ABSTRACT
Previous experiments carried out in a sheep scrapie model demonstrated that the transfusion of 200 μl of prion-infected whole blood has an apparent 100% efficacy for disease transmission. These experiments also indicated that, despite the apparent low infectious titer, the intravenous administration of white blood cells (WBC) resulted in efficient disease transmission. In the study presented here, using the same transmissible spongiform encephalopathy (TSE) animal model, our aim was to determine the minimal number of white blood cells and the specific abilities of mononucleated cell populations to transmit scrapie by the transfusion route. Our results confirmed that the transfusion of 100 μl, but not 10 μl, of fresh whole blood collected in asymptomatic scrapie-infected donor sheep can transmit the disease. The data also show that the intravenous administration of 105 WBCs is sufficient to cause scrapie in recipient sheep. Cell-sorted CD45R+ (predominantly B lymphocytes), CD4+/CD8+ (T lymphocytes), and CD14+ (monocytes/macrophages) blood cell subpopulations all were shown to contain prion infectivity by bioassays in ovine PrP transgenic mice. However, while the intravenous administration of 106 CD45+ or CD4+/8+ living cells was able to transmit the disease, similar numbers of CD14+ cells failed to infect the recipients. These data support the contention that mononucleated blood cell populations display different abilities to transmit TSE by the transfusion route. They also represent an important input for the risk assessment of blood-borne prion disease transmission and for refining the target performance of leukoreduction processes that currently are applied to mitigate the transmission risk in transfusion medicine.
IMPORTANCE Interindividual variant Creutzfeldt-Jakob disease (vCJD) transmission through blood and blood-derived products is considered a major public health issue in transfusion medicine. Over the last decade, TSE in sheep has emerged as a relevant model for assessing the blood-borne vCJD transmission risk. In this study, using a sheep TSE model, we characterized the ability of different peripheral blood mononucleated cell populations to infect TSE-free recipients by the transfusion route. Our results indicate that as little as 105 WBC and 100 μl of blood collected from asymptomatic scrapie infected animals can transmit the disease. They also demonstrate unambiguously that peripheral blood mononuclear cell subpopulations display dramatically different abilities to transmit the disease. These data represent an important input for the risk assessment of blood-borne prion disease transmission and for refining the target performance of leukoreduction processes that currently are applied to mitigate the transmission risk in transfusion medicine.
INTRODUCTION
Transmissible spongiform encephalopathies (TSE), or prion diseases, are fatal neurodegenerative disorders occurring in sheep (scrapie), cattle (bovine spongiform encephalopathy [BSE]), and humans (Creutzfeldt-Jakob disease [CJD]). In 1996, a new form of TSE, named variant CJD (vCJD), was identified in humans. Variant CJD was demonstrated to be due to the same TSE agent as the one that causes cattle BSE, and its emergence in humans was considered to be the consequence of a dietary exposure to BSE-contaminated products (1, 2). To date, four vCJD cases have been reported in individuals within the United Kingdom who had received red blood cell concentrates (RBCs) (3–6). In addition, abnormal prion protein was detected in a postmortem spleen sample from a hemophilic patient who had received purified factor VIII prepared from plasma batches that included donations from individuals who later developed vCJD (3). Collectively, these particular cases of vCJD infection strongly support the view that this condition can be transmitted by blood transfusion.
A decade ago, transmission of experimental BSE and natural scrapie were reported to occur following transfusion of whole blood and/or buffy coat collected from asymptomatic experimentally infected sheep (7, 8). These observations opened new opportunities for investigating the potential role of blood in the pathogenesis of the TSE agent and provided an alternative model to rodents for studying the biology of prions in blood. In addition, the possibility to collect blood volumes comparable to those used in transfusion medicine and the similarities in the pathogenesis of sheep TSE and vCJD in humans have reinforced the utility of the sheep transfusion model (9).
Recently, infectivity measurements in blood fractions collected from a vCJD-infected patient and those from a scrapie-infected sheep indicated that in both situations prion levels and the partition of infectivity in blood compartments are similar (10, 11). This further reinforced the pertinence of data collected in TSE-infected sheep for assessing the vCJD blood-borne transmission risk.
The relative ability of whole blood, plasma, and white blood cells (WBCs) to transmit prion disease upon transfusion into recipients has been assessed (12). It was shown that the intravenous administration of 200 μl of whole blood harvested during the asymptomatic preclinical phase of experimental sheep scrapie was sufficient to infect recipient scrapie-free sheep. However, this experiment failed to determine what would be the minimal volume of blood that was able to transmit the disease. TSE transmission experiments in sheep by the transfusion route also showed that despite similar infectious titers, as determined by intracerebral inoculation in rodents, plasma and cellular blood products displayed substantially different capacities to transmit the disease by the transfusion route. The intravenous administration of 200 ml fresh crude plasma to sheep transmitted the disease with limited efficacy, but no transmission was observed in animals that received 20 ml of plasma. In contrast, the WBCs displayed an ability similar to that of whole blood in its efficacy at infecting recipient sheep (13, 14). These results demonstrated that the ability of blood and blood-derived products to transmit prion disease by the transfusion route does not depend solely on their infectious titer and pointed out the importance that WBC populations might play in the transmission of the disease by the transfusion route (12).
In the study presented here, using the scrapie transfusion model in sheep, our aim was to determine the minimal volume of blood and the minimal number of white blood cells that are able to transmit the disease. We further characterized infectivity levels in different peripheral blood mononucleated cell populations and their ability to infect TSE-free recipients by the transfusion route. Our results indicate that as little as 105 WBC and 100 μl of blood collected in asymptomatic scrapie-infected sheep can transmit the disease. They also demonstrate unambiguously that despite their comparable infectious titers, peripheral blood mononuclear cell (PBMC) subpopulations display dramatically different abilities to transmit the disease when administered by the intravenous route.
MATERIALS AND METHODS
Ethics statement.
All animal experiments have been performed in compliance with the INRA Toulouse/ENVT ethics committee (MP/05/05/01/12) and in accordance with the European Community Council Directive 86/609/EEC.
PG127 classical scrapie oral inoculation model.
The classical scrapie isolate used here was derived from experimentally infected VRQ/VRQ sheep (PG127 isolate). Six- to 10-month-old TSE-free cheviot sheep were orally challenged with 2-g equivalents of brain material (5% brain homogenate in glucose). This scrapie isolate previously has been endpoint titrated in mice expressing the VRQ variant of the ovine prion protein (tg338 mice) (15). Inoculated sheep were observed until the occurrence of clinical signs of scrapie (around 200 days postinoculation) and culled when they exhibited locomotor signs of disease that impaired their feeding capacities. After culling, each animal was necropsied and a variety of lymphoid tissues (spleen, third eye lid, mesenteric lymph node, prescapular lymph node, and tonsil) and central nervous system (CNS) were collected and stored at −20°C prior to analysis.
TSE-free recipient sheep.
TSE-free VRQ/VRQ cheviot sheep were produced in the DEFRA TSE-free flock, which is a unique source able to provide VRQ/VRQ animals that can be considered free from classical scrapie (16). The sheep included in our experiments were imported into France and, prior to their use in infectivity experiments, housed in a dedicated scrapie-free farm situated 30 km from the experimental facilities. To avoid the possibility of cross contamination, a dedicated staff (who had no contact with the infected animals) was used. Sheep PrP genotype was verified by sequencing exon 3 of the ovine PrP gene as previously described (17, 18).
Blood collection and WBC preparation.
Sheep whole blood was collected from the jugular vein using citrate dextrose (35 ml) into each 250-ml blood collection pouch (MSE3500Q; Macopharm). Six hundred milliliters of blood was collected from each donor. Whole-blood collection and handling were carried out by following the standards applied to human transfusion medicine.
For WBC preparation, 45 ml whole blood was transferred in 15-ml conical tubes before centrifugation at 3,600 rpm for 10 min at room temperature. Plasma was removed and buffy coat was collected using a disposable hard-bulb pipette. The buffy coat was mixed with an equal volume of ACK solution (0.15 MNH4CL, 1 mM KHCO3, 0.1 mM Na2EDTA, pH 7.4) for 5 min at room temperature to lyse red blood cells, and the isolated WBCs then were washed 3 times with PBS and their concentration established by counting using either a Mallasez's cell or an automatic cell counter. WBCs were aliquoted and, when not used for transfusion, stored at −80°C for freezing.
PBMC preparation and cell sorting.
Blood from the same whole-blood pouch collected for WBC preparation and the whole-blood dilution experiment was used to prepare PBMC. The whole blood was diluted in an equal amount of Hanks' balanced salt solution (HBSS), and 25 ml of diluted blood was decanted into 50-ml conical tubes before the addition of 12.5 ml of 1.077g/ml Ficoll solution (Ficoll-Paque plus; 17-440-03; GE Health Care Life Sciences). Tubes were centrifuged for 20 min at 1,000 × g and 20°C. PBMC at the density medium interface were collected by pipette, washed twice in HBSS, and then stored on ice.
Cells were labeled using fluorescein isothiocyanate (FITC)-coupled anti-ovine CD4 (MCA2213F; 1/100 diluted; Serotec), anti-CD8 (MCA837F; 1/100 diluted; Serotec), CD45R (MC2221F; 1/50 diluted; Serotec), and anti-CD14 (clone VPM65; 1 μg/ml). Labeled cells then were incubated using anti-FITC monoclonal antibody coupled to magnetic beads (130-048-701; Miltenyi Biotec) according to the manufacturer's recommendations. Positive selection was achieved using two successive passages on MS separation columns (130-048-701; Miltenyi Biotec) according to the manufacturer's recommendations. The purity of the sorted cell subpopulations and their viability (propidium iodide labeling) was assessed by flow cytometry using 2 × 104 events per cell fraction, established by counting using a Mallasez's cell. After counting, the cells required for transfusion were transferred into a 100-ml 5% glucose perfusion pouch, and the remaining cells were divided into aliquots of 106 cells, pelleted, and stored at −80°C.
Whole blood, WBC, and PBMC transfusion.
Whole blood, WBC, and PBMC were transfused/intravenously administered into individual recipient sheep. Recipient sheep were anesthetized by intravenous administration of ketamine/diazepam (2 mg and 0.1 mg per kg, respectively), and a catheter (16 gauge) was installed in the jugular vein. This catheter was used for transfusions or intravenous administrations. For experiments involving whole-blood transfusion, the required whole-blood volumes were sampled from the collection bag using a sterile syringe and either transferred into a new pouch or gently resuspended in a sterile 5% glucose solution (100-ml final volume) before administration (gravity) to recipients. In the WBC/PBMC experiment, WBC were gently resuspended in a 200-ml sterile pouch of isotonic (5%) glucose before infusion (gravity) over a 20- to 30-min period. All transfusions were performed within the 6 h following blood collection.
Bioassay.
Mouse bioassays were carried out in ovine VRQ PrP transgenic mice (tg338), which are considered to be highly efficient for the detection of sheep scrapie infectivity (19, 20). High sensitivity of tg338 mice for detection of PG127 scrapie was reported previously (15). At least six mice were intracerebrally inoculated with each sample (20 μl). Mice were clinically monitored until the occurrence of clinical signs of murine prion disease, at which time they were culled. CNS and spleen samples were collected individually and analyzed by Western blotting (WB).
Detection of PrPSc by WB.
A WB kit (TeSeE Western blot; Bio-Rad) was used by following the manufacturer's recommendations. For each sample, 250 μl of 10% brain homogenate was submitted to PrPSc extraction. The obtained pellet was denatured in Laemmli's buffer (15 μl) before being loaded, undiluted or diluted, on a 12% acrylamide gel and then was subjected to electrophoresis and blotting. Immunodetection was performed using Sha31, which recognizes the 145 to 152 sequence of PrP (YEDRYYRE) (21), conjugated to horseradish peroxidase (at 0.06 μg per ml). Peroxidase activity was revealed using ECL substrate (Pierce).
Infectious prion titer estimates.
The titer of prion infectivity in each sample tested was estimated using the method developed by Arnold et al. (22). The model uses both the probability of survival (attack rate at each dilution) and the individual mouse incubation periods at each dilution to estimate infectious load; thus, it is able to provide a more accurate estimation of titer than using either attack rate or incubation period data alone. The model assumes (i) a normal distribution for the relationship between dose and incubation period and (ii) that the probability of infection versus dose follows a logistic regression curve.
RESULTS
Four VRQ/VRQ sheep were orally inoculated with the PG127 scrapie isolate, and all developed experimentally induced clinical scrapie, with incubation periods ranging from 196 days postinfection (dpi) to 219 dpi. Six hundred milliliters of blood was collected from each of these animals at the late preclinical stage of the incubation period (180 dpi).
Infectivity in the collected whole-blood pouches was measured by the intracerebral inoculation of mice (n = 18) expressing the VRQ variant of the ovine PrP (tg338) (Table 1). In all four donor sheep, the infectious prion titer was lower than 10 infectious doses (ID) per ml. In one sheep (D4), the apparent infectious titer was below the sensitivity limit of the bioassay (8.81 ID/ml). These values were comparable to those previously measured in the blood of PG127 scrapie sheep using the same tg338 mice (12).
TABLE 1.
Infectivity in whole blood of VRQ/VRQ sheep orally challenged with PG127 scrapie agent as determined by intracerebral inoculation in tg338 micea
| Donor | Total inoculated volume (whole blood; μl) | Positive mice (no. positive/total no.) | ID per ml of whole blood (95% CI) |
|---|---|---|---|
| D1 | 340 | 0/17 | <8.81b |
| D2 | 340 | 2/17 | 6.3 (1–19.4) |
| D3 | 360 | 1/18 | 2.9 (0.2–12.6) |
| D4 | 360 | 1/18 | 2.9 (0.2–12.6) |
Blood was collected from the four VRQ/VRQ donor sheep that had been orally challenged with PG127 scrapie (D1, D2, D3, and D4) 180 days postinoculation. Donor sheep developed clinical scrapie 2 to 5 weeks following blood collection and were euthanized at 207, 219, 217, and 196 dpi, respectively. Whole blood from donor sheep was inoculated intracerebrally (i.c.) into 18 tg338 mice (20 μl per mouse). Two mice (inoculated with blood from donors D1 and D2) died within the first few days following i.c. inoculation. Mice were euthanized when they showed clinical signs of prion infection or after 250 dpi. Mice were considered prion infected when abnormal PrP deposition was detected in the brain. Infectious prion titers were estimated using the limiting dilution titration method (application of Poisson's model) as described by Brown et al. (28). Infectious titers are given as the most likely values.
When no transmission was observed, the detection limit of the bioassay is indicated (upper boundary of the 95% CI).
One hundred milliliters, 10 ml, 1 ml, 0.1 ml, and 0.01 ml of the fresh whole blood from each donor was transfused shortly after collection into VRQ/VRQ scrapie-free recipient sheep (Table 2). Whatever the donor, sheep transfused with ≥1 ml of blood developed clinical scrapie with incubation periods varying between 190 and 274 days. In contrast, in sheep that were transfused with 100 μl of whole blood, only one individual (blood from donor D2) developed clinical scrapie. According to infectious titer measured by IC inoculation in tg338 mice (Table 1), 100 μl of blood from donor D2 contained about 0.63 ID (95% confidence interval [CI], 0.1 ID to 1.94 ID). None of the sheep that received 10 μl of whole blood developed clinical scrapie or displayed a detectable amount of abnormal PrP in their peripheral tissues or central nervous system. These results are consistent with the view that the minimal volume of whole blood that is able to transmit the disease was between 10 μl and 100 μl.
TABLE 2.
Incubation period of VRQ/VRQ TSE-free recipient sheep inoculated with PG127 classical scrapie isolatea
| Blood population and volume or cell no. | Incubation periodb (dpi) and presence of PrPSc |
|||||||
|---|---|---|---|---|---|---|---|---|
| D1 |
D2 |
D3 |
D4 |
|||||
| Incub | PrPSc | Incub | PrPSc | Incub | PrPSc | Incub | PrPSc | |
| Whole blood | ||||||||
| 10 ml | 183 | + | 177 | + | 207 | + | 184 | + |
| 1 ml | 213 | + | 190 | + | 274 | + | 215 | + |
| 0.1 ml | >450 | − | 232 | + | >450 | − | >450 | − |
| 0.01 ml | >450 | − | >450 | − | >450 | − | >450 | − |
| WBC | ||||||||
| 106 cells | >450 | + | 230 | + | 218 | + | 220 | + |
| 105 cells | >450 | − | 254 | + | >450 | − | >450 | − |
| 104 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| 103 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| CD4+/CD8+ | ||||||||
| 106 cells | >450 | − | 195 | + | >450 | + | >450 | − |
| 105 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| 104 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| CD45R+ | ||||||||
| 106 cells | 305 | + | 224 | + | >450 | + | 240 | + |
| 105 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| 104 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| CD14+ | ||||||||
| 106 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| 105 cells | >450 | − | >450 | − | >450 | − | >450 | − |
| 104 cells | >450 | − | >450 | − | >450 | − | >450 | − |
Shown are the incubation periods of VRQ/VRQ TSE-free recipient sheep that received, by the intravenous route, whole blood or one of the WBC or cell-sorted PBMC subpopulations collected from four VRQ/VRQ sheep orally inoculated with PG127 classical scrapie isolate.
The four susceptible VRQ/VRQ sheep between 6 and 10 months of age (identified as D1, D2, D3, and D4) were orally challenged with 2 g of brain homogenate (106.6 50% infectious doses [ID50]/g intracranially in tg338 mice). The sheep were euthanized at 207, 219, 217, and 196 dpi, respectively. At 180 dpi, whole blood was collected from each donor, and a 200-ml volume was transfused into scrapie-free VRQ/VRQ recipients within the 6 h following collection. Recipient sheep were observed until the occurrence of clinical signs of scrapie. The incubation periods (Incub) of recipients are presented (in days). Recipient sheep that apparently were still healthy 450 days posttransfusion were killed. The occurrence of scrapie was confirmed by histopathology (vacuolar change in central nervous system) and the detection of abnormal PrP deposits (PrPSc) in the central nervous system and lymphoid tissues.
In each donor, the blood enumeration and leukocyte formula were established using an aliquot of the initial blood donations (Table 3). According to enumeration, 1 ml of whole blood (i.e., the volume that was able to transmit disease to all recipients) in the four donors contained between 4.5 × 106 and 7.3 × 106 WBC. In addition, 100 μl of whole blood from donor 2 (the lowest volume of whole blood that transmitted the disease) contained 5.9 × 105 WBC cells.
TABLE 3.
Cell counts and blood formula in the sheep orally inoculated with PG127 classical scrapie isolate
| Cell type (vol) | Cell counta |
|||
|---|---|---|---|---|
| D1 | D2 | D3 | D4 | |
| Red blood cells (109/ml) | 11.97 | 10.5 | 10.55 | 12.11 |
| Platelets (106/ml) | 357 | 359 | 281 | 496 |
| White blood cells (106/ml) | 5.56 | 5.93 | 7.34 | 4.45 |
| Neutrophils (106/ml) | 1.73 | 1.86 | 1.87 | 1.29 |
| Lymphocytes (106/ml) | 3.38 | 3.32 | 4.45 | 2.84 |
| Monocytes (106/ml) | 0.23 | 0.21 | 0.25 | 0.10 |
| Eosinophils (106/ml) | 0.19 | 0.52 | 0.72 | 0.21 |
| Basophils (106/ml) | 0.03 | 0.02 | 0.05 | 0.01 |
Four susceptible VRQ/VRQ sheep between 6 and 10 months of age (identified as D1, D2, D3, and D4) were orally challenged with 2 g of brain homogenate (106.6 ID50/g intracranially in tg338 mice). The sheep were euthanized at 207, 219, 217, and 196 dpi, respectively. Whole blood (600 ml) was collected from each donor at 180 days postinoculation.
In order to determine the minimal number of WBC required to transmit scrapie disease by the transfusion route, cells were prepared using a fraction of the whole-blood pouches collected from each donor animal. WBC were washed extensively (three times) to eliminate platelets and were resuspended in 5% glucose solution before counting. One hundred milliliters of 5% glucose solution containing 103 to 106 WBC from each donor was prepared and administered (within the 4 h following the initial blood collection) by the intravenous route to VRQ/VRQ scrapie-free recipients (Table 2). Clinical scrapie or abnormal PrP accumulation was observed in sheep that received 106 WBC prepared from the four donor animals and in one of the recipients that received 105 cells. No clinical signs or abnormal PrP accumulation was observed in animals that received 103 or 104 WBC.
To assess potential differences in the abilities of white blood cell populations to transmit prion disease by the transfusion route, different PBMC subpopulations were sorted using magnetic bead technology. PBMC were prepared using the same blood pouches as those used for establishing the minimal volume of blood that was able to transmit scrapie disease. Cells were labeled using antibodies raised against different leukocyte phenotypic markers (CD14, CD4/8, and CD45R). A double-positive selection was used to ensure that the sorted cell fractions had purity greater than 92% as assessed by flow cytometry (Table 4). A range of 104 to 106 cells from each purified fraction were intravenously administered to TSE-free recipient sheep within the 6 h following blood collection (Table 2).
TABLE 4.
Intracerebral inoculation of tg338 mice with WBC and cell-sorted mononuclear cell subpopulations prepared from VRQ/VRQ sheep orally inoculated with PG127 scrapiea
| Cell type | Infection characteristicsb |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 |
D2 |
D3 |
D4 |
|||||||||||||
| Fraction purity (%) | tg338 transmission |
Infectious titerc | Fraction purity (%) | tg338 transmission |
Infectious titerc | Fraction purity (%) | tg338 transmission |
Infectious titerc | Fraction purity (%) | tg338 transmission |
Infectious titerc | |||||
| No. positive/total no. | Incubation period (dpi) | No. positive/total no. | Incubation period (dpi) | No. positive/total no. | Incubation period (dpi) | No. positive/total no. | Incubation period (dpi) | |||||||||
| PBMC | 6/6 | 110 ± 12 | 102.28 | 6/6 | 92 ± 5 | 103.8 | 6/6 | 90 ± 2 | 103.8 | 6/6 | 103 ± 7 | 102.79 | ||||
| CD14+ | 98.2 | 6/6 | 110 ± 9 | 102.27 | 96.4 | 6/6 | 96 ± 10 | 103.47 | 95.1 | 6/6 | 107 ± 9 | 103.47 | 95.2 | 4/6 | 118 ± 9 | 101.67 |
| CD4+/8+ | 97.1 | 6/6 | 90 ± 5 | 104 | 96.1 | 6/6 | 90 ± 2 | 103.95 | 95.3 | 6/6 | 106 ± 5 | 103.95 | 95.5 | 5/6 | 111 ± 17 | 102.22 |
| CD45R+ | 95.3 | 5/6 | 121 ± 7 | 101.56 | 98.2 | 6/6 | 116 ± 12 | 101.91 | 99.2 | 3/6 | 100, 104, 106* | 101.91 | 97.3 | 2/6 | 110, 117 | 101.62 |
Four susceptible VRQ/VRQ sheep aged between 6 and 10 months (identified as D1, D2, D3, and D4) were orally challenged with 2 g of brain homogenate (106.6 ID50/g intracranially in tg338 mice). These animals were sampled at 180 dpi. The donor sheep were euthanized at 207, 219, 217, and 196 dpi, respectively.
Classical scrapie occurrence was confirmed by histopathology (vacuolar change in central nervous system) and detection of abnormal PrP deposits in central nervous system and lymphoid tissues. A fraction of the collected whole blood (150 ml) was used to prepare PBMC. CD14+ (FITC-labeled VPM65), CD4+/8+ (FITC-labeled SBU/T4 and SBU/T8), and CD45R+ (FITC-labeled 20.29) subpopulations were sorted using anti-FITC-coated magnetic beads and two passages on magnetically activated cell sorting minicolumns. The purity of sorted cells was assessed by flow cytometry. After their preparation, cells were counted and aliquots of 5 × 106 cells were homogenized in 200 μl of a 5% glucose solution. Each homogenate was inoculated intracerebrally into tg338 mice (n = 6; 20 μl per mouse, i.e., 5 × 105 cells per mouse). Mice then were observed until the occurrence of clinical signs or were killed after 200 dpi. Mice were considered positive when abnormal PrP deposition was detected in brain of prion disease mice. Incubation periods are presented as means ± standard deviations, except for the dilution with which less than 50% of mice were found to be prion disease positive. In that case (indicated by an asterisk), incubation times of the positive mice are individually presented.
Individual incubation periods were used to estimate the infectious titers (ID50 per ml of cell homogenate) in each cell population according to the method developed by Arnold et al. (22).
In parallel, aliquots of the different cell populations prepared from each donor sheep were homogenized and intracerebrally inoculated into tg338 mouse groups (n = 6 or n = 7 per sample) (Table 4). These bioassays indicated the presence of infectivity in all three tested PBMC subpopulations.
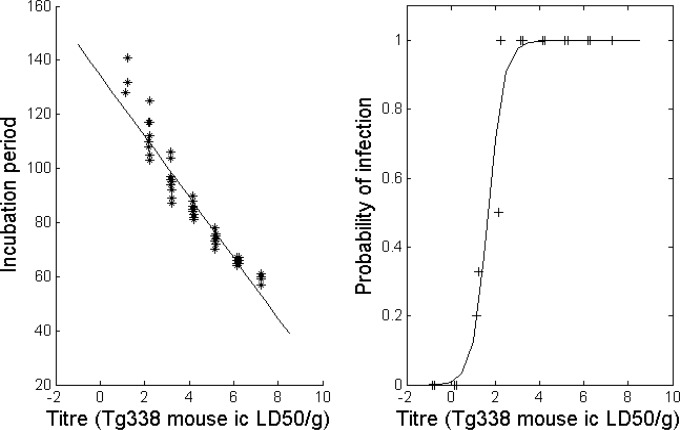
In order to estimate prion infectivity levels in each different PMBC cell population, we used the method developed by Arnold et al. (22). The approach combines the probability of survival (attack rate) and the individual mouse incubation period to provide an estimation of the infectious titers. Data corresponding to endpoint titration in tg338 mice of posterior brain stems collected from two VRQ/VRQ sheep inoculated with PG127S scrapie (clinical stage of the disease) (Table 5) were used to derive the relationship between the prion titer of inoculum and the probability of infection and length of incubation (Fig. 1). According to this model and to the data recorded in tg388 mice inoculated with the different PBMC populations, CD45R+ cell homogenates displayed lower infectious titers than CD14+ and CD4+/8+ homogenates (Table 4). Surprisingly, while the intravenous administration of 106 CD45R+ cells prepared from each of the four donors resulted in scrapie transmission in all of the recipient sheep, the same number of CD14+ cells failed to infect any of the recipients (Table 2). Only some of the recipients that received 106 CD4+/8+ cells were positive. None of the sheep that received 105 cells (CD45+, CD4+/8+, or CD14+) developed clinical scrapie or accumulated PrPSc in their tissues.
TABLE 5.
Endpoint titration in tg338 mice of two 10% brain homogenates collected from VRQ/VRQ sheep orally inoculated with PG127 scrapiea
| Dilution | Brain homogenate 1 |
Brain homogenate 2 |
||
|---|---|---|---|---|
| No. positive mice/total no. | Incubation period (dpi; means ± SD) | No. positive mice/total no. | Incubation period (dpi; means ± SD) | |
| Undiluted | 6/6 | 65 ± 1 | 6/6 | 59 ± 1 |
| 10−1 | 6/6 | 74 ± 4 | 6/6 | 66 ± 1 |
| 10−2 | 6/6 | 86 ± 2 | 6/6 | 74 ± 2 |
| 10−3 | 6/6 | 99 ± 5 | 6/6 | 84 ± 2 |
| 10−4 | 3/6 | 112 ± 5 | 6/6 | 89 ± 3 |
| 10−5 | 1/6 | 128* | 6/6 | 104 ± 9 |
| 10−6 | 0/6 | >200 | 2/6 | 132, 141* |
| 10−7 | 0/6 | >200 | 0/6 | >200 |
| 10−8 | 0/6 | >200 | 0/6 | >200 |
A homogenate (10%, wt/vol) was prepared using posterior brainstem from two VRQ/VRQ sheep inoculated with the PG127 scrapie isolate at the terminal stage of disease. Groups of 6 tg338 mice were intracerebrally inoculated with 20 μl of successive 1/10 dilutions of this homogenate. Mice were considered positive when abnormal PrP deposition was detected in brain of prion disease mice. Incubation periods are presented as means ± standard deviations; asterisks indicate dilutions at which less than 50% of mice were found to be prion disease positive.
FIG 1.
Dose-response relationship for the incubation period and probability of infection of tg338 mice inoculated (20 μl, intracerebral route) with 10% (wt/vol) homogenate, prepared using posterior brainstem from two VRQ/VRQ sheep inoculated with the PG127 scrapie isolate and at the terminal stage of disease. The model values are represented by the solid line, and observed are values given as crosses. ic, intracranial; LD50, 50% lethal dose.
DISCUSSION
In this study, scrapie transmission was observed in all recipient sheep that were transfused with 1 ml of blood from scrapie-affected donor animals. The transfusion of 100 μl of whole blood collected from a scrapie-infected but asymptomatic donor sheep can be sufficient to transmit the disease. No transmission was observed in recipients that received 10 μl whole-blood transfusions. The infectious titers that we measured in the whole blood used for the transfusion experiment were lower than 10 ID/ml. These infectious titer values are similar to those established in a previous study in similar animals (VRQ/VRQ sheep) infected with the same scrapie isolate (12, 13). These data complement and expand the previously reported results and confirm that the risk of transmitting prion disease by blood transfusion is higher than expected from the measurement of blood infectious titers by the intracerebral inoculation of mice (12).
Approximately 15 years ago, a significant proportion of European countries decided to implement leukocyte reduction treatment within their human blood supplies in order to mitigate the risk of human prion disease transmission (10). According to the standards of transfusion medicine applied in most countries, red blood cell units that are aimed at transfusion contain less than 106 WBC (6). Current epidemiological studies indicate that leukocyte reduction has had a positive impact on reducing the risk of vCJD transmission by transfusion (10).
Various experimental studies carried out in animal models of prion disease have confirmed that leukocyte reduction, as applied in human transfusion medicine, provides significant but incomplete protection against the transmission of prion disease by blood transfusion (13, 23).
Our study demonstrates that 105 WBC harvested from a sheep during the preclinical phase of scrapie incubation is sufficient to transmit the disease. This value is lower than the current leukoreduction standard target for red blood cell concentrate (106 WBC per unit). This is a likely explanation for the imperfect efficacy of leukoreduction to prevent prion disease transmission by RBC transfusion in sheep TSE models (13, 23). Under the hypothesis that the sheep scrapie model is relevant for the evaluation of the vCJD transmission risk by the transfusion route in humans, these results support the contention that RBC leukodepletion is insufficient in the prevention of vCJD blood-borne transmission.
The presence of prion infectivity in various peripheral blood mononucleated cell populations, such as lymphocytes and monocytes/macrophages, has been reported in both CWD-infected cervids and scrapie-infected sheep (14, 24). In addition to the detection of prions in PBMC subpopulations, our study assessed the relative capacity of different cell populations to transmit scrapie disease when administered by the intravenous route. CD14+ cells (monocyte/macrophage lineage) displayed infectious titers that were similar to those observed in CD4+/8+ (T lymphocyte) subpopulations and superior to those of CD45R+ (B lymphocyte and activated T lymphocyte) subpopulations (Table 4). However, the intravenous administration of 106 CD14+ cells failed to transmit scrapie, while the administration of the same number of CD45R+ cells caused the onset of the disease. These findings demonstrate that, independent of their infectious prion load, PBMC populations display different abilities to transmit scrapie disease.
While all of the sheep that received 106 WBC were found to be positive for scrapie disease, only a fraction of the sheep that received 106 CD45+ or CD4+/8+ cells (and none of those who received 105 of these cells) were positive for the disease. Considering the fact that lymphocytes represent about 5% of the total WBC (Table 3), this result supports the notion that CD45+ and CD4+/8+ cells are not responsible for the entire capacity of WBC to transmit blood-borne prion disease and implies that other cell populations participate in this process. In this context, the potential role of polynucleated cells appears to be an interesting topic for future investigations.
At this stage, the reasons for the higher capacity displayed by lymphocytes above that of monocytes to transmit scrapie disease by the transfusion route in the model we have examined remain unknown. The ability of allogeneic lymphocytes to relocate in a recipient's lymph nodes following transfusion has been reported in humans (25). Considering the central role of the lymphoreticular system in the early pathogenesis of peripherally acquired TSE, the redistribution of transfused lymphocytes within lymph nodes and the lymphoreticular system as a whole following transfusion of blood cells could partly explain their ability to transmit the disease (26, 27).
In conclusion, our results support the view that in addition to their infectious load, the specific properties of white blood cell populations play a crucial role in their efficacy to transmit prion disease by the transfusion route. Moreover, although in Europe no vCJD case has been observed in people who received blood products prepared after the implementation of leukodepletion (10), our data indicate that the standard of leukodepletion applied during red blood cell concentrate preparation is insufficiently stringent to prevent disease transmission by the residual white blood cells.
REFERENCES
- 1.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. 1997. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 2.Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. 1996. Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature 383:685–690. [DOI] [PubMed] [Google Scholar]
- 3.Peden A, McCardle L, Head MW, Love S, Ward HJ, Cousens SN, Keeling DM, Millar CM, Hill FG, Ironside JW. 2010. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia 16:296–304. doi: 10.1111/j.1365-2516.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 4.Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG. 2004. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 5.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. 2004. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 6.Lefrere JJ, Hewitt P. 2009. From mad cows to sensible blood transfusion: the risk of prion transmission by labile blood components in the United Kingdom and in France. Transfusion 49:797–812. [DOI] [PubMed] [Google Scholar]
- 7.Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. 2000. Transmission of BSE by blood transfusion in sheep. Lancet 356:999–1000. doi: 10.1016/S0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- 8.Hunter N, Houston F. 2002. Can prion diseases be transmitted between individuals via blood transfusion: evidence from sheep experiments. Dev Biol 108:93–98. [PubMed] [Google Scholar]
- 9.Ironside JW, Head MW. 2004. Variant Creutzfeldt-Jakob disease: risk of transmission by blood and blood products. Haemophilia 10(Suppl 4):S64–S69. [DOI] [PubMed] [Google Scholar]
- 10.Douet JY, Bujdoso R, Andreoletti O. 2015. Leukoreduction and blood-borne vCJD transmission risk. Curr Opin Hematol 22:36–40. doi: 10.1097/MOH.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 11.Douet JY, Zafar S, Perret-Liaudet A, Lacroux C, Lugan S, Aron N, Cassard H, Ponto C, Corbiere F, Torres JM, Zerr I, Andreoletti O. 2014. Detection of infectivity in blood of persons with variant and sporadic Creutzfeldt-Jakob disease. Emerg Infect Dis 20:114–117. doi: 10.3201/eid2001.130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreoletti O, Litaise C, Simmons H, Corbiere F, Lugan S, Costes P, Schelcher F, Vilette D, Grassi J, Lacroux C. 2012. Highly efficient prion transmission by blood transfusion. PLoS Pathog 8:e1002782. doi: 10.1371/journal.ppat.1002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacroux C, Bougard D, Litaise C, Simmons H, Corbiere F, Dernis D, Tardivel R, Morel N, Simon S, Lugan S, Costes P, Weisbecker JL, Schelcher F, Grassi J, Coste J, Andreoletti O. 2012. Impact of leucocyte depletion and prion reduction filters on TSE blood borne transmission. PLoS One 7:e42019. doi: 10.1371/journal.pone.0042019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacroux C, Vilette D, Fernandez-Borges N, Litaise C, Lugan S, Morel N, Corbiere F, Simon S, Simmons H, Costes P, Weisbecker JL, Lantier I, Lantier F, Schelcher F, Grassi J, Castilla J, Andreoletti O. 2012. Prionemia and leukocyte-platelet-associated infectivity in sheep transmissible spongiform encephalopathy models. J Virol 86:2056–2066. doi: 10.1128/JVI.06532-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreoletti O, Orge L, Benestad SL, Beringue V, Litaise C, Simon S, Le Dur A, Laude H, Simmons H, Lugan S, Corbiere F, Costes P, Morel N, Schelcher F, Lacroux C. 2011. Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathog 7:e1001285. doi: 10.1371/journal.ppat.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons HA, Simmons MM, Spencer YI, Chaplin MJ, Povey G, Davis A, Ortiz-Pelaez A, Hunter N, Matthews D, Wrathall AE. 2009. Atypical scrapie in sheep from a UK research flock which is free from classical scrapie. BMC Vet Res 5:8. doi: 10.1186/1746-6148-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arsac JN, Andreoletti O, Bilheude JM, Lacroux C, Benestad SL, Baron T. 2007. Similar biochemical signatures and prion protein genotypes in atypical scrapie and Nor98 cases, France and Norway. Emerg Infect Dis 13:58–65. doi: 10.3201/eid1301.060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno CR, Moazami-Goudarzi K, Laurent P, Cazeau G, Andreoletti O, Chadi S, Elsen JM, Calavas D. 2007. Which PrP haplotypes in a French sheep population are the most susceptible to atypical scrapie? Arch Virol 152:1229–1232. doi: 10.1007/s00705-007-0956-7. [DOI] [PubMed] [Google Scholar]
- 19.Le Dur A, Beringue V, Andreoletti O, Reine F, Lai TL, Baron T, Bratberg B, Vilotte JL, Sarradin P, Benestad SL, Laude H. 2005. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc Natl Acad Sci U S A 102:16031–16036. doi: 10.1073/pnas.0502296102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douet JY, Lacroux C, Corbiere F, Litaise C, Simmons H, Lugan S, Costes P, Cassard H, Weisbecker JL, Schelcher F, Andreoletti O. 2014. PrP expression level and sensitivity to prion infection. J Virol 88:5870–5872. doi: 10.1128/JVI.00369-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feraudet C, Morel N, Simon S, Volland H, Frobert Y, Creminon C, Vilette D, Lehmann S, Grassi J. 2005. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem 280:11247–11258. [DOI] [PubMed] [Google Scholar]
- 22.Arnold ME, Hawkins SA, Green R, Dexter I, Wells GA. 2009. Pathogenesis of experimental bovine spongiform encephalopathy (BSE): estimation of tissue infectivity according to incubation period. Vet Res 40:8. doi: 10.1051/vetres:2008046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCutcheon S, Alejo Blanco AR, Houston EF, de Wolf C, Tan BC, Smith A, Groschup MH, Hunter N, Hornsey VS, Macgregor IR, Prowse CV, Turner M, Manson JC. 2011. All clinically-relevant blood components transmit prion disease following a single blood transfusion: a sheep model of vCJD. PLoS One 6:e23169. doi: 10.1371/journal.pone.0023169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathiason CK, Hayes-Klug J, Hays SA, Powers J, Osborn DA, Dahmes SJ, Miller KV, Warren RJ, Mason GL, Telling GC, Young AJ, Hoover EA. 2010. B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease. J Virol 84:5097–5107. doi: 10.1128/JVI.02169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read EJ, Keenan AM, Carter CS, Yolles PS, Davey RJ. 1990. In vivo traffic of indium-111-oxine labeled human lymphocytes collected by automated apheresis. J Nuclear Med 31:999–1006. [PubMed] [Google Scholar]
- 26.Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen JM, Lantier F. 2000. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol 81(Part 12):3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 27.Aguzzi A, Nuvolone M, Zhu C. 2013. The immunobiology of prion diseases. Nat Rev Immunol 13:888–902. doi: 10.1038/nri3553. [DOI] [PubMed] [Google Scholar]
- 28.Brown P, Cervenakova L, McShane LM, Barber P, Rubenstein R, Drohan WN. 1999. Further studies of blood infectivity in an experimental model of transmissible spongiform encephalopathy, with an explanation of why blood components do not transmit Creutzfeldt-Jakob disease in humans. Transfusion 39:1169–1178. doi: 10.1046/j.1537-2995.1999.39111169.x. [DOI] [PubMed] [Google Scholar]