Abstract
A panel of influenza A viruses expressing chimeric hemagglutinins (cHA) with intragroup or intergroup head/stalk combinations was generated. Viruses were characterized for growth kinetics and preservation of stalk epitopes. With a few notable exceptions, cHA viruses behaved similarly to wild-type viruses and maintained stalk epitopes, which indicated their potential as vaccine candidates to induce stalk-specific antibodies.
TEXT
An annually formulated vaccine against influenza viruses which is comprised of two subtypes of influenza A viruses (H1N1 and H3N2) and one or two strains of influenza B virus(es) is currently available (1, 2). The efficacy of the vaccine is greatly diminished when the circulating strain does not match its vaccine counterpart due to mutations. Furthermore, vaccine production is lengthy, and it may take around 6 months to manufacture a new vaccine stock against a novel pandemic strain (2).
Developing a universal influenza virus vaccine appears to be the best strategy to address the inherent problems of current vaccines. The majority of antibody (Ab) responses to influenza virus are aimed at the HA glycoprotein on the surface of the virion (3). Within influenza A viruses, the HA subtypes cluster into group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, and H18) and group 2 (H3, H4, H7, H10, H14, and H15) (4). The HA protein is composed of an immunodominant, more plastic, head domain and an immunosubdominant, but more conserved, stalk domain. These regions are divided by a disulfide bridge between cysteines 52 and 277 (H3 numbering) (5). Most of the escape mutations that occur within HA are found in the head region, which has recently been demonstrated to be more tolerant of changes than the stalk domain (6). Thus, a potential universal vaccine approach has been proposed to focus the humoral responses on the more antigenically stable stalk domain (7).
Levels of stalk-specific antibodies can be boosted when influenza viruses containing the same stalk or similar stalks but different HA heads are administered, as shown in nature during the 2009 H1N1 pandemic situation (8) and in laboratory settings (7, 9–12). Administration of sequential immunizations is the key to induction of protective levels of stalk-specific antibodies, and this vaccination regimen requires a panel of viruses expressing chimeric hemagglutinins (cHA). Here, we evaluated a large panel of novel recombinant viruses expressing cHAs in addition to those that had already been generated (7, 8, 13). Briefly, sequence-based alignment was performed for the HA proteins of influenza A viruses in which the head domains, flanked by cysteines 52 and 277 (H3 numbering), were identified. Influenza A virus HA heads were attached by cloning to either group 1 A/California/04/09 (Cal09) or A/Puerto Rico/8/34 (PR8) HA stalk or group 2 A/Perth/16/09 (Perth) HA stalk with corresponding packaging signals. Rescue of viruses with cHAs was performed in 293T cells and amplified in embryonated chicken eggs (8). The chimeric viruses that were generated are listed in Table 1 along with the abbreviated naming scheme.
TABLE 1.
Chimeric virusesa
All strains were rescued on a PR8 background. Group 1 HA heads, green; group 1 HA stalks, red; group 2 HA heads, purple; group 2 HA stalks, blue. HA sequences were amplified from the reference strains, which included A/Puerto Rico/8/34 (H1), A/California/04/09 (H1), A/Singapore/1/57 (H2), A/Perth/16/09 (H3), A/Hong Kong/1/68 (H3), A/duck/Czech/56 (H4), A/VN/1203/04 (H5), A/mallard/Sweden/81/02 (H6), A/mallard/Alberta/24/01 (H7), A/mallard/Sweden/24/2002 (H8), A/guinea fowl/Hong Kong/WF10/99 (H9), A/mallard/Interior Alaska/10BM01929/2010 (H10), A/shoveler/Netherlands/18/1999 (H11), A/mallard/Interior Alaska/7MP0167/2007 (H12), A/black headed gull/Sweden/1/1999 (H13), A/mallard/Gurjev/263/1982 (H14), A/shearwater/West Australia/2576/1979 (H15), and A/black headed gull/Sweden/5/1999 (H16) viruses. HA sequences were confirmed by Sanger sequencing. All recombinant viruses contained the regular low-pathogenicity H1 or H3 cleavage sites. Asterisks denote cross-group head/stalk combinations.
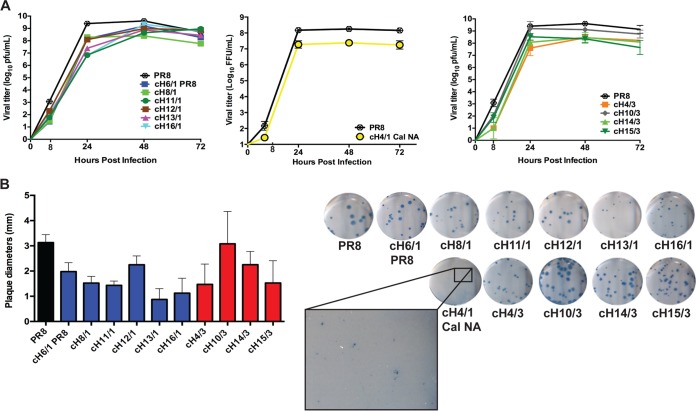
Viruses expressing cHAs replicated similarly to wild-type (WT) PR8 virus, typically reaching peak titers at 48 h postinfection (Fig. 1A). Additionally, all viruses formed plaques with variations in size compared to those seen with WT virus, with the notable exception of cH4/1, which formed barely visible pinpoint-sized plaques (Fig. 1B). In order to assess whether the stalk of cHAs expressed by the recombinant viruses retained parental WT stalk epitopes, we evaluated binding and inhibition of these viruses with previously characterized stalk-specific monoclonal antibodies (MAbs). MDCK cells were infected with WT or cHA viruses and were stained with stalk-specific MAbs for the corresponding group. Stalk-specific MAbs (KB2 [14] and 6F12 [15]) and polyclonal anti-headless serum (16) were used to assess preservation of stalk-specific epitopes in chimeric viruses with a group 1 HA stalk. Serum against PR8 virus served as a positive control for all of the viruses. Chimeric viruses with a group 1 stalk were bound by KB2 and both polyclonal antibodies (Fig. 2A). Monoclonal antibody 6F12 bound chimeric viruses with a group 1 stalk, with the exception of cH16/1 and cH13/1, in which binding was qualitatively lower than that of other viruses. To assess binding for chimeric viruses expressing a group 2 stalk, staining was performed using MAbs 12D1 (17) and 9H10 (18). Similarly to what was observed with group 1 stalk analysis, all cHAs with a group 2 stalk, except cH10/3, can be detected by both 12D1 and 9H10 MAbs. Interestingly, MAb 12D1 was able to bind to cH10/3 under denaturing conditions in a Western blot analysis (Fig. 2C).
FIG 1.
Characterization of growth properties of viruses expressing cHA. (A) Multicycle growth of chimeric and WT viruses was evaluated in embryonated chicken eggs. Error bars are based on standard deviations of results from three independent samples at each time point. (B) Plaque morphology of chimeric viruses was determined by immunostaining (21) and by measuring the size of plaques formed in ImageJ. Images are representative of results of two plaque assays per virus, and error bars are based on standard deviations of results from comparisons between 20 plaques counted per two wells for each virus. Plaques could not be counted for cH4/1 Cal NA virus.
FIG 2.
Conformational stalk epitopes are preserved in cHA of influenza viruses. Stalk-specific antibodies or control serum (PR8 serum) samples were used to probe infected MDCK cells after 16 to 24 h. (A and B) Binding was evaluated using immunofluorescence staining (A) and flow cytometry (B). Immunofluorescence images are representative of results from three samples. Flow cytometry images are representative of results of three experiments for chimeric viruses and two experiments for control viruses Cal09 and Perth. Mean fluorescence intensity (MFI) values for the representative histograms depicted are listed in tables below the respective panels. MFI values were normalized to the “no virus” control value for each panel. ND, not determined. (C) Binding of cHA with group 2 stalks was assessed under denaturing conditions using Western blot analysis performed with antibody 12D1.
Binding of stalk-specific MAbs to all chimeric influenza viruses was further confirmed by flow cytometry (Fig. 2B). All chimeric viruses with a group 1 stalk were bound by mouse polyclonal (headless) or monoclonal (KB2 and 6F12) antibodies with the exception that 6F12 did not bind cH16/1 (Fig. 2B). Human MAb FI6 (19, 20), which recognizes 16 subtypes of influenza A virus HA, bound all chimeric viruses with a group 1 stalk. The results indicate that most cHAs retain their original stalk epitopes whereas others can exhibit a loss of binding, as seen with cH16/1 and MAb 6F12. Viruses with a group 2 stalk were bound by mouse MAbs 12D1 and 9H10, as well as by human MAb FI6. Interestingly, cH10/3 was not bound by 12D1 or 9H10 but was recognized by FI6. Overall, the results of flow cytometry confirm the results of the immunofluorescence assay and demonstrate that most cHAs recapitulate the native stalk epitopes of the parental HA on the surface of infected cells.
In order to further confirm that cHA antigenic epitopes remain intact, neutralization of chimeric viruses with stalk-specific antibodies was assessed by a microneutralization assay (15). Monoclonal antibodies KB2 and 6F12 were used to evaluate viruses with a group 1 stalk (Fig. 3A and B). Both MAbs inhibited the replication of cH6/1, cH8/1, cH11/1, and cH12/1 viruses. Viruses cH13/1 and cH16/1 were neutralized by KB2 only at the highest concentration and were not neutralized by 6F12. In addition, the intergroup cH4/1 virus was neutralized by both KB2 and 6F12 (Fig. 3C). Viruses with a group 2 stalk were neutralized by 9H10, with the exception of cH10/3, which was neutralized only at the highest concentration (Fig. 3D).
FIG 3.
Chimeric HA influenza viruses can be neutralized by stalk-specific antibodies. Stalk-specific antibodies KB2, 6F12, and 9H10 were tested for their ability to neutralize WT and chimeric viruses by microneutralization assay for viruses with a group 1 (A, B, and C) or group 2 (D) stalk. Error bars are based on standard deviations of results from an average of triplicate samples from three independent experiments. (E to H) An ELISA was performed with select monoclonal antibodies and viruses. OD490, optical density at 490 nm.
To further investigate the diminished binding of MAbs 6F12 and KB2 to cH13/1 and cH16/1 as well as the loss of binding of 9H10 to cH10/3, we performed enzyme-linked immunosorbent assays (ELISAs) (Fig. 3E to H). MAb KB2 showed diminished binding to both cH13/1 and cH16/1 viruses compared to cH8/1 or wild-type Cal09 virus. Diminished binding was also observed with 6F12 and cH13/1. 6F12 binding to cH16/1 was completely lost. Similarly, no binding of MAb 9H10 to cH10/3 was detected, whereas this virus was recognized reasonably well by stalk MAb CR9114. In general, these quantitative data are in agreement with results from flow cytometry, neutralization assays, and cell surface staining.
Here, recombinant viruses have been generated for all of the intragroup combinations of HA heads with an H1 HA stalk or an H3 HA stalk. Our data suggest that combinations with different group 1 heads with an H1 (group 1) stalk or group 2 heads with an H3 (group 2) stalk are well tolerated (8, 13). For intergroup head/stalk combinations of cHA, we have generated cH4/1 virus, the first rescued chimeric virus with a group 2 head on a group 1 stalk. All cHA viruses replicated similarly to WT virus and can be bound or inhibited by a series of stalk-specific antibodies. Interestingly, for two group 1 cHAs (cH13/1 and cH16/1) and one group 2 cHA (cH10/3), we found reduced or completely abolished stalk-antibody binding. We hypothesize that these changes could be caused by structural changes in the epitope of the tested antibody or by steric hindrance from the head domains or by both mechanisms at the same time. H13 and H16 HAs are closely related, and their head domains are very similar (1). It is conceivable that structural changes caused by the H13 head would also be caused by the H16 head domain, which is exactly what we observed. Since MAb FI6, which has a footprint similar to that of 6F12, shows no reduced binding to these two strains, it is likely that small changes in the epitope itself caused this loss of binding (Fig. 2B). This change seems to have had a greater impact on 6F12 binding than on KB2 binding. Ablation of MAb 12D1 binding to the H3 stalk of cH10/3 was observed only under native conditions (Fig. 2A and B). Under denaturing conditions (e.g., in Western blot analyses—Fig. 2C), the MAb recognized the stalk domain. The epitope of 12D1 is located at a relatively high point in the stalk domain on the long alpha helix of HA2, partially covered by the head domain. The head domain of cH10/3 might therefore sterically hinder 12D1 binding. Overall, this information not only is valuable for the design of future influenza virus vaccines but also sheds light on the intricate interactions between the head and stalk domains of the hemagglutinin.
ACKNOWLEDGMENTS
We thank Daniel Perez (University of Georgia) for providing us with the A/mallard/Alberta/24/01 (H7) and A/guinea fowl/Hong Kong/WF10/99 (H9) HA constructs, Ron Fouchier (Erasmus MC) for sharing the A/mallard/Sweden/81/02 (H6) HA construct, Jonathan Runstadler (MIT) for sharing avian influenza virus isolates (H10N7 and H12N5), and Patrick C. Wilson (University of Chicago) for sharing MAb FI6.
This work was partially supported by NIH grants P01 AI097092 (P.P.) and U19 AI109946 (P.P. and F.K.) and by the NIH Centers of Excellence in Influenza Virus Research and Surveillance (CEIRS; contract number HHSN272201400008C; P.P. and F.K.).
Funding Statement
This work was partially supported by NIH grants P01 AI097092 (P.P.) and U19 AI109946 (P.P. and F.K.) and by the NIH Centers of Excellence in Influenza Virus Research and Surveillance (CEIRS; contract number HHSN272201400008C; P.P. and F.K.).
REFERENCES
- 1.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. September 22, 2014. Selecting the viruses in the seasonal influenza (Flu) vaccine. http://www.cdc.gov/flu/professionals/vaccination/virusqa.htm Accessed July 1, 2014.
- 3.Petrie JG, Ohmit SE, Johnson E, Truscon R, Monto AS. 26 May 2015. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J Infect Dis doi: 10.1093/infdis/jiv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latorre-Margalef N, Grosbois V, Wahlgren J, Munster VJ, Tolf C, Fouchier RA, Osterhaus AD, Olsen B, Waldenstrom J. 2013. Heterosubtypic immunity to influenza A virus infections in mallards may explain existence of multiple virus subtypes. PLoS Pathog 9:e1003443. doi: 10.1371/journal.ppat.1003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waterfield M, Scrace G, Skehel J. 1981. Disulphide bonds of haemagglutinin of Asian influenza virus. Nature 289:422–424. doi: 10.1038/289422a0. [DOI] [PubMed] [Google Scholar]
- 6.Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. 2013. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A 110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krammer F, Albrecht RA, Tan GS, Margine I, Hai R, Schmolke M, Runstadler J, Andrews SF, Wilson PC, Cox RJ, Treanor JJ, Garcia-Sastre A, Palese P. 2014. Divergent H7 immunogens offer protection from H7N9 virus challenge. J Virol 88:3976–3985. doi: 10.1128/JVI.03095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. 2014. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol 88:2340–2343. doi: 10.1128/JVI.03183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krammer F, Pica N, Hai R, Tan GS, Palese P. 2012. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J Virol 86:10302–10307. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, Garcia-Sastre A, Albrecht RA. 2014. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 88:3432–3442. doi: 10.1128/JVI.03004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaton NS, Leyva-Grado VH, Tan GS, Eggink D, Hai R, Palese P. 2013. In vivo bioluminescent imaging of influenza a virus infection and characterization of novel cross-protective monoclonal antibodies. J Virol 87:8272–8281. doi: 10.1128/JVI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. 2012. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol 86:6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohlbold TJ, Nachbagauer R, Margine I, Tan GS, Hirsh A, Krammer F. 2015. Vaccination with soluble headless hemagglutinin protects mice from challenge with divergent influenza viruses. Vaccine 33:3314–3321. doi: 10.1016/j.vaccine.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P. 2010. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog 6:e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan GS, Lee PS, Hoffman RM, Mazel-Sanchez B, Krammer F, Leon PE, Ward AB, Wilson IA, Palese P. 2014. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. J Virol 88:13580–13592. doi: 10.1128/JVI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekiert DC, Wilson IA. 2012. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr Opin Virol 2:134–141. doi: 10.1016/j.coviro.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 21.Hai R, Martinez-Sobrido L, Fraser KA, Ayllon J, Garcia-Sastre A, Palese P. 2008. Influenza B virus NS1-truncated mutants: live-attenuated vaccine approach. J Virol 82:10580–10590. doi: 10.1128/JVI.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]