Abstract
Scaffold proteins play a critical role in cellular homeostasis by anchoring signaling enzymes in close proximity to downstream effectors. In addition to anchoring static enzyme complexes, some scaffold proteins also form dynamic signalosomes that can traffic to different subcellular compartments upon stimulation. Gravin (AKAP12), a multivalent scaffold, anchors PKA and other enzymes to the plasma membrane under basal conditions, but upon [Ca2+]i elevation, is rapidly redistributed to the cytosol. Because gravin redistribution also impacts PKA localization, we postulate that gravin acts as a calcium “switch” that modulates PKA-substrate interactions at the plasma membrane, thus facilitating a novel crosstalk mechanism between Ca2+ and PKA-dependent pathways. To assess this, we measured the impact of gravin-V5/His expression on compartmentalized PKA activity using the FRET biosensor AKAR3 in cultured cells. Upon treatment with forskolin or isoproterenol, cells expressing gravin-V5/His showed elevated levels of plasma membrane PKA activity, but cytosolic PKA activity levels were reduced compared with control cells lacking gravin. This effect required both gravin interaction with PKA and localization at the plasma membrane. Pretreatment with calcium-elevating agents thapsigargin or ATP caused gravin redistribution away from the plasma membrane and prevented gravin from elevating PKA activity levels at the membrane. Importantly, this mode of Ca2+/PKA crosstalk was not observed in cells expressing a gravin mutant that resists calcium-mediated redistribution from the cell periphery. These results reveal that gravin impacts subcellular PKA activity levels through the spatial targeting of PKA, and that calcium elevation modulates downstream β-adrenergic/PKA signaling through gravin redistribution, thus supporting the hypothesis that gravin mediates crosstalk between Ca2+ and PKA-dependent signaling pathways. Based on these results, AKAP localization dynamics may represent an important paradigm for the regulation of cellular signaling networks.
Keywords: A-kinase anchoring protein, gravin, calcium, AKAR3, protein kinase A, isoproterenol
1. Introduction
Intracellular signal transduction requires precise physical interactions between specific signaling proteins within a receptor-directed signaling cascade. It is now clear that many of these protein-protein interactions are facilitated by scaffold proteins and not by random diffusion [1]. A-Kinase Anchoring Proteins (AKAPs) play an integral role in this by compartmentalizing cAMP-dependent protein kinase (PKA) and other enzymes to specific subcellular locations. AKAPs share a conserved amphipathic helical domain that binds the regulatory subunit PKA and a subcellular targeting domain that serves to anchor PKA and often additional kinases, phosphatases, and other regulatory enzymes to a diverse array of subcellular compartments [reviewed in 2]. Interestingly, some AKAPs are more than static “anchors”, but can traffic to alternative subcellular compartments in response to stimuli [3–6].
Gravin (AKAP12), a 300 kDa AKAP with dramatic spatial targeting dynamics, anchors PKA and a host of other signaling enzymes to the plasma membrane through an N-myristoylation site and three polybasic domains (PB1-3). In response to either PKC activation or intracellular calcium ([Ca2+]i) elevation, gravin is redistributed away from the membrane along with PKA that is bound to gravin. Gravin redistribution by PKC activation was shown by Yan et al. [7] to redirect gravin and PKA to a juxtanuclear vesicular compartment. In response to [Ca2+]i elevation, Tao et al. [8] showed that gravin redistributes to the cytosol through a mechanism thought to involve Ca2+/calmodulin binding to gravin’s membrane-associated polybasic domains, PB1-3. A recent study from our laboratory further revealed that Ca2+-mediated gravin redistribution triggers the relocalization of PKA away from the membrane, and a fourth putative calmodulin binding domain which we call CB4 may also be critical in this event [9]. Furthermore, we also showed that receptor-mediated signaling triggers gravin/PKA redistribution to the cytosol through a mechanism involving both calcium and PKC [9]. These findings raise the interesting possibility that gravin serves as a membrane-localized “switch” that can direct PKA away from the plasma membrane to alternative subcellular compartments in response to Ca2+- and/or PKC signaling, thus facilitating crosstalk between these ubiquitous signaling pathways. However, gravin’s impact on subcellular PKA activity, both basally and following Ca2+ mediated redistribution, is poorly understood. This could have important implications for disease contexts that utilize crosstalk between Ca2+/PKC-dependent and PKA-dependent signaling pathways, such as cellular migration [10–12], cancer [reviewed in 13], learning and memory [14], cardiac function [15], and vascular biology [16, 17].
In the current study, we investigated the role of gravin in shaping subcellular PKA activity levels and in mediating crosstalk between Ca2+ and PKA-dependent signaling pathways. Gravin’s role in targeting PKA to the plasma membrane suggests that gravin potentiates PKA signaling at the plasma membrane. This in turn implies that Ca2+ elevation may diminish plasma membrane PKA activity by triggering the redistribution of gravin/PKA into the cytosol. We hypothesize that through this mechanism of redistribution, gravin mediates cross-talk between calcium and PKA-dependent signaling pathways. We tested this hypothesis by targeting the genetically encoded FRET-based PKA biosensor AKAR3 to the plasma membrane and to the cytosol [18] and measuring the impact of exogenous gravin expression on compartmentalized PKA activity within these compartments. In addition, we tested the impact of calcium-mediated gravin redistribution on plasma membrane PKA activity.
2. Materials and Methods
2.1 Cell culture and transfection
AN3 CA cells (Manassas, VA, ATCC number: HTB-111), a human endometrial metastatic cancer cell line that does not express endogenous gravin, and HEC 1A cells (ATCC HTB-112) were maintained at 37°C with 5% CO2 in low glucose Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 100 units/ml penicillin and 100ug/ml streptomycin. Growth medium was replaced three times each week and cells were split 1:25 upon reaching confluence.
Cells were plated at 25,000 cells per cm2 onto glass coverslips in a 6-well plate (9.6 cm2 per well). Upon 70% confluence (2–3 days), cells were transfected for an additional 24 hours in a solution containing 3μl/ml GeneJammer (Agilent) and 1μg/ml total plasmid DNA. For experiments involving co-transfection with AKAR3 and gravin constructs, it was critical that cells expressing AKAR3 were also expressing gravin. A 1:3 molar ratio of AKAR3:gravin plasmid DNA in the transfection mixture yielded transfected cultures in which roughly 90% of cells expressing AKAR3 were also positive for gravin expression (See Fig. 1F).
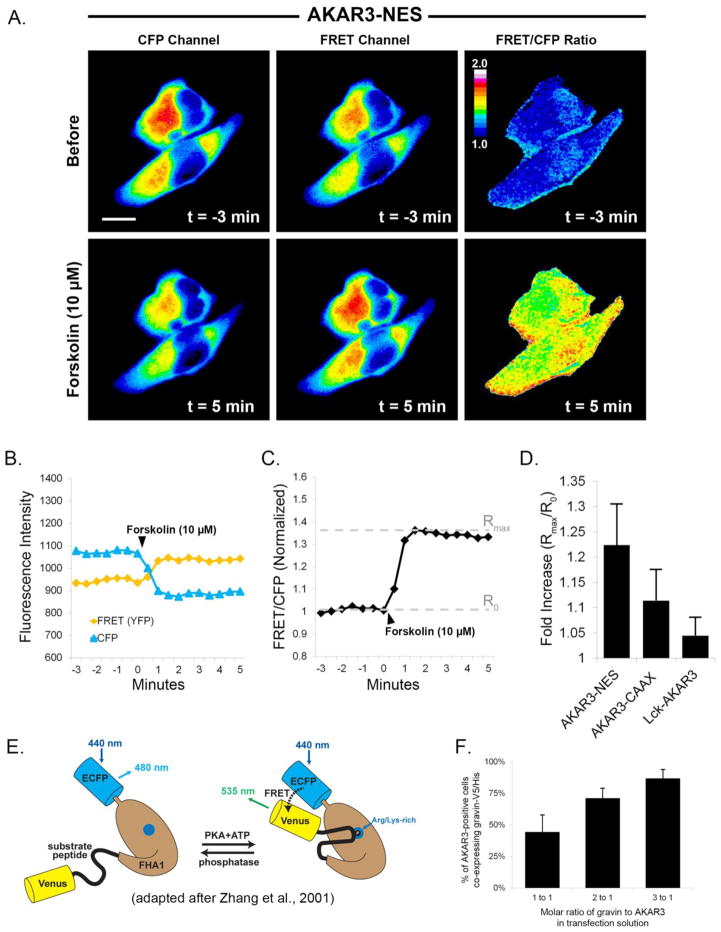
Fig 1.
Quantification of PKA dynamics using AKAR3. Images in part A show that upon treatment with forskolin (10 μM), fluorescence in the CFP channel decreased while fluorescence in the FRET channel increased. The ratiometric image shows the consequent increase in FRET/CFP emissions after treatment with forskolin. Graph B shows the quantification of CFP and FRET emissions over time. FRET intensity was corrected for CFP crossover (44%) at each time point. In graph C, the normalized ratio of FRET/CFP intensity was plotted over time, and the fold increase was calculated by dividing the maximal ratio value after treatment (Rmax) by the mean ratio value prior to treatment (R0). Graph D represents the mean fold increase in each of the AKAR3 constructs after forskolin treatment. Scale bar = 10 μm; Cells were incubated in SES with 10 μM IBMX for 30 minutes prior to forskolin treatment. The diagram in E [adapted from ref. 54] illustrates the principle underlying how the FRET biosensor works. PKA dependent phosphorylation of the substrate peptide results in binding of the peptide to a phosphoamino acid binding site on the FHA1 region of the biosenor and a change in the positioning of the fluorescent proteins relative to each other. Graph F illustrates the effect of different molar ratios of AKAR3:gravin plasmid DNA in the transfection mixture on the percentatge coexpression of the gravin construct in cells expressing AKAR3.
2.2 Construction of expression vectors
Gravin-EGFP, gravin-V5/His, (ΔCB4) gravin-EGFP, and PKA RII-ECFP were constructed and described previously [7, 9]. This study utilized four additional gravin constructs which were made as follows: A mutant gravin construct termed “(mutCB4) gravin-EGFP” was generated with three substitutions in the CB4 domain which replaced valine 672, valine 676, and leucine 681 each with alanine (V672A, V676A, L681A) using site-directed mutagenesis (Phusion®, New England Biolabs, product F-541; forward primer 5′ CATCTTGGGAAGCTGCAATTTGTGTG; reverse primer 5′CTGAGGTATCCGCCTTTCGCTTTGGT; mutagenic nucleotides underlined). The mutation indicated at the T position in the reverse primer is a silent mutation introduced to optimize primer design. A gravin-V5/His construct with the mutations in the CB4 domain, termed (mutCB4) gravin-V5/His, was constructed by removing the mutated DNA fragment in the (mutCB4) gravin-EGFP construct (located between the SacII and EcoRV restriction sites) and inserting it at the same location in a full-length gravin-V5/His construct. A gravin-V5/His construct missing the PKA-binding domain, termed (ΔPKA) gravin-V5/His, was constructed by swapping a DNA fragment between two XbaI restriction sites of full-length gravin-V5/His with that of (ΔPKA) gravin-EYFP. Finally, AKAP18α-gravin V5/His and EGFP chimera constructs were generated by replacing residues 1-1660 of full length gravin (entire gravin membrane localization domain including the CB4 region) with the membrane localization domain of AKAP18α (residues 1–12).
The FRET PKA biosensors AKAR3-NES and AKAR3-CAAX (also called pmAKAR3) were kind gifts of Dr. Jin Zhang (Johns Hopkins) and were constructed as described previously [18]. Lck-AKAR3 was made by inserting a linker containing the N-terminal membrane localization domain of Lck between the HindIII and BamHI restriction sites of AKAR3-NES (Primers: 5′AGCTTGCCACCATGGGCTGTGTCTGCAGCTCAAACCCTGAAAAG; 5′GATCCTTTTCAGGGTTTGAGCTGCAGACACAGCCCATGGTGGCA).
2.3 Ratiometric FRET imaging and quantification
For imaging, AN3 CA or HEC 1A cells expressing AKAR3 constructs were incubated at 37°C in standard extracellular solution (SES; 1.5 mM CaCl2 dihydrate, 145 mM NaCl, 5 mM KCl, 1 mM MgCl2 anhydrous, 10 mM glucose, 10 mM Hepes, pH 7.3) with or without a 30 min pretreatment with IBMX (3-isobutyl-1-methylxanthine; Sigma I-7018; 10 μM), thapsigargin (Tocris 11381; 10 μM) or ATP (Sigma A-2383; 10 mM). CFP and FRET images of AKAR3 biosensors were acquired at 30-second intervals using either a Nikon TE300 or an Olympus IX83 inverted microscope. The Nikon TE300 was equipped with a 40x oil objective (1.3NA), a Hamamatsu ORCA CCD camera, an excitation filter for CFP (440AF21) with a 455DRLP dichroic mirror, and emission filters for CFP (480AF30) and FRET (535AF26). Similarly, the Olympus IX83 was equipped with a 40x oil objective (1.35NA), a Hamamatsu Flash 4.0 CMOS camera, a CFP excitation filter (436/20) with a 445DLRP dichroic mirror, and emission filters for CFP (480/40) and FRET (535/30). Excitation light intensity was optimized for minimal photobleaching, and acquisition parameters were the same for each experiment (200 ms exposure). Images were subjected to background subtraction, fluorescence intensity values in the FRET and CFP channels were quantified using ImageJ, and then FRET channel intensity values were corrected for CFP channel crossover (44% based on images of cells expressing CFP only). Following this, ratios of FRET/CFP intensity were plotted over time for each cell measured.
2.4 Fluorescence and Confocal microscopy
For immunofluorescence labeling, cells were fixed in 3.7% paraformaldehyde in PBS, rinsed with PBS, permeabilized with digitonin, treated with 5% normal goat serum to block non-specific antibody binding, and then treated with an anti-gravin monoclonal mouse antibody as previously described [7]. Primary antibody labeling was detected using CY3 conjugated donkey anti-mouse secondary antibody (Jackson Immunoresearch, Inc., West Grove, PA). Images of immunolabeled cells were obtained using a Zeiss 510 META laser scanning confocal microscope, with a Zeiss 100x Plan-Fluar oil objective lens (NA 1.45).
To determine the impact of calcium elevation on the subcellular distribution of the AKAP18α-gravin chimera vs WT gravin, cells were transfected with EGFP-tagged constructs and imaged at 2 min intervals for 30 min following thapsigargin treatment using an Olympus IX83 fluorescence microscope. The ratio of membrane to cytosolic fluorescence was calculated in a manner similar to that described previously [9]. Following background subtraction, fluorescence intensity was measured at several points across the cell periphery using a line profile 3 pixels wide and approximately 60 pixels long. From this, we calculated the ratio of membrane intensity (mean of three brightest pixels at the cell periphery) to cytosolic intensity (mean of a ten-pixel cytosolic region). The fold change in gravin distribution was determined by dividing the membrane to cytosolic ratios at the beginning and end of the imaging series.
3. Results
3.1 Forskolin Treatment Stimulates AKAR3 Dynamics
Fluorescent micrographs of AN 3CA cells in Figure 1A illustrate the dynamics of the AKAR3 biosensor following treatment with the agonist forskolin, which stimulates PKA activation through adenylyl cyclase-mediated elevation of cAMP. In response to forskolin treatment, AKAR3 undergoes a conformational change that positions CFP and Venus closer to one another (Fig. 1E), and this triggers a drop in CFP intensity and a corresponding increase in FRET intensity (Fig 1A,B). These changes were measured as a ratio of FRET/CFP (Fig. 1A, third column). As seen in Figure 1C, FRET/CFP ratios were plotted over time, and the maximal ratio value after treatment (Rmax) was divided by the mean ratio value prior to treatment (R0) to quantify the fold increase in forskolin-stimulated PKA activity for each of AKAR3 construct (Fig. 1D).
3.2 Gravin expression shapes plasma membrane and cytosolic PKA activity
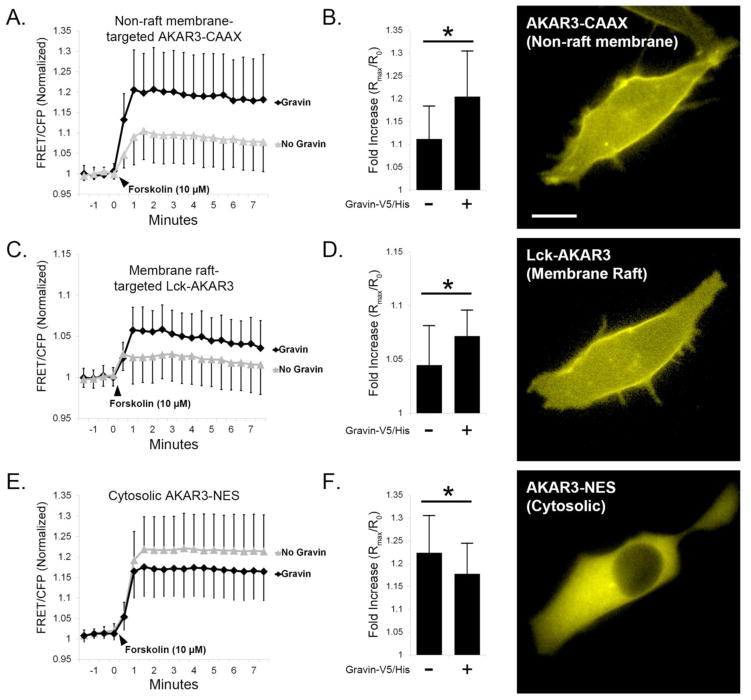
To measure the impact of gravin-V5/His expression on compartmentalized PKA activity, we used AN 3CA cells expressing AKAR3 constructs targeted to the plasma membrane (AKAR3-CAAX and Lck-AKAR3) or cytosol (AKAR3-NES). Fluorescence micrographs in Figure 2 show the relative distribution of each of the AKAR3 constructs used. AKAR3-CAAX contains a motif that is reported to preferentially target non-raft plasma membrane microdomains, whereas Lck-AKAR3 contains a motif reported to target membrane rafts [18–23]. AKAR3-NES contains a nuclear export signal that restricts this biosensor to the cytosol [18]. To prevent cAMP degradation, cells were equilibrated for 30 minutes in standard extracellular solution (SES) containing the phosphodiesterase inhibitor IBMX (10 μM) prior to treatment with forskolin. As seen in Figure 2, forskolin triggered a sustained increase in the ratio of AKAR3 FRET/CFP fluorescence which reached its peak value by roughly 60 seconds with each construct. Forskolin-stimulated PKA activity levels in cells expressing AKAR3-CAAX were significantly elevated in the presence of gravin-V5/His (1.205, SD=0.100, n=16 cells) compared with control cells lacking gravin (1.112, SD=0.072, n=15) (Fig. 2A,B). In cells expressing Lck-AKAR3, forskolin-stimulated PKA activity levels were significantly elevated in the presence of gravin-V5/His (1.072, SD=0.024, n=42) compared with cells without gravin (1.045, SD=0.037, n=40) (Fig. 2C,D), suggesting that gravin expression directs the potentiation of PKA activity at the plasma membrane. As seen in Figure 2E–F, cytosolic PKA levels measured by AKAR3-NES displayed a modest but significant reduction in forskolin-stimulated PKA activity in the presence of gravin (1.178, SD=0.067, n=115) compared with control cells lacking gravin (1.223, SD=0.082, n=146). From these results, we conclude that gravin expression potentiated PKA activity at the plasma membrane, but gravin decreased PKA activity levels in the cytosol. It is likely that gravin expression accomplished this by anchoring PKA to the plasma membrane and sequestering PKA away from the cytosol.
Fig 2.
Gravin-V5/His expression alters subcellular PKA activity. AN3 CA cells were co-transfected with gravin-V5/His and AKAR3 constructs containing targeting sequences for non-raft membrane (CAAX), membrane raft (Lck), or cytosolic (NES) localization. Representative fluorescent images show the distribution the AKAR3 constructs. Expression of gravin increased forskolin-stimulated PKA activity in both non-raft (A,B), and raft (C,D) targeted AKAR3 constructs. Conversely, gravin reduced PKA activity in the cytosol (E,F). Mann-Whitney Rank Sum tests revealed significant differences between treatments as indicated by asterisks (p < 0.05); error bars denote SD. Scale bar = 10 μm.
3.3 Gravin-PKA interaction is required for gravin-mediated changes in subcellular PKA activity
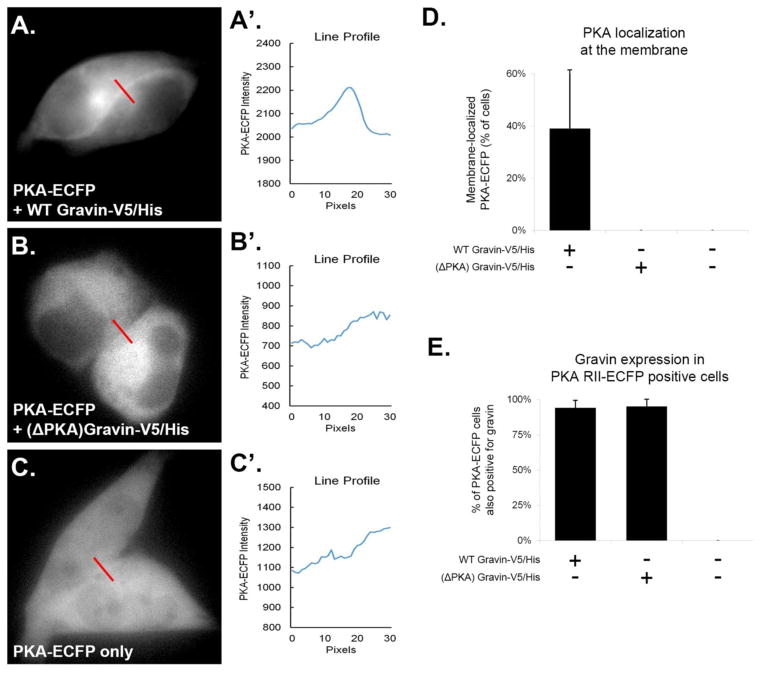
To determine if gravin’s ability to shape plasma membrane and cytosolic PKA activity required gravin-PKA interaction, we generated a gravin mutant which lacks its PKA-binding domain. First, we verified that this gravin mutant did not direct PKA to the cell periphery in AN3 CA cells by quantifying the percentage of cells showing PKA RII-ECFP at the cell periphery. As previously described [9] and as seen in Figure 3A–C, representative micrographs and corresponding line profiles show that PKA was localized to the cell periphery only in cells co-expressing WT gravin, and not in cells co-expressing (ΔPKA) gravin or in cells lacking gravin. Quantification of PKA localization revealed that ~40% of cells co-expressing WT gravin-V5/His and PKA RII-ECFP showed ECFP localization along the cell periphery. In contrast, no ECFP was seen to localize at the cell periphery in cells co-expressing (ΔPKA) gravin-V5/His and PKA RII-ECFP, or in cells expressing PKA RII-ECFP only (Fig. 3D). As seen in Figure 3E, the observed difference in PKA localization was not due to a difference in co-transfection between the two gravin constructs, and no endogenous gravin was detected in cells that were not transfected with gravin. These results demonstrate that WT gravin-V5/His directed PKA RII-ECFP to the cell periphery, and this localization of PKA was not observed in cells expressing (ΔPKA) gravin-V5/His or in cells lacking gravin.
Fig. 3.
PKA RII-ECFP was localized to the cell periphery in AN3 CA cells only when full-length (WT) gravin was present. Representative images and corresponding line-scans (A–C′) show that PKA RII-ECFP localized along the cell periphery only when co-transfected with WT gravin-V5/His. Graph D shows that ~40% of cells expressing PKA RII-ECFP showed ECFP localization at the cell periphery when co-transfected with WT gravin-V5/His. No cells showed PKA RII-ECFP localization at the cell periphery when co-transfected with (ΔPKA) gravin-V5/His, or when expressed in cells containing no gravin. Graph E shows the percentage of PKA RII-ECFP positive cells coexpressing gravin following cotransfection with and without gravin-V5/His constructs. PKA-RII-ECFP positive cells showed a high rate of coexpression with gravin when transfected with gravin-V5/His constructs as indicated by immunofluorescence labeling with a gravin antibody (94% with WT gravin-V5/His; 95% with (ΔPKA) gravin-V5/His). Cells transfected with PKA RII-ECFP only showed no immunofluorescence labeling the gravin antibody. Error bars denote SD.
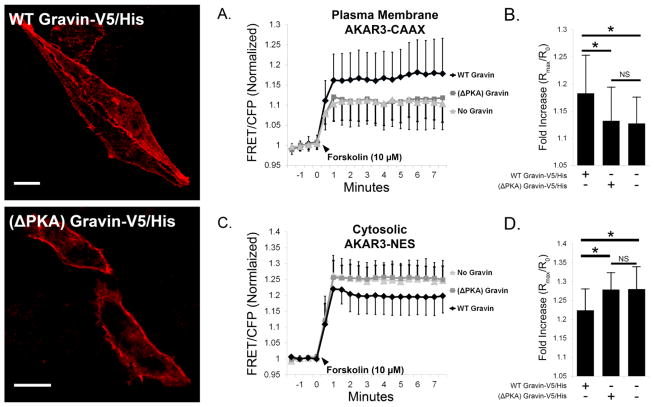
To test the role of gravin-PKA interaction in plasma membrane and cytosolic PKA activity levels, we cotransfected cells with AKAR3 constructs and either full-length (WT) gravin-V5/His or (ΔPKA) gravin-V5/His. As seen in figure 4, representative confocal micrographs of transfected AN3 CA cells immunolabeled with a gravin antibody show that both full-length (WT) gravin-V5/His and (ΔPKA) gravin-V5/His were localized to the plasma membrane. With plasma membrane AKAR3-CAAX, cells expressing gravin-V5/His showed an elevation in forskolin-stimulated PKA activity levels (1.183±0.070, n=25 cells) compared with control cells with no gravin (1.127±0.049, n=33), but this increase was not observed in cells expressing (ΔPKA) gravin-V5/His (1.132±0.062, n=30) (Fig. 4A,B). With cytosolic AKAR3-NES, cells expressing gravin-V5/His had reduced forskolin-stimulated PKA activity levels (1.224±0.057, n=14) compared with control cells with no gravin (1.280±0.059, n=17), but this reduction was not observed in cells expressing (ΔPKA) gravin-V5/His (1.279±0.045, n=16) (Fig. 4C,D). These results demonstrate that that gravin-PKA interaction is required for gravin-mediated potentiation of plasma membrane PKA activity and decrease in forskolin-stimulated PKA activity in the cytosol.
Fig. 4.
Forskolin-stimulated PKA activity at the plasma membrane (AKAR3-CAAX) and cytosol (AKAR3-NES) in AN3 CA cells co-expressing AKAR3 and either full-length (WT) gravin-V5/His or ΔPKA gravin which lacks the PKA binding domain. Representative confocal micrographs show the distribution of transfected gravin-V5/His constructs in fixed cells immunolabeled with a gravin antibody. Graphs A,B show that while WT gravin expression increased forskolin-stimulated PKA activity levels at the plasma membrane, this increase was not observed in cells expressing (ΔPKA) gravin. Graphs C,D show that the gravin-mediated decrease in cytosolic PKA activity levels was not observed in cells expressing ΔPKA gravin. One-way ANOVA with Holm-Sidak post hoc tests revealed significant differences between treatments as indicated by asterisks (p < 0.05); error bars denote SD. Scale bar = 10 μm
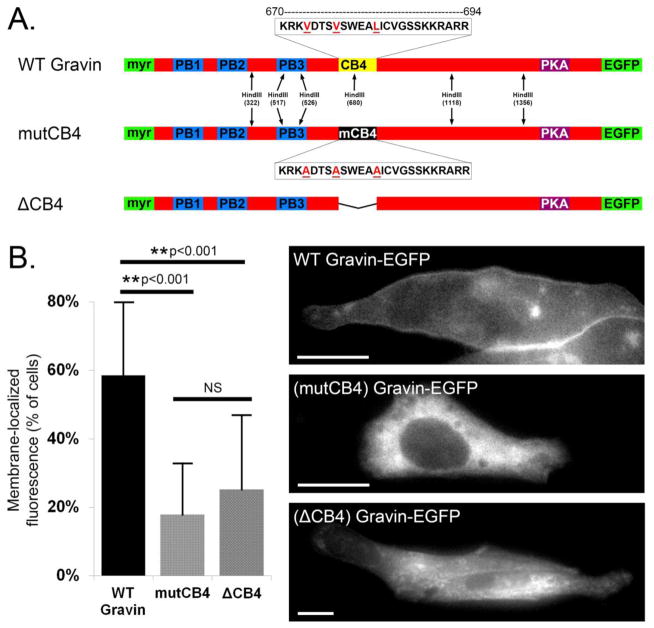
3.4 Mutation of gravin’s CB4 domain inhibits membrane localization and gravin-mediated changes in subcellular PKA activity
Gravin localization at the plasma membrane is aided in part by an N-myristoylation sequence and three polybasic domains (PB1-3) located in gravin’s N-terminal half [7]. Tao et al. (2006) reported that gravin is redistributed away from the plasma membrane upon Ca2+ elevation, and proposed that Ca2+/calmodulin binding to PB1-3 is responsible for this change in localization [8]. However, more recently we reported that a gravin mutant missing PB1-3 still underwent Ca2+-mediated redistribution identical to that of full-length gravin [9]. Moreover, we found that gravin localization at the cell periphery was severely hindered by the deletion of a fourth putative calmodulin binding domain, CB4 [9], a region that contains a 1-5-10 consensus motif for Ca2+/calmodulin interaction [24]. To test the role of this Ca2+/calmodulin consensus sequence within the CB4 domain in the membrane localization of gravin, we substituted the 1-5-10 amino acids in this sequence for alanine (Fig. 5A), similar to the strategy used by other investigators [25–27]. Expression of this mutant construct, (mutCB4) gravin-EGFP, revealed a dramatic reduction in basal membrane localization compared with WT gravin-EGFP, but was not different from (ΔCB4) gravin-EGFP (Fig. 5B). From these results, we conclude that the 1-5-10 amino acids within gravin’s CB4 domain are required for normal membrane localization. This suggests a novel role for Ca2+/calmodulin in supporting gravin localization at the membrane, which contrasts with previous reports that suggest that Ca2+/calmodulin promotes gravin relocalization away from the membrane by binding to PB1-3.
Fig. 5.
Role of CB4 domain in subcellular gravin localization. Part A illustrates the sequence and location of gravin’s CB4 domain downstream of the membrane targeting regions (myr, PB1-3). Amino acids shown in red comprise a calmodulin-binding consensus sequence and were substituted for alanine. Mutagenesis was confirmed by HindIII restriction digest (not shown). In graph B, either mutation or deletion of the CB4 domain caused a significant reduction in membrane localization compared with WT gravin, while the observed difference between the two mutant constructs was not significant. (ANOVA with Holm–Sidak post-hoc test; asterisks denote p < 0.001). Representative fluorescent images show the localization of these constructs in AN3 CA cells. Error bars denote SD. Scale bar = 20 μm.
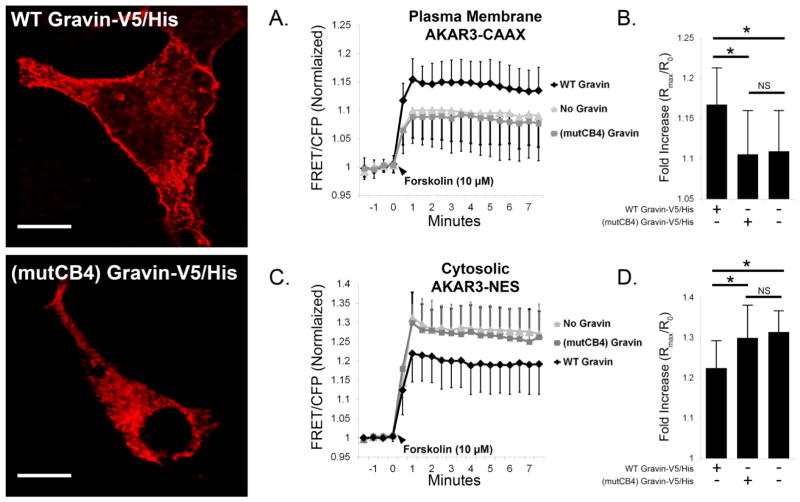
Using the (mutCB4) gravin-V5/His construct, we tested the impact of gravin localization at the membrane in gravin’s ability to shape subcellular PKA activity both at the plasma membrane and within the cytosol. In figure 6, representative confocal micrographs of transfected AN3 CA cells immunolabeled with a gravin antibody illustrate that full-length (WT) gravin localized along the cell periphery, but (mutCB4) gravin did not. In AN3 CA cells expressing plasma membrane AKAR3-CAAX, gravin-V5/His expression elevated forskolin-stimulated PKA activity levels (1.168±0.046, n=13) compared with control cells lacking gravin (1.109±0.051, n=19), but this increase was not observed in cells expressing (mutCB4) gravin-V5/His (1.106±0.054, n=16) (Fig. 6A–B). Similarly, in cells expressing cytosolic AKAR3-NES, the gravin-V5/His reduced forskolin-stimulated PKA activity levels in the cytosol (1.224±0.068, n=16) compared with control cells lacking gravin (1.314±0.052, n=15), but this reduction was not observed in cells expressing (mutCB4) gravin-V5/His (1.300±0.081, n=14) (Fig. 6C–D). These results demonstrate that gravin localization has a profound effect on subcellular PKA activity both at the plasma membrane and in the cytosol.
Fig. 6.
Forskolin-stimulated PKA activity at the plasma membrane (AKAR3-CAAX) and cytosol (AKAR3-NES) in AN3 CA cells co-expressing AKAR3 and either WT or (mutCB4) gravin. Representative confocal micrographs show the distribution of transfected gravin-V5/His constructs in fixed cells immunolabeled with a gravin antibody. Graphs A,B show that gravin expression increased forskolin-stimulated PKA activity levels at the plasma membrane compared with control cells lacking gravin, but this increase was not observed in cells expressing mutCB4 gravin. Graphs C,D show that forskolin-stimulated PKA activity levels in the cytosol were reduced in cells expressing WT gravin-V5/His compared with control cells lacking gravin, but this was not observed in cells expressing mutCB4 gravin. One-way ANOVA with Holm-Sidak post hoc tests revealed significant differences between treatments as indicated by asterisks. Error bars denote SD. Scale bar = 10 μm.
3.5 Ca2+ elevation reduces plasma membrane PKA activity levels through gravin redistribution
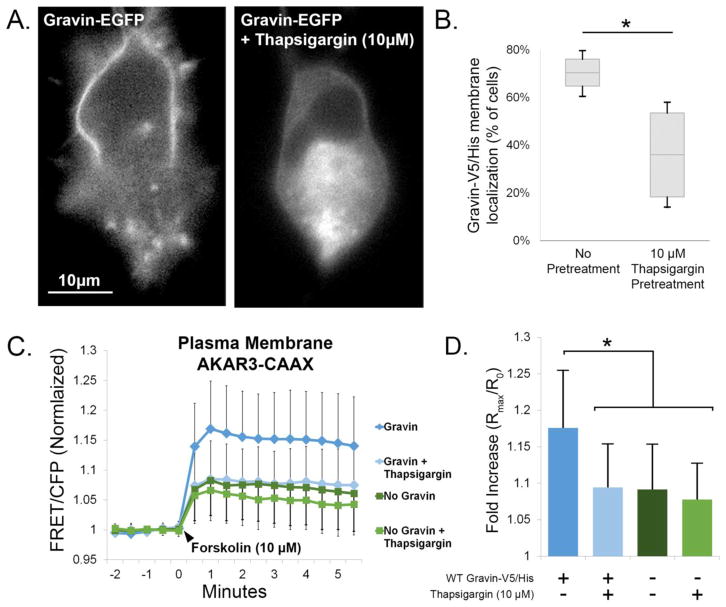
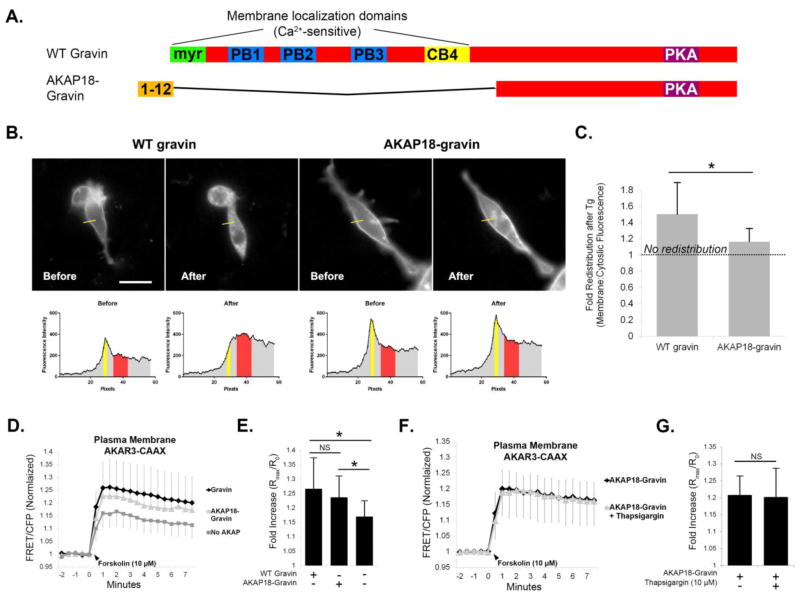
Intracellular Ca2+ elevation causes the redistribution of gravin away from the plasma membrane and into the cytosol [8, 9], additionally causing the relocalization of PKA away from the cell periphery [9]. This suggests that gravin intersects Ca2+ and PKA dependent signaling by serving as a Ca2+-dependent “switch” to modulate PKA activity at the plasma membrane. To test this, we pre-treated AN3 CA cells expressing AKAR3-CAAX with thapsigargin (10 μM), a calcium-elevating agent that causes gravin redistribution. First, we confirmed thapsigargin-mediated gravin redistribution as seen in representative micrographs of AN3 CA cells expressing gravin-EGFP (Fig. 7A) and quantification of the percentage of cells showing gravin localization at the cell periphery (Fig. 7B). As seen in Figure 7C–D, forskolin-stimulated PKA activity levels at the plasma membrane were elevated by gravin-V5/His expression in the absence of thapsigargin pre-treatment (1.176±0.079, n=43 cells) compared with control cells lacking gravin (1.091±0.062, n=65). However, 30 minute pre-treatment with thapsigargin inhibited this elevation (1.094±0.059, n=36). To confirm that thapsigargin did not impact PKA independently, as has been shown in previous work [28], thapsigargin pre-treatment in control cells lacking gravin showed no significant difference in forskolin-stimulated PKA activity levels (1.078±0.049, n=37). To confirm that thapsigargin decreased PKA activity through gravin redistribution, we generated a gravin construct fused with the membrane-targeting motif of AKAP18α (Fig. 8A). This AKAP18α-gravin chimera significantly resisted thapsigargin-mediated redistribution from the cell periphery compared with WT gravin (Fig. 8B–C). Like WT gravin, the AKAP18-gravin chimera caused a significant elevation in PKA activity levels at the plasma membrane (Fig. 8D–E), but thapsigargin had no effect on plasma membrane PKA activity levels in cells expressing AKAP18-gravin. In parallel experiments (not shown), we confirmed the efficacy of thapsigargin in WT gravin-expressing cells as seen in Fig. 7C–D, 9C–D. Overall these results demonstrate that Ca2+ can modulate PKA activity levels at the plasma membrane by triggering gravin redistribution.
Fig. 7.
Thapsigargin triggers gravin redistribution and abolishes gravin-mediated elevation in plasma membrane PKA activity. Representative fluorescent micrographs (A) illustrate the redistribution of gravin-EGFP away from the plasma membrane by thapsigargin. Gravin-V5/His redistribution was confirmed after FRET experiments by quantifying the percentage of cells showing gravin localization at the membrane, shown in the box plot represented in B (Mann-Whitney Rank Sum Test, p < 0.05). Graphs C,D show that gravin expression increased forskolin-stimulated PKA activity at the membrane, but this increase was not observed in cells expressing gravin and pretreated with the calcium-elevating agent thapsigargin (10 μm) for 30 min. Thapsigargin pretreatment had no effect on forskolin-stimulated PKA activity in the absence of gravin. One-way ANOVA on ranks followed by Kruskal-Wallis post hoc showed significant differences between treatments as indicated by asterisks. Error bars denote SD.
Fig. 8.
Ca2+/PKA crosstalk does not occur in the presence of a Ca2+-resistant gravin mutant. Cartoons depict the AKAP18α-gravin chimera, which replaces gravin’s membrane localization domains with the first twelve amino acids of AKAP18α. Images and corresponding linescans of AN3 CA cells before and after thapsigargin treatment show that while WT gravin undergoes redistribution, the AKAP18-gravin chimera does not. Graph C shows the quantification of the fold change in membrane:cytosolic fluoresence intensity before and after thapsigargin treatment as depicted in the corresponding linescans. Note that a value of 1.0 depicts no redistribution after thapsigargin treatment, and values higher than 1.0 depict a greater extent of redistribution. Graphs D–E show that both WT gravin and AKAP18-gravin caused elevated PKA activity levels at the plasma membrane compared with controls lacking gravin. Graphs F–G show that thapsigargin had no effect on PKA activity levels at the plasma membrane in cells expressing AKAP18-gravin. Statistical differences were determined by a T-test in C, G or one-way ANOVA with Holm-Sidak post hoc in E and are indicated by asterisks. Error bars denote SD. Scale bar = 20 μm.
Fig. 9.
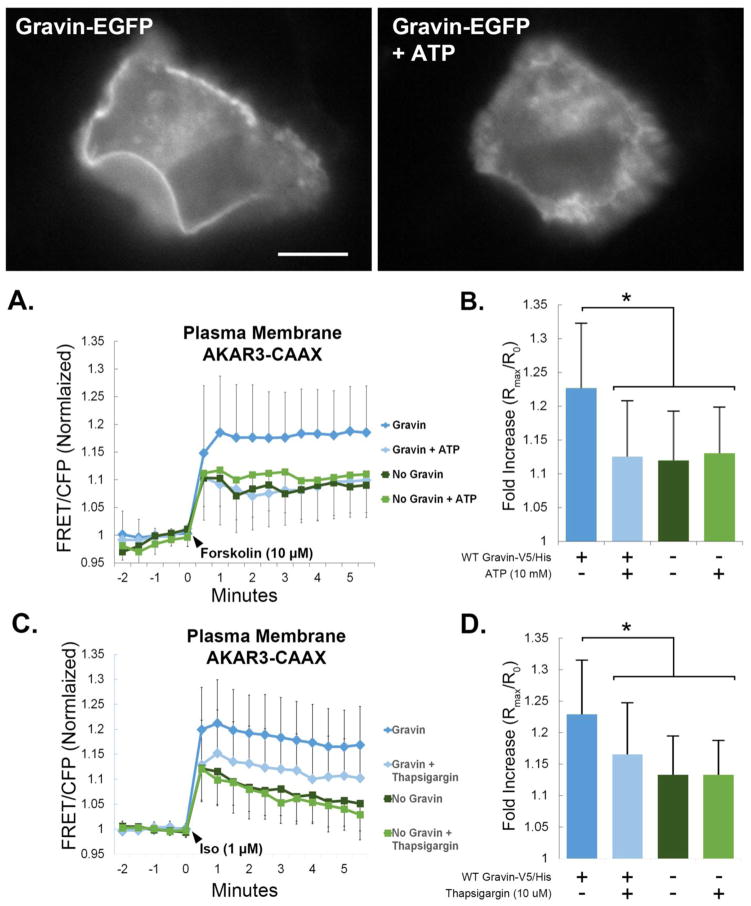
Receptor-mediated Ca2+ elevation modulates PKA activity by gravin redistribution, and gravin regulates β-adrenergic receptor mediated PKA activity at the plasma membrane. Fluorescent images show that ATP caused the redistribution of gravin away from the plasma membrane. Graphs A,B show that gravin raised forskolin-stimulated PKA activity at the membrane, but this increase was not observed in cells expressing gravin and pretreated with ATP (10mM) for 30 min. ATP pretreatment had no effect on forskolin-stimulated PKA activity in the absence of gravin. Graphs C,D show that gravin increased isoproterenol-stimulated PKA activity levels at the plasma membrane compared with control cells lacking gravin. This increase was not observed in cells expressing gravin but pretreated with thapsigargin (10 μm for 30 min). Thapsigargin pretreatment had no effect on isoproterenol-stimulated PKA activity in the absence of gravin. One-way ANOVA with Kruskal-Wallis post hoc showed significant differences between the treatments as indicated by asterisks. Error bars denote SD. Scale bar = 10 μm.
3.6 Gravin/PKA axis is sensitive to receptor-mediated Ca2+ elevation and shapes β-adrenergic eceptor mediated PKA activation
To confirm that gravin mediates Ca2+/PKA crosstalk in the context of receptor stimulation, we tested the role of gravin in modulating β-adrenergic receptor mediated PKA activity levels, and determined the impact of receptor-mediated Ca2+ elevation by ATP (10mM) in mediating changes in PKA activity through gravin redistribution. Consistent with our previous work [9], fluorescent micrographs in figure 9 show that ATP treatment caused gravin redistribution away from the cell periphery in HEC 1A cells. In HEC 1A cells expressing both AKAR3-CAAX and gravin, forskolin-stimulated PKA activity levels were significantly reduced by ATP pre-treatment (1.227±0.095 vs 1.120±0.073, n=36 and 27 respectively). However, in cells lacking gravin, ATP pre-treatment had no effect on PKA activity levels after forskolin treatment (1.126±0.081 vs 1.131±0.068, n=38 and 14 respectively) (Fig. 9A–B). To test the impact of gravin redistribution on β-adrenergic receptor signaling, figure 9C–D shows that gravin-V5/His expression in AN3 CA cells significantly raised isoproterenol-stimulated PKA activity levels (1.229±0.086, n=52) compared with control cells lacking gravin (1.133±0.061, n=38) or in cells expressing gravin-V5/His but pre-treated with thapsigargin (1.166±0.081, n=31). In addition, thapsigargin pre-treatment in the absence of gravin had no effect on isoproterenol-stimulated PKA activity (1.133±0.054, n=34). From these data, we conclude that receptor-mediated calcium elevation impacts subcellular PKA activity through gravin redistribution. Furthermore, gravin expression potentiated β-adrenergic receptor-mediated PKA activity at the plasma membrane but lost this effect upon Ca2+ elevation. These results support the hypothesis that gravin mediates crosstalk between calcium and PKA-dependent signaling pathways.
4. Discussion
In the current study, we investigated the role of gravin in shaping compartmentalized PKA activity and in mediating crosstalk between Ca2+ and PKA-dependent signaling pathways. We showed that upon stimulation with forskolin or isoproterenol, cells expressing gravin had elevated levels of plasma membrane (PM) PKA activity and reduced levels of cytosolic PKA activity. Gravin-PKA interaction at the PM was required for these alterations, as mutant gravin constructs lacking PKA interaction ([ΔPKA] gravin) or normal membrane localization ([mutCB4] gravin) had no impact on PM or cytosolic PKA activity. Finally, pre-treatment with Ca2+-elevating agents thapsigargin and ATP, which triggered gravin redistribution away from the cell periphery, blocked the increase in PKA activity levels mediated by gravin expression. Importantly, Ca2+ elevation had no impact on PKA activity in the absence of gravin or in the presence of a Ca2+-resistant gravin mutant. This study shows a novel mechanism whereby intracellular calcium elevation regulates β-adrenergic receptor signaling through gravin redistribution and supports the hypothesis that gravin mediates crosstalk between calcium and PKA-dependent signaling pathways.
Although the main advance of the current study was describing a novel Ca2+/PKA crosstalk mechanism, our assays using overexpression of gravin constructs in AN3 CA cells lacking endogenous gravin also revealed noteworthy “proof of principle” mechanisms that are likely to occur in vivo. First, gravin expression preferentially shifted PKA activity to the plasma membrane, but unexpectedly reduced cytosolic PKA activation presumably by sequestering PKA from of the cytoplasm. This “sequestration” phenomenon by A-Kinase Anchoring Proteins has been previously proposed in a study of AKAP79 overexpression in HEK293 cells [29]. Furthermore, gravin was shown to reduce FAK phosphorylation through the proposed sequestration of Src [30], and gravin has also been shown to sequester cyclin D1 away from the nucleus [31]. Our findings that PKA activity was reduced in the cytosol may be an important consequence of gravin expression or upregulation in vivo. This possibility was recently highlighted in a study by Finger et al. (2015), where hypoxia-induced gravin upregulation in human melanoma cells changed the phosphorylation profile of a wide variety of intracellular proteins [32]. Interestingly, the findings of Finger et al. (2015) also support a second mechanistic observation from the current study, namely that gravin may direct the phosphorylation of a wider range of PKA substrates at or near the membrane than has been previously reported. Currently, the known phosphorylation targets dependent on gravin/PKA interaction are gravin itself [33], β2-adrenergic receptor [14, 33], and possibly PDE4D [34, 35]. The specificity with which gravin directs PKA to target substrates is currently unknown, although some AKAPs require very high submolecular specificity in the range of 10–20nm, where both PKA and its substrate are tethered to the same AKAP [36]. Our observation that gravin increased the phosphorylation of AKAR3, a non-endogenous protein, suggests that colocalization of gravin and PKA substrates at the PM may be sufficient for phosphorylation to occur at nearby substrates such as ion channels [37–39], cytoskeletal regulators [40, 41], and junctional proteins [17, 42–44]. Understanding the mechanisms underlying specificity in gravin-PKA signaling will require further identification of additional substrates beyond those already known, as well as studies of gravin-substrate binding, gravin structural dynamics, and mobility in the membrane under normal physiologic conditions.
The current study showed that gravin localization at the cell periphery is impaired by the mutation of a consensus binding motif for Ca2+/calmodulin (CaM) within gravin’s fourth CaM-binding, or CB4, domain. This suggests a novel role for CaM interaction with gravin. Previously, CaM interaction at polybasic domains 1-3 (PB1-3) upstream of the CB4 domain was shown to promote dissociation of gravin from the PM [8], but unexpected findings from our recent work also shows that PM dissociation can occur by disrupting CaM interaction with CB4, either by deletion [9] or mutation of this domain. This suggests that CaM binding to CB4 strengthens gravin/membrane interaction, but the reason for this is currently unclear. One possibility is that the CB4 domain may influence gravin’s tertiary structure and regulate the accessibility of the myristoylation site to the PM. Evidence for this comes from our previous work showing that the myristoylation site is sufficient to retain normal PM localization in a mutant lacking PB1-3 [7], but the myristoylation site is no longer sufficient for membrane localization in the absence of both PB1-3 and CB4 [9]. It is also interesting to note that the CB4 domain (a.a. 669–693) lies directly upstream of three PKA-phosphorylation sites (ser696-698) that are known to enhance gravin association with β2 adrenergic receptor upon phosphorylation [33]. In addition, a putative PKC phosphorylation site also lies within the CB4 domain. It is possible that CaM binding to CB4 may influence either the phosphorylation/dephosphorylation of these sites or the interaction of these sites with other proteins at the PM.
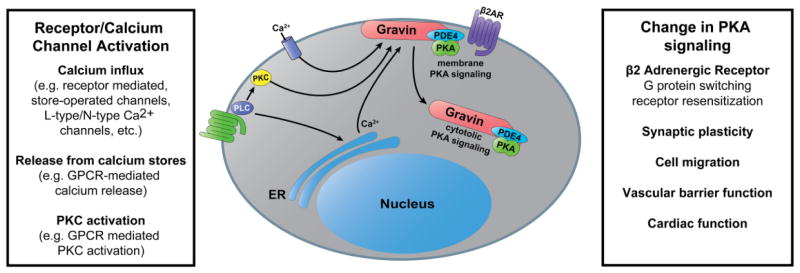
Our studies strongly support the hypothesis that gravin mediates crosstalk between Ca2+- and PKA-dependent signaling pathways. Although it is known that gravin/PKA undergo redistribution away from the cell periphery in response to PKC activation [7, 45, 46] and cytosolic Ca2+ elevation [8, 9], the current study defines a novel crosstalk mechanism whereby Ca2+ regulates GPCR-mediated subcellular PKA activity levels via gravin redistribution. First, calcium elevation by thapsigargin or ATP reduced plasma membrane PKA activity levels only in the presence of WT gravin. Secondly, calcium elevation had no impact on membrane PKA activity levels in the absence of gravin or in the presence of a gravin mutant that resisted Ca2+-mediated redistribution. Thus Ca2+ only impacted PKA activity under conditions where gravin underwent redistribution, clearly demonstrating a novel mode of Ca2+/PKA crosstalk. This is distinct from other known crosstalk mechanisms [reviewed in 47] and lays groundwork for future studies to investigate the impact of gravin-mediated Ca2+/PKA crosstalk in vivo. For example, while agonists such as thrombin, bradykinin, and histamine stimulate G protein pathways linked to [Ca2+]i elevation and regulate endothelial permeability, gravin is also known to support endothelial barrier strength through cAMP-dependent pathways [17, 44]. Our finding that ATP treatment impacts PKA activity suggests that receptor-mediated Ca2+ agonists that stimulate endothelial permeability may accomplish this in part by redirecting gravin/PKA away from the cell periphery. Another area of relevance may be the hippocampal neurons, where Ca2+ and PKA are paramount in postsynaptic signaling within dendritic spines. Given that gravin is already known to play a role in synaptic plasticity in the hippocampus [14], it is possible that this may occur through an interplay between different Ca2+ coupled and cAMP coupled postsynaptic receptors, or broadly within dendritic Ca2+ and PKA signaling as is the case with AKAP79 [48–53]. Many questions remain to be addressed before the role of gravin in Ca2+/PKA signaling crosstalk is fully understood. Although many different Ca2+ signaling pathways are possible candidates for the dynamic spatial regulation of PKA by gravin (Fig. 10), the types of agonists and receptors involved in these systems still need to be defined. In addition, the types of Ca2+ signals that affect gravin-membrane dynamics as well as the mechanism underlying Ca2+ mediated changes in gravin distribution require further characterization before a complete picture of gravin’s role in signaling is established. However, given the wide array of physiological functions to which gravin has been linked, understanding its role in signaling crosstalk mechanisms and its integration into signaling networks will be critically important to understanding the regulation of these physiological processes.
Fig. 10.
Illustration depicting the hypothesis that a variety of Ca2+-dependent signaling inputs may alter PKA activity levels at the plasma membrane by inducing gravin redistribution and, in turn, impact gravin’s role in regulating β2-adrenergic receptors, synaptic plasticity, cellular migration, and vascular barrier function.
5. Conclusions
In conclusion, we report that gravin expression modulates both plasma membrane and cytosolic PKA activity levels and mediates a novel crosstalk mechanism between Ca2+ and PKA-dependent signaling pathways. This mechanism occurs through the Ca2+ mediated redistribution of gravin/PKA away from the plasma membrane, which in turn impacts PKA activity levels at the membrane. The current study lays important groundwork for future investigation of gravin-mediated Ca2+/PKA crosstalk in physiological contexts such as learning and memory and endothelial barrier function.
Highlights.
The FRET biosensor AKAR3 was used to investigate gravin’s impact on PKA activity.
Gravin elevated plasma membrane PKA activity and reduced cytosolic PKA activity.
Gravin is redistributed away from the cell periphery upon Ca2+ elevation.
Ca2+ elevation reduced plasma membrane PKA activity through gravin redistribution.
Acknowledgments
This work was supported by NIH P30GM103329. We gratefully acknowledge Dr. Jin Zhang (Johns Hopkins University) for providing AKAR3-CAAX and AKAR3-NES constructs. In addition, the authors acknowledge use of the Edward C. Carlson Imaging and Image Analysis Core Facility which is also supported in part by NIH grant P30GM103329 and NIH Shared Instrumentation Grant 1S100D016250-01.
Abbreviations
- AKAP
A-Kinase anchoring protein
- AKAR3
A-Kinase activity reporter 3
- β2AR
β2-adrenergic receptor
- [Ca2+]i
intracellular calcium concentration
- CaM
calcium-calmodulin
- cAMP
cyclic adenosine monophosphate
- CB4
calmodulin binding domain 4
- CFP
cyan fluorescent protein
- ECFP
enhanced cyan fluorescent protein
- EGFP
enhanced green fluorescent protein
- EYFP
enhanced yellow fluorescent protein
- FRET
Förster’s resonance energy transfer
- Fsk
forskolin
- GPCR
G protein coupled receptor
- kD
kilodaltons
- IBMX
3-isobutyl-1-methylxanthine
- Iso
isoproterenol
- NES
nuclear export signal
- PB1-3
polybasic domains 1 through 3
- PDE4
phosphodiesterase type 4
- PKA
protein kinase A
- PKC
protein kinase C
- PM
plasma membrane
- RII
regulatory subunit of type II PKA
- SES
standard extracellular solution
- SSeCKS
Src-Suppressed C-Kinase Substrate
- Tg
thapsigargin
- WT
wild-type/full-length
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong W, Scott JD. AKAP signalling complexes: Focal points in space and time. Nature Reviews Molecular Cell Biology. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 3.Smith KE, Gibson ES, Dell’Acqua ML. cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J Neurosci. 2006;26:2391–2402. doi: 10.1523/JNEUROSCI.3092-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawe VY, Payne C, Navara C, Schatten G. WAVE1 intranuclear trafficking is essential for genomic and cytoskeletal dynamics during fertilization: cell-cycle-dependent shuttling between M-phase and interphase nuclei. Dev Biol. 2004;276:253–267. doi: 10.1016/j.ydbio.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Adamik R, Pacheco-Rodriguez G, Moss J, Vaughan M. Protein kinase A-anchoring (AKAP) domains in brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) Proc Natl Acad Sci U S A. 2003;100:1627–1632. doi: 10.1073/pnas.0337678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eide T, Coghlan V, Orstavik S, Holsve C, Solberg R, Skalhegg BS, Lamb NJ, Langeberg L, Fernandez A, Scott JD, Jahnsen T, Tasken K. Molecular cloning, chromosomal localization, and cell cycle-dependent subcellular distribution of the A-kinase anchoring protein, AKAP95. Exp Cell Res. 1998;238:305–316. doi: 10.1006/excr.1997.3855. [DOI] [PubMed] [Google Scholar]
- 7.Yan X, Walkiewicz M, Carlson J, Leiphon L, Grove B. Gravin dynamics regulates the subcellular distribution of PKA. Experimental Cell Research. 2009;315:1247–1259. doi: 10.1016/j.yexcr.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao J, Shumay E, McLaughlin S, Wang HY, Malbon CC. Regulation of AKAP-membrane interactions by calcium. J Biol Chem. 2006;281:23932–23944. doi: 10.1074/jbc.M601813200. [DOI] [PubMed] [Google Scholar]
- 9.Schott MB, Grove B. Receptor-mediated Ca2+ and PKC signaling triggers the loss of cortical PKA compartmentalization through the redistribution of gravin. Cell Signal. 2013;25:2125–2135. doi: 10.1016/j.cellsig.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelman IH, Tombler E, Vargas J., Jr A role for SSeCKS, a major protein kinase C substrate with tumour suppressor activity, in cytoskeletal architecture, formation of migratory processes, and cell migration during embryogenesis. Histochem J. 2000;32:13–26. doi: 10.1023/a:1003950027529. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Guan M, Hu T, Gu X, Lu Y. Re-Expression of AKAP12 inhibits progression and metastasis potential of colorectal carcinoma in vivo and in vitro. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akakura S, Gelman IH. Pivotal Role of AKAP12 in the Regulation of Cellular Adhesion Dynamics: Control of Cytoskeletal Architecture, Cell Migration, and Mitogenic Signaling. Journal of signal transduction. 2012;2012:529179. doi: 10.1155/2012/529179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelman IH. Suppression of tumor and metastasis progression through the scaffolding functions of SSeCKS/Gravin/AKAP12. Cancer and Metastasis Reviews. 2012;31:493–500. doi: 10.1007/s10555-012-9360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havekes R, Canton DA, Park AJ, Huang T, Nie T, Day JP, Guercio LA, Grimes Q, Luczak V, Gelman IH, Baillie GS, Scott JD, Abel T. Gravin orchestrates protein kinase A and beta2-adrenergic receptor signaling critical for synaptic plasticity and memory. J Neurosci. 2012;32:18137–18149. doi: 10.1523/JNEUROSCI.3612-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillory AN, Yin X, Wijaya CS, Diaz Diaz AC, Rababa’h A, Singh S, Atrooz F, Sadayappan S, McConnell BK. Enhanced cardiac function in Gravin mutant mice involves alterations in the beta-adrenergic receptor signaling cascade. PLoS One. 2013;8:e74784. doi: 10.1371/journal.pone.0074784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grove BD, Bruchey AK. Intracellular distribution of gravin, a PKA and PKC binding protein, in vascular endothelial cells. J Vasc Res. 2001;38:163–175. doi: 10.1159/000051043. [DOI] [PubMed] [Google Scholar]
- 17.Weissmuller T, Glover LE, Fennimore B, Curtis VF, MacManus CF, Ehrentraut SF, Campbell EL, Scully M, Grove BD, Colgan SP. HIF-dependent regulation of AKAP12 (gravin) in the control of human vascular endothelial function. FASEB J. 2014;28:256–264. doi: 10.1096/fj.13-238741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun. 2006;348:716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- 19.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal SR, Yang PC, Rice M, Singer CA, Nikolaev VO, Lohse MJ, Clancy CE, Harvey RD. Role of membrane microdomains in compartmentation of cAMP signaling. PLoS One. 2014;9:e95835. doi: 10.1371/journal.pone.0095835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Depry C, Allen MD, Zhang J. Visualization of PKA activity in plasma membrane microdomains. Mol Biosyst. 2011;7:52–58. doi: 10.1039/c0mb00079e. [DOI] [PubMed] [Google Scholar]
- 22.Gao X, Lowry PR, Zhou X, Depry C, Wei Z, Wong GW, Zhang J. PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci U S A. 2011;108:14509–14514. doi: 10.1073/pnas.1019386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 24.Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- 25.Ahn EY, Lim ST, Cook WJ, McDonald JM. Calmodulin binding to the Fas death domain. Regulation by Fas activation. J Biol Chem. 2004;279:5661–5666. doi: 10.1074/jbc.M311040200. [DOI] [PubMed] [Google Scholar]
- 26.Suever JD, Chen Y, McDonald JM, Song Y. Conformation and free energy analyses of the complex of calcium-bound calmodulin and the Fas death domain. Biophysical journal. 2008;95:5913–5921. doi: 10.1529/biophysj.108.130542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fancy RM, Wang L, Napier T, Lin J, Jing G, Lucius AL, McDonald JM, Zhou T, Song Y. Characterization of calmodulin-Fas death domain interaction: an integrated experimental and computational study. Biochemistry. 2014;53:2680–2688. doi: 10.1021/bi500228h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 29.Ndubuka C, Li Y, Rubin CS. Expression of a kinase anchor protein 75 depletes type II cAMP-dependent protein kinases from the cytoplasm and sequesters the kinases in a particulate pool. J Biol Chem. 1993;268:7621–7624. [PubMed] [Google Scholar]
- 30.Su B, Gao L, Meng F, Guo LW, Rothschild J, Gelman IH. Adhesion-mediated cytoskeletal remodeling is controlled by the direct scaffolding of Src from FAK complexes to lipid rafts by SSeCKS/AKAP12. Oncogene. 2013;32:2016–2026. doi: 10.1038/onc.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnworth B, Pippin J, Karna P, Akakura S, Krofft R, Zhang G, Hudkins K, Alpers CE, Smith K, Shankland SJ, Gelman IH, Nelson PJ. SSeCKS sequesters cyclin D1 in glomerular parietal epithelial cells and influences proliferative injury in the glomerulus. Laboratory Investigation. 2012;92:499–510. doi: 10.1038/labinvest.2011.199. [DOI] [PubMed] [Google Scholar]
- 32.Finger EC, Castellini L, Rankin EB, Vilalta M, Krieg AJ, Jiang D, Banh A, Zundel W, Powell MB, Giaccia AJ. Hypoxic induction of AKAP12 variant 2 shifts PKA-mediated protein phosphorylation to enhance migration and metastasis of melanoma cells. Proc Natl Acad Sci U S A. 2015;112:4441–4446. doi: 10.1073/pnas.1418164112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao J, Wang HY, Malbon CC. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the beta2-adrenergic receptor. EMBO J. 2003;22:6419–6429. doi: 10.1093/emboj/cdg628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DMF. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO Journal. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raymond DR, Wilson LS, Carter RL, Maurice DH. Numerous distinct PKA-, or EPAC-based, signalling complexes allow selective phosphodiesterase 3 and phosphodiesterase 4 coordination of cell adhesion. Cell Signal. 2007;19:2507–2518. doi: 10.1016/j.cellsig.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Smith FD, Reichow SL, Esseltine JL, Shi D, Langeberg LK, Scott JD, Gonen T. Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. eLife. 2013;2:e01319. doi: 10.7554/eLife.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiological reviews. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown DA, Yule DI. Protein kinase A regulation of P2X(4) receptors: requirement for a specific motif in the C-terminus. Biochimica et biophysica acta. 2010;1803:275–287. doi: 10.1016/j.bbamcr.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Advances in second messenger and phosphoprotein research. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]
- 40.Howe AK, Baldor LC, Hogan BP. Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc Natl Acad Sci U S A. 2005;102:14320–14325. doi: 10.1073/pnas.0507072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Chen Y, Chen M, Xu W. AKAPs competing peptide HT31 disrupts the inhibitory effect of PKA on RhoA activity. Oncol Rep. 2006;16:755–761. [PubMed] [Google Scholar]
- 42.Kwon HB, Choi YK, Lim JJ, Kwon SH, Her S, Kim HJ, Lim KJ, Ahn JC, Kim YM, Bae MK, Park JA, Jeong CH, Mochizuki N, Kim KW. AKAP12 regulates vascular integrity in zebrafish. Experimental and Molecular Medicine. 2012;44:225–235. doi: 10.3858/emm.2012.44.3.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You QH, Sun GY, Wang N, Chen S, Luo QL. Role of src-suppressed C kinase substrate in rat pulmonary microvascular endothelial hyperpermeability stimulated by inflammatory cytokines. Inflammation research : official journal of the European Histamine Research Society .. [et al.] 2010;59:949–958. doi: 10.1007/s00011-010-0207-3. [DOI] [PubMed] [Google Scholar]
- 44.Radeva MY, Kugelmann D, Spindler V, Waschke J. PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation. PLoS One. 2014;9:e106733. doi: 10.1371/journal.pone.0106733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piontek J, Brandt R. Differential and regulated binding of cAMP-dependent protein kinase and protein kinase C isoenzymes to gravin in human model neurons: Evidence that gravin provides a dynamic platform for the localization for kinases during neuronal development. J Biol Chem. 2003;278:38970–38979. doi: 10.1074/jbc.M306749200. [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Tombler E, Nelson PJ, Ross M, Gelman IH. A novel src- and ras-suppressed protein kinase C substrate associated with cytoskeletal architecture. Journal of Biological Chemistry. 1996;271:28430–28438. doi: 10.1074/jbc.271.45.28430. [DOI] [PubMed] [Google Scholar]
- 47.Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harbor perspectives in biology. 2011;3:a004143. doi: 10.1101/cshperspect.a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez LL, Alam S, Smith KE, Horne E, Dell’Acqua ML. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dell’Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveria SF, Dittmer PJ, Youn DH, Dell’Acqua ML, Sather WA. Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels. J Neurosci. 2012;32:15328–15337. doi: 10.1523/JNEUROSCI.2302-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy JG, Sanderson JL, Gorski JA, Scott JD, Catterall WA, Sather WA, Dell’Acqua ML. AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell reports. 2014;7:1577–1588. doi: 10.1016/j.celrep.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuller MD, Fu Y, Scheuer T, Catterall WA. Differential regulation of CaV1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. J Gen Physiol. 2014;143:315–324. doi: 10.1085/jgp.201311075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]