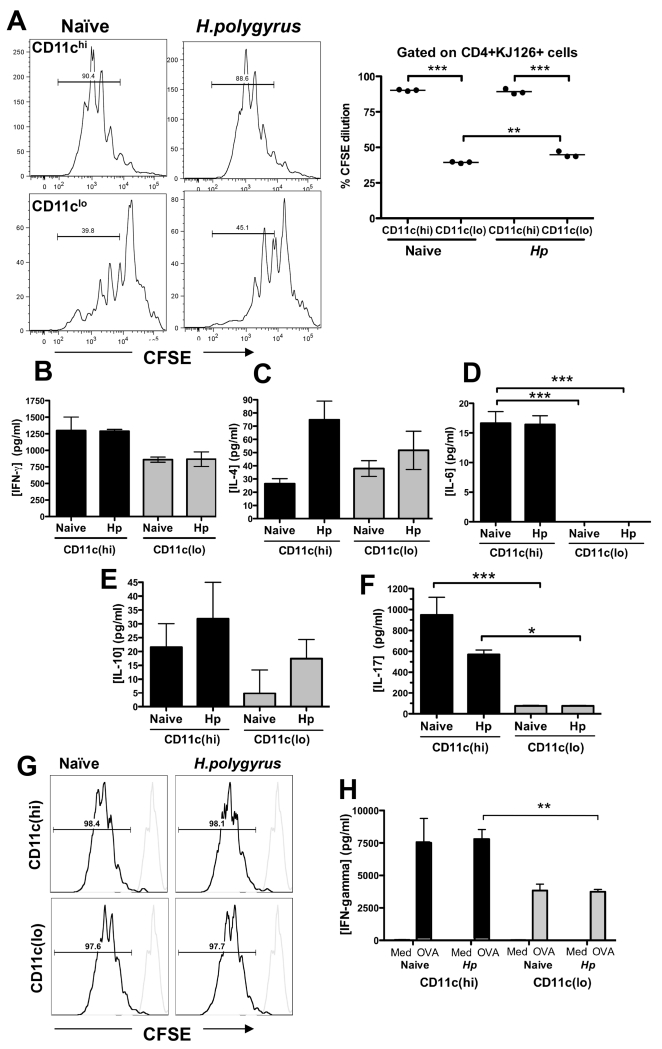
Figure 7. Ability of CD11chi and CD11clo DCs to stimulate T cell responsiveness.
A. Proliferation of CFSE-labeled DO11.10 CD4+CD25– T cells following incubation with pooled CD11chi or CD11clo cells from naïve and H. polygyrus-infected mice and 0.03 μg/ml OVA peptide; a representative analysis from each DC type is shown in the left hand set of panels, and the right hand panel presents data from 3 individual wells. Gating was set on unstimulated labeled cells. Results are representative of 2 separate experiments. Significant differences in this and other panels are denoted by * (p ≤ 0.05), ** (p ≤ 0.01) and ***(p ≤ 0.001).
B-F. Induction of IFN-γ(B), IL-4 (C), IL-6 (D), IL-10 (E) and IL-17 (F) from CD4+CD25– DO11.10 T cells incubated with pooled CD11chi and CD11clo cells from naïve and H. polygyrus infected mice and 0.03μg/ml OVA peptide. Supernatants were harvested after 5 days of co-culture and analyzed for cytokines by ELISA. Results are representative of 2 separate experiments.
G-H. Presentation of ovalbumin protein to CD4+CD25– DO11.10 T cells incubated with pooled CD11chi and CD11clo cells from naïve and H. polygyrus infected mice, measured as dilution of CFSE label following proliferation (G) and antigen-dependent release of IFN-γ in the presence of OVA protein compared to medium alone (MED) control (H). Data are representative of 2 separate experiments.
Because the CD11clo DC subset was less effective at presenting peptide to T cells, we tested whether they were functional at the level of antigen processing. Flow-sorted DCs of each phenotype were incubated with ovalbumin protein antigen, and the response of co-cultured CFSE-labelled CD4+CD25− DO11.10 T cells measured by CFSE dilution (Fig. 7 G) and cytokine release (Fig. 7 H). The results showed that CD11clo DCs are capable of processing and presenting intact protein antigen, although stimulating a significantly weaker IFN-γ response from naive transgenic T cells.
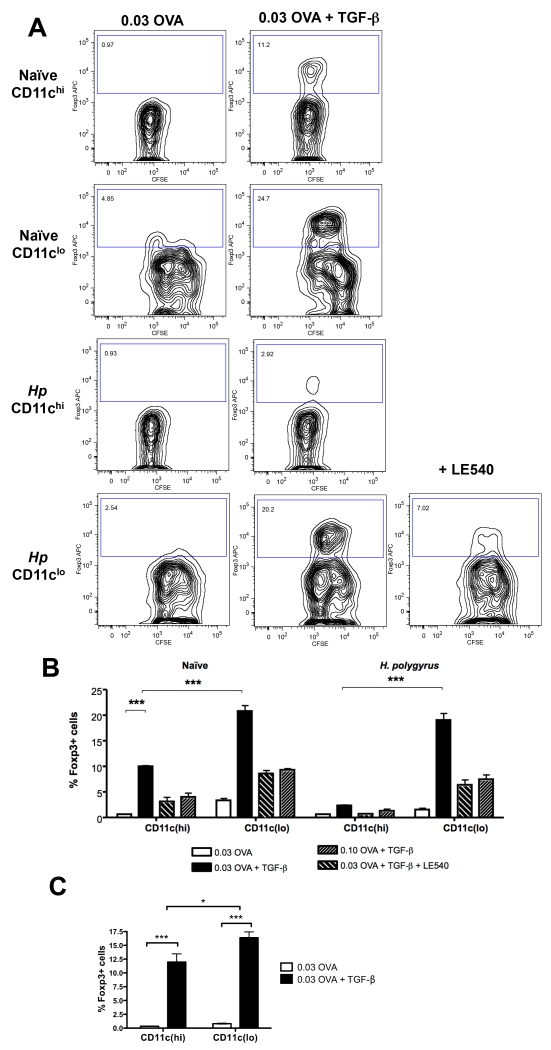
CD11clo DCs preferentially induce regulatory T cells
Because CD11clo DCs were found to be ineffective stimulators of the effector T cell response, we aimed to determine whether this reflected the absence of an activated phenotype (Fig. 5) or if they were able to induce regulatory T cell activity in vitro, potentially suppressing antigen-specific T cell proliferative and cytokine responses. We found that CD11clo cells, from either naïve or infected mice, showed dramatically enhanced ability to induce antigen-specific CD4+Foxp3+ T cells in culture compared to CD11chi cells when tested in the presence of exogenous TGF-β. Conversely, induction of CD4+Foxp3+ cells was significantly reduced by including the retinoic acid receptor antagonist LE540, or by increasing the concentration of OVA antigen (Fig. 8 A, B). The requirement for exogenous TGF-β reflected the low levels of TGF-β1 mRNA detected by RT-PCR in the CD11clo population (data not shown).
Figure 8. Induction of Foxp3+ T cells by CD11clo DCs.
A. Foxp3 expression by DO11.10 CD4+CD25– T cells following incubation with FACS-sorted CD11chi and CD11clo cells from naïve and H. polygyrus-infected mice, with 0.03 μg/ml OVA peptide and 2 ng/ml TGF-β in the presence or absence of 1 μM LE540.
B. Percentage of DO11.10 CD4+ Foxp3+ T cells under conditions of 0.03 μg/ml OVA peptide + 2 ng/ml TGF-β, 0.1 μg/ml OVA peptide + 2 ng/ml TGF-β, 0.03μg/ml OVA peptide + 2 ng/ml TGF-β + 1 μM LE540 or 0.03 μg/ml OVA peptide alone. Data shown are representative of three separate experiments. Significant differences in this and panel C are denoted by * (p ≤ 0.05) and ***(p ≤ 0.001).
C. Percentage of DO11.10 CD4+ Foxp3+ T cells following incubation of B- and T-cell depleted, FACS-sorted CD11chi and CD11clo cells from naïve and H. polygyrus- infected mice, with or without 0.03 μg/ml OVA peptide and 2 ng/ml TGF-β. Data shown are representative of two separate experiments.
To exclude any influence of either B or T lymphocytes that may be present in the CD11clo population, further experiments were undertaken in which these cell types were depleted by magnetic bead negative selection using anti-CD3, anti-IgM and anti-IgD prior to flow sorting by CD11c expression levels. B- and T-cell-depleted CD11c DCs from H. polygyrus-infected mice were then tested for their ability to induce a regulatory T cell phenotype and were found to strongly drive Foxp3 expression in naive CD4+CD25− DO11.10 T cells, with a significantly greater effect induced by the CD11clo subset.
CD11c DC depletion in vivo
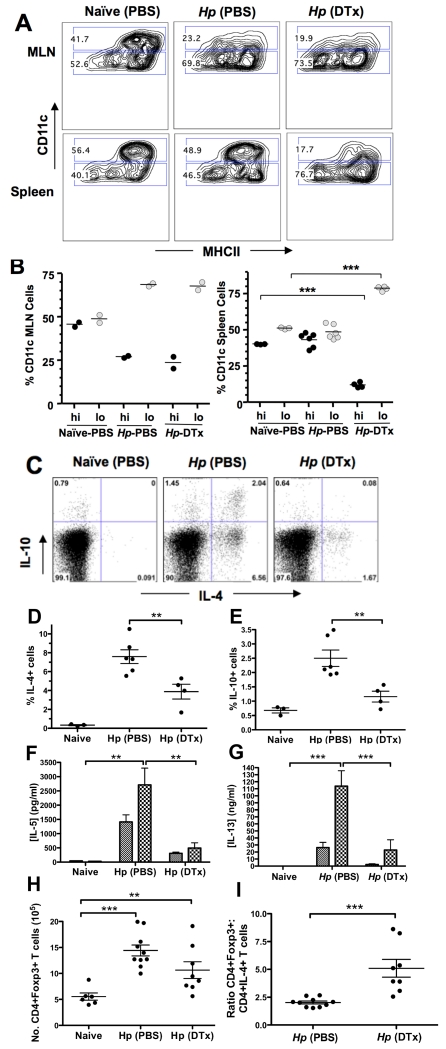
In order to test the in vivo role of CD11clo DCs, we altered the ratio of CD11clo:CD11chi DCs in vivo through the use of CD11c.DOG mice, depleting CD11chi MHC class II+ DCs using daily administration of 8 ng/g DTx (50). This regime resulted in effective depletion of CD11chi DCs from the MLN and spleen of H. polygyrus-infected mice (Fig. 9 A), and an increase in the proportion of CD11clo to CD11chi DCs in both the MLN and spleen (Fig. 9 B). Notably, DTx treatment achieved a systemic depletion, whereas in H. polygyrus infection the loss of CD11chi DCs occurred only in the MLN. Following 7 days of H. polygyrus infection, intracellular cytokine staining demonstrated that CD11chi MHC class II+ depletion resulted in a significant reduction in the percentage of IL-4 and IL-10 cytokine-synthesizing CD4+ T cells in the MLN (Fig. 9 C-E). Antigen-specific re-stimulation of total MLN cells also demonstrated dramatic reduction in IL-5 and IL-13 secretion following CD11chi MHC class II+ depletion (Fig. 9 F, G), confirming that the effector Th2 response had been greatly diminished in the absence of CD11chi DCs. In contrast, depletion with DTx did not abolish the expansion in CD4+Foxp3+ Treg MLN cell numbers resulting from H. polygyrus infection (Fig. 9 H). The combination of these effects resulted in a markedly higher regulatory:effector ratio as measured by Foxp3 and IL-4 expression respectively (Fig. 9 I).
Figure 9. In vivo depletion of CD11chi and predominance of CD11clo DCs in CD11c.DOG mice.
A. CD11chi depletion in the MLN and spleen of day 7 H. polygyrus-infected, DTx-treated or PBS control treated mice, confirmed by flow cytometry based on surface expression of CD11c and MHC class II.
B. Percentage of CD11chi and CD11clo DCs in the MLNs and spleens of infected, DTx-treated or PBS control mice.
C-E Cytoplasmic IL-4 and IL-10 expression by CD4+ T cells shown by intracellular cytokine staining of MLN cells from infected, DTx-treated or PBS control mice. Panel C shows representative bivariate plot for intracellular IL-4 and IL-10, with data from individual animals shown if D for IL-4 and E for IL-10. Significant differences in this and other panels are denoted by **(p ≤ 0.01) and ***(p≤0.0005)
F, G Antigen-specific Th2 cytokine responsivness in cultures of MLN cells from infected, DTx-treated or PBS control mice, restimulated with H. polygyrus HES antigen and assayed for IL-5 (G) and IL-13 (H) production.
H, I Numbers of CD4+Foxp3+ MLN T cells, 7 days following infection in the presence and absence of CD11chiMHCII+ DC depletion (H)., and ratio of Foxp3+:intracellular IL-4+ CD4+ T cells in the MLN of H. polygyrus-infected CD11c.DOG mice receiving control or DTx administration (I).
Discussion
Many parasitic infections are accompanied by, and may owe their longevity to, heightened immunoregulatory responses in their host (58-60). Regulatory cell populations may be directly stimulated by pathogens for their survival (61), by commensal organisms in the steady state (62) or activated by host mechanisms to forestall pathology, depending on the particular setting in question (63). Here, we studied a gastrointestinal nematode, H. polygyrus, in which a marked immunoregulatory response develops, including the expansion of Foxp3+ regulatory T cells (43, 44, 46) during chronic infection.
Dendritic cell populations play a decisive role T cell subset differentiation in response to pathogens (8), and are of integral importance for intestinal immunity (64) and homeostasis (65). DCs pulsed with helminth products selectively drive the development of Th2 cells (41, 42, 66-68), and the depletion of DCs dramatically impairs Th2 induction by Schistosoma mansoni (13) and, as we now confirm, H. polygyrus. While a critical role for basophils, rather than DCs, has been suggested for Th2 reactivity to the colon-dwelling nematode Trichuris muris (69), this does not hold for other infections: the magnitude and balance of Th2 responsiveness to S. mansoni (13) and N. brasiliensis (11) are unaffected by basophil depletion, as we have also found for H. polygyrus infection (Smith, K.A. et al, unpublished data). Hence, it is appropriate to place due emphasis on how host DC subsets, and their ability to induce regulatory T cells, change with infection. For this reason, we analyzed the DC population within the immune tissue most affected by gastrointestinal infection, the MLN.
A significant transformation takes place within the MLN DC population, with important regulatory consequences. First, preferential expansion of a non-plasmacytoid DC subset, with a CD11cloCD103– phenotype, occurs within 7 days of infection and eclipses conventional CD11chi subsets. Not only is the CD11clo:CD11chi ratio reversed, but the remaining CD11chi cells show diminished expression of functional markers, including CD103. Secondly, the CD11clo cells are poor stimulators of T cell proliferation or cytokine production, while being potent inducers of de novo Foxp3+expression. Reflecting this, in vivo depletion of the CD11chiMHCII+ population accentuates CD11clo predominance, resulting in a greatly reduced effector response and a higher percentage of regulatory T cells. Thus, chronic infection is associated with the emergence of a CD11clo DC population which is directly implicated in the induction of regulatory T cell responses in vitro and in vivo.
CD11clo DCs are mostly, in naïve mice, classified as plasmacytoid by their B220+PDCA-1+Siglec-H+ phenotype. However, in H. polygyrus infection, the frequency of pDCs declines and CD11clo cells do not mount an IFN-α response to TLR ligation (data not shown). Further, pDCs do not appear to play a critical role in H. polygyrus infection, as their depletion with anti-120G8-mediated impacts on neither Th2 responsiveness nor CD4+Foxp3+ T reg expansion. The induced CD11clo subset expresses low levels of F4/80, CD11b and CD8 and is therefore distinct from tolerogenic lymphoid-derived CD8+ DCs (70). They do not express NK1.1 or high levels of Ly6C, and so are unlikely to represent a population of interferon-producing killer DCs (71), pulmonary inflammatory DCs (72), or the Ly6C+CD11clo DCs found in Leishmania infection (73). Interestingly, CD11clo populations are quite apparent in published studies of Peyer’s patches (74), lamina propria (36) and MLNs (75).
A diverse range of DC types have been demonstrated to be capable of driving Treg induction and/or expansion (18). In steady-state conditions, where many DCs are immature and activation signals are lacking, most DC subsets are more tolerogenic and able to induce regulatory T cell gene expression (28, 33). Certain phenotypes are endowed with intrinsic pro-regulatory properties, such as the CD11chiCD103+ cells from MLN and lamina propria which produce endogenous TGF-β and retinoic acid to induce Foxp3 expression in T cells in vitro (20, 76). In non-mucosal systems, both plasmacytoid (39) and non-plasmacytoid (32, 77) CD11clo cells are tolerogenic and elicit regulatory cytokines, while in the skin Tregs are induced by CD103-negative CD11chi DCs (78). Hence, our finding of regulatory T cell induction by mucosal CD11clo DCs highlights a novel function of a subset which shares some, but not all, phenotypic properties of previously described pro-regulatory cells.
Consistent with their attenuated co-stimulatory profile, CD11clo DCs can weakly induce T cell proliferation, but may also influence T cell polarization by eliciting low levels of IFN-γ, IL-4 and IL-10 while failing to stimulate any IL6 or IL-17 release by CD4+ T cells. More strikingly, these cells efficiently induce CD4+Foxp3+ cells in vitro. This ability requires exogenous TGF-β and is inhibited by excess antigen and the retinoic acid receptor inhibitor LE540 in a similar manner to mucosal CD103+CD11chi dendritic cells (20). However, CD103 expression is downregulated on CD11chi cells following H. polygyrus infection, in tune with their reduced propensity to induce CD4+Foxp3+ T cells in vitro. Thus, although CD11chiCD103+ cells contribute to CD4+Foxp3+ T cell induction, this capacity declines following infection and is surpassed by the ability of CD11clo cells to perform this function.
Due to the lack of a unique marker for the CD11clo population, we were restricted in determining their function in vivo. However, we were able to model the predominance of the CD11clo population described following H. polygyrus infection by administrating diphtheria toxin (DTx) into CD11c.DOG mice. Here, we find effective depletion of CD11chi DCs with a compensatory increase in CD11clo frequency in the MLN and spleen of DTx-treated mice, which is more extensive than the MLN-restricted effect observed with active H. polygyrus infection. CD11chi depletion of infected mice decreased effector T cell activation while raising the percentage of CD4+Foxp3+ T cells. Interestingly, in mice with transgenic genetic ablation of CD11chi DCs, generation of Foxp3+ Tregs was found to be intact (79), again implying a pro-regulatory function for CD11clo DCs of one or another type.
Several key biological questions now need to be addressed. The primary pro-regulatory pathway invoked by CD11clo DCs we have identified is that of TGF-β/retinoic acid dependent induction of Foxp3, but as these are required exogenously, the intrinsic pro-regulatory characteristics of the CD11clo DC population remain to be identified. A second issue is whether the numerical expansion of DCs during infection represents increased migration of gut-derived antigen-laden cells, the accumulation of CD103– blood-borne precursors, or expansion in situ of resident cells (80, 81). Within the steady-state MLN, CD103+ DCs, and not CD103– cells, are those arriving from the mucosal sites (38, 82) suggesting that CD11clo cells differentiate from the precursor population, consistent with the fact that pre-DCs retain B220 expression prior to completing their maturation (83). Further, we have previously reported that the CD8αint population, which is restricted to cells migrating from the mucosa to the MLN (74), is diminished in H. polygyrus infection. If antigen-loaded DC migration to the MLN is impaired, while recruitment of tolerogenic DC is enhanced, the balance of response to antigen in the lymph node may primarily be a regulatory one.
Finally, it would be interesting to determine if the dominance of CD11clo DCs is a result of a parasite immune modulation strategy, or a host mechanism to control potentially damaging responses in the gut. H. polygyrus is known to secrete products that interfere with the pro-inflammatory functions of DCs (84, 85), and blocking mucosal DC migration to the MLN may be one result of this. On the other hand, the expansion of CD11clo DCs can occur in mice exposed to LPS (32) as well as other pathogens, and may represent a physiological response to infection stress. In combination, these two factors may result in the outcome we describe here. Further work is now in progress to resolve these issues.
Acknowledgements
The authors are grateful to Yvonne Harcus and Kara Filbey for maintenance of the H. polygyrus and N. brasiliensis life cycles; to Martin Waterfall and Andrew Sanderson for their flow sorting assistance, and to Alex-Phythian Adams for generous help with the transgenic lines. We thanks the Wellcome Trust and the Medical Research Council for support.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Biggelaar A, van Ree R, Roderigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 3.Maizels RM. Infections and allergy – helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Smits HH, Yazdanbakhsh M. Chronic helminth infections modulate allergen-specific immune responses: Protection against development of allergic disorders? Annals of medicine. 2007;39:428–439. doi: 10.1080/07853890701436765. [DOI] [PubMed] [Google Scholar]
- 5.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 6.Fleming J, Fabry Z. The hygiene hypothesis and multiple sclerosis. Ann Neurol. 2007;61:85–89. doi: 10.1002/ana.21092. [DOI] [PubMed] [Google Scholar]
- 7.Zaccone P, Burton OT, Cooke A. Interplay of parasite-driven immune responses and autoimmunity. Trends Parasitol. 2008;24:35–42. doi: 10.1016/j.pt.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Bravo M, Ardavin C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29:343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010 doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hämmerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 16.Rescigno M. Dendritic cells in tolerance induction for the treatment of autoimmune diseases. Eur J Immunol. 2010;40:2119–2123. doi: 10.1002/eji.201040474. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3CD25CD4 regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314–329. doi: 10.1111/j.0105-2896.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grainger JR, Hall JA, Bouladoux N, Oldenhove G, Belkaid Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol Rev. 2010;234:305–316. doi: 10.1111/j.0105-2896.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, Thomson AW. “;Alternatively activated”; dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–5877. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110:4293–4302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 26.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 29.McGuirk P, McCann C, Mills KHG. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams VC, Hunt JR, Martinelli R, Palmer R, Rook GA, Brunet LR. Mycobacterium vaccae induces a population of pulmonary CD11c+ cells with regulatory potential in allergic mice. Eur J Immunol. 2004;34:631–638. doi: 10.1002/eji.200324659. [DOI] [PubMed] [Google Scholar]
- 31.Bonifazi P, Zelante T, D’Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, Fallarino F, Puccetti P, Romani L. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362–374. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- 32.Wong KA, Rodriguez A. Plasmodium infection and endotoxic shock induce the expansion of regulatory dendritic cells. J Immunol. 2008;180:716–726. doi: 10.4049/jimmunol.180.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahnke K,E, Schmitt L. Bonifaz, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 36.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 37.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban JF, Madden KB, Sveti’c A, Cheever A, Trotta PP, Gause WC, Katona IM, Finkelman FD. The importance of Th2 cytokines in protective immunity to nematodes. Immunol. Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8– Dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 42.Balic A, Harcus Y, Holland MJ, Maizels RM. Selective maturation of dendritic cells by Nippostrongylus brasiliensis secreted proteins drives T helper type 2 immune responses. Eur. J. Immunol. 2004;34:3047–3059. doi: 10.1002/eji.200425167. [DOI] [PubMed] [Google Scholar]
- 43.Finney CAM, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rausch S, Huehn J, Kirchhoff D, Rzepecka J, Schnoeller C, Pillai S, Loddenkemper C, Scheffold A, Hamann A, Lucius R, Hartmann S. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect. Immun. 2008;76:1908–1919. doi: 10.1128/IAI.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tetsutani K, Ishiwata K, Ishida H, Tu L, Torii M, Hamano S, Himeno K, Hisaeda H. Concurrent infection with Heligmosomoides polygyrus suppresses anti-Plasmodium yoelii protection partially by induction of CD4+CD25+Foxp3+ Treg in mice. Eur J Immunol. 2009;39:2822–2830. doi: 10.1002/eji.200939433. [DOI] [PubMed] [Google Scholar]
- 46.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CAM, Greenwood EJD, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C-C, Louie S, McCormick BA, Walker WA, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J Immunol. 2006;176:472–483. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balic A, Smith KA, Harcus Y, Maizels RM. Dynamics of CD11c+ dendritic cell subsets in lymph nodes draining the site of intestinal nematode infection. Immunol Lett. 2009;127:68–75. doi: 10.1016/j.imlet.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hochweller K, Miloud T, Striegler J, Naik S, Hammerling GJ, Garbi N. Homeostasis of dendritic cells in lymphoid organs is controlled by regulation of their precursors via a feedback loop. Blood. 2009;114:4411–4421. doi: 10.1182/blood-2008-11-188045. [DOI] [PubMed] [Google Scholar]
- 50.Hochweller K, Striegler J, Hammerling GJ, Garbi N. A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur J Immunol. 2008;38:2776–2783. doi: 10.1002/eji.200838659. [DOI] [PubMed] [Google Scholar]
- 51.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 2000;30:1977–1987. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Harcus Y, Nicoll G, Murray J, Filbey K, Gomez-Escobar N, Maizels RM. C-type lectins from the nematode parasites Heligmosomoides polygyrus and Nippostrongylus brasiliensis. Parasitol Int. 2009;58:461–470. doi: 10.1016/j.parint.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 54.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, Coyle A, Clausen BE, Hoogsteden HC, Lambrecht BN, Hammad H. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol. 2009;183:1074–1082. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 55.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 57.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King CL, Kumaraswami V, Poindexter RW, Kumari S, Jayaraman K, Alling DW, Ottesen EA, Nutman TB. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B cell lymphocyte precursor frequency in the microfilaremic state. J. Clin. Invest. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor M, Allen JE. Helminth parasites: masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 60.Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J Clin Invest. 2009;119:1019–1028. doi: 10.1172/JCI36534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor M, Le Goff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 62.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions. Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 63.D’Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T cells: a role in the control of helminth driven intestinal pathology and worm survival. J Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fahlen-Yrlid L, Gustafsson T, Westlund J, Holmberg A, Strombeck A, Blomquist M, MacPherson GG, Holmgren J, Yrlid U. CD11c(high)dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J Immunol. 2009;183:5032–5041. doi: 10.4049/jimmunol.0803992. [DOI] [PubMed] [Google Scholar]
- 65.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 2000;164:6453–6460. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 67.Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, van der Bosch J, Mohrs K, Haas H, Mohrs M, Yazdanbakhsh M, Schramm G. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, Pardoll DM, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 72.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 74.Anjuère F, Luci C, Lebens M, Rousseau D, Hervouet C, Milon G, Holmgren J, Ardavin C, Czerkinsky C. In vivo adjuvant-induced mobilization and maturation of gut dendritic cells after oral administration of cholera toxin. J Immunol. 2004;173:5103–5111. doi: 10.4049/jimmunol.173.8.5103. [DOI] [PubMed] [Google Scholar]
- 75.Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujita S, Seino K, Sato K, Sato Y, Eizumi K, Yamashita N, Taniguchi M. Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood. 2006;107:3656–3664. doi: 10.1182/blood-2005-10-4190. [DOI] [PubMed] [Google Scholar]
- 78.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 79.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 81.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Segura E, Wong J, Villadangos JA. Cutting edge: B220+CCR9-dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J Immunol. 2009;183:1514–1517. doi: 10.4049/jimmunol.0901524. [DOI] [PubMed] [Google Scholar]
- 84.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: A potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 85.Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, Harris NL. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]