ABSTRACT
Human T cell lymphotropic virus type 1 (HTLV-1) Tax-1, a key protein in HTLV-1-induced T cell transformation, deregulates diverse cell signaling pathways. Among them, the NF-κB pathway is constitutively activated by Tax-1, which binds to NF-κB proteins and activates the IκB kinase (IKK). Upon phosphorylation-dependent IκB degradation, NF-κB migrates into the nucleus, mediating Tax-1-stimulated gene expression. We show that the transcriptional regulator of major histocompatibility complex class II genes CIITA (class II transactivator), endogenously or ectopically expressed in different cells, inhibits the activation of the canonical NF-κB pathway by Tax-1 and map the region that mediates this effect. CIITA affects the subcellular localization of Tax-1, which is mostly retained in the cytoplasm, and this correlates with impaired migration of RelA into the nucleus. Cytoplasmic and nuclear mutant forms of CIITA reveal that CIITA exploits different strategies to suppress Tax-1-mediated NF-κB activation in both subcellular compartments. CIITA interacts with Tax-1 without preventing Tax-1 binding to both IKKγ and RelA. Nevertheless, CIITA affects Tax-1-induced IKK activity, causing retention of the inactive p50/RelA/IκB complex in the cytoplasm. Nuclear CIITA associates with Tax-1/RelA in nuclear bodies, blocking Tax-1-dependent activation of NF-κB-responsive genes. Thus, CIITA inhibits cytoplasmic and nuclear steps of Tax-1-mediated NF-κB activation. These results, together with our previous finding that CIITA acts as a restriction factor inhibiting Tax-1-promoted HTLV-1 gene expression and replication, indicate that CIITA is a versatile molecule that might also counteract Tax-1 transforming activity. Unveiling the molecular basis of CIITA-mediated inhibition of Tax-1 functions may be important in defining new strategies to control HTLV-1 spreading and oncogenic potential.
IMPORTANCE HTLV-1 is the causative agent of human adult T cell leukemia-lymphoma (ATLL). The viral transactivator Tax-1 plays a central role in the onset of ATLL, mostly by deregulating the NF-κB pathway. We demonstrate that CIITA, a key regulator of adaptive immunity, suppresses Tax-1-dependent activation of NF-κB by acting at several levels: it retains most of Tax-1 and RelA in the cytoplasm and inhibits their residual functional activity in the nucleus. Importantly, this inhibition occurs in cells that are targets of HTLV-1 infection. These findings are of interest in the field of virology because they expand the current knowledge of the functional relationship between viral products and cellular interactors and provide the basis for a better understanding of the molecular countermeasures adopted by the host cell to antagonize HTLV-1 spreading and transforming properties. Within this framework, our results may contribute to the establishment of novel strategies against HTLV-1 infection and virus-dependent oncogenic transformation.
INTRODUCTION
The onset of adult T cell leukemia/lymphoma (ATLL), a malignant disorder of CD4+ T lymphocytes, has been associated with infection with human T cell lymphotropic virus type 1 (HTLV-1), the first oncogenic retrovirus discovered in humans (1–3). It is currently estimated that HTLV-1 affects approximately 15 to 20 million of people in the world, 2 to 5% of whom develop leukemia following many years of clinical latency (4, 5). HTLV-1 is also the causative agent of a neurological disease called tropical spastic paraparesis/HTLV-1-associated myelopathy (6). In contrast, HTLV-2, a closely related retrovirus originally isolated from a case of atypical hairy T cell leukemia, has not been epidemiologically linked to lymphoproliferative disorders (7). Both the HTLV-1 and HTLV-2 genomes encode homologous transcription activators, designated Tax-1 and Tax-2, respectively, that control viral gene expression and viral replication (8–17). Besides promoting proviral transcription, Tax-1 is a pivotal player in HTLV-1-induced T cell transformation, modulating the expression of cellular genes and deregulating cell signaling pathways involved in cell proliferation, cell cycle control, DNA damage repair, and apoptosis (4, 8, 18–23). The oncogenic potential of Tax-1 is due mostly to its ability to constitutively activate the nuclear factor kappa B (NF-κB) pathway (24–28). Two distinct NF-κB signaling pathways, the canonical and the noncanonical, are activated by different immune stimuli (29, 30). Antigens and cytokines activate the canonical route via the trimeric IκB kinase (IKK), composed of two catalytic subunits, α and β, and the regulatory IKKγ subunit (NEMO). Inactive canonical NF-κB heterodimer, composed of p50 and RelA subunits, is sequestered in the cytoplasm complexed with the IκB inhibitor. Following phosphorylation by activated IKK, IκB is ubiquitinated and degraded. The noncanonical pathway is induced by several tumor necrosis factor family members and requires the NF-κB-inducing kinase (NIK) and downstream kinase IKKα, which causes the phosphorylation-dependent processing of precursor protein p100, whose C-terminal portion acts as an NF-κB inhibitor, trapping the NF-κB heterodimer of the noncanonical pathway, p52/RelB, in the cytoplasm (31). Free from the inhibitors, the NF-κB heterodimers migrate into the nucleus and activate the transcription of NF-κB target genes. Tax-1 activates both axes of the NF-κB signaling network by stimulating the different IKK complexes. In the canonical pathway, Tax-1 interacts directly with IKKγ and triggers the activation of IKK (25, 32, 33). In the noncanonical pathway, Tax-1–IKKγ interaction facilitates assembly of the Tax-1/IKKα complex, bypassing the requirement of NIK for IKK kinase activation (34–37). Unlike Tax-1, Tax-2 is able to activate only the canonical NF-κB route (38, 39). Besides binding to IKKγ in the cytoplasm, Tax-1 promotes NF-κB activation in the nucleus by interacting with RelA and stabilizing the binding of p50/RelA to NF-κB-responsive promoters (24, 40). In addition, Tax-1 colocalizes with RelA, p50, and IKKγ in discrete nuclear structures called nuclear speckles or nuclear bodies (NB) (26, 41–43). These structures contain many cellular factors, among which RNA polymerase II, splicing factors, and CDK8 and are sites of Tax-1 transcriptional activity (28, 44). Several posttranslational modifications of Tax-1 regulate its ability to activate NF-κB (reviewed in references 28, 44, and 45), although for some of them, there is no consensus as yet. For instance, it has been shown that ubiquitin-conjugated Tax-1 activates IKKγ in the cytoplasm, whereas sumoylation of Tax-1 is required for its assembly in NB and activation of NF-κB-responsive genes (26). However, more recent papers have reported that the formation of Tax-1 NB and Tax-1 sumoylation are not strictly required for NF-κB-driven gene induction (46, 47). Similarly, the requirement of Tax-2 ubiquitination and sumoylation for the activation of NF-κB is still debated (28, 48, 49).
We have demonstrated that transactivation of the HTLV long terminal repeat (LTR) by both Tax-1 and Tax-2 is inhibited by the cellular host factor CIITA (class II transactivator). Importantly, this inhibition correlates with the suppression of HTLV-1 and HTLV-2 replication, respectively (50–54). Similarly, we previously found that CIITA also inhibits HIV-1 replication by targeting the viral transactivator Tat (55, 56). CIITA, originally discovered for its function as a transcriptional activator of major histocompatibility complex class II (MHC-II) genes (57–59), plays a central role in antigen presentation to and activation of CD4+ T helper cells and thus in the triggering of adaptive immunity. Importantly, CIITA may be expressed in activated human T cells, the specific targets of HTLV-1. Thus, CIITA is an example of a versatile molecule able to counteract retroviral infections in a dual manner, not only because of its capacity to trigger the adaptive immune response against pathogens but also for its intrinsic antiviral properties that enable it to act as a bona fide viral restriction factor (53, 60).
In this study, we investigated whether CIITA, besides inhibiting the transactivation of the viral LTR promoter by Tax-1, may also affect the molecular mechanisms involved in the HTLV-1-mediated oncogenic transformation of infected cells. We focused our attention on Tax-1-driven activation of the NF-κB pathway. We found that activation of the canonical NF-κB pathway by Tax-1 is inhibited in cells expressing CIITA both ectopically and endogenously. We also mapped the region of CIITA that mediates this effect. This inhibition is brought about by several distinct actions of CIITA exerted at both the cytoplasmic and nuclear levels, indicating a pleiomorphic effect of CIITA on the viral transactivator. Overall, our results disclose new scenarios in the mechanisms through which HTLV-1 and its cellular host interact and may lead to a better interpretation of the virus-mediated oncogenic process and possibly to alternative strategies to counteract it.
MATERIALS AND METHODS
Plasmids.
The pNF-κB-Luc plasmid contains the firefly luciferase reporter gene driven by a basic promoter element (TATA box) plus a cis enhancer element including five NF-κB binding sites (Stratagene). In the phRL-CMV vector (Promega), the expression of Renilla luciferase is under the control of the cytomegalovirus promoter. The pCMV-Tax-1 and Tax-2 plasmids were previously described (51, 53). The pACH plasmid, containing the entire HTLV-1 genome, has been previously described (53). Expression vectors for FLAG-tagged full-length (amino acids 1 to 1130) CIITA (pcfCIITA) and for the deletion-containing forms of CIITA (pcf1-252, pcf253-1130, pcf253-410, pcfΔ253-410, pcf64-200) have been described elsewhere (51, 53, 61). The CIITA-5aa expression vector (here pcfCIITAΔ955-959) was kindly donated by J. Ting, University of North Carolina. Expression vectors pJFETax-1 and pJFETax-1 F9 were a kind gift from F. Bex, Universitè Libre de Bruxelles, Brussels, Belgium.
Cells and treatments.
Human embryonic kidney 293T cells were grown in Dulbecco's modified Eagle medium containing 5 mM l-glutamine and supplemented with 10% fetal calf serum. Where indicated, the cells were incubated with 20 nM leptomycin B (LMB; Sigma) or the vehicle methanol for the last 3 h of transfection. To detect P-IκB, 293T cells were transfected with pTax-1 (100 ng) alone or together with pcfCIITA (1 μg) and treated with the proteasome inhibitor MG132 (30 μM; Calbiochem) for 7 h before harvesting. Cells were then lysed and analyzed by Western blotting with anti-phospho-IκBα (Ser 32/36) and anti-IκBα (Cell Signaling Technology) antibodies and anti-FLAG M2 (Sigma) and anti-α-tubulin (Sigma) antibodies. Jurkat and Jurkat GFPfCIITA cells and monocytic U937 isogenic cell clones 10 and 34 and U937 cl10-fCIITA cells were previously described (51, 53).
NF-κB luciferase assay and Western blotting.
293T cells were seeded into 60-mm-diameter plates and cotransfected with 0.15 μg of the reporter plasmid pNF-κB-Luc with either 12.5 ng of pCMV-Tax-1 or 50 ng of pTax-2 with increasing amounts (0.2, 0.4, and 0.8 μg) of the expression vectors for full-length CIITA or deletion mutant forms of CIITA by using Lipofectamine reagent (3 μl/μg DNA; Life Technology) as described previously (62). For the experiments with pJFETax-1 and pJFETax-1 F9, 50 ng was transfected into 293T cells. All transfections were carried out in the presence of 5 ng of phRL-CMV. Empty backbone vector was used as stuffer DNA to maintain a constant total amount of transfected DNA. Cell extracts were prepared 24 h posttransfection and assayed for luciferase activities by using the dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions. Mean luciferase values, normalized to Renilla luciferase values, of at least three independent experiments performed in duplicate are expressed as percentages of Tax-1- or Tax-2-dependent luciferase activity, which was set to 100%. Error bars represent the standard deviation (SD). Cell lysates were analyzed for the expression of recombinant proteins by SDS-PAGE and Western blotting with the anti-FLAG M2 (Sigma) monoclonal antibody to detect FLAG-tagged CIITA proteins. Horseradish peroxidase-conjugated anti-mouse Ig secondary antibody was used (Thermo Scientific). Blots were developed by chemiluminescence assay (SuperSignal West Pico; Thermo Scientific). Assays of NF-κB luciferase activity in Jurkat and U937 cells were performed by cotransfecting 2 × 106 cells with 1 μg of pNF-κB-Luc, 100 ng of phRL-CMV, and 2 μg of pACH by using FuGENE HD (Promega). Cells were harvested after 48 h and tested for luciferase activity. Experiments were performed in duplicate, and mean luciferase values were normalized to Renilla luciferase values.
Immunoprecipitation.
For protein binding studies, 293T cells were seeded into 100-mm-diameter plates and transfected with expression vectors coding for FLAG-tagged CIITA (2 μg) or FLAG-tagged mutant forms of CIITA (2 μg) and Tax-1 (2 μg) with Lipofectamine. Empty pcDNA3 vector was used as stuffer DNA. Twenty-four hours after transfection, cells were washed once with 1× phosphate-buffered saline (PBS) and lysed with 500 μl of lysis buffer (1% NP-40, 10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA) supplemented with 0.1% protease inhibitor cocktail (Sigma) while incubating on ice for 45 min. Precleared cell lysates were immunoprecipitated overnight at 4°C with anti-FLAG M2 agarose beads (Sigma). After extensive washing with lysis buffer, precipitated proteins were resolved by 8% SDS-PAGE and analyzed by immunoblotting with anti-Tax-1 hybridoma (clone 168 A51-2 from the NIH AIDS Research and Reference Reagent Program). To detect Tax-1 interacting with endogenous RelA or IKKγ, 293T cells were transfected with pTax-1 (2 μg) with or without pcfCIITA (2 μg). Twenty-four hours after transfection, cells were lysed as described above. After preclearing, cell extracts were incubated with anti-RelA or anti-IKKγ rabbit polyclonal antibodies (Santa Cruz Biotechnology) and protein A-Sepharose beads overnight at 4°C. Immune complexes bound to the beads were extensively washed with the lysis buffer. Precipitated proteins were resolved by 8% SDS-PAGE and analyzed by Western blotting with anti-RelA or anti-IKKγ rabbit polyclonal antibodies, anti-CIITA 7-1H antibodies (Santa Cruz Biotechnology), and anti-Tax-1 clone 168 A51-2 antibodies. Ten percent of the total cell extract was used for the detection of protein expression by Western blotting (input).
IF assay and confocal microscopy.
293T cells cultured on glass coverslips precoated with poly-l-lysine were transfected with 0.2 μg of each plasmid expressing untagged Tax-1 and FLAG-tagged full-length CIITA or mutant forms of CIITA. At 24 h posttransfection, cells were fixed in methanol for 6 min at −20°C and blocked with 0.5% BSA in 1× PBS for 1 h at room temperature (RT). Cells were stained overnight with anti-Tax-1 clone 168A51-2, anti-RelA rabbit polyclonal antibody (Santa Cruz Biotechnology), and anti-FLAG M2 monoclonal antibody (Sigma). The slides were then washed five times with cold 1× PBS and incubated in the dark for 1 h at RT with the following secondary antibodies from Life Technology: goat anti-mouse IgG2a conjugated to Alexa Fluor 488 to detect Tax-1, goat anti-rabbit IgG conjugated to Alexa Fluor 546 to detect RelA, and goat anti-mouse IgG1 coupled to Alexa Fluor 633 to detect CIITA. After extensive washings with 1× PBS, slides were mounted on coverslips with the Fluor Save reagent (Calbiochem) and examined by a confocal laser scanning microscope (Leica TCS SP5; HCX PL APO objective lenses, ×63 original magnification, numerical aperture 1.25). Images were acquired and analyzed by LAS AF software. U937 cell clones were transfected with 2 μg of plasmid pACH for 48 h and processed as described above for confocal analysis. Anti-CIITA 7-1H mouse monoclonal antibody (Santa Cruz Biotechnology) and goat anti-mouse IgG1 coupled to Alexa Fluor 633 were used to detect endogenous CIITA.
RESULTS
CIITA inhibits Tax-dependent activation of the NF-κB pathway, and this suppression occurs independently of the phosphorylation status of Tax-1.
To investigate whether CIITA is able to counteract the persistent activation of the NF-κB pathway by Tax-1, 293T cells were cotransfected with the NF-κB luciferase gene reporter plasmid and the expression vector of Tax-1 with or without increasing amounts of the plasmid coding for CIITA. We found that CIITA inhibits the NF-κB-dependent luciferase activity induced by Tax-1 in a dose-dependent manner (Fig. 1A, columns 4 to 6 versus column 3), but it does not significantly affect the basal activation of NF-κB (Fig. 1A, column 2 versus column 1). It has been demonstrated that Tax-1 constitutively activates both the canonical and the noncanonical pathways of NF-κB, whereas Tax-2 activates only the canonical pathway. Therefore, in order to verify whether the observed inhibitory effect of CIITA depends on the specific inhibition of the canonical NF-κB pathway, we analyzed whether Tax-2-mediated activation of NF-κB is also suppressed by CIITA. We found that, similarly to Tax-1, activation of the NF-κB pathway by Tax-2 is suppressed by CIITA in a dose-dependent manner (Fig. 1A, columns 8 to 10 versus column 7). Thus, we conclude that CIITA inhibits at least Tax-mediated activation of the canonical NF-κB pathway. It has been shown that the phosphorylation of Tax-1 is required for the activation of the NF-κB pathway and that the Tax-1 F9 mutant protein, with serines in positions 300 and 301 replaced with aspartic acid, still activates NF-κB (63). This phosphomimetic mutant protein was used to understand whether CIITA inhibits Tax-1-mediated activation of NF-κB by affecting the phosphorylation of Tax-1, because in this case, CIITA should not inhibit the F9 mutant protein. As previously shown (63) and confirmed here, Tax-1 F9 activates the NF-κB–luciferase gene reporter less efficiently than wild-type Tax-1 does (Fig. 1B, column 7 versus column 3). Importantly, CIITA still inhibits this activation in a dose-dependent manner (Fig. 1B, columns 8 to 10 versus column 7), indicating that CIITA inhibition of Tax-1-dependent activation of NF-κB does not operate via modification of the phosphorylation status of the viral transactivator.
FIG 1.
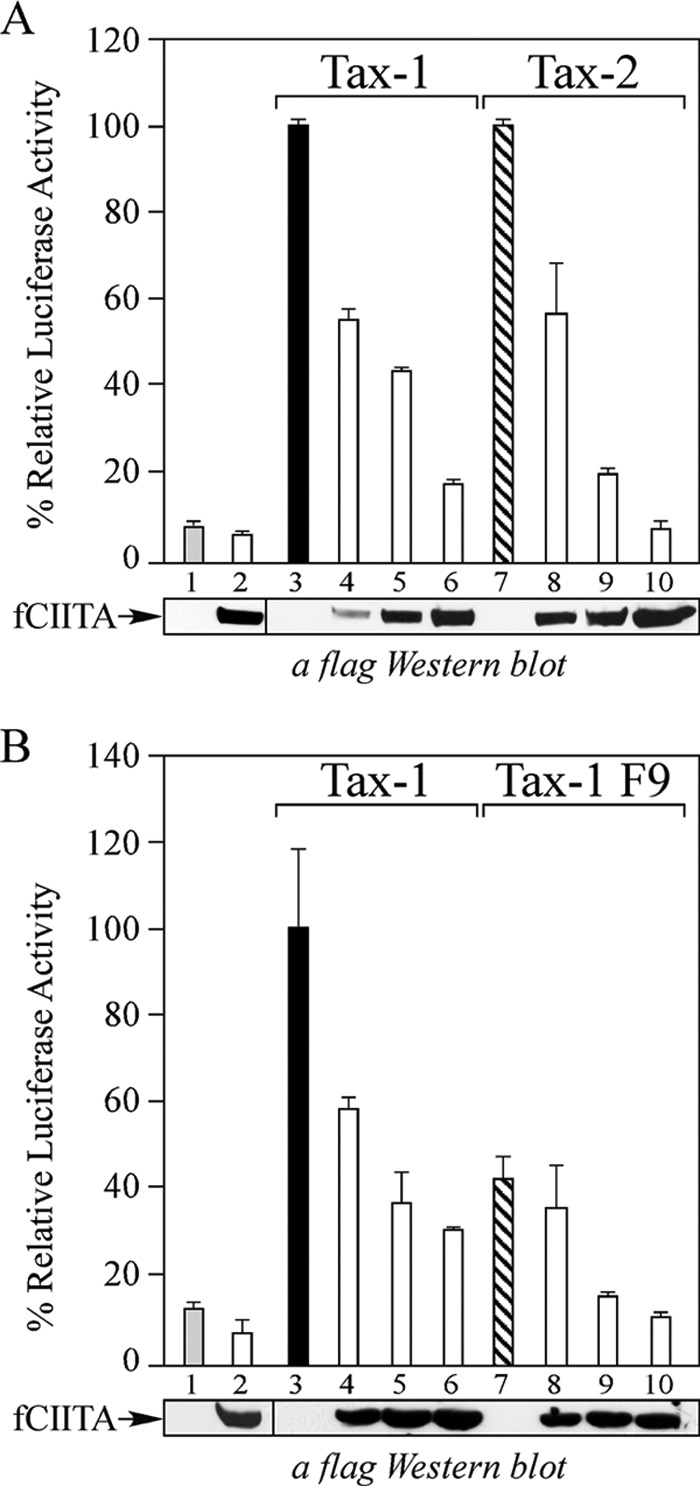
CIITA inhibits activation of the NF-κB pathway induced by HTLV-1 Tax-1, the phosphomimetic mutant form Tax-1 F9, and HTLV-2 Tax-2. (A) 293T cells were cotransfected with fixed amounts of pNF-κB-Luc, phRL-CMV, and pTax-1 (black column 3) or pTax-2 (hatched column 7) plus increasing amounts (0.2, 0.4, and 0.8 μg) of the plasmid coding for FLAG-tagged CIITA (columns 4 to 6 and 8 to 10). Mean luciferase activities, normalized to Renilla luciferase activity, are presented as percentages of the activation by Tax-1 and Tax-2, which is set to 100% (columns 3 and 7, respectively). (B) 293T cells were cotransfected with fixed amounts of pNF-κB-Luc, phRL-CMV, and pJFETax-1 (black column 3) or pJFETax-1 F9 (hatched column 7) plus increasing amounts (0.2, 0.4, and 0.8 μg) of pcfCIITA (columns 4 to 6 and 8 to 10). Mean luciferase activities, normalized to Renilla luciferase activity, are presented as percentages of the activation by Tax-1, which is set to 100% (column 3). Columns 1 and 2 represent the NF-κB activity of 293T cells transfected with the empty backbone vector or with the maximum amount of plasmid pcfCIITA (0.8 μg), respectively. Error bars represent the SD. fCIITA protein expression in cell extracts was evaluated by anti-FLAG Western blotting.
Both nuclear and cytoplasmic mutant forms of CIITA containing the N-terminal region inhibit Tax-1-dependent activation of NF-κB.
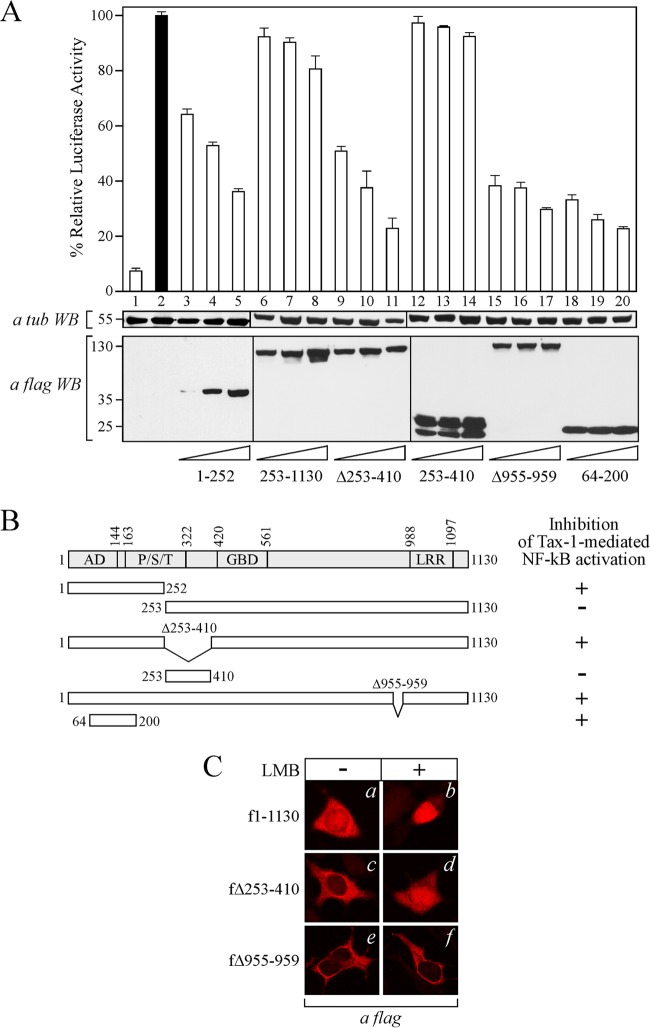
To define the region of CIITA involved in the inhibition of Tax-1-dependent activation of NF-κB, several deletion mutant forms of CIITA were analyzed for the ability to suppress the NF-κB-dependent luciferase activity induced by Tax-1. We found that two CIITA N-terminal truncations (1 to 252 and 64 to 200) having predominant nuclear localization (53) inhibited NF-κB activation in a dose-dependent manner (Fig. 2A, columns 3 to 5 and 18 to 20 versus column 2, respectively). Thus, the region encompassing amino acids 64 to 200, including the activation domain of CIITA, is crucial for inhibition of NF-κB activation by Tax-1. Accordingly, CIITA fragments encompassing amino acids 253 to 1130 and 253 to 410 did not inhibit Tax-1-dependent NF-κB activation (Fig. 2A, columns 6 to 8 and 12 to 14 versus column 2, respectively), confirming the requirement of the N terminus of CIITA for its inhibitory function. Because CIITA is a shuttling protein with dual cytoplasmic and nuclear distribution and is sensitive to LMB, an inhibitor of CRM1-dependent nuclear export, causing the accumulation of CIITA in the nucleus (53, 64, 65) (Fig. 2C, b versus a), we next investigated whether cytoplasmic mutant forms of CIITA, containing the region encompassing amino acids 64 to 200, were able to suppress the activation of NF-κB by Tax-1. We found that the dimerization-deficient CIITAΔ253-410 mutant form (61), with steady-state localization in the cytoplasm, inhibited NF-κB activation by Tax-1 (Fig. 2A, columns 9 to 11 versus column 2). However, this mutant protein partially accumulated in the nucleus after LMB treatment (Fig. 2C, d versus c) and did not allow clear demonstration of a possible inhibitory action of CIITA at the cytoplasmic level. To clarify this issue, we searched for a CIITA mutant protein with exclusive cytoplasmic localization. We found that the CIITAΔ955-959 mutant protein carrying an in-frame deletion of residues 955 to 959 containing a functional nuclear localization signal (NLS) (66) is insensitive to LMB and thus may be considered a bona fide cytoplasmic mutant form of CIITA (Fig. 2C, f versus e). Of note, CIITAΔ955-959 was still able to inhibit the activation of NF-κB by Tax-1 (Fig. 2A, columns 15 to 17 versus column 2) and maintained the ability to suppress Tax-1-dependent transcriptional activation of the viral LTR promoter similarly to full-length CIITA (data not shown). Altogether, these data demonstrate that CIITA may abrogate Tax-1-induced activation of NF-κB at the cytoplasmic level.
FIG 2.
Inhibition of Tax-1-dependent NF-κB activation requires the N-terminal region of CIITA. (A) 293T cells were cotransfected with fixed amounts of pNF-κB-Luc, phRL-CMV, and pTax-1 and increasing amounts (0.2, 0.4, and 0.8 μg) of the plasmid coding for each of the FLAG-tagged deletion mutant forms of CIITA indicated at the bottom. The results of a representative experiment are shown. Mean luciferase activities, normalized to Renilla luciferase activity, are presented as percentages of the activation by Tax-1, which is set to 100% (black column 2). Column 1 represents the basal activity of cells transfected with the pcDNA3 vector. The expression of recombinant fCIITA proteins in cell extracts was evaluated by anti-FLAG Western blotting (WB). As a loading control, the expression of α-tubulin was analyzed by Western blotting. (B) Schematic representation of the results of the gene reporter assay illustrated in panel A. The CIITA proteins used for mapping are shown, along with their capacity to inhibit Tax-1-dependent activation of the NF-κB pathway (+). The endpoints of full-length CIITA, mutant forms of CIITA, and the internal deleted region are indicated. At the top is a diagram of CIITA with its domains labeled as follows: AD, activation domain; P/S/T, proline/serine/threonine-rich domain; GBD, GTP-binding domain; LRR, leucine-rich repeats. (C) The subcellular localization of FLAG-tagged full-length CIITA (amino acids 1 to 1130) and the Δ253-410 and Δ955-959 mutant forms was analyzed in 293T cells by IF assay and confocal microscopy analysis before (panels a, c, and e) and after (b, d, and f) LMB treatment as described in Materials and Methods.
CIITA affects the subcellular localization of Tax-1.
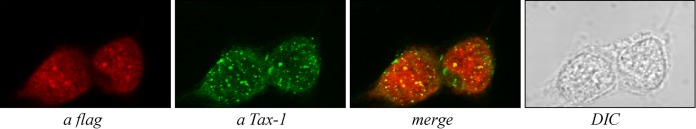
To further investigate the cellular correlates of CIITA-mediated inhibition of NF-κB activation by Tax-1, we assessed whether CIITA affects the subcellular localization of Tax-1. For this purpose, we first analyzed the distribution of Tax-1 by transfecting 293T cells with the expression vector of untagged Tax-1 because the presence of a tag has been shown to influence the nucleus/cytoplasm ratio of Tax-1 distribution (67). At least 200 Tax-1-expressing cells were analyzed by immunofluorescence (IF) assay and confocal microscopy. We found that 70% of the cells express Tax-1 in nuclear punctate structures (NB), 20% express Tax-1 in both NB and the cytoplasm, and the remaining 10% express Tax-1 only in the cytoplasm (Fig. 3A). Similar results were reported by other investigators (26). We then analyzed the localization of Tax-1 in 293T cells coexpressing CIITA. The images relative to the entire population of transfected cells and enlarged fields representative of the Tax-1 distribution observed are shown in Fig. 3B and C, respectively. We found a subcellular localization of Tax-1 in cells coexpressing Tax-1 and CIITA that was different from that in cells expressing Tax-1 alone. Sixty percent of the cotransfected cells expressed Tax-1 only in the cytoplasm. The remaining 40% of the cells expressed Tax-1 in both the cytoplasm and NB. Remarkably, no cells with exclusive localization of Tax-1 in NB were detected. The overexpression of Tax-1 did not affect the subcellular distribution of CIITA, which exhibits its canonical nuclear and cytoplasmic localization. However, in many cells, we observed a more granular staining of nuclear CIITA overlapping Tax-1-containing dots (Fig. 3C). Overall, these results indicate that CIITA drastically affects the typical distribution of Tax-1, modifying the relative percentages of cells expressing Tax-1 in the different subcellular compartments.
FIG 3.
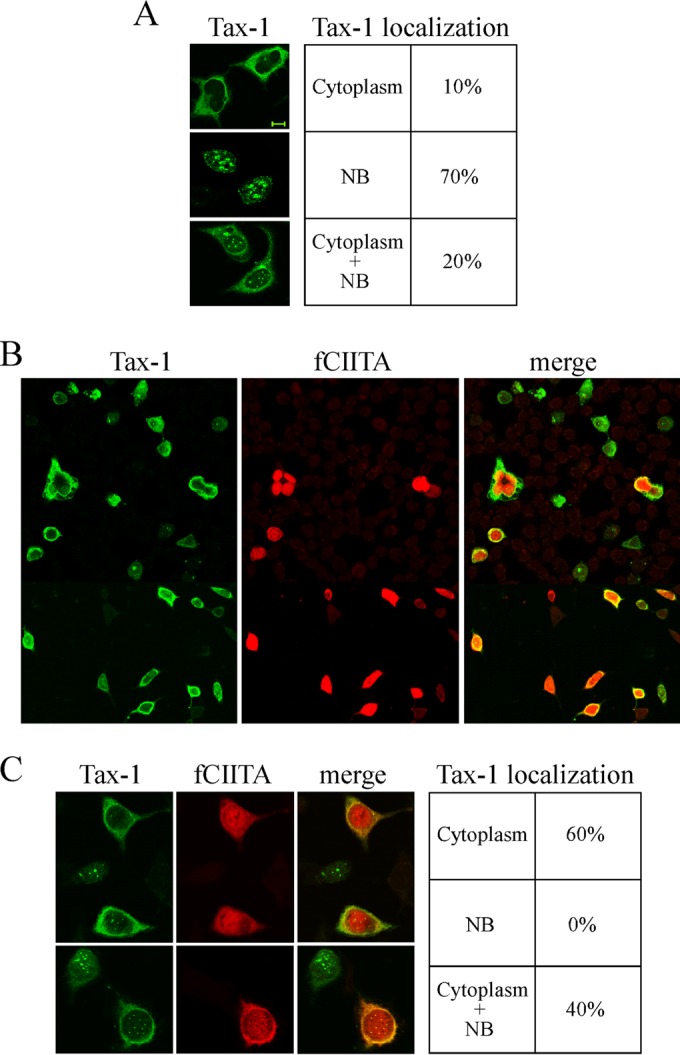
The subcellular distribution of Tax-1 is affected by CIITA. 293T cells were transfected with 0.2 μg of Tax-1 expression vector alone (A) or in combination with 0.2 μg of plasmid pfCIITA (B and C) and analyzed by IF assay and confocal microscopy for Tax-1 and CIITA subcellular localization. In panels A and C, the tables show the percentages of cells expressing Tax-1 in the different subcellular compartments. Bar, 10 μm.
CIITA prevents the nuclear translocation of RelA induced by Tax-1.
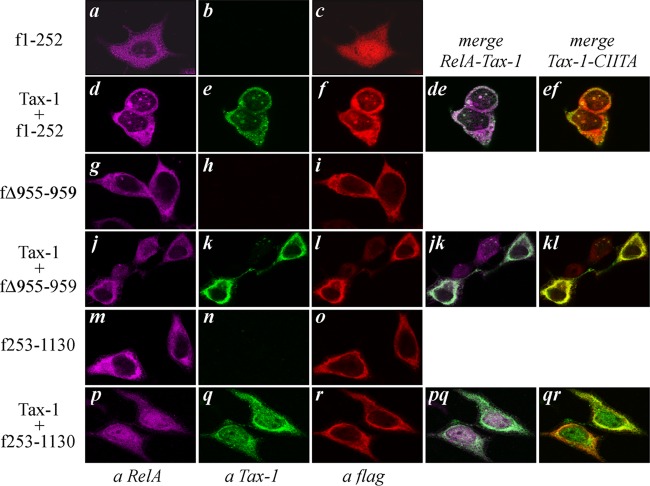
To verify whether the abilities of CIITA to inhibit Tax-1-mediated NF-κB activation and to delocalize Tax-1 in the cytoplasm correlate with impaired migration of RelA into the nucleus, we analyzed the cellular distribution of endogenous RelA in 293T cells expressing Tax-1 with or without CIITA. In untransfected cells (NT), RelA shows exclusive diffuse cytoplasmic staining (Fig. 4a). In contrast, the expression of Tax-1 induces the nuclear translocation of RelA and its accumulation in NB (Fig. 4d) containing Tax-1 (Fig. 4e and de), as previously reported (40, 41). Remarkably, in cells expressing both CIITA and Tax-1, RelA is retained in the cytoplasm (Fig. 4j) and colocalizes with Tax-1 (Fig. 4k and jk). The overexpression of CIITA does not affect the cytoplasmic distribution of endogenous RelA (Fig. 4g). These results indicate that a major component of CIITA-mediated inhibition of NF-κB activation by Tax-1 consists in the capacity of CIITA to prevent the nuclear translocation of RelA and its accumulation in Tax-1-containing NB.
FIG 4.
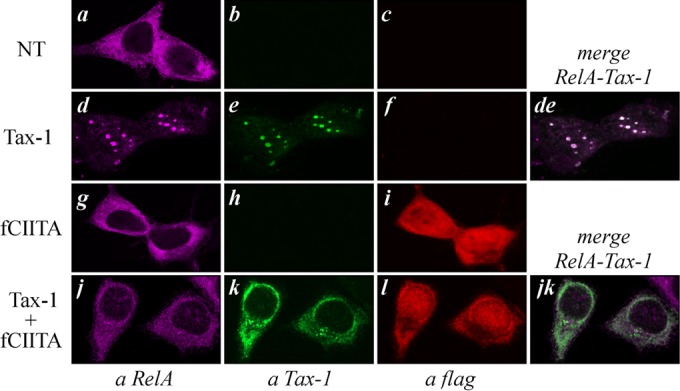
CIITA blocks Tax-1-dependent nuclear translocation of RelA. 293T cells were transfected with the Tax-1 or FLAG-tagged CIITA (fCIITA) expression vector or with both plasmids. The cells were fixed, stained with anti-Tax-1, anti-FLAG, and anti-RelA antibodies and analyzed by confocal microscopy as described in Materials and Methods. Images related to cells expressing Tax-1 or Tax-1 plus fCIITA show the best-represented distribution of the three factors in the total population.
Activation of the NF-κB pathway by Tax-1 is inhibited in cells that are natural targets of HTLV-1 infection and express CIITA.
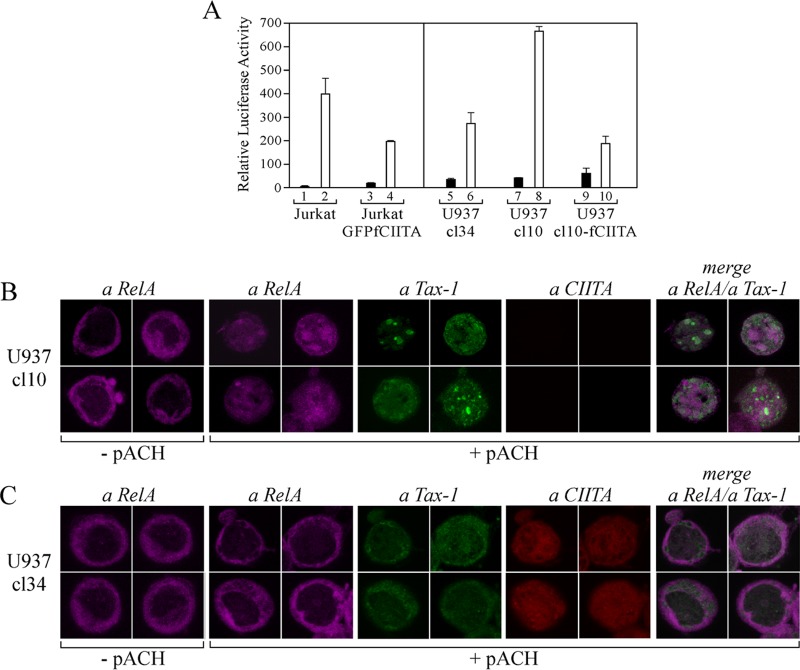
To assess whether the inhibitory effect of CIITA on Tax-1-mediated NF-κB activation observed in 293T cells also occurs in cells that are natural targets of HLTV-1 infection (T cells and monocytes), we performed the NF-κB–luciferase gene reporter assay by cotransfecting the cells with the pACH HTLV-1 proviral clone (53). We first assessed the activation of NF-κB by virus-derived Tax-1 in Jurkat T cells and in Jurkat cells stably transfected with a green fluorescent protein (GFP)-fCIITA vector (51) expressing CIITA at levels similar to that of the Raji B cell line (data not shown). We found that in Jurkat cells expressing CIITA, activation of NF-κB is strongly inhibited compared to that in parental Jurkat cells (Fig. 5A, column 4 versus column 2). More importantly, we then verified whether activation of NF-κB by Tax-1 is suppressed in cells expressing endogenous CIITA. We performed the functional NF-κB luciferase assay with two isogenic U937 monocytic clones, CIITA-positive clone 34 and CIITA-negative clone 10 (53). We demonstrated that in U937 clone 10, the NF-κB pathway was strongly activated by virus-derived Tax-1 (Fig. 5A, column 8 versus column 7). In contrast, in U937 clone 34, activation of NF-κB was significantly reduced with respect to that in clone 10 (Fig. 5A, column 6 versus column 8). To confirm that the distinct behavior of the two cell clones was indeed due to their differential expression of CIITA, we performed our functional assay with U937 clone 10 cells stably transfected with CIITA vector (U937 cl10-fCIITA) (53). Remarkably, upon the overexpression of CIITA in U937 clone 10, we observed a major reduction of NF-κB activation compared to that in untransfected clone 10 cells (Fig. 5A, column 10 versus column 8). This reduction was even greater with respect to that observed in U937 clone 34 cells expressing endogenous CIITA (Fig. 5A, column 10 versus column 6). To define whether the functional inhibition of Tax-1-promoted activation of NF-κB by endogenous CIITA correlates with the delocalization of RelA, confocal microscopy analysis was carried out with the two U937 clones before and after transfection with the pACH vector. Endogenous RelA localizes in the cytoplasm of both untransfected cell clones (Fig. 5B and C, − pACH). Upon pACH transfection, the pattern of localization of RelA diverged dramatically between CIITA-negative clone 10 and CIITA-positive clone 34. Indeed, RelA accumulated in the nucleus of clone 10 cells but remained cytoplasmic in clone 34 cells, and this correlated with the subcellular localization of Tax-1, which was nuclear in clone 10 cells and mostly cytoplasmic in clone 34 cells (Fig. 5B and C, respectively). Overall, these findings indicate that not only overexpression of CIITA but, most importantly, physiological levels of CIITA are sufficient to cause the subcellular delocalization of both Tax-1 and RelA and, consequently, the inhibition of NF-κB activation by Tax-1.
FIG 5.
CIITA expressed in T and monocytic cells inhibits Tax-1-mediated NF-κB activation. (A) Jurkat, Jurkat-GFPfCIITA, U937 cl10, U937 cl34, and U937 cl10-fCIITA cells were transfected with pNF-κB-Luc and phRL-CMV in the absence (black columns) or in the presence of proviral plasmid pACH (white columns). At 48 h posttransfection, the luciferase activity of the cell lysates was measured. (B and C) U937 clone 10 and 34 cells, respectively, were transfected with plasmid pACH. At 48 h posttransfection the cells were fixed; stained with anti-RelA, anti-Tax-1, and anti-CIITA antibodies; and analyzed by confocal microscopy as described in Materials and Methods.
CIITA uses different strategies to suppress Tax-1-dependent activation of the NF-κB pathway in the cytoplasmic and nuclear compartments.
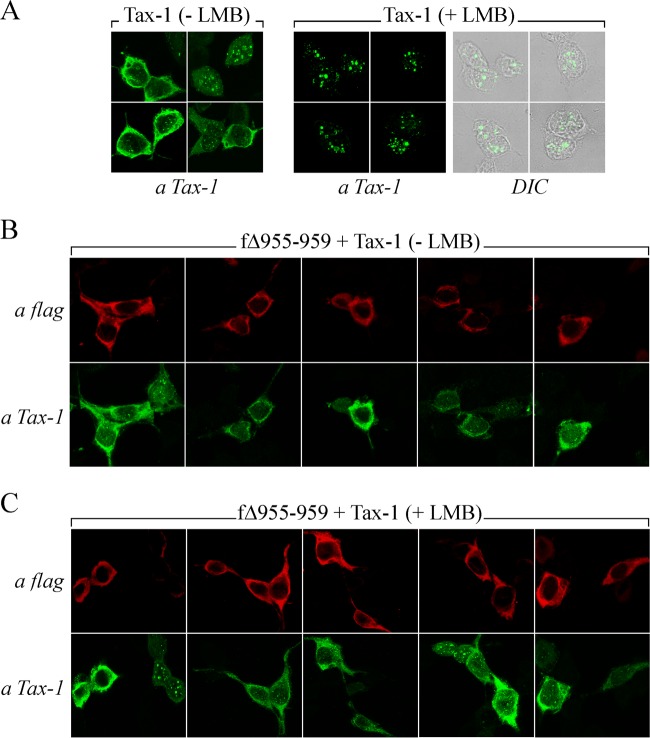
The above findings indicate that a major mechanism through which CIITA inhibits Tax-1-mediated NF-κB activation is retention of both Tax-1 and RelA in the cytoplasm (Fig. 4 and 5). To finally confirm this point, we investigated whether this effect also occurs with selected cytoplasmic and nuclear mutant forms of CIITA. First, we analyzed whether the inhibiting cytoplasmic CIITAΔ955-959 mutant protein impaired Tax-1-induced migration of RelA into the nucleus. We found that in the presence of this mutant protein, both Tax-1 and RelA were trapped in the cytoplasm, where the three factors colocalize (Fig. 6k, j, jk, and kl). CIITAΔ955-959 expressed alone did not affect the distribution of endogenous RelA (Fig. 5g). On the contrary, the noninhibiting cytoplasmic CIITA fragment encompassing amino acids 253 to 1130, which is also LMB insensitive (53), did not impair Tax-1 and Tax-1-induced RelA migration into the nucleus (Fig. 6q and p, respectively). Overall, these findings indicate that the inhibitory effect of cytoplasmic CIITA on Tax-1-dependent NF-κB activation is due to the retention of Tax-1 and RelA in the cytoplasm and that cytoplasmic localization of CIITA per se is not sufficient to redistribute Tax-1 and RelA in the cytoplasm. The cytoplasmic retention of Tax-1 observed in cells coexpressing wild-type CIITA (Fig. 3) and CIITAΔ955-959 (Fig. 6) might be due to impaired nuclear import or increased nuclear export of the viral transactivator. Because wild-type CIITA responds to LMB, whereas the CIITAΔ955-959 mutant protein is LMB insensitive (Fig. 2C), in order to discriminate between the two above possibilities, we assessed whether, in the presence of CIITAΔ955-959, Tax-1 is retained in the cytoplasm even after treatment with the nuclear export inhibitor LMB. It must be stressed that, in agreement with a previous study (26), under our experimental conditions, cumulatively 30% of the 293T cells transfected with Tax-1 express the viral transactivator in the cytoplasm (Fig. 3A) and they can be potentially sensitive to LMB. Thus, we first compared the subcellular distribution of Tax-1 in 293T cells treated with LMB (+ LMB) with that in cells treated with the vehicle methanol (− LMB). We found that LMB treatment caused nuclear accumulation of Tax-1 with no cells expressing Tax-1 in the cytoplasm (Fig. 7). Interestingly, the presence of CIITAΔ955-959 caused cytoplasmic retention of Tax-1 and abrogated the effect of LMB on Tax-1 localization (Fig. 7B and C, respectively). Overall, these findings indicate that cytoplasmic CIITA inhibiting Tax-1-mediated activation of NF-κB abrogates the nuclear import of Tax-1.
FIG 6.
CIITA uses different modalities to inhibit Tax-1-dependent activation of NF-κB at the cytoplasmic and nuclear levels. The expression vectors of the indicated FLAG-tagged mutant forms of CIITA were transfected alone or together with plasmid pTax-1 into 293T cells. After fixation, the cells were stained by triple IF assay with anti-Tax-1, anti-FLAG, and anti-RelA antibodies and analyzed by confocal microscopy as described in Materials and Methods.
FIG 7.
LMB-induced nuclear retention of Tax-1 is abrogated by CIITAΔ955-959. 293T cells transfected with plasmid pTax-1 alone (A) or together with plasmid pfΔ955-959 were treated with LMB (+ LMB) (C) or the vehicle methanol (− LMB) (B). Cells were fixed and stained with anti-FLAG or anti-Tax-1 antibodies and analyzed by confocal microscopy as described in Materials and Methods. DIC, differential interference contrast.
Subsequently, we investigated whether the CIITA1-252 fragment, with relevant nuclear localization (Fig. 6c) and displaying strong inhibition of Tax-1-mediated NF-κB activation, could alter the localization of RelA. In the absence of Tax-1, CIITA1-252 did not affect the distribution of RelA (Fig. 6a). In cells coexpressing Tax-1 and CIITA1-252, RelA staining overlapped that of Tax-1. They were both diffused in the cytoplasm but also accumulated in NB (Fig. 6d and e, respectively, and de). Interestingly, the cellular distribution of CIITA1-252 overlapped not only that of Tax-1 but also that of RelA. In particular, the nuclear fraction of CIITA1-252 was no longer diffused but rather localized in the same NB containing Tax-1 and RelA (Fig. 6f and ef). This finding suggests that although RelA is recruited to NB by Tax-1, RelA transcriptional activity is inhibited by CIITA1-252, strongly indicating that the nuclear fraction of full-length CIITA also exerts its inhibitory function when recruited to NB by Tax-1 (Fig. 3C). CIITA localization in Tax-1-containing NB was even more evident when the nuclear accumulation of both factors was forced by treatment with LMB (Fig. 8). We conclude that CIITA might exploit different mechanisms to inhibit Tax-1-mediated NF-κB transcriptional activation, depending on its relative expression in the nuclear and cytoplasmic compartments.
FIG 8.
Nuclear CIITA accumulates in Tax-1-containing NB in 293T cells treated with LMB. 293T cells cotransfected with plasmids pTax-1 and pfCIITA were treated with LMB. Cells were fixed and stained with anti-FLAG antibody to detect FLAG-tagged CIITA or with anti-Tax-1 antibody and analyzed by confocal microscopy as described in Materials and Methods. DIC, differential interference contrast.
Mutant forms of CIITA inhibiting Tax-1-driven activation of NF-κB interact with the viral transactivator.
We previously showed that CIITA interacts in vivo with Tax-1 (53). Here we confirm that Tax-1 binds not only to wild-type CIITA but also to CIITA1-252 (Fig. 9, lanes 2 and 5, respectively). Moreover, we analyzed whether the two cytoplasmic mutant forms CIITAΔ253-410 and CIITAΔ955-959 maintain the ability to interact with the viral transactivator. We found that Tax-1 coimmunoprecipitated with both mutant proteins when coexpressed in 293T cells (Fig. 9, lanes 3 and 4, respectively). This association was specific because Tax-1 was not precipitated by the anti-FLAG antibody when expressed alone (Fig. 9, lane 1). We conclude that the binding of CIITA to Tax-1 occurs both in the nucleus and in the cytoplasm and that this interaction might affect the function of the viral transactivator. Nevertheless, binding of CIITA to Tax-1 is necessary but not sufficient to inhibit Tax-1-mediated activation of NF-κB, because the noninhibitory amino acid 253 to 1130 fragment can still bind Tax-1, as we have previously shown (53).
FIG 9.
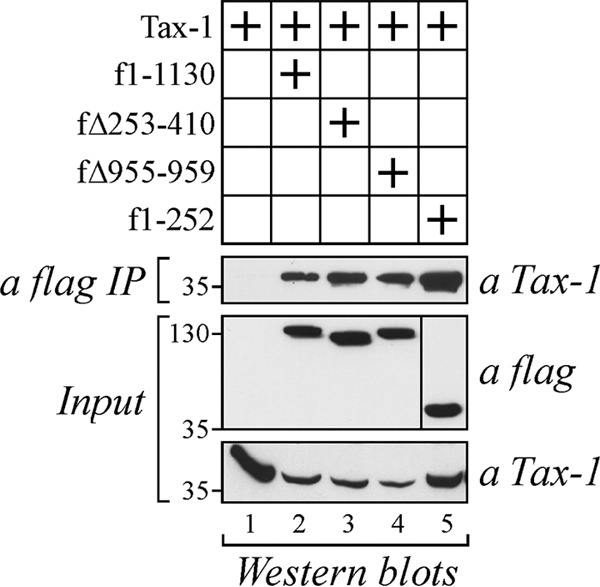
Tax-1 coprecipitates with inhibiting fragments of CIITA in 293T cells. 293T cells were transfected with plasmid pTax-1 alone or in combination with vectors expressing FLAG-tagged full-length CIITA or mutant forms of CIITA. Cell extracts were immunoprecipitated with anti-FLAG antibody (a FLAG IP), and the immunoprecipitated complexes were analyzed by Western blotting with anti-Tax-1 antibody. The input corresponding to 10% of the whole-cell extract was analyzed by Western blotting for the expression of Tax-1 and CIITAs. The values to the left of the gels are molecular sizes in kilodaltons.
CIITA does not affect the association of Tax-1 with endogenous RelA and IKKγ.
It has been demonstrated that Tax-1 interacts with RelA and IKKγ to promote NF-κB activation (32, 36, 40). To further detail the molecular mechanisms of CIITA-mediated inhibition of NF-κB activation by Tax-1, we asked whether the interaction between CIITA and Tax-1 could affect the association of the viral transactivator with endogenous RelA and IKKγ. To test this hypothesis, Tax-1 and FLAG-tagged CIITA were coexpressed in 293T cells. Cell lysates were immunoprecipitated with anti-RelA or anti-IKKγ antibodies (Fig. 10A and B, respectively). RelA- and IKKγ-bound proteins were assessed for the presence of Tax-1 and CIITA by Western blotting with specific antibodies. As a control for protein expression, 10% of the total lysate was analyzed by Western blotting (Fig. 10A and B, input). Results show that Tax-1 coprecipitates with endogenous RelA (Fig. 10A, top, lane 1) and with endogenous IKKγ (Fig. 10B, top, lane 1) and both of these interactions were not affected by the overexpression of CIITA (Fig. 10A and B, top, lanes 2, respectively). Taken together, these results indicate that CIITA-mediated inhibition of NF-κB activation by Tax-1 cannot be attributed to a defective interaction between Tax-1 and two major components of the canonical NF-κB cascade, RelA and IKKγ.
FIG 10.
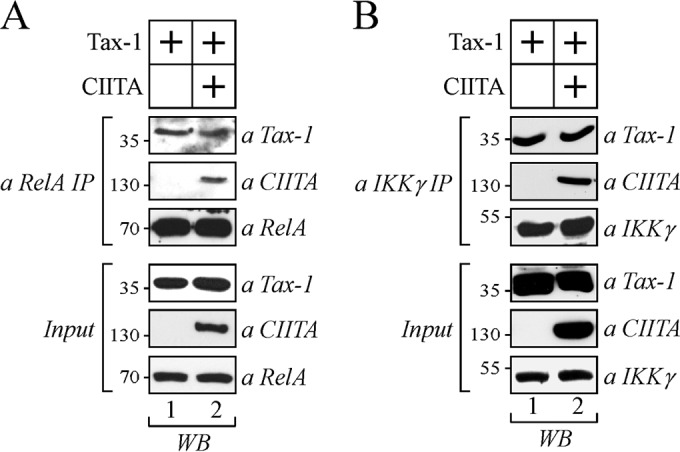
The interaction of Tax-1 with RelA and IKKγ is not impaired by CIITA. 293T cells were transfected with vectors expressing Tax-1 alone or with FLAG-tagged CIITA. (A) Cell extracts were immunoprecipitated with anti-RelA antibody (a RelA IP), and the immunoprecipitated complexes were analyzed by Western blotting with anti-Tax-1, anti-CIITA, and anti-RelA antibodies. (B) Cell extracts were immunoprecipitated with anti-IKKγ antibody (a IKKγ IP), and the immunoprecipitated complexes were analyzed by Western blotting (WB) with anti-Tax-1, anti-CIITA, and anti-IKKγ antibodies. The values to the left of the gels are molecular sizes in kilodaltons.
CIITA suppresses Tax-1-induced phosphorylation of IκB.
The above results showing that CIITA does not affect Tax-1-IKKγ interaction do not rule out the possibility that the kinase activity of IKK is impaired in the presence of CIITA. Thus, we used Western blotting to analyze the phosphorylation of the IκB inhibitor in 293T cells transfected with the pCDNA3 empty vector or with Tax-1 in the presence or absence of CIITA. After transfection, the cells were treated with dimethyl sulfoxide (DMSO) as a control or with the proteasome inhibitor MG132 to prevent phospho-IκB (P-IκB) degradation. The expression of total IκB and P-IκB was assessed. As expected, Tax-1 increased the basal level of IκB phosphorylation (Fig. 11, middle, lane 5 versus lane 4). In contrast, in the presence of CIITA, no specific P-IκB band was detected (Fig. 11, middle, lane 6) and no accumulation of P-IκB occurred in the absence of MG132 (Fig. 11, middle, lanes 1 to 3). We conclude that CIITA inhibits Tax-1-mediated nuclear translocation of RelA by inhibiting, at least in part, the phosphorylation, and thus the degradation, of its physiologic inhibitor IκB.
FIG 11.
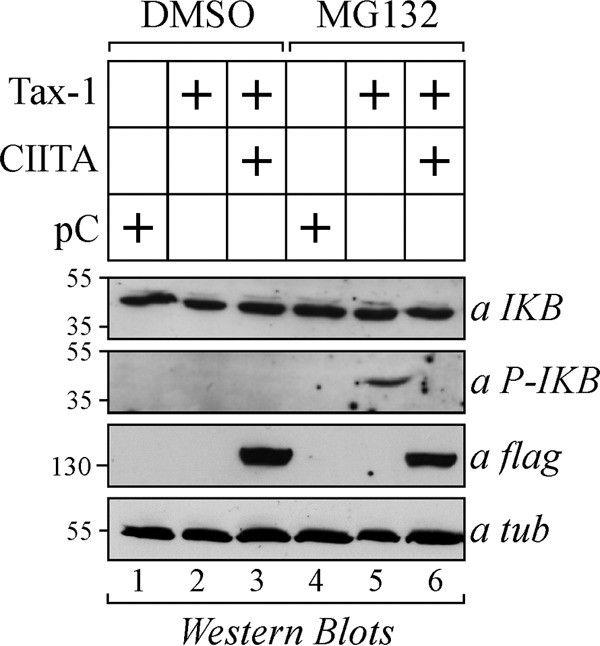
The phosphorylation of IκB induced by Tax-1 is inhibited by CIITA. 293T cells were transfected with the empty pCDNA3 vector (pC) or with Tax-1 in the presence or absence of FLAG-tagged CIITA. Cells were treated with the proteasome inhibitor MG132 or with the vehicle DMSO. The expression of total IκB, P-IκB, and FLAG-tagged CIITA was assessed by Western blotting with the antibodies indicated. As a control for loading, the expression of α-tubulin (a tub) was also evaluated. The values to the left of the gels are molecular sizes in kilodaltons.
DISCUSSION
CIITA, the master regulator of MHC-II gene transcription, has been previously found to inhibit HTLV-1 replication by targeting the viral transactivator Tax-1 (53). In the present study, we demonstrate that CIITA also has the ability to inhibit the persistent activation of the canonical NF-κB pathway by Tax-1, thus counteracting one of the major strategies used by HTLV-1 to induce T cell transformation. Remarkably, the inhibitory action of CIITA on Tax-1-mediated activation of NF-κB was observed not only with CIITA exogenously expressed in 293T and Jurkat T cells but also with endogenous CIITA expressed in monocytic U937 clone 34 cells, demonstrating the biological relevance of the finding in cell types that are common targets of HTLV-1 infection. It is known that stimulation of T cells via their T cell receptor results in the activation of the canonical NF-κB pathway. On the other hand, HTLV-1-infected T cells display activation of both canonical and noncanonical NF-κB pathways mediated by Tax-1. Thus, future efforts will be focused to investigate if CIITA also inhibits the Tax-1-mediated noncanonical NF-κB route. Functional mapping of the CIITA region involved in the inhibition of Tax-1-dependent NF-κB activation showed that the N-terminal region of CIITA was responsible for this effect. Interestingly, this region corresponds to that inhibiting Tax-1-mediated LTR transactivation as well (53). Importantly, we found that in 293T cells, CIITA dramatically affects the nucleocytoplasmic distribution of Tax-1 in that the best-represented Tax-1 localization in NB was no longer observed. Conversely, in the presence of CIITA, the fraction of cells expressing Tax-1 in the cytoplasm increased comparatively more (60% versus 10%) than that expressing Tax-1 in both the cytoplasm and NB (40% versus 20%). Importantly, in monocytic U937 clone 34 cells, physiological levels of CIITA were able to affect the subcellular localization of Tax-1, preventing its translocation into the nucleus. Overall, these findings indicate that CIITA perturbs the relative distribution of Tax-1 by favoring its retention in the cytoplasm, an event that has been correlated with defective transcriptional and transforming activities (44).
It has been shown that Tax-1 subcellular distribution is cell type dependent (67) and that in the cytoplasm the viral transactivator exhibits different patterns of distribution, being diffused throughout the cytoplasm or concentrated in punctate structures containing TAB2, calreticulin, RelA, IKK, and TAX1-BP1 (reviewed in references 39, 42, and 44). Tax-1 has also been found in Golgi compartment-associated lipid raft microdomains and in the centrosome (68, 69). Within this frame, future investigation will focus on precisely defining the cytoplasmic compartment(s) where CIITA and Tax-1 colocalize. It has also been reported that Tax localization and its ability to activate NF-κB are regulated by posttranslational modifications. Of these, Tax-1 phosphorylation, a prerequisite for Tax-1 acetylation, is certainly important (70). The use of the phosphomimetic F9 mutant form of Tax-1, which retains a partial ability to activate NF-κB with respect to that of wild-type Tax-1 (63) (Fig. 1B), was instrumental in understanding whether CIITA inhibits the activation of NF-κB by affecting the phosphorylation of Tax-1. In this regard, two scenarios could be envisaged. In the first, CIITA does not inhibit the activation of NF-κB by Tax-1 F9, indicating that its inhibitory action might occur via the alteration of Tax-1 phosphorylation. In the second, CIITA inhibits the activation of NF-κB by Tax-1 F9, excluding a possible role for CIITA in Tax-1 phosphorylation. We found that CIITA inhibits the activation of NF-κB by Tax-1 F9, suggesting that CIITA does not suppress Tax-1-mediated activation of NF-κB by affecting the phosphorylation of the viral transactivator. Besides phosphorylation, p300-induced acetylation of Tax-1 has also been found to promote the activation of NF-κB (70). In this regard, we previously reported that Tax-1 cooperates with both the histone acetyltransferases PCAF (p300/CBP-associated factor) and p300 to promote HTLV-I LTR activation and that CIITA inhibits this activation by squelching PCAF but not p300 (53). Thus, it is unlikely that sequestration of p300 by CIITA may cause impaired acetylation of Tax-1 and, consequently, defective activation of NF-κB. Sumoylation and ubiquitination are additional posttranslational modifications that regulate the localization of Tax and its ability to activate NF-κB (26, 48, 71). CIITA might delocalize Tax-1 by altering sumoylation and/or ubiquitination. In this study, we have not addressed this point. However, recent papers have challenged the strategic importance of Tax-1 sumoylation, because Tax-1 sumoylation and the formation of Tax-1 NB do not seem to be strictly required for NF-κB-promoted gene expression (46, 47). Moreover, in contrast to the findings of Turci et al. (48), Tax-2-mediated activation of NF-κB has been reported to be independent of sumoylation and ubiquitination (49). These observations, together with our finding that Tax-2-mediated activation of NF-κB is also inhibited by CIITA, suggest that CIITA does not modify the levels of sumoylation and ubiquitination of Tax to inhibit the activation of NF-κB.
A hallmark of the aberrant activation of NF-κB by HTLV-1 is Tax-1-induced translocation of RelA in the nucleus independently of external stimuli (25, 41, 44). A major finding of the present work is the demonstration that CIITA-mediated sequestration of Tax-1 in the cytoplasm correlates with impaired migration of RelA into the nucleus. This event was observed both in 293T cells ectopically expressing CIITA and, most importantly, in natural targets of HTLV-1 infection such as monocytic U937 cells expressing endogenous CIITA, underlying the biological relevance of our findings.
In studying the biochemical basis of the above findings, we found that CIITA inhibits Tax-1-induced phosphorylation of IκB, implying defective activity of IKK. This result supports the idea that, in the presence of CIITA, the NF-κB heterodimer (p50-RelA), complexed with its physiologic inhibitor IκB, is retained in the cytoplasm. Of note, the failure of IKK kinase activity cannot be ascribed to an impaired interaction between Tax-1 and IKKγ caused by CIITA. Instead, because CIITA and Tax-1 have been found to physically associate in vivo, it is possible that CIITA, Tax-1, and IKKγ form a trimolecular complex that is unable to activate the catalytic subunits of IKK. Alternatively, we cannot exclude the possibility that, in the presence of CIITA, Tax-1 recruits the IKKγ regulatory subunit but not the two catalytic subunits IKKα and IKKβ, destroying the enzymatic activity of IKK. Moreover, recent findings have indicated that Tax directly activates the ubiquitin E3-conjugating enzyme RNF8 to assemble K63-linked polyubiquitin chains to activate TAK1 and many downstream kinases, including IKK (72). Thus, interaction between Tax and IKK may also be indirect and mediated via RNF8 and the K63-linked polyubiquitin chains. In this case, CIITA-Tax interaction may functionally interfere with upstream mechanisms governing IKK kinase activity.
Overall, these findings suggest a suppressive effect of CIITA at the cytoplasmic level targeting the initial steps of the NF-κB pathway activated by Tax-1.
CIITA is a shuttling protein with dual cytoplasmic and nuclear localization, and a priori, it could exert its inhibitory effect on Tax-1 in both compartments. We have previously shown that CIITA inhibits Tax-1-dependent LTR transactivation by acting in the nucleus, but we could not exclude the possibility that parallel mechanisms operating in the cytoplasm might contribute to CIITA-mediated abrogation of Tax-1 activities (53). To clarify this issue, two crucial cytoplasmic mutant forms of CIITA, one that does not (CIITA253-1130) and one that does (CIITAΔ955-959) inhibit Tax-1-driven NF-κB activation, have been analyzed for the ability to delocalize both Tax-1 and RelA in the cytoplasm. In the presence of CIITA253-1130, both Tax-1 and RelA migrated to the nucleus without accumulating in NB and maintained their functional activities. This result is in line with the work of Bonnet et al. (46), showing that the formation of Tax-1 NB is dispensable for Tax-1-induced RelA nuclear translocation and the triggering of NF-κB-responsive gene expression. In contrast, CIITAΔ955-959 inhibits the activation of NF-κB by Tax-1 and, similarly to full-length CIITA, interacts with Tax-1 and retains both Tax-1 and RelA in the cytoplasm, where the three factors colocalize. Interestingly, CIITAΔ955-959 lacks a functional NLS, and although it contains several other NLSs identified within the CIITA molecule (64, 65, 73), it remains cytoplasmic even after treatment with LMB. This result shed new light on the cell biology of CIITA, indicating that the NLS, consisting of amino acids 955 to 959, plays a major role in dictating the nuclear import of CIITA. In contrast to previous reports (67, 74), we found that LMB inhibits the nuclear export of Tax-1, which is known to contain a CRM-1-binding nuclear export signal (NES) (67). This discrepancy might be due to the different cellular system adopted and to the use, in our investigation, of untagged Tax-1 instead of GFP-tagged Tax-1. The GFP itself could mask the accessibility to NES determining Tax-1 insensitivity to LMB or affect posttranslational modifications and protein-protein interactions crucial for Tax-1 nucleocytoplasmic shuttling (75). In accordance with our finding that Tax-1 responds to LMB, it has previously been demonstrated that Tax-1 follows CRM1-dependent nuclear export in rat embryo fibroblasts exposed to genotoxic and cellular stress (76). Of note, Tax-1 becomes refractory to LMB in the presence of CIITAΔ955-959, strengthening the idea that CIITA entraps most of the viral transactivator in the cytoplasm by overcoming the physiological mechanism controlling its nuclear import. Furthermore, Tax-1 trapped by CIITA in the cytoplasm could mimic cytoplasmic Tax-1 mutant forms that have been shown to form a ternary complex with NF-κB and CBP in the cytoplasm rather than in the nucleus. The resulting complex is retained in the cytoplasm and is therefore devoid of transcriptional activity (77).
Interestingly, we found that mutant forms of CIITA with predominant nuclear accumulation (CIITA64-200 and CIITA1-252) inhibit the activation of NF-κB by Tax-1, indicating that CIITA may also exert its inhibitory action at the nuclear level. In this regard, it is important to mention that in the presence of CIITA, 40% of cells still express Tax-1 and RelA in NB and the cytoplasm. Thus, the possibility exists that the fraction of Tax-1 and RelA accumulating in NB in the presence of CIITA is nonfunctional. This interpretation is supported by our finding that Tax-1 drives the redistribution of nuclear CIITA in NB. Although CIITA does not abrogate the binding of Tax-1 to RelA, it may contribute to the formation of a nonfunctional trimolecular complex with Tax-1 and RelA in NB, blocking the activation of the NF-κB pathway. The inhibitory effect of CIITA in the nucleus is also corroborated by the observation that increasing doses of CIITA determine stronger inhibition of NF-κB activation by Tax-1 but do not proportionally elevate the amount of cells expressing Tax-1 exclusively in the cytoplasm. Taken together, these findings indicate that CIITA uses different mechanisms to inhibit Tax-1-dependent activation of NF-κB at the cytoplasmic and nuclear levels.
The persistent and constitutive activation of NF-κB has been implicated in the inhibition of the transcriptional activity of the tumor suppressor p53 by Tax-1 (78, 79). Thus, it will be interesting to assess whether CIITA, by blocking Tax-1-mediated NF-κB activation, can restore the functional activity of p53 inhibited by Tax-1. Considering that the Tax-1 oncoprotein has pleiotropic functions that are mediated by direct and indirect interactions with many cellular proteins (reviewed in references 20 and 80), it is possible that the CIITA–Tax-1 association might avoid some of these interactions, counteracting Tax-1 transforming activity. If this were the case, CIITA might have a broader effect on HTLV-1 infection, inhibiting not only viral replication but also the oncogenic potential of the virus. Within this context, it can be argued that HTLV-1-infected cells often display a phenotype of activated T cells with the expression of several activation markers, including MHC-II cell surface molecules (reviewed in reference 81) and, as a consequence, their transcriptional regulator CIITA. Thus, in the frame of the natural history of HTLV-1 infection and neoplastic transformation, the role of CIITA may be underestimated. Several considerations, however, reinforce the notion that CIITA may indeed play a crucial role. First, it should be stressed that, unlike MHC class II molecules, which have a half-life of 48 h, the expression of their regulator CIITA in inducible cells, like activated T cells, is temporally short, with a half-life of 30 to 45 min, and quantitatively very limited (82). Second, even in antigen- or mitogen-activated human T cells, MHC-II expression is a transient event, it may be observed only in a limited subpopulation of cells, and it displays variable kinetics, depending on the nature and intensity of the activation stimulus, as well as on the functional maturation of the cells (83, 84). Finally, and of relevance, we demonstrate here that the stability and relative amount of CIITA expressed in physiological cellular settings such as monocytic U937 clone 34 cells correlate with the inhibition of Tax-1-mediated NF-κB activation, as well as with the previously shown inhibition of Tax-1-mediated HTLV-1 replication (53).
Thus, on the basis of previous knowledge and the results presented in this report, it is tempting to speculate that sustained expression of CIITA in infected cells, which may be generated in the inflammatory environment of viral infection, may represent an inhibitory mechanism not only for viral replication and spreading but also for the initiation of HTLV-1 Tax-1-dependent oncogenic transformation. Future investigation will be directly focused on verifying the above hypothesis.
ACKNOWLEDGMENT
We thank Luisa Guidali (DISTA, University of Insubria) for excellent assistance with confocal microscopy analysis.
REFERENCES
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A 77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida M, Miyoshi I, Hinuma Y. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A 79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchiyama T. 1997. Human T-cell leukemia virus type 1 (HTLV-1) and human diseases. Annu Rev Immunol 15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka M, Jeang KT. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer 7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 5.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de Thè G. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407–10. [DOI] [PubMed] [Google Scholar]
- 7.Kalyanaraman VS, Sarngadharan MG, Robert-Guroff M, Miyoshi I, Golde D, Gallo RC. 1982. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 218:571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- 8.Franchini G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619–3639. [PubMed] [Google Scholar]
- 9.Goren I, Semmes OJ, Jeang KT, Moelling K. 1995. The amino terminus of Tax is required for interaction with the cyclic AMP response element binding protein. J Virol 69:5806–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok RP, Laurance ME, Lundblad JR, Goldman PS, Shih H, Connor LM, Marriott SJ, Goodman RH. 1996. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 11.Harrod R, Tang Y, Nicot C, Lu HS, Vassilev A, Nakatani Y, Giam CZ. 1998. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol 18:5052–5061. doi: 10.1128/MCB.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashanchi F, Duvall JF, Kwok RPS, Lundblad J, Goodman R, Brady JN. 1998. The coactivator CBP stimulates human T-cell lymphotropic virus type I Tax transactivation in vitro. J Biol Chem 273:34646–34652. doi: 10.1074/jbc.273.51.34646. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Lu H, Schiltz RL, Pise-Masison CA, Ogryzko VV, Nakatani Y, Brady JN. 1999. PCAF interacts with Tax and stimulates Tax transactivation in a histone acetyltransferase-independent manner. Mol Cell Biol 19:8136–8145. doi: 10.1128/MCB.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrod R, Kuo YL, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam CZ. 2000. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type 1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J Biol Chem 275:11852–11857. doi: 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- 15.Georges SA, Giebler HA, Cole PA, Luger K, Laybourn PJ, Nyborg JK. 2003. Tax recruitment of CBP/p300, via the KIX domain, reveals a potent requirement for acetyltransferase activity that is chromatin dependent and histone tail independent. Mol Cell Biol 23:3392–3404. doi: 10.1128/MCB.23.10.3392-3404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuer G, Green PL. 2005. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 24:5996–6004. doi: 10.1038/sj.onc.1208971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuoka M, Jeang KT. 2011. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ and therapy. Oncogene 30:1379–1389. doi: 10.1038/onc.2010.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franchini G, Nicot C, Johnson JM. 2003. Seizing of T cells by human T-cell leukemia/lymphoma virus type 1. Adv Cancer Res 89:69–132. doi: 10.1016/S0065-230X(03)01003-0. [DOI] [PubMed] [Google Scholar]
- 19.Jeang KT, Giam CZ, Majone F, Aboud M. 2004. Life, death, and Tax: role of HTLV-1 oncoprotein in genetic instability and cellular transformation. J Biol Chem 279:31991–31994. doi: 10.1074/jbc.R400009200. [DOI] [PubMed] [Google Scholar]
- 20.Hall WW, Fujii M. 2005. Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene 24:5965–5975. doi: 10.1038/sj.onc.1208975. [DOI] [PubMed] [Google Scholar]
- 21.Peloponese JM, Kinjo T, Jeang KT. 2007. Human T-cell leukemia virus type 1 Tax and cellular transformation. Int J Hematol 86:101–106. doi: 10.1532/IJH97.07087. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JM, Nicot C. 2008. HTLV-1 and apoptosis: role in cellular transformation and recent advances in therapeutic approaches. Apoptosis 13:733–747. doi: 10.1007/s10495-008-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chlichlia K, Khazaie K. 2010. HTLV-1 Tax: linking transformation, DNA damage and apoptotic T-cell death. Chem Biol Interact 188:359–365. doi: 10.1016/j.cbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Petropoulos L, Lin R, Hischott J. 1996. Human T cell leukemia virus type 1 Tax protein increases an NF-kappa B dimer formation and antagonized the inhibitory activity of the I kappa B alpha regulatory protein. Virology 225:52–64. doi: 10.1006/viro.1996.0574. [DOI] [PubMed] [Google Scholar]
- 25.Sun SC, Yamaoka S. 2005. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene 24:5952–5964. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- 26.Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F. 2005. Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-kappaB activation by the human T-cell leukemia virus tax oncoprotein. Mol Cell Biol 25:10391–10406. doi: 10.1128/MCB.25.23.10391-10406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Journo C, Filipe J, About F, Chevalier SA, Afonso PV, Brady JN, Flynn D, Tangy F, Israël A, Vidalain PO, Mahieux R, Weil R. 2009. NRP/optineurin cooperates with TAX1BP1 to potentiate the activation of NF-kappa B by human T-lymphotropic virus type 1 Tax protein. PLoS Pathog 5:e1000521. doi: 10.1371/journal.ppat.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirinian M, Kfoury Y, Dassouki Z, El-Hajj H, Bazarbachi A. 2013. Tax-1 and Tax-2 similarities and differences: focus on post-translational modifications and NF-κB activation. Front Microbiol 4:231. doi: 10.3389/fmicb.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karin M, Lin A. 2002. NF-kappaB at the crossroads of life and death. Nat Immunol 3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 30.Pomerantz JL, Baltimore D. 2002. Two pathways to NF-kappaB. Mol Cell 10:693–695. doi: 10.1016/S1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 31.Sun SC. 2011. Non-canonical NF-κB signaling pathway. Cell Res 21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harhaj EW, Sun SC. 1999. IKKgamma serves as a docking subunit of IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem 274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 33.Xiao G, Harhaj EW, Sun SC. 2000. Domain-specific interactin with the I kappa B kinase (IKK) regulatory subunit IKK gamma is an essential step in Tax-mediated activation of IKK. J Biol Chem 275:34060–34067. doi: 10.1074/jbc.M002970200. [DOI] [PubMed] [Google Scholar]
- 34.Chu ZL, DiDonato JA, Hawiger J, Ballard DW. 1998. The Tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkappaB kinases containing IKKalpha and IKKbeta. J Biol Chem 273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 35.Chu ZL, Shin YA, Yang JM, DiDonato JA, Ballard DW. 1999. IKK gamma mediates the interaction of cellular IkappaB kinases with the Tax transforming protein of human T cell leukemia virus type 1. J Biol Chem 274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 36.Jin DY, Giordano V, Kibler KV, Nakano H, Jeang KT. 1999. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J Biol Chem 274:17402–17405. [DOI] [PubMed] [Google Scholar]
- 37.Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC. 2001. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J 20:6805–6815. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuchi M, Tsubata C, Kondo R, Yoshida S, Takahashi M, Oie M, Tanaka Y, Mahieux R, Matsuoka M, Fujii M. 2007. Cooperation of NF-κB2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV) Tax but not HTLV-2 Tax-2 is crucial for interleukin-2-independent growth transformation of a T-cell line. J Virol 81:11900–11907. doi: 10.1128/JVI.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertazzoni U, Turci M, Avesani F, Di Gennaro G, Bidoia C, Romanelli MG. 2011. Intracellular localization and cellular factors interaction of HTLV-1 and HTLV-2 Tax proteins: similarities and functional differences. Viruses 3:541–560. doi: 10.3390/v3050541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki T, Hirai H, Yoshida M. 1994. Tax protein of HTLV-1 interacts with the Rel homology domain of NF-kappa b p65 and c-Rel proteins bound to NF-kappa B binding site and activates transcription. Oncogene 9:3099–3105. [PubMed] [Google Scholar]
- 41.Bex F, McDowall A, Burny A, Gaynor R. 1997. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-κB proteins. J Virol 71:3484–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avesani F, Romanelli MG, Turci M, Di Gennaro M, Sampaio C, Bidoia C, Bertazzoni U, Bex F. 2010. Association of HTLV Tax proteins with TAK1-binding protein 2 and RelA in calreticulin-containing cytoplasmic structures participates in Tax-mediated NF-kappaB activation. Virology 408:39–48. doi: 10.1016/j.virol.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Kfoury Y, Setterblad N, El-Sabban M, Zamborlini A, Dassouki Z, El Hajj H, Hermine O, Pique C, de The H, Saib A, Bazarbachi A. 2011. Tax ubiquitylation and SUMOylation control the dynamic shuttling of Tax and NEMO between Ubc9 nuclear bodies and the centrosome. Blood 117:190–199. doi: 10.1182/blood-2010-05-285742. [DOI] [PubMed] [Google Scholar]
- 44.Lodewick J, Lamsoul I, Bex F. 2011. Move or die: the fate of the Tax oncoprotein of HTLV-1. Viruses 3:829–857. doi: 10.3390/v3060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kfoury Y, Nasr R, Journo C, Mahieux R, Pique C, Bazarbachi A. 2012. The multifaceted oncoprotein Tax: subcellular localization, posttranslational modifications, and NF-κB activation. Adv Cancer Res 113:85–120. doi: 10.1016/B978-0-12-394280-7.00003-8. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet A, Randrianarison-Huetz V, Voahangy R-H, Nzounza P, Nedelec M, Chazal M, Waast L, Pène S, Bazarbachi A, Mahieux R, Benit L, Pique C. 2012. Low nuclear body formation and Tax sumoylation do not prevent NF-kappaB promoter activation. Retrovirology 9:77. doi: 10.1186/1742-4690-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pène S, Waast L, Bonnet A, Benit L, Pique C. 2014. A non-SUMOylated Tax protein is still functional for NF-κB pathway activation. J Virol 88:10655–10661. doi: 10.1128/JVI.01827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turci M, Lodewich J, Di Gennaro G, Rinaldi AS, Marin O, Diani E, Sanpaio C, Bex F, Bertazzoni U, Romanelli MG. 2012. Ubiquitination and sumoylation of the HTLV-2 Tax-2B protein regulate its NF-κB activity: a comparative study with the HTLV-1 Tax-1 protein. Retrovirology 9:102. doi: 10.1186/1742-4690-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Journo C, Bonnet A, Favre-Bovin A, Turpin J, Vinera J, Coté E, Chevalier SA, Kfoury Y, Bazarbachi A, Pique C, Mahieux R. 2013. Human T cell leukemia virus type 2 Tax-mediated NF-κB activation involves a mechanism independent of Tax conjugation to SUMO. J Virol 87:1123–1136. doi: 10.1128/JVI.01792-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casoli C, De Lerma Barbaro A, Pilotti E, Bertazzoni U, Tosi G, Accolla RS. 2004. The MHC class II transcriptional activator (CIITA) inhibits HTLV-2 viral replication by blocking the function of the viral transactivator Tax-2. Blood 103:995–1001. [DOI] [PubMed] [Google Scholar]
- 51.Tosi G, Pilotti E, Mortara L, De Lerma Barbaro A, Casoli C, Accolla RS. 2006. Inhibition of human T cell leukemia virus type 2 replication by the suppressive action of class II transactivator and nuclear factor Y. Proc Natl Acad Sci U S A 103:12861–12866. doi: 10.1073/pnas.0601589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tosi G, Bozzo L, Accolla RS. 2009. The dual function of the MHC class II transactivator CIITA against HTLV retroviruses. Front Biosci (Landmark Ed) 14:4149–4156. [DOI] [PubMed] [Google Scholar]
- 53.Tosi G, Forlani G, Andresen V, Turci M, Bertazzoni U, Franchini G, Poli G, Accolla RS. 2011. Major histocompatibility complex class II transactivator CIITA is a viral restriction factor that targets human T-cell lymphotropic virus type 1 Tax-1 function and inhibits viral replication. J Virol 85:10719–10729. doi: 10.1128/JVI.00813-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orlandi C, Forlani G, Tosi G, Accolla RS. 2011. Molecular and cellular correlates of the CIITA-mediated inhibition of HTLV-2 Tax-2 transactivator function resulting in loss of viral replication. J Transl Med 9:106. doi: 10.1186/1479-5876-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Accolla RS, De Lerma Barbaro A, Mazza S, Casoli C, De Maria A, Tosi G. 2001. The MHC class II transactivator: prey and hunter in infectious diseases. Trends Immunol 22:560–563. doi: 10.1016/S1471-4906(01)02003-8. [DOI] [PubMed] [Google Scholar]
- 56.Accolla RS, Mazza S, De Lerma Barbaro A, De Maria A, Tosi G. 2002. The HLA class II transcriptional activator blocks the function of HIV-1 Tat and inhibits viral replication. Eur J Immunol 32:2783–2791. doi:. [DOI] [PubMed] [Google Scholar]
- 57.Accolla RS, Jotterand-Bellomo M, Scarpellino L, Maffei A, Carra G, Guardiola J. 1986. air-1, a newly found locus on mouse chromosome 16 encoding a trans-acting activator factor for MHC class II gene expression. J Exp Med 164:369–374. doi: 10.1084/jem.164.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steimle V, Otten LA, Zufferey M, Mach B. 1993. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 75:135–146. doi: 10.1016/S0092-8674(05)80090-X. [DOI] [PubMed] [Google Scholar]
- 59.Fontes JD, Kanazawa S, Nekrep N, Peterlin BM. 1999. The class II transactivator CIITA is a transcriptional integrator. Microbes Infect 1:863–869. doi: 10.1016/S1286-4579(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 60.Forlani G, Abdallah R, Accolla RS, Tosi G. 2013. The MHC-II transactivator CIITA, a restriction factor against oncogenic HTLV-1 and HTLV-2 retroviruses: similarities and differences in the inhibition of Tax-1 and Tax-2 viral transactivators. Front Microbiol 4:234. doi: 10.3389/fmicb.2013.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tosi G, Jabrane-Ferrat N, Peterlin BM. 2002. Phosphorylation of CIITA directs its oligomerization, accumulation, and increased activity on MHC-II promoters. EMBO J 21:5467–5476. doi: 10.1093/emboj/cdf557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forlani G, Accolla RS, Tosi G. 2014. Investigating human T cell lymphotropic retrovirus (HTLV) Tax functions with molecular and immunophenotypic techniques. Methods Mol Biol 1087:299–313. doi: 10.1007/978-1-62703-670-2_24. [DOI] [PubMed] [Google Scholar]
- 63.Bex F, Murphy K, Wattiez R, Burny A, Gaynor R. 1999. Phosphorylation of the human T-cell leukemia virus type 1 transactivator Tax on adjacent serine residues is critical for Tax activation. J Virol 73:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cressman DE, O'Connor WJ, Greer SF, Zhu XS, Ting JPY. 2001. Mechanisms of nuclear import and export that control the subcellular localization of class II transactivator. J Immunol 167:3626–3634. doi: 10.4049/jimmunol.167.7.3626. [DOI] [PubMed] [Google Scholar]
- 65.Kretsovali A, Spilianakis C, Dimakopoulos A, Makatounakis T, Papamatheakis J. 2001. Self-association of class II transactivator correlates with its intracellular localization and transactivation. J Biol Chem 276:32191–32197. doi: 10.1074/jbc.M103164200. [DOI] [PubMed] [Google Scholar]
- 66.Cressman DE, Chin KC, Taxman DJ, Ting JPY. 1999. A defect in the nuclear translocation of CIITA causes a form of type II bare lymphocyte syndrome. Immunity 10:163–171. doi: 10.1016/S1074-7613(00)80017-5. [DOI] [PubMed] [Google Scholar]
- 67.Alefantis T, Barmak K, Harhaj EW, Grant C, Wigdahl B. 2003. Characterization of a nuclear export signal within the human T cell leukemia virus type I transactivator protein Tax. J Biol Chem 278:21814–21822. doi: 10.1074/jbc.M211576200. [DOI] [PubMed] [Google Scholar]
- 68.Huang J, Ren T, Guan H, Jiang Y, Cheng H. 2009. HTLV-1 Tax is a critical lipid raft modulator that hijacks IκB kinases to the microdomains for persistent activation of NF-kappaB. J Biol Chem 284:6208–6217. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 69.Kfoury Y, Nasr R, Favre-Bovin A, El-Sabban M, Renault N, Giron ML, Setterblad N, Hajj HE, Chiari E, Mikati AG, Hermine O, Saib A, de The H, Pique C, Bazarbachi A. 2008. Ubiquitylated Tax targets and binds the IKK signalosome at the centrosome. Oncogene 27:1665–1676. doi: 10.1038/sj.onc.1210804. [DOI] [PubMed] [Google Scholar]
- 70.Lodewick J, Lamsoul I, Polania A, Lebrun S, Burny A, Ratner L, Bex F. 2009. Acetylation of human T-cell leukemia virus type 1 Tax oncoprotein by p300 promotes activation of the NF-κB pathway. Virology 386:68–78. doi: 10.1016/j.virol.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasr R, Chiari E, El-Sabban M, Mahieux R, Kfoury Y, Abdulhay M, Yazbeck V, Hermine O, de The H, Pique C, Bazarbachi A. 2006. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-κB activation. Blood 107:4021–4029. doi: 10.1182/blood-2005-09-3572. [DOI] [PubMed] [Google Scholar]
- 72.Ho Y-K, Zhi H, Bowlin T, Dorjbal B, Philip S, Zahoor A, Shih H-M, Semmes OJ, Schaefer B, Glover JNM, Giam C-Z. 2015. HTLV-1 Tax stimulates ubiquitin E3 ligase, ring finger protein 8, to assemble lysine 63-linked polyubiquitin chains for TAK1 and IKK activation. PLoS Pathog 11:e1005102. doi: 10.1371/journal.ppat.1005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raval A, Weissman JD, Howcroft TK, Singer DS. 2003. The GTP-binding domain of class II transactivator regulates its nuclear export. J Immunol 170:922–930. doi: 10.4049/jimmunol.170.2.922. [DOI] [PubMed] [Google Scholar]
- 74.Meertens L, Chevalier S, Weil R, Gessain A, Mahieux R. 2004. A 10-amino acid domain within human T-cell leukemia virus type 1 and type 2 Tax protein sequences is responsible for their divergent subcellular distribution. J Biol Chem 279:43307–43420. doi: 10.1074/jbc.M400497200. [DOI] [PubMed] [Google Scholar]
- 75.Gatza ML, Dayaram T, Marriot SJ. 2007. Ubiquitination of HTLV-1 Tax in response to DNA damage regulates nuclear complex formation and nuclear export. Retrovirology 4:95. doi: 10.1186/1742-4690-4-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gatza ML, Marriot SJ. 2006. Genotoxic stress and cellular stress alter the subcellular distribution of human T-cell leukemia virus type 1 Tax through a CRM1-dependent mechanism. J Virol 80:6657–6668. doi: 10.1128/JVI.02270-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azran I, Jeang KT, Aboud M. 2005. High levels of cytoplasmic HTLV-1 Tax mutant proteins retain a Tax-NF-κB-CBP ternary complex in the cytoplasm. Oncogene 24:4521–4530. doi: 10.1038/sj.onc.1208645. [DOI] [PubMed] [Google Scholar]
- 78.Pise-Masison CA, Mahieux R, Jiang H, Ashcroft M, Radonovich M, Duvall J, Guillerm C, Brady JN. 2000. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation. Mol Cell Biol 20:3377–3386. doi: 10.1128/MCB.20.10.3377-3386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tabakin-Fix Y, Azran I, Schavibky-Khrapunsky Y, Levy O, Aboud M. 2006. Functional inactivation of p53 by human T-cell leukemia virus type 1 Tax protein: mechanisms and clinical implications. Carcinogenesis 27:673–681. [DOI] [PubMed] [Google Scholar]
- 80.Boxus M, Twizere J-C, Legros S, Dewulf J-F, Kettmann R, Willems L. 2008. The HTLV-1 Tax interactome. Retrovirology 5:76–99. doi: 10.1186/1742-4690-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kress AK, Grassmann R, Fleckenstein B. 2011. Cell surface markers in HTLV-1 pathogenesis. Viruses 3:1439–1459. doi: 10.3390/v3081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schnappauf F, Hake SB, Camacho Carvajal MM, Bontron S, Lisowska-Grospierre B, Steimle V. 2003. N-terminal destruction signals lead to rapid degradation of the major histocompatibility complex class II transactivator CIITA. Eur J Immunol 33:2337–2347. doi: 10.1002/eji.200323490. [DOI] [PubMed] [Google Scholar]
- 83.Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. 1979. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen and antigen-activated T cells. J Exp Med 150:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reinherz EL, Kung PC, Pesando JM, Ritz J, Goldstein C, Schlossman SF. 1979. Ia determinants on human T cell subsets defined by monoclonal antibodies. Activation stimuli required for expression. J Exp Med 150:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]