ABSTRACT
Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus, and, as such, its genome becomes chromosomally integrated following infection. The resulting provirus contains identical 5′ and 3′ peripheral long terminal repeats (LTRs) containing bidirectional promoters. Antisense transcription from the 3′ LTR regulates expression of a single gene, hbz, while sense transcription from the 5′ LTR controls expression of all other viral genes, including tax. Both the HBZ and Tax proteins are implicated in the development of adult T-cell leukemia (ATL), a T-cell malignancy caused by HTLV-1 infection. However, these proteins appear to harbor opposing molecular functions, indicating that they may act independently and at different time points prior to leukemogenesis. Here, we used bidirectional reporter constructs to test whether transcriptional interference serves as a mechanism that inhibits simultaneous expression of Tax and HBZ. We found that sense transcription did not interfere with antisense transcription from the 3′ LTR and vice versa, even with strong transcription emanating from the opposing direction. Therefore, bidirectional transcription across the provirus might not restrict hbz or tax expression. Single-cell analyses revealed that antisense transcription predominates in the absence of Tax, which transactivates viral sense transcription. Interestingly, a population of Tax-expressing cells exhibited antisense but not activated sense transcription. Consistent with the ability of Tax to induce cell cycle arrest, this population was arrested in G0/G1 phase. These results imply that cell cycle arrest inhibits Tax-mediated activation of sense transcription without affecting antisense transcription, which may be important for long-term viral latency.
IMPORTANCE The chromosomally integrated form of the retrovirus human T-cell leukemia virus type 1 (HTLV-1) contains identical DNA sequences, known as long terminal repeats (LTRs), at its 5′ and 3′ ends. The LTRs modulate transcription in both forward (sense) and reverse (antisense) directions. We found that sense transcription from the 5′ LTR does not interfere with antisense transcription from the 3′ LTR, allowing viral genes encoded on opposite DNA strands to be simultaneously transcribed. Two such genes are tax and hbz, and while they are thought to function at different times during the course of infection to promote leukemogenesis of infected T cells, our results indicate that they can be simultaneously transcribed. We also found that the ability of Tax to induce cell cycle arrest inhibits its fundamental function of activating viral sense transcription but does not affect antisense transcription. This regulatory mechanism may be important for long-term HTLV-1 infection.
INTRODUCTION
Retroviruses express their proviral genome by taking advantage of the host cell transcription machinery. Following reverse transcription and integration within the infected cell genome, the 5′ and 3′ flanking regions of provirus comprise identical regions, termed long terminal repeats (LTRs) (1). Recent studies have shown that LTRs contain bidirectional promoters that are able to initiate transcription in both the sense and antisense orientations (2–4). With respect to expression of viral genes, a promoter within the 5′ LTR of the provirus regulates sense transcription and, therefore, production of the viral gene products necessary for viral particle synthesis (gag, pro, pol, and env) as well as regulatory and accessory genes (5). For all human T-cell leukemia virus (HTLV) family members (6–15), as well as for the human immunodeficiency virus type 1 (HIV-1) (16–18), a separate promoter or promoters within the 3′ LTR regulate antisense transcription. In all five of these viruses, the antisense transcripts are translated into proteins (7, 8, 14, 15, 19).
For HTLV type 1 (HTLV-1), the protein produced by the single antisense gene, HTLV-1 basic leucine zipper bZIP factor (HBZ), opposes many of the functions of the HTLV-1 regulatory protein, Tax, whose gene is encoded on the sense strand. For example, Tax activates transcription from the 5′ LTR promoter through formation of complexes with cellular bZIP factors in the ATF/CREB family and the coactivator p300/CBP (20), while HBZ represses transcription by binding to and sequestering these cellular proteins away from the viral promoter (7, 21, 22). In addition, Tax constitutively activates NF-κB signaling, which can induce cellular senescence (23, 24), whereas HBZ blocks this effect by inhibiting canonical NF-κB signaling (25–27). Also, Tax and HBZ have opposing effects on AP-1, nuclear factor of activated T cells (NFAT), and transforming growth factor β (TGF-β) signaling (28).
Despite these divergent molecular functions, both Tax and HBZ appear to contribute to certain pathological effects that arise from HTLV-1 infection. One of the main diseases caused by HTLV-1 is a rare and fatal malignancy known as adult T-cell leukemia (ATL) that is characterized by the uncontrolled proliferation of often highly invasive CD4+ T cells (29). Tax and HBZ have each been implicated in the development of ATL as both proteins stimulate T-cell proliferation, exhibit oncogenic properties in cell culture models, and cause phenotypic effects in transgenic mice that parallel certain clinical features of ATL (30, 31). However, leukemic cells from patients with severe subtypes of ATL often express only HBZ due to modification of the 5′ LTR of the HTLV-1 provirus that inactivates sense transcription or due to mutations in the tax gene (32–36). Consequently, one model has emerged in which Tax acts during early stages of infection to stimulate the initial events required for the development of ATL, while HBZ functions later in infection and plays a role in maintaining the ATL phenotype (31).
In conjunction with the proposed sequential roles of each protein, certain lines of evidence suggest that sense transcription of Tax and antisense transcription of HBZ may oppose one another. Indeed, when HAM/TSP cells are cultured ex vivo, the level of Tax mRNA peaks at 24 h and then rapidly declines, while the level of HBZ mRNA is initially low and begins to increase only after 24 h (37). In a rabbit infection model, temporal changes in Tax and HBZ mRNA levels exhibit a similar divergent pattern (38). It is possible that this apparent inverse pattern of Tax and HBZ expression is caused by transcriptional interference in which sense transcription from the 5′ LTR of the HTLV-1 provirus obstructs antisense transcription from the 3′ LTR and vice versa (3).
In this study, we designed a set of HTLV-based reporter plasmids containing reporter genes placed in opposite orientations between the 5′ and 3′ LTRs. This system allowed us to analyze the balance between sense and antisense transcription in cell populations as well as in single cells. Unexpectedly, we found that transcription in one direction had no effect on the level of transcription in the opposite direction, suggesting that transcriptional interference does not occur between the HTLV-1 LTRs. We also found that Tax did not influence the level of transcription from the 3′ LTR and, strikingly, that Tax did not activate sense transcription from the 5′ LTR in all cells. Specifically, Tax was unable to activate sense transcription in a subset of cells exhibiting constitutive antisense transcription. These cells appeared to be arrested in the G0/G1 phase of the cell cycle, which may relate to the ability of Tax to induce cell cycle arrest and senescence (23, 24). Therefore, inhibition of Tax-mediated activation of sense transcription due to the onset of cellular senescence may contribute to the long-term survival of cells infected with latent virus.
MATERIALS AND METHODS
Plasmid constructs.
The core dual-luciferase reporter plasmid pLuc(Reni)-AsLuc(Fire) was constructed by amplifying by PCR the Renilla luciferase gene from pRL-SV40 (where SV40 is simian virus 40) (Promega), cloning the product into SacI/KpnI of pUC19, and then amplifying the firefly luciferase poly(A) cassette from pGL3-basic vector (Promega) and cloning the product into BamHI/PstI. Promoters (HTLV-1 LTRs and cytomegalovirus [CMV] promoter) as well as the 3′ polyadenylation signal were cloned into the EcoRI (sense 5′ promoters) or HindIII site [antisense 3′ promoters and poly(A) signal]. The HTLV-1 LTR was PCR amplified from pAsLuc(Fire)-HTLV-Luc(Reni) (2). The CMV promoter and late SV40 polyadenylation signal sequence were amplified from pcDNA3.1 (Life Technologies). Plasmids constructed through permutations in this cloning approach included pHTLV-Luc(Reni)-AsLuc(Fire)-HTLV, pHTLV-Luc(Reni)-AsLuc(Fire)-CMV, pCMV-Luc(Reni)-AsLuc(Fire)-HTLV, pHTLV-Luc(Reni)-AsLuc(Fire)-pA (where pA indicates the polyadenylation signal), and pnull-Luc(Reni)-AsLuc(Fire)-HTLV. Similar DsRed2-AsEGFP (where DsRed is Discosoma sp. red fluorescent protein and EGFP is enhanced green fluorescent protein) vectors were constructed by replacing the antisense firefly luciferase and sense Renilla luciferase genes with EGFP and DsRed2, respectively. The EGFP gene was PCR amplified from pEGFP-N1 (Clontech), and the product was cloned into NcoI/XbaI. The DsRed2 gene was amplified from pDsRed2-N1 (Clontech), and the product was cloned into NheI/KpnI. The pSG-Tax and β-galactosidase expression vectors have been described previously (2, 39). The plasmid pSG-Tax-His was prepared by PCR amplification of the tax gene from pSG-Tax using a reverse primer with a 6×His tag sequence. The product was cloned into EcoRI/BamHI of pSG5 (Agilent). All constructs were sequenced and found to be correct. All primers used in this study are available upon request.
Cell lines and transfection.
HEK293T/17 (ATCC) and CEM cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich) and Iscove's modified Dulbecco's medium (IMDM; Sigma-Aldrich), respectively. Media were supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies). CEM cells were electroporated as described previously (2). Briefly, 5 × 106 cells were washed twice with serum-free IMDM combined with 5 μg of β-galactosidase-expressing vector, 5 μg of reporter plasmid, and 500 ng of either pSG-Tax or pcDNA3.1 and electroporated using a Gene Pulser Xcell (Bio-Rad). Jurkat cells were electroporated in RPMI medium containing 10 mM dextrose and 0.1 mM dithiothreitol (DTT) using the same amount of plasmid DNA as used above for luciferase assays or 10 μg of reporter plasmid and 5 μg of pSG-Tax or pcDNA3.1 for cell cycle analyses. HEK293T cells were transfected using TurboFect reagent (Life Technologies) according to the manufacturer's instructions. Briefly, 24 h prior to transfection, cells were plated at 5 × 105 cells/well in six-well plates for fluorescence-activated cell sorting (FACS) analyses and for establishing clonal cell lines, at 1 × 105 cells/well in 12-well plates for luciferase assays, or at 1.5 × 107 cells/150-cm2 dish for sorting experiments. For luciferase assays 400 ng of reporter plasmid, 500 ng of β-galactosidase-expressing vector, and 100 ng of either pSG-Tax or pcDNA3.1 were used. For FACS analyses 1 μg of reporter plasmid and 100 ng of pSG-Tax or pcDNA3.1 were used. For sorting experiments 30 μg of reporter plasmid and 3 μg of pSG-Tax-His or pcDNA3.1 were used. Clonal cell lines were established by cotransfecting HEK293T cells with 1 μg of ApaLI-linearized pHTLV-Luc(Reni)-AsLuc(Fire)-HTLV or pLuc(Reni)-AsLuc(Fire) and 100 ng of SspI-linearized pCMV-Hyg vector (18) using TurboFect reagent. Cells were supplemented with 100 μg/ml of hygromycin B (Invitrogen) at 72 h posttransfection. Hygromycin-resistant colonies were later isolated, expanded, and tested for luciferase activity. To confirm the activities of wild-type Tax and the Tax mutant M22, HEK293T cells were transfected with a pminLuc-viral CRE (vCRE-Luc) (21) or pNF-κB-Luc (27) and pSG-Tax or pSG-M22 (39) plasmids.
Luciferase assays.
HEK293T and CEM cells were lysed 48 h posttransfection in 1× Passive Lysis Buffer (Promega); luciferase activities were measured using a TD-20/20 luminometer (Turner Design) and normalized to β-galactosidase activity (2) and protein concentration (Pierce bicinchoninic acid [BCA] protein assay kit), or activities were normalized to pRL-TK (Promega). Two-tailed Student's t tests were used to determine significant differences between sample sets, and differences were considered significant at a P value of <0.01 and highly significant at a P value of <0.001.
Flow cytometry experiments.
For cell cycle analyses, harvested cells were washed in ice-cold phosphate-buffered saline (PBS) and then fixed with PBS (137 mM NaCl, 2.7 mM KCl, 3 mM Na2HPO4, and 1.5 mM KH2PO4)–2% paraformaldehyde (PFA) at 4°C for 30 m. Cells were then washed in ice-cold PBS and incubated for 30 min in PBS–0.1% Triton X-100–1 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI) in the dark. Stained cells were analyzed for DNA content and EGFP/DsRed expression using an LSR II flow cytometer (BD Biosciences). FlowJo (Tree Star, Inc.) was used for population gating, and ModFit LT (Verity Software House) was used to construct cell cycle profiles for each of the populations. For other flow cytometry experiments, harvested cells were washed in PBS and then resuspended in PBS–5 mM EDTA prior to FACS acquisition and analysis with FlowJo. Two-tailed Student's t tests were used to determine significant differences between sample sets, and differences were considered significant at a P value of <0.01 and highly significant at a P value of <0.001.
PCR analysis of the reporter construct.
Harvested HEK293T cells were washed in ice-cold PBS–5 mM EDTA and sorted based on their DsRed/EGFP expression profiles using a FACSVantage SE cell sorter (BD Biosciences). DNA was purified from each cell population by phenol-chloroform extraction and ethanol precipitation. Relative quantities of the reporter construct in each population were determined by real-time PCR amplification of a region of the plasmid DNA within the EGFP gene and a region of genomic DNA within the CDK6 gene. DNA was amplified using a CFX96 real-time PCR detection system (Bio-Rad), and data were analyzed using Bio-Rad CFX Manager, version 3.1. Values were determined by the calculation 2(CDK6 CT − EGFP CT), in which CT denotes the threshold cycle.
Western blot assays.
Harvested HEK293T cells were washed in ice-cold PBS–5 mM EDTA and sorted based on their DsRed/EGFP expression profiles using a FACSVantage SE cell sorter (BD Biosciences). Approximately 5 × 105 sorted cells from each profile were lysed directly in SDS loading dye and resolved by SDS-PAGE. Proteins were transferred to nitrocellulose membranes that were then probed with 6×His tag (ab9108; Abcam), actin (MAB1501R; Chemicon International), cyclin B1 (D5C10; Cell Signaling), and Skp2 (D3G5; Cell Signaling) antibodies. A supernatant from the Tax hybridoma line 168B17-46-92 (NIH AIDS Research and Reference Reagent Program) was used for Western blot analysis of luciferase assay lysates. Blots were developed using ECL 2 chemiluminescence (Thermo Scientific Pierce) and scanned using a Typhoon 9410 (GE Healthcare).
RESULTS
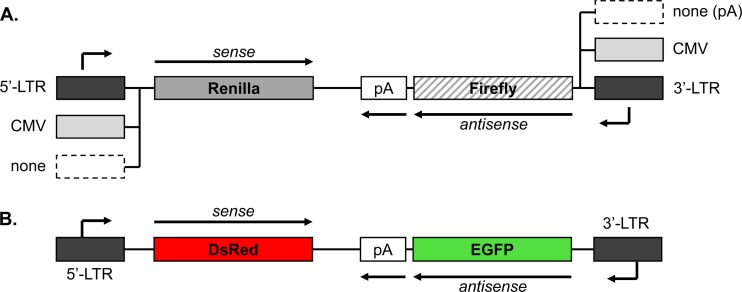
We were interested in determining whether transcriptional interference contributes to the relative outputs of HTLV-1 sense and antisense transcription, based on the distinct kinetics of Tax and HBZ expression observed previously (37, 38). For this analysis we generated the pHTLV-Luc(Reni)-AsLuc(Fire)-HTLV dual promoter/reporter plasmid, containing the Renilla and firefly luciferase genes in opposite orientations between the flanking LTRs (Fig. 1A). Specifically, the 5′ LTR (sense transcription) controls expression of the Renilla luciferase gene, and the 3′ LTR (antisense transcription) controls expression of the firefly luciferase gene. In addition, we cloned the SV40 late polyadenylation signal immediately downstream of the antisense-oriented firefly gene. This modification was made based on the observation that hbz gene transcripts terminate 1,450 nucleotides downstream of the HBZ stop codon and, therefore, do not extend into the 5′ LTR further downstream (8). A similar modification was not required for sense transcripts, which utilize polyadenylation signals located within the 3′ LTR for termination (40). The size of the luciferase construct encompassing the two LTRs and reporter genes is approximately 5 kb. Variants of the reporter system were also engineered, in which either the 5′ or 3′ LTR was removed to eliminate sense or antisense transcription, respectively, and/or one of the LTRs was replaced with the CMV promoter to generate strong, constitutive transcription. For the construct lacking the 3′ LTR, a polyadenylation signal was added for the sense transcript. In addition to the pHTLV-Luc(Reni)-AsLuc(Fire)-HTLV construct, we generated a pHTLV-DsRed-AsEGFP-HTLV construct in which the Renilla and firefly luciferase genes were replaced with the DsRed and EGFP genes, respectively (Fig. 1B).
FIG 1.
Schematic representation of dual-luciferase/dual-fluorescent reporter constructs. (A) The core pLuc(Reni)-AsLuc(Fire) plasmid contains genes for Renilla luciferase and firefly luciferase that are translated from the sense and antisense transcripts, respectively, as indicated. Variations in the DNA regions flanking the core are indicated. pA denotes the SV40 late polyadenylation signal. A polyadenylation signal for the sense transcript is located in the 3′ LTR. (B) Schematic representation of the bidirectional pHTLV-DsRed-AsEGFP-HTLV reporter construct.
Antisense transcription from the 3′ LTR is unaffected by the level of sense transcription.
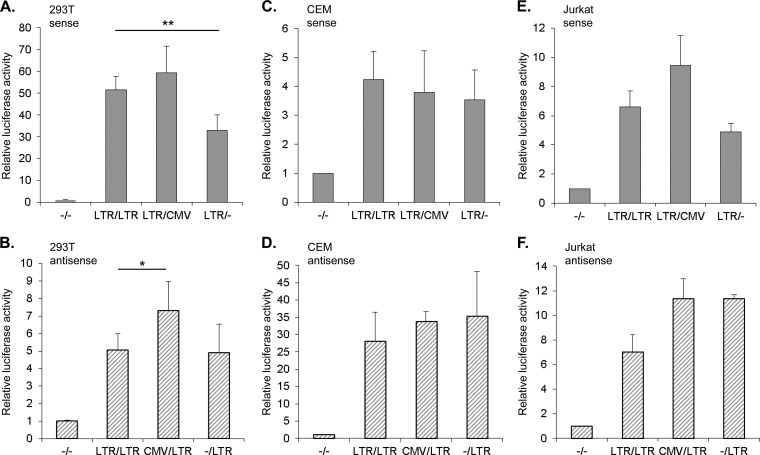
We first analyzed the effects of sense and antisense transcription on one another in the absence of the viral transactivator Tax, using the first set of constructs shown in Fig. 1A. In HEK293T cells, we found that the level of transcription from the 3′ LTR was not affected by deletion of the 5′ LTR, and deletion of the 3′ LTR caused only a slight reduction in basal transcription from the 5′ LTR (Fig. 2A and B). Considering that deletion of either LTR failed to augment transcription from the opposing LTR promoter, basal levels of transcription do not appear to lead to transcriptional interference. Consequently, we tested whether the activity of each LTR promoter would be affected by strong transcription from the opposite direction by individually replacing the LTRs with CMV promoter. As with the LTR deletions these permutations did not drastically affect levels of transcription from the 5′ and 3′ LTR promoters (Fig. 2A and B). Similar results were obtained using CEM T cells (Fig. 1C and D); however, the ratio of antisense to sense transcription was substantially higher in these cells than in the HEK293T cells. To address this variation, we performed the same experiments with Jurkat T cells. These cells produced similar levels of antisense and sense transcription, which led to a ratio of antisense to sense transcription that was distinct from the ratios obtained using HEK293T and CEM cells (Fig. 1E and F). Therefore, antisense and sense transcriptional output from the LTRs appears to vary among cell lines. Despite these differences, the overall results again indicate that the basal sense transcription from the 5′ LTR and strong sense transcription from a CMV promoter do not significantly influence the level of antisense transcription from the 3′ LTR (and vice versa).
FIG 2.
Sense and antisense transcription are unaffected by the transcriptional output from the opposite direction. Luciferase activities corresponding to sense and antisense transcription are represented with solid and hatched bars, respectively. (A, C, and E) The graphs show a comparison of the relative luciferase activities of sense transcription between the construct containing both LTRs (LTR/LTR), those in which the 5′ or 3′ LTR was replaced with the CMV promoter (CMV/LTR or LTR/CMV, respectively), and those with a deletion of the 5′ (−/LTR) or 3′ LTR (LTR/−) from HEK293T, CEM, and Jurkat cells as indicated. (B, D, and F) The graphs show a comparison of the relative luciferase activities of antisense transcription between the construct containing both LTRs (LTR/LTR), those in which the 5′ or 3′ LTR was replaced with the CMV promoter (CMV/LTR or LTR/CMV, respectively), and those with a deletion of the 5′ (−/LTR) or 3′ LTR (LTR/−) from HEK293T, CEM, and Jurkat cells as indicated. All graphs display mean luciferase activities relative to the β-galactosidase internal control with values normalized to luciferase activities from the reporter construct with a deletion of both LTRs (−/−; set to 1). Data from HEK293T, CEM, and Jurkat cells are from six, four, and three independent experiments, respectively. Error bars denote standard deviations. Significant results as determined by Student's t test are indicated (*, P < 0.01; **, P < 0.001).
Tax does not affect antisense transcription from the 3′ LTR.
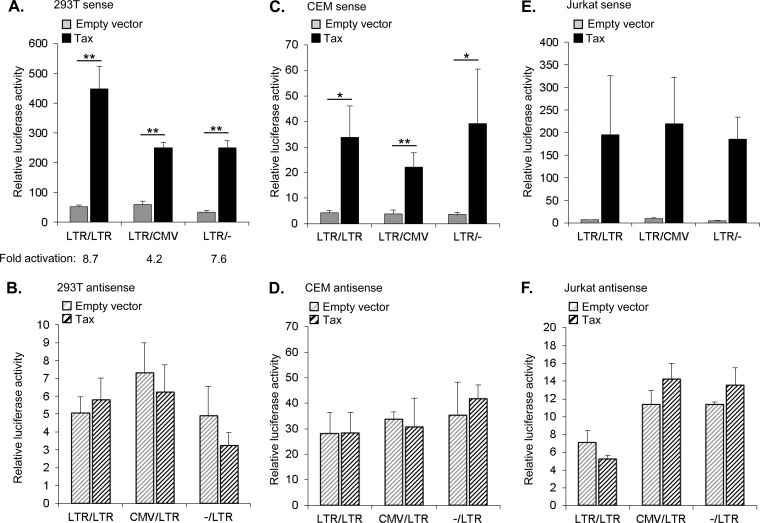
We next examined whether Tax affects the level of antisense transcription from the 3′ LTR either through transcriptional interference caused by activation of sense transcription from the 5′ LTR or through a direct effect on the 3′ LTR promoter. Tax transactivates viral sense transcription by forming complexes with a variety of cellular transcriptional regulatory proteins at multiple Tax-responsive elements within the 5′ LTR promoter (20). In addition, results from some studies show that Tax enhances antisense transcription from the 3′ LTR (41); however, this effect has not been uniformly documented (2, 6). In HEK293T cells we found that Tax induced a substantial increase in sense transcription from the 5′ LTR promoter, as expected (Fig. 3A). Transcriptional activation by Tax was retained in the constructs lacking the 3′ LTR or carrying the CMV promoter in place of the 3′ LTR. Replacement of the 3′ LTR with CMV promoter reduced the level of activation in HEK293T cells but not in the other cell lines (Fig. 3C and E), suggesting that the reduction is specific to the HEK293T cell line. In contrast to the 5′ LTR, Tax did not significantly alter the level of basal antisense transcription from the 3′ LTR in any of the three construct variants (Fig. 3B). Identical experiments in CEM and Jurkat T cells produced similar results (Fig. 3C to F), suggesting that Tax does not affect the level of antisense transcription.
FIG 3.
Tax activates sense transcription without affecting the level of antisense transcription. Luciferase activities corresponding to sense and antisense transcription are represented with solid and hatched bars, respectively. (A, C, and E) The graphs show relative luciferase activities corresponding to activation of 5′ LTR-controlled sense transcription by Tax from constructs containing the 3′ LTR (LTR/LTR) or the CMV promoter in place of the 3′ LTR (LTR/CMV) or with a with a deletion of the 3′ LTR (LTR/−) in HEK293T, CEM, or Jurkat cells as indicated. Fold activation denotes the average Tax activation over basal transcription levels. (B, D, and F) The graphs show relative luciferase activities corresponding to 3′ LTR-controlled antisense transcription in the absence or presence of Tax from constructs containing the 5′ LTR (LTR/LTR) or the CMV promoter in place of the 5′ LTR (CMV/LTR) or with a deletion of the 5′ LTR (−/LTR) in HEK293T, CEM, or Jurkat cells as indicated. All graphs display mean luciferase activities relative to the β-galactosidase internal control with values normalized to luciferase activities from the reporter construct with a deletion of both LTRs (−/−; set to 1). Data from HEK293T, CEM, and Jurkat cells are from six, four, and three independent experiments, respectively. Error bars denote standard deviations. Significant results as determined by Student's t test are indicated (*, P < 0.01; **, P < 0.001).
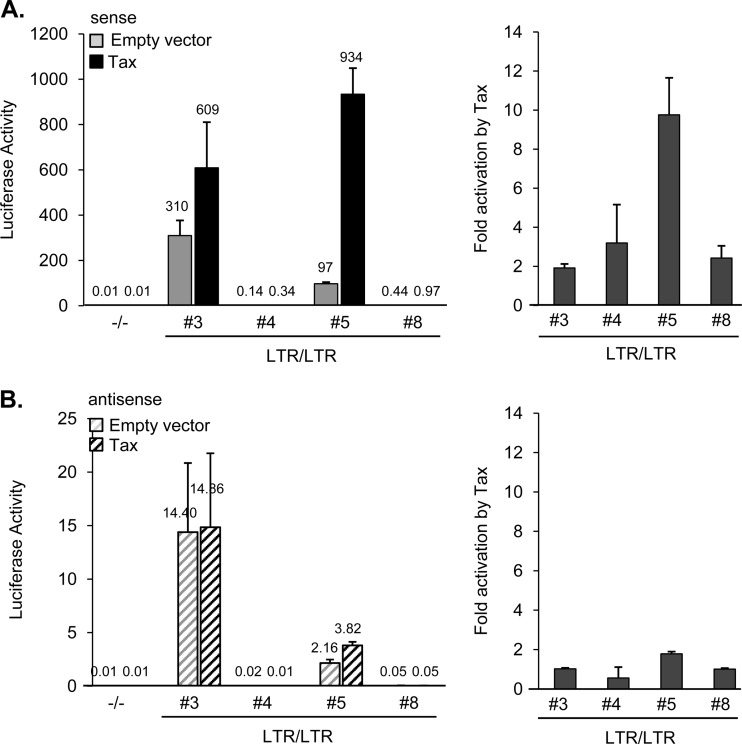
Given that HTLV-1 transcription occurs from an integrated provirus, we also analyzed levels of sense and antisense transcription from reporter constructs that were genomically integrated in 293T cells. Four clonal cell lines were analyzed that contained the 5′ and 3′ LTRs. For comparison, averaged results from eight clonal cell lines containing reporter constructs with deletions of both LTRs were used. In all four cell lines carrying constructs with the 5′ and 3′ LTRs, Tax augmented sense transcription; however the extent of activation as well as the level of basal sense transcription varied among the cell lines (Fig. 4A). These differences may reflect distinct integration sites and variable integrated reporter construct copies among these cell lines. Levels of basal antisense transcription also differed among the cell lines and paralleled the variable pattern of basal sense transcription among these lines; however, Tax did not significantly increase basal antisense transcription (Fig. 4B). These results match those obtained using transiently transfected reporter constructs in the prior analyses.
FIG 4.
Tax activates sense transcription but does not affect antisense transcription from chromosomally integrated 5′ LTR/3′ LTR reporter constructs. Graphs on the left of each panel show luciferase activities corresponding to sense (A) or antisense (B) transcription in the presence and absence of Tax from constructs containing the 5′ and 3′ LTRs (LTR/LTR) using four independent clonal cell lines derived from HEK293T cells. Also shown are the mean luciferase activities from eight clonal cell lines in which both LTRs are deleted (−/−). Graphs on the right of each panel show the levels of activation of sense (A) and antisense (B) transcription by Tax in the four clonal cell lines. Luciferase activities are the mean values from three independent experiments. Error bars denote standard deviations.
HTLV-1 antisense transcription is primarily expressed in the absence of Tax.
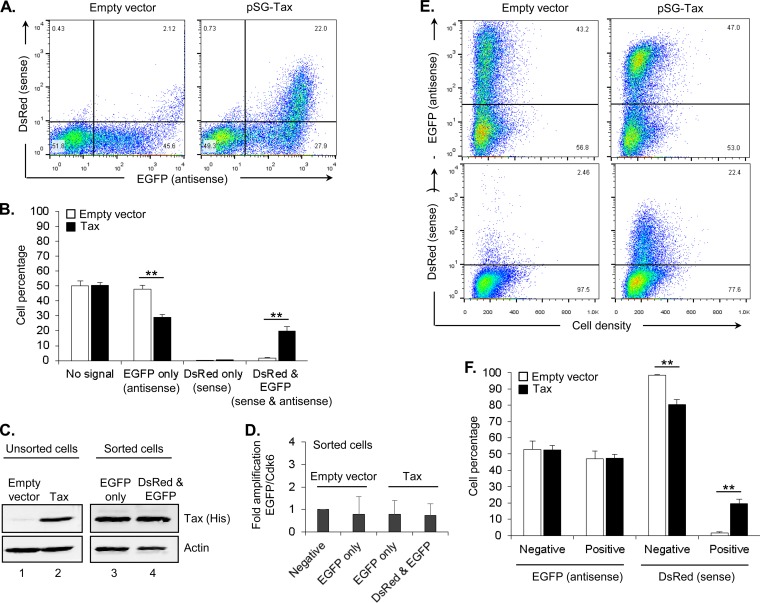
Given that the above results were derived from a population of cells as a whole, we were also interested in analyzing sense and antisense transcription in single cells. For this purpose, we used pHTLV-DsRed2-AsEGFP-HTLV (Fig. 1B), which spans approximately 4 kb from the 5′ LTR to the 3′ LTR and produces DsRed from sense transcription and EGFP from antisense transcription. This construct was transfected into HEK293T cells, and expression of EGFP and DsRed was evaluated by FACS. In the absence of Tax, almost 50% of cells expressed EGFP, while only about 2% of cells expressed DsRed, which occurred only in cells coexpressing EGFP (Fig. 5A, left panel). Tax expression led to a specific increase in cells coexpressing EGFP and DsRed but not to the formation of a cell population expressing DsRed alone (Fig. 5A and B). This observation suggests that Tax-mediated activation of viral sense transcription from the 5′ LTR occurs in the context of constitutively active antisense transcription. We confirmed that the Tax protein was indeed present in the cell population coexpressing EGFP and DsRed and, interestingly, was also present in the population expressing EGFP alone (Fig. 5C). Therefore, Tax appears unable to induce significant sense transcription from the 5′ LTR in the latter population. Quantitative PCR analysis showed that all cell populations, including the one lacking both EGFP and DsRed expression, carried the reporter construct (Fig. 5D). The absence of expression of both EGFP and DsRed (the negative population) may arise through a transcriptional mechanism or damage to the reporter plasmid that prevents expression of the fluorescent proteins. We were unable to distinguish between these possibilities due to technical challenges in amplifying the 4-kb region of the plasmid encompassing the LTRs and the reporter genes. An analysis of each fluorescent channel separately revealed that, while Tax expression led to an increase in the percentage of cells expressing DsRed, it did not alter the percentage of cells expressing EGFP (Fig. 5E and F). This pattern is consistent with the luciferase assay results, further indicating that Tax does not affect antisense transcription from the 3′ LTR.
FIG 5.
Tax fails to activate sense transcription in all cells. (A) Flow cytometry analysis of sense/antisense profiles of HEK293T cells cotransfected with the reporter construct and pcDNA3.1 (left plot) or pSG-Tax (right plot). Plots show one representative experiment from four independent experiments. (B) The graph shows mean cell percentages in each plot quadrant from the four independent experiments including those in panel A. Error bars denote standard deviations, and significant results as determined by Student's t test are indicated (**, P < 0.001). (C) The Western blot shows Tax expression in HEK293T cells transfected with the reporter construct and an empty vector (lane 1) or pSG-Tax-His (lanes 2, 3, and 4). The left panels show Tax and actin expression in unsorted cells. The right panel shows Tax and actin expression in cells sorted as a function of EGFP or EGFP/DsRed expression. (D) The graph shows quantitative real-time PCR amplification of DNA from cells transfected with pcDNA3.1 or pSG-Tax that were sorted as a function of their fluorescent signals. Values were normalized to those of the fluorescence-negative population (set to 1). The graph shows data averaged from three independent experiments. (E) Plots show separate analyses of antisense (EGFP) and sense (DsRed) transcription of the plots in panel A. (F) The graph shows mean cell percentages in each plot section from the four independent experiments. Error bars denote the standard deviations, and significant results as determined by Student's t test are indicated (*, P < 0.01; **, P < 0.001).
Tax fails to activate sense transcription in cells arrested in G0/G1 phase.
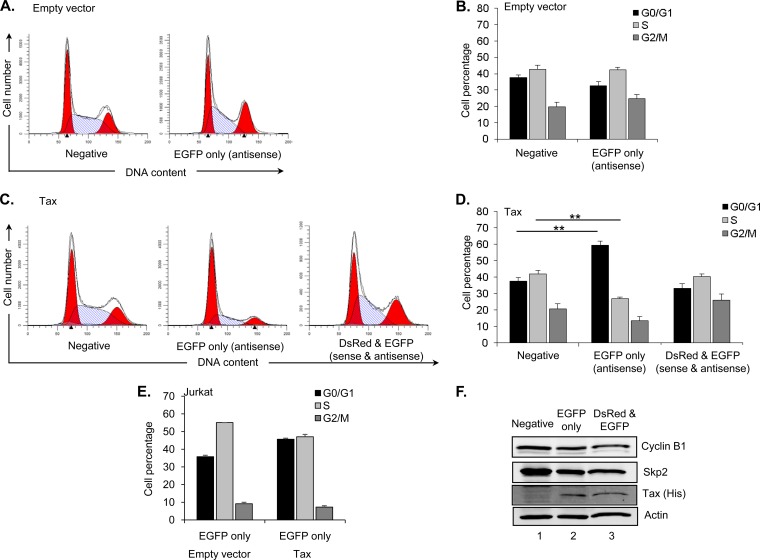
One explanation for the different populations revealed by the single-cell analysis was possible cell cycle-mediated regulation of HTLV-1 sense and antisense transcription. To test this hypothesis, we compared the cell cycle profiles of the negative, EGFP-positive, and EGFP/DsRed-positive populations in the absence and presence of Tax. As shown in Fig. 5A, only the negative and EGFP-positive cell populations are present in the absence of Tax, and we found that the G0/G1, S, and G2/M distributions of cells in each population did not differ significantly (Fig. 6A and B). In the presence of Tax, a similar pattern was observed in the negative and EGFP/DsRed-positive populations (Fig. 6C and D). However, the majority of cells expressing EGFP alone were shifted into G0/G1 in the presence of Tax (Fig. 6C and D). Using Jurkat T cells, we similarly found that Tax expression also increased the proportion of cells in G0/G1 within the EGFP-alone population (Fig. 6E).
FIG 6.
Cells arrested in G0/G1 phase are unresponsive to Tax-mediated activation of sense transcription. (A) Histograms show the cell cycle distribution of the fluorescence-negative and EGFP-only (antisense transcription) populations from HEK293T cells cotransfected with pHTLV-DsRed-AsEGFP-HTLV and pcDNA3.1 from one representative experiment of four independent experiments. Solid red peaks denote G0/G1 and G2/M phases. Hatched blue areas denote S phase. (B) The graph shows mean percentages of cells in G0/G1, S, or G2/M phase from the fluorescence-negative and EGFP populations for the four independent experiments. Error bars denote standard deviations. (C) Histograms show the cell cycle distribution of the fluorescence-negative, EGFP-only (antisense transcription), and DsRed/EGFP (both sense and antisense transcription) populations from HEK293T cells cotransfected with the reporter construct and pSG-Tax from one representative experiment of four independent experiments. (D) The graph shows mean percentages of cells in G0/G1, S, or G2/M phase from the different populations for the four independent experiments. Error bars denote standard deviations. Significant results as determined by Student's t test are indicated (**, P < 0.001). (E) The graph shows mean percentages of cells in G0/G1, S, or G2/M phase from the different populations for two independent experiments with Jurkat cells. (F) The Western blot shows cyclin B1, Skp2, Tax, and actin expression in HEK293T cells transfected with the reporter construct and pSG-Tax-His. Cells were sorted as a function of fluorescence.
Given that these results are consistent with the ability of Tax to induce cell cycle arrest and senescence (23, 24), we analyzed the senescence markers, cyclin B1 and Skp2, in the sorted HEK293T cell populations. Following transfection with the Tax expression plasmid, we detected Tax in cells expressing EGFP alone or DsRed and EGFP but not in cells lacking expression of both fluorescent proteins (Fig. 6F). Consistent with this pattern, cells with EGFP alone or DsRed/EGFP exhibited a slight decrease in levels of Skp2 and cyclin B1 compared to cells lacking the fluorescent proteins. However, no difference in the abundance of Skp2 and cyclin B1 occurred between the population expressing EGFP alone and the population expressing DsRed/EGFP (Fig. 6F). Therefore, the Tax-mediated increase of cells in G0/G1 within the EGFP-alone population did not specifically correlate with a reduction in these senescence markers. We attempted to analyze additional markers, including p21 and p27, but were unable to detect these proteins in the sorted cell populations (data not shown).
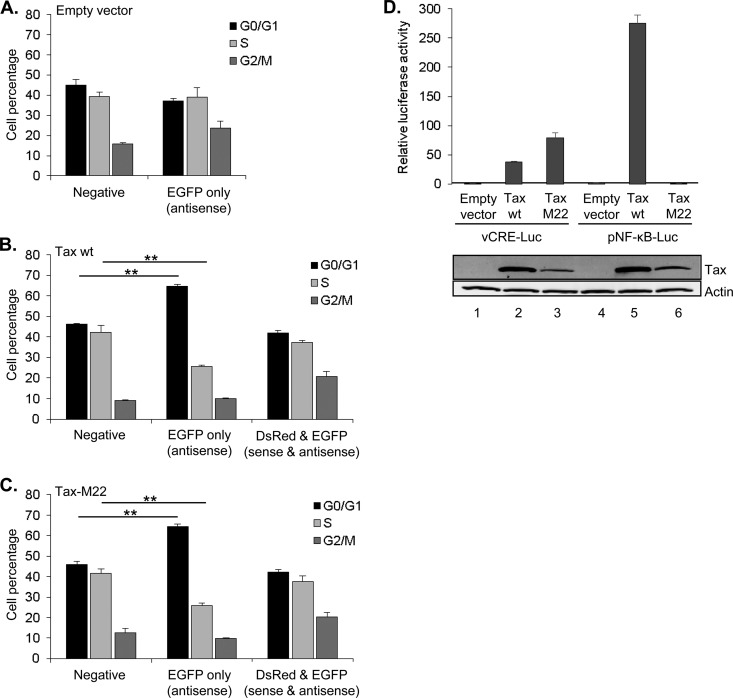
We also tested whether NF-κB activation by Tax in HEK293T cells played a role in G0/G1 phase accumulation of cells expressing EGFP alone as this pathway has been shown to contribute to cellular senescence (25). We specifically compared the effects of wild-type Tax and the Tax mutant M22 on the cell cycle distribution of the different fluorescent populations. M22 is defective for activation of NF-κB signaling but retains the ability to activate transcription through CREB (42). Consistent with the previous results, wild-type Tax increased the number of cells in G0/G1 in the EGFP-only population (Fig. 7A and B). Interestingly, M22 produced the same effect (Fig. 7C). Using a luciferase assay, we confirmed that M22 was defective for NF-κB signaling in HEK293T cells. Therefore, the shift to G0/G1 appears to involve a mechanism distinct from Tax-mediated NF-κB signaling. Considering that Tax fails to activate sense transcription in these cells, it is possible that cell cycle arrest suppresses activation of transcription from the 5′ LTR by Tax.
FIG 7.
Activation of NF-κB by Tax is not required for G0/G1 arrest in HEK293T cells. Cell cycle distribution of the fluorescence-negative, EGFP-only (antisense transcription), and DsRed/EGFP (sense and antisense transcription) populations from HEK293T cells cotransfected with pHTLV-DsRed-AsEGFP-HTLV and pcDNA3.1 (A), pSG-Tax (B), or pSG-TaxM22 (C). The graph shows mean percentages of cells in G0/G1, S, and G2/M phase from the fluorescence-negative, EGFP-only, and DsRed/EGFP populations for three independent experiments. Transfection of pcDNA3.1 does not produce a DsRed/EGFP population. Error bars denote standard deviations. (D) HEK293T cells were cotransfected with vCRE-Luc (200 ng) or pNF-κB-Luc and pSG-Tax (200 ng) or pSG-TaxM22 (200 ng). The graph shows average luminescence values ± standard deviations from one experiment performed in triplicate and is representative of three independent experiments. Cellular lysates used for the luciferase assay were also analyzed by Western blotting using the antibodies indicated.
DISCUSSION
In this study, we found that HTLV-1 sense transcription from the 5′ LTR and antisense transcription from the 3′ LTR occur concurrently and do not interfere with one another. According to this mechanism, Tax and HBZ may be simultaneously expressed from a single provirus at some point during infection. Such a possibility was not apparent from an analysis of HAM/TSP cells cultured ex vivo or from HTLV-1-infected rabbits as in both cases mRNA levels of Tax and HBZ exhibited somewhat divergent patterns (37, 38). However, the abundance of these transcripts may not reflect the outputs of HTLV-1 sense and antisense transcription in that complex events occurring during infection and involving multiple HTLV-1 genes likely influence the abundance of viral RNA (43–45). Furthermore, the cellular environment may influence viral mRNA stability, which is supported by a substantial increase in the stability of the Tax transcript following T-cell stimulation (46).
Contrasting results have been obtained regarding the role of Tax in the regulation of HTLV-1 antisense transcription. Specifically, Tax has not been shown to affect antisense transcription from an HTLV-1 LTR lacking additional proviral sequence (2, 6); however, it has been found to activate antisense transcription from a proviral construct with a 5′ deletion (41). These observations suggest that the ability of Tax to augment antisense transcription involves a region of DNA located outside the 3′ LTR. Consistent with these observations, Tax did not regulate antisense transcription in our system as our analysis was focused on the interplay between sense and antisense transcription strictly mediated by the 5′ and 3′ LTRs, respectively. Any possible effect of Tax on antisense transcription is unlikely to interfere with sense transcription as strong antisense transcription driven by a CMV promoter in our system did not interfere with sense transcription from the 5′ LTR.
The fact that sense transcription did not interfere with antisense transcription in our system suggests the importance of maintaining expression of HBZ at times during the course of infection when sense transcription and corresponding Tax expression are induced. For example, Tax is highly immunogenic, and too much Tax expression may lead to elimination of infected cells in the host. It is possible that HBZ counteracts this effect by binding to and sequestering cellular transcriptional regulators utilized by Tax, thereby dampening Tax-mediated activation of sense transcription from the 5′ LTR (7, 21, 22). The importance of preventing the accumulation of Tax is also illustrated by the function of a separate viral protein, p30, which inhibits Tax protein expression by sequestering Tax mRNA in the nucleus (47). In addition to triggering an immune response, high Tax expression may hinder the HTLV-1 infection by activating apoptosis. Indeed, despite its ability to target multiple cell cycle regulators and stimulate proliferation, Tax has also been shown to induce apoptosis in T cells through activation of NF-κB signaling and the corresponding accumulation of reactive oxygen species (48, 49). Interestingly, HBZ inhibits the classical NF-κB pathway (25–27), which may prevent infected cells that express high levels of Tax from undergoing apoptosis. In support of this hypothesis, recent studies have shown that HBZ counteracts Tax-induced senescence of HeLa cells, which also involves activation NF-κB signaling and the accumulation of reactive oxygen species (25). Considering that Tax alone is able to immortalize primary T cells, albeit inefficiently (50), it would be interesting to examine whether coexpression of HBZ augments this process or possibly leads to T-cell transformation.
Consistent with the ability of Tax to promote cell cycle arrest and senescence in HeLa cells (23, 24), we identified a population of Tax-expressing cells that appeared to be arrested in G0/G1 phase, and, interestingly, these cells lacked activated sense transcription. This observation suggests that while Tax is able to directly transactivate sense transcription from the 5′ LTR, it may also be capable of indirectly repressing this effect by promoting cell cycle arrest and senescence. Multiple cellular transcription factors are known to participate with Tax in activating sense transcription, and it is possible that one or more of these are downregulated during cell cycle arrest, leading to the loss of activated sense transcription. We explored this possibility by analyzing the levels of CREB, CBP, p300, and p/CAF, which have established roles in Tax-dependent transcriptional (20). However, we did not observe noticeable differences in the abundances of any of these proteins between the cell populations in which Tax activated and failed to activate sense transcription (data not shown). This result does not rule out the possibility that another key regulator is downregulated or that one of these proteins is modified and unable to participate in HTLV-1 sense transcription during cell cycle arrest. Future studies will aim to address these issues.
ACKNOWLEDGMENTS
We thank Pamela Cook for constructing the pSG-Tax-His plasmid, Jean-Michel Mesnard for providing the pAsLuc(Fire)-HTLV-Luc(Reni)- and β-galactosidase-expressing vectors, and Ethan Anderson for providing the pDsRed2 plasmid.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Dhar R, McClements WL, Enquist LW, Vande Woude GF. 1980. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A 77:3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpin-Andre C, Laverdure S, Barbeau B, Gross A, Mesnard JM. 2014. Construction of a reporter vector for analysis of bidirectional transcriptional activity of retrovirus LTR. Plasmid 74:45–51. doi: 10.1016/j.plasmid.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Barbeau B, Mesnard JM. 2015. Does chronic infection in retroviruses have a sense? Trends Microbiol 23:367–375. doi: 10.1016/j.tim.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen MH, Ballarin-Gonzalez B, Liu J, Lassen LB, Fuchtbauer A, Fuchtbauer EM, Nielsen AL, Pedersen FS. 2010. Antisense transcription in gammaretroviruses as a mechanism of insertional activation of host genes. J Virol 84:3780–3788. doi: 10.1128/JVI.02088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrowski MC, Berard D, Hager GL. 1981. Specific transcriptional initiation in vitro on murine type C retrovirus promoters. Proc Natl Acad Sci U S A 78:4485–4489. doi: 10.1073/pnas.78.7.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larocca D, Chao LA, Seto MH, Brunck TK. 1989. Human T-cell leukemia virus minus strand transcription in infected T-cells. Biochem Biophys Res Commun 163:1006–1013. doi: 10.1016/0006-291X(89)92322-X. [DOI] [PubMed] [Google Scholar]
- 7.Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. 2002. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J Virol 76:12813–12822. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanagh M, Landry S, Audet B, Arpin-Andre C, Hivin P, Pare M-E, Thete J, Wattel E, Marriott SJ, Barbeau B, Mesnard J-M. 2006. HTLV-I antisense transcripts initiate in the 3′ LTR and are alternatively spliced and polyadenylated. Retrovirology 3:15. doi: 10.1186/1742-4690-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata K, Hayashibara T, Sugahara K, Uemura A, Yamaguchi T, Harasawa H, Hasegawa H, Tsuruda K, Okazaki T, Koji T, Miyanishi T, Yamada Y, Kamihira S. 2006. A novel alternative splicing isoform of human T-cell leukemia virus type 1 bZIP factor (HBZ-SI) targets distinct subnuclear localization. J Virol 80:2495–2505. doi: 10.1128/JVI.80.5.2495-2505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. 2006. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A 103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usui T, Yanagihara K, Tsukasaki K, Murata K, Hasegawa H, Yamada Y, Kamihira S. 2008. Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells. Retrovirology 5:34. doi: 10.1186/1742-4690-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, Satou Y, Yasunaga J, Fujisawa J, Matsuoka M. 2008. Transcriptional control of spliced and unspliced human T-cell leukemia virus type 1 bZIP factor (HBZ) gene. J Virol 82:9359–9368. doi: 10.1128/JVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Green PL. 2007. Detection and quantitation of HTLV-1 and HTLV-2 mRNA species by real-time RT-PCR. J Virol Methods 142:159–168. doi: 10.1016/j.jviromet.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halin M, Douceron E, Clerc I, Journo C, Ko NL, Landry S, Murphy EL, Gessain A, Lemasson I, Mesnard JM, Barbeau B, Mahieux R. 2009. Human T-cell leukemia virus type 2 produces a spliced antisense transcript encoding a protein that lacks a classic bZIP domain but still inhibits Tax2-mediated transcription. Blood 114:2427–2438. doi: 10.1182/blood-2008-09-179879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larocque E, Halin M, Landry S, Marriott SJ, Switzer WM, Barbeau B. 2011. Human T-cell lymphotropic virus type 3 (HTLV-3)- and HTLV-4-derived antisense transcripts encode proteins with similar Tax-inhibiting functions but distinct subcellular localization. J Virol 85:12673–12685. doi: 10.1128/JVI.05296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RH. 1988. Human immunodeficiency virus may encode a novel protein on the genomic DNA plus strand. Science 239:1420–1422. doi: 10.1126/science.3347840. [DOI] [PubMed] [Google Scholar]
- 17.Michael NL, Vahey MT, d'Arcy L, Ehrenberg PK, Mosca JD, Rappaport J, Redfield RR. 1994. Negative-strand RNA transcripts are produced in human immunodeficiency virus type 1-infected cells and patients by a novel promoter downregulated by Tat. J Virol 68:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landry S, Halin M, Lefort S, Audet B, Vaquero C, Mesnard JM, Barbeau B. 2007. Detection, characterization and regulation of antisense transcripts in HIV-1. Retrovirology 4:71. doi: 10.1186/1742-4690-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clerc I, Laverdure S, Torresilla C, Landry S, Borel S, Vargas A, Arpin-Andre C, Gay B, Briant L, Gross A, Barbeau B, Mesnard JM. 2011. Polarized expression of the membrane ASP protein derived from HIV-1 antisense transcription in T cells. Retrovirology 8:74. doi: 10.1186/1742-4690-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polakowski N, Lemasson I. 2010. Regulation of HTLV-1 transcription by viral and cellular proteins, p 129–169. In Lever AML, Jeang KT (ed), Recent advances in human retroviruses: principles of replication and pathogenesis Advances in retroviral research. World Scientific Publishing Co., Singapore. [Google Scholar]
- 21.Lemasson I, Lewis MR, Polakowski N, Hivin P, Cavanagh MH, Thebault S, Barbeau B, Nyborg JK, Mesnard JM. 2007. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol 81:1543–1553. doi: 10.1128/JVI.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clerc I, Polakowski N, Andre-Arpin C, Cook P, Barbeau B, Mesnard JM, Lemasson I. 2008. An interaction between the human T cell leukemia virus type 1 basic leucine zipper factor (HBZ) and the KIX domain of p300/CBP contributes to the down-regulation of tax-dependent viral transcription by HBZ. J Biol Chem 283:23903–23913. doi: 10.1074/jbc.M803116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, Yang L, Zhang L, Liu B, Merling R, Xia Z, Giam CZ. 2008. Human T-cell leukemia virus type 1 infection leads to arrest in the G1 phase of the cell cycle. J Virol 82:8442–8455. doi: 10.1128/JVI.00091-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho YK, Zhi H, DeBiaso D, Philip S, Shih HM, Giam CZ. 2012. HTLV-1 tax-induced rapid senescence is driven by the transcriptional activity of NF-κB and depends on chronically activated IKKα and p65/RelA. J Virol 86:9474–9483. doi: 10.1128/JVI.00158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, Giam CZ. 2011. NF-κB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog 7:e1002025. doi: 10.1371/journal.ppat.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao T, Yasunaga J, Satou Y, Nakao M, Takahashi M, Fujii M, Matsuoka M. 2009. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-κB. Blood 113:2755–2764. doi: 10.1182/blood-2008-06-161729. [DOI] [PubMed] [Google Scholar]
- 27.Wurm T, Wright DG, Polakowski N, Mesnard JM, Lemasson I. 2012. The HTLV-1-encoded protein HBZ directly inhibits the acetyl transferase activity of p300/CBP. Nucleic Acids Res 40:5910–5925. doi: 10.1093/nar/gks244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuoka M, Yasunaga J. 2013. Human T-cell leukemia virus type 1: replication, proliferation and propagation by Tax and HTLV-1 bZIP factor. Curr Opin Virol 3:684–691. doi: 10.1016/j.coviro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Ishitsuka K, Tamura K. 2014. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol 15:e517–526. doi: 10.1016/S1470-2045(14)70202-5. [DOI] [PubMed] [Google Scholar]
- 30.Yasunaga J, Matsuoka M. 2011. Molecular mechanisms of HTLV-1 infection and pathogenesis. Int J Hematol 94:435–442. doi: 10.1007/s12185-011-0937-1. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka M, Jeang KT. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer 7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 32.Koiwa T, Hamano-Usami A, Ishida T, Okayama A, Yamaguchi K, Kamihira S, Watanabe T. 2002. 5′-Long terminal repeat-selective CpG methylation of latent human T-cell leukemia virus type 1 provirus in vitro and in vivo. J Virol 76:9389–9397. doi: 10.1128/JVI.76.18.9389-9397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda S, Maeda M, Morikawa S, Taniguchi Y, Yasunaga J, Nosaka K, Tanaka Y, Matsuoka M. 2004. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int J Cancer 109:559–567. doi: 10.1002/ijc.20007. [DOI] [PubMed] [Google Scholar]
- 34.Taniguchi Y, Nosaka K, Yasunaga J, Maeda M, Mueller N, Okayama A, Matsuoka M. 2005. Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology 2:64. doi: 10.1186/1742-4690-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki M, Yasunaga J, Taniguchi Y, Tamiya S, Nakahata T, Matsuoka M. 2007. Preferential selection of human T-cell leukemia virus type 1 provirus lacking the 5′ long terminal repeat during oncogenesis. J Virol 81:5714–5723. doi: 10.1128/JVI.02511-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibon D, Zane L, Idrissi ME, Delfau-Larue MH, Gessain A, Gout O, Mortreux F, Wattel E. 2013. Mosaicism of HTLV-1 5′ LTR CpG methylation in the absence of malignancy. Virus Res 178:452–461. doi: 10.1016/j.virusres.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Belrose G, Gross A, Olindo S, Lezin A, Dueymes M, Komla-Soukha I, Smadja D, Tanaka Y, Willems L, Mesnard JM, Peloponese JM Jr, Cesaire R. 2011. Effects of valproate on Tax and HBZ expression in HTLV-1 and HAM/TSP T lymphocytes. Blood 118:2483–2491. doi: 10.1182/blood-2010-11-321364. [DOI] [PubMed] [Google Scholar]
- 38.Li M, Kesic M, Yin H, Yu L, Green PL. 2009. Kinetic analysis of human T-cell leukemia virus type 1 gene expression in cell culture and infected animals. J Virol 83:3788–3797. doi: 10.1128/JVI.02315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rousset R, Desbois C, Bantignies F, Jalinot P. 1996. Effects on NF-κB1/p105 processing of the interaction between the HTLV-1 transactivator Tax and the proteasome. Nature 381:328–331. doi: 10.1038/381328a0. [DOI] [PubMed] [Google Scholar]
- 40.Bar-Shira A, Panet A, Honigman A. 1991. An RNA secondary structure juxtaposes two remote genetic signals for human T-cell leukemia virus type I RNA 3′-end processing. J Virol 65:5165–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landry S, Halin M, Vargas A, Lemasson I, Mesnard JM, Barbeau B. 2009. Upregulation of human T-cell leukemia virus type 1 antisense transcription by the viral tax protein. J Virol 83:2048–2054. doi: 10.1128/JVI.01264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith MR, Greene WC. 1990. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev 4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 43.Mocquet V, Neusiedler J, Rende F, Cluet D, Robin JP, Terme JM, Duc Dodon M, Wittmann J, Morris C, Le Hir H, Ciminale V, Jalinot P. 2012. The human T-lymphotropic virus type 1 tax protein inhibits nonsense-mediated mRNA decay by interacting with INT6/EIF3E and UPF1. J Virol 86:7530–7543. doi: 10.1128/JVI.07021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philip S, Zahoor MA, Zhi H, Ho YK, Giam CZ. 2014. Regulation of human T-lymphotropic virus type I latency and reactivation by HBZ and Rex. PLoS Pathog 10:e1004040. doi: 10.1371/journal.ppat.1004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choudhary G, Ratner L. 2011. The HTLV-1 hbz antisense gene indirectly promotes tax expression via down-regulation of p30(II) mRNA. Virology 410:307–315. doi: 10.1016/j.virol.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin H-C, Simon P, Rabson AB. 2014. A role for RNA stability in the induction of HTLV-1 expression in chronically infected CD4+ T cells. Retrovirology 11(Suppl 1):P114. doi: 10.1186/1742-4690-11-S1-P114. [DOI] [Google Scholar]
- 47.Nicot C, Dundr M, Johnson J, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. 2004. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat Med 10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- 48.Chlichlia K, Khazaie K. 2010. HTLV-1 Tax: linking transformation, DNA damage and apoptotic T-cell death. Chem Biol Interact 188:359–365. doi: 10.1016/j.cbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Nicot C, Harrod R. 2000. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 tax protein. Mol Cell Biol 20:8580–8589. doi: 10.1128/MCB.20.22.8580-8589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellon M, Baydoun HH, Yao Y, Nicot C. 2010. HTLV-I Tax-dependent and -independent events associated with immortalization of human primary T lymphocytes. Blood 115:2441–2448. doi: 10.1182/blood-2009-08-241117. [DOI] [PMC free article] [PubMed] [Google Scholar]