1. Introduction
Over one million Americans currently have a lower-limb amputation, and this number is projected to double by 2050 [1] due to dysvascular pathologies (e.g. diabetes mellitus (DM)) [2]. Patients with dysvascular amputation commonly have multiple comorbidities and 40-50% have limited physical function [3], which require different treatments apart from patients with traumatic amputation. Although patients with dysvascular amputation differ in age, BMI, prosthetic use time, and comorbidities from patients with traumatic amputation [3,4], it is common to combine them into a single group when investigating how amputation affects functional movement characteristics [5,6]. Because patients with DM prior to amputation move differently than healthy controls [7], differences in movement compensations between patients with dysvascular amputation to patients with DM alone could be used as physical rehabilitation targets for movement retraining following amputation.
Patients with unilateral transtibial amputation (TTA) are at increased risk of developing low back pain (LBP) [8], which may relate to necessary movement compensations to achieve forward progression and balance during walking. For example, to accomplish forward progression in the absence of an ankle plantar flexor, patients with unilateral TTA increase hip extensor power during the stance period of the residual limb [9]. Patients with unilateral TTA demonstrate exaggerated lateral trunk lean toward the amputated limb (compensated Trendelenburg) [10] and altered foot placement of the intact limb, which leads to uneven step length, swing time, and stance time [9]. While these compensations may be necessary to accomplish mobility, asymmetric movements are linked to the development of LBP [11]. This coordination of excessive trunk and pelvic motion during walking likely contributes to step-to-step asymmetric loading at the low back previously measured in patients with unilateral TTA [12], and may increase the risk of developing LBP, which was previously demonstrated in patients with transfemoral amputation [13,14].
Clinicians rely on observational gait analysis to identify movement compensations which is highly subjective and unreliable for identifying consequential movement compensations in amputees [15]. Although laboratory-based gait analysis is valid and reliable for quantitatively measuring movement, it is accompanied by high computational and economic expenses, and currently impractical in the vast majority of clinical settings. Because clinicians use observational gait analysis to guide interventions and gait retraining in patients with unilateral TTA, the ability to obtain accurate measures of trunk and pelvis movement patterns could help tailor treatment to patients and ultimately prevent injuries, such as LBP.
Identification of segmental strategies used to generate and arrest segmental angular momentum can provide insight into muscle demands following unilateral dysvascular TTA. During walking, muscles are used concentrically and eccentrically as the primary mechanisms to generate and arrest segment angular momentum [16]. Measuring and understanding segmental angular momentum is a promising approach to bridge the gap between observational and quantitative gait analysis. We previously demonstrate a framework to describe clinical movement compensations during gait using separation of translational angular momentum referenced to the stance foot [17]. Total segmental angular momentum can be separated into two components, each with a unique interpretation: 1) Translational Angular Momentum (TAM): angular momentum created by linear velocity of the segment with mass with respect to a point and 2) Rotational Angular Momentum (RAM): angular momentum created by the rotational velocity of an object with inertia [18].
The objective of this investigation was to assess movement compensations in patients with unilateral dysvascular TTA and patients with DM by examining translational angular momentum and rotational angular momentum of the trunk and pelvis during walking for patterns of generating/arresting momentum. We hypothesized that patients with unilateral dysvascular TTA, patients with DM, and healthy control participants would demonstrate similar patterns of generating/arresting TAM of the trunk and pelvis when walking at similar speeds. We also hypothesized that patients with unilateral dysvascular TTA would demonstrate higher RAM of the trunk and pelvis than the other groups, which illustrates potentially consequential movement compensations that can be retrained through clinical intervention.
2. Methods
2.1 Participants
Ten patients with DM and unilateral TTA 1-3 years post amputation (AMP) (Table 1) (10 M; age: 56.8 ± 4.3 years; mass: 97.6 ± 15.2 kg; height: 1.8 ± 0.1 m), 11 patients with DM (2F, 9 M; age: 61.4 ± 8.0 years; mass: 94.3 ± 22.0 kg; height: 1.7 ± 0.1 m), and 13 healthy control patients (HC) (3 F, 10 M; age: 63.1 ± 7.7 years; mass: 77.7 ± 13.2 kg; height: 1.7 ± 0.1 m) were enrolled. Eligibility criteria included: age: 50-85 years; BMI ≤ 40 kg/m2; independent community ambulation (ability to walk for four minutes without rest or assistive device); 1-3 years post amputation (AMP group); controlled Type-II diabetes mellitus (AMP and DM groups); no traumatic or cancer-related amputation (AMP group); no major amputation on contralateral limb (AMP group); no cardiovascular, orthopaedic, neurologic, wounds, or ulcers that limit physical function; no history of LBP (HC group); no diagnosed rheumatoid arthritis (HC group); no diagnosed osteoarthritis (HC group); and no total hip/knee joint arthroplasty (HC group). Each participant provided a written, informed consent in accordance with the Colorado Multiple Institutional Review Board prior to the start of the experimental session and completed one data collection in which whole body kinematics were collected.
Table 1.
Participant characteristics for patients with dysvascular unilateral transtibial amputation (AMP) group.
| Time since Amputation (Months) | Residual Limb Length (cm) | Socket Type | Prosthetic Foot |
|---|---|---|---|
| 17.4 ± 5.1 | 14.8 ± 2.5 | Total contact carbon fiber | Dynamic elastic response |
2.2 Motion Analysis
Each participant was instrumented with 63 reflective markers used to obtain whole-body kinematics during gait. Motion was recorded from eight infrared cameras (Vicon) sampled at 100 Hz. Each participant performed three gait trials at 1.0 m/s (± 0.05 m/s) on a 10-m walkway. Motions were averaged across the three trials and used for group comparisons.
2.3 Data Analysis
Kinematic data were low-pass filtered with a 4th-order Butterworth filter (6 Hz cutoff frequency). A 15-segment subject-specific model (head, upper arms, forearms, hands, trunk, pelvis, thighs, shanks, and feet) was created in Visual 3D (C-Motion, Inc.). Segment masses were based on a percentage of total body weight and segment inertias were based on segment geometry [19]. For the AMP group, mass the center of mass position, and inertial properties of the prosthetic shank (residual limb + prosthetic socket) and prosthetic foot were determined using a reaction board technique and oscillation method [20].
TAM (angular momentum of a segment with respect to the stance foot) is described as:
| (1) |
where ri and rFoot are the position vectors of the ith segment and foot, respectively, mi is the mass of the ith segment, and vi and vFoot are the velocities of the ith segment and foot respectively. RAM (angular moment of a segment with respect to its center of mass) is described as:
| (2) |
where Ii is the moment of inertia tensor and ωi is the angular velocity of the segment. To facilitate planar analyses, all angular momenta vectors were expressed in a path reference frame, that is defined by the velocity vector of the body COM: efrontal (tangent to the horizontal path of the body COM), etransverse (opposite direction of the gravity vector), and esagittal (efrontal × etransverse). Within the path reference frame, positive momenta values in each plane are defined as: sagittal – posterior rotation away from stance foot, frontal – medial-lateral rotation toward stance foot, transverse – rotation away from stance foot.
2.4 Statistical Analysis
Patient anthropometrics (mass and height) were compared across groups using a one-way ANOVA followed by Tukey HSD for post hoc comparison (α = 0.05).
All momenta were calculated during one gait cycle (AMP: amputated limb heel strike to amputated limb heel strike; DM and HC: right heel strike to right heel strike). TAM (hi/Foot), was calculated with respect to the stance foot was analyzed during the stance period. RAM (hi) was analyzed during the entire gait cycle. To quantify generation and arresting of trunk and pelvis angular momentum, global minimums and maximums were determined.
Magnitudes of the global minima and maxima in each segmental angular momentum variable (TAM and RAM) were compared across the three groups using an ANCOVA (covariates: mass and height) followed by pairwise comparisons using Tukey HSD (α = 0.05). Qualitative analysis was performed to assess when peak momenta values occurred throughout the functional phases of gait: weight acceptance (0-12%), single limb support (12-50%), swing limb advancement (50-100%) [21].
3. Results
3.1 Patient Anthropometrics
Body mass was larger in the AMP group than the HC group (P = 0.03). No differences in height existed across groups.
3.2 Translational Angular Momentum
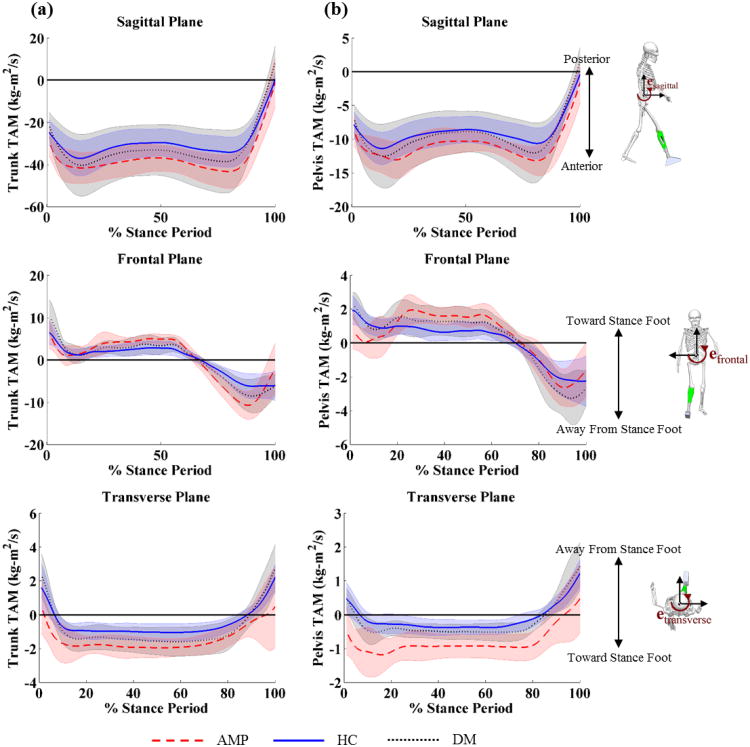
In the sagittal plane, peak posterior trunk and pelvis TAM was lower in the AMP group than the DM group (trunk: P = 0.01, pelvis: P = 0.01) at the end of single limb support (Figure 1a, Table 1). In the sagittal plane, peak anterior trunk TAM was lower in the DM group than the HC group (P = 0.03) at the beginning of single limb support (Figure 1a, Table 2).
Figure 1.
Translational angular momentum (TAM) of the (a) trunk and (b) pelvis with respect to the stance foot in the sagittal, frontal, and transverse plane healthy controls (blue solid line), patients with diabetes mellitus (DM) (black dotted line), and patients with DM and transtibial amputation (AMP) (red dashed line).
Table 2.
Mean ± SD peak (minimum and maximum) translational angular momentum (TAM) of the trunk and pelvis (hTrunk/Foot and hPelvis/Foot) during the stance period for patients with dysvascular transtibial amputation (AMP), diabetes mellitus (DM), and healthy control (HC) groups.
| Trunk TAM (kg·m2/s) | Pelvis TAM (kg·m2/s) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| AMP | DM | HC | AMP | DM | HC | ||
|
|
|||||||
| Sagittal Plane | Min (Anterior) | -45.6 ± 9.0 | -40.7 ± 14.7 | -37.3 ± 8.3 | -14.5 ± 2.7 | -12.7 ± 5.8 | -11.5 ± 2.4 |
| Max (Posterior) | -0.9 ± 10.8 | 8.4 ± 7.8 | 0.5 ± 3.3 | -1.6 ± 2.8 | 1.3 ± 2.4 | -0.4 ± 1.0 | |
| Frontal Plane | Min (Away from Stance Foot) | -11.5 ± 3.1 | -9.5 ± 4.2 | -8.6 ± 2.4 | -3.2 ± 1.1 | -3.6 ± 2.0 | -3.0 ± 0.9 |
| Max (Toward Stance Foot) | 8.1 ± 2.2 | 10.2 ± 4.2 | 7.6 ± 4.0 | 2.6 ± 0.7 | 2.7 ± 1.2 | 2.3 ± 1.1 | |
| Transverse Plane | Min (Toward Stance Foot) | -2.3 ± 0.9 | -1.8 ± 0.9 | -1.2 ± 0.5 | -1.4 ± 0.6 | -0.8 ± 0.4 | -0.4 ± 0.3 |
| Max (Away from Stance Foot) | 1.8 ± 1.7 | 3.1 ± 1.4 | 2.5 ± 1.0 | 0.8 ± 0.7 | 1.5 ± 0.9 | 1.2 ± 0.4 | |
|
|
|||||||
In the frontal plane, peak lateral trunk TAM toward the stance foot was lower in the AMP group than the DM group (P < 0.001) during weight acceptance (Figure 1a, Table 2).
In the transverse plane, peak trunk and pelvis TAM toward the stance foot was higher in the AMP group than the DM group (trunk: P = 0.03, pelvis: P = 0.01) at the beginning of single limb support (Figure 1a, Table 2). Peak pelvis TAM away from the stance foot was lower in the AMP group than both the DM group (P < 0.001) and the HC group (P < 0.001) at the end of single limb support (Figure 1a, Table 2). All other comparisons were not statistically significant.
3.3 Rotational Angular Momentum
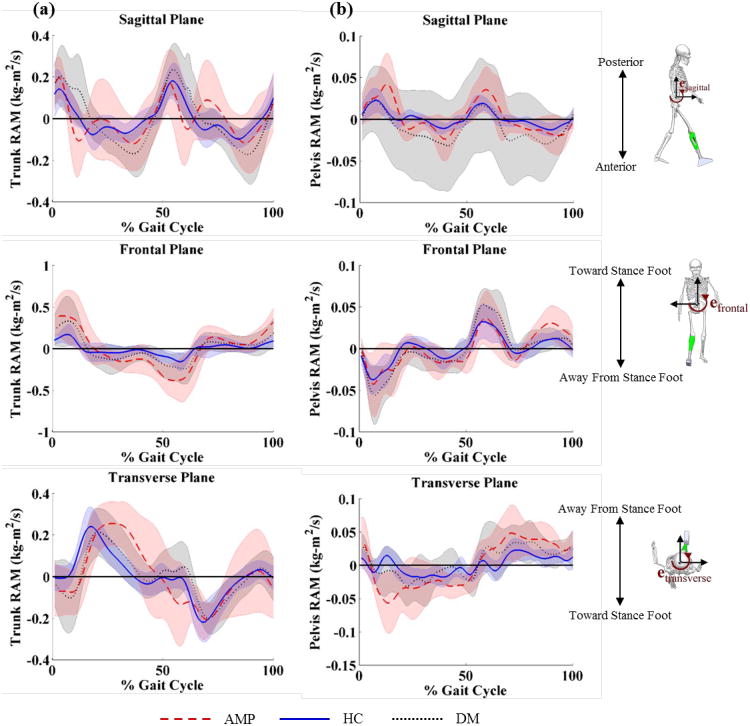
In the sagittal plane, peak anterior trunk RAM was higher in the AMP group than both the DM group (P = 0.02) and HC group (P = 0.01) at the beginning of single limb support (Figure 2a, Table 3). Peak posterior trunk RAM was lower in the AMP group than both the DM group (P = 0.04) and HC group (P = 0.05) at the beginning of swing limb advancement (Figure 2a, Table 2). Peak anterior pelvis RAM was higher in the AMP group than both the DM group (P = 0.04) and the HC group (P = 0.04) at the beginning of single limb support (Figure 2b, Table 3).
Figure 2.
Rotational angular momentum (RAM) of the (a) trunk and (b) pelvis with respect to the stance foot in the sagittal, frontal, and transverse plane healthy controls (blue solid line), patients with diabetes mellitus (DM) (black dotted line), and patients with DM and transtibial amputation (AMP) (red dashed line).
Table 3.
Mean ± SD peak (minimum and maximum) rotational angular momentum (RAM) of the trunk and pelvis (hTrunk and hPelvis) during the gait cycle for patients with dysvascular amputation (AMP), diabetes mellitus (DM), and healthy control (HC) groups.
| Trunk RAM (kg·m2/s) | Pelvis RAM (kg·m2/s) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| AMP | DM | HC | AMP | DM | HC | ||
|
|
|||||||
| Sagittal Plane | Min (Anterior) | -0.34 ± 0.12 | -0.24 ± 0.12 | -0.16 ± 0.05 | -0.05 ± 0.02 | -0.03 ± 0.02 | -0.02 ± 0.01 |
| Max (Posterior) | 0.39 ± 0.12 | 0.33 ± 0.15 | 0.25 ± 0.08 | 0.07 ± 0.04 | 0.05 ± 0.03 | 0.03 ± 0.01 | |
| Frontal Plane | Min (Away from Stance Foot) | -0.47 ± 0.22 | -0.33 ± 0.21 | -0.20 ± 0.08 | -0.07 ± 0.05 | -0.06 ± 0.04 | -0.04 ± 0.01 |
| Max (Toward Stance Foot) | 0.47 ± 0.27 | 0.37 ± 0.29 | 0.22 ± 0.10 | 0.06 ± 0.03 | 0.07 ± 0.03 | 0.04 ± 0.01 | |
| Transverse Plane | Min (Toward Stance Foot) | -0.33 ± 0.11 | -0.26 ± 0.11 | -0.24 ± 0.09 | -0.08 ± 0.04 | -0.06 ± 0.03 | -0.04 ± 0.01 |
| Max (Away from Stance Foot) | 0.34 ± 0.13 | 0.27 ± 0.12 | 0.26 ± 0.09 | 0.08 ± 0.04 | 0.07 ± 0.04 | 0.04 ± 0.01 | |
|
|
|||||||
In the frontal plane, peak lateral trunk RAM toward the stance foot was higher in the AMP group than the DM group (P = 0.04) during swing limb advancement (Figure 2a, Table 3).
In the transverse plane, peak pelvis RAM toward the stance foot was higher for the AMP group than both the DM group (P = 0.02) and the HC group (P = 0.03) at the beginning of single limb support (Figure 2b, Table 3). All other comparisons were not statistically significant.
4. Discussion
The objective of this investigation was to identify and compare movement patterns in patients with dysvascular transtibial amputation (AMP), patients with diabetes mellitus (DM), and healthy controls (HC) using patterns of generating and arresting trunk and pelvis angular momentum during walking. We observed differences in translational angular momentum in all three planes between the AMP, DM, and HC groups, which indicates unique movement patterns adopted by each group during walking. Loss of ankle function in the AMP group is linked to different movement compensations, and results in higher generation of trunk and pelvis RAM in all three planes compared to the DM and HC groups. Large trunk angular momentum with small pelvis momentum is a compensation in the AMP group that may result in high paraspinal muscle demand, which leads leading to LBP. The identification of movement compensations through analysis of segmental RAM has potential important clinical applications in a gait retraining setting through wearable sensors.
Patterns of trunk and pelvis TAM indicate the use of a postural compensation by the AMP group to maintain balance and achieve forward progression without ankle function. TAM is a function of position and linear momentum of each segment relative to the stance foot (Eq. 1). In the sagittal plane, trunk and pelvis anterior TAM is generated about the stance foot during weight acceptance, is slightly arrested throughout single limb support, and then arrested completely at the transition to swing limb advancement (Figure 1). Without active plantar flexion at the end of single limb support, the AMP group generated smaller posterior angular momentum when compared to the DM group, which is adopted to maintain forward progression when unloading the amputated limb. In the frontal plane, trunk and pelvis TAM toward the stance limb is rapidly arrested during loading response and then is gradually arrested throughout the remainder of single limb support until angular momentum is generated away from the stance limb during the preparation of swing limb advancement as weight is transferred between limbs. In the transverse plane, trunk and pelvis TAM were arrested during loading response and then remained constant throughout the duration of single limb stance. Remarkably, trunk and pelvis TAM at initial foot contact in the AMP group were directed toward the stance (amputated) limb, which is opposite of both the HC and DM groups. This difference is likely a result of excessive propulsion by the intact limb, which creates a transverse rotation toward the amputated limb. Because each group walked at the same speed, the large transverse TAM toward the stance foot throughout the duration of single limb support in the AMP group occurs by a more medial position of the segment with respect to the stance foot. In the frontal plane, this corresponds to a wider step width, which is a commonly observed finding in amputee gait [9].
Segment rotational angular momentum provides a unique framework for identifying differences in movement patterns by highlighting the motion of the segment, which can assist in characterizing and interpreting movement compensations observed in the clinic. In the sagittal plane, large anterior rotational angular momentum in the AMP group leads to a forward trunk lean that is frequently observed during single limb support, and represents an adaptive strategy to maintain forward progression in light of ankle plantar flexor loss [22]. The hip and trunk extensor demands needed to arrest the large anterior trunk rotational angular momentum, which occurs at approximately 10% of the gait cycle, may contribute to overuse injuries in the lower extremity and the low back [23,24].
In the frontal plane, the AMP group generated larger trunk RAM toward the amputated limb during weight acceptance, and arrested trunk angular momentum later in the gait cycle, compared to the DM and HC groups, corresponding to large trunk displacement toward the stance limb. To prevent a fall at this point in the gait cycle, the AMP group must quickly arrest a large amount of angular momentum that has been generated in the trunk, which creates high paraspinal muscle demand [25]. The lateral trunk posture over the stance limb (compensated Trendelenburg posture) corresponds with increased loading in the low back, and is linked to the development of LBP [26].
In the transverse plane, the AMP group generated substantially larger pelvis RAM toward the amputated than the HC and DM groups limb during weight acceptance. This large RAM, due to excessive angular speed, may be linked to the large ankle power in the intact limb needed to achieve forward progression [27]. Therefore, pelvis RAM must be arrested following peak generation to maintain balance during swing and continue progression during gait.
Our results indicate that the AMP group generates and arrests trunk and pelvis momenta differently than either the DM or HC groups, and the associated muscle demands with the observed movement patterns after amputation may be linked to LBP [8,12]. Because a patient with unilateral TTA cannot create propulsive ankle joint moments, the generating demands shifts higher in the kinetic change. Our results show that movement compensations occur in the pelvis and trunk, and indicates that demand on local muscles (e.g. multifidus, erector spinae, obliques, etc.) is likely higher for patients with amputation than other populations, which potentially could be consequential in the development for LBP.
Identifying movement compensations using segmental RAM may have important clinical applications. RAM combines inertia with angular speed of the segment, which is a parameter that can be interpreted in light of the effort needed to generate or arrest the measured momentum. Because observational analysis is based on presence of events and postures (e.g. compensated Trendelenburg sign), a clinician can gain insight into the effort needed to accomplish the observed event by supplementing with angular momentum. Measuring rotational angular momentum is easily facilitated by wearable sensors such as gyroscopes, and would not require additional instrumentation (e.g. force platforms). Use of low-cost wearable sensors have emerged in biomechanics that facilitate spatiotemporal gait characteristics [28,29] as well as segment and joint kinematics [30]. With additional research, angular momentum may provide clinicians and patients with immediate and accurate information on their ability understand when movement compensations occur and increase the efficacy of targeted movement retraining following amputation.
Several limitations should be considered. First, the analysis did not consider consecutive gait cycles; therefore, repeatability of movement compensations was not characterized in these measures. In future investigations we will extend this analysis using repeated over ground trials or a treadmill. Second, we do not know how segmental angular momentum variables correspond with traditional biomechanical variables. In future investigations we will associate how these movement compensations correlate with traditional quantitative biomechanical analyses (e.g. joint moments, joint loading, etc.). Third, neither the AMP and DM groups were screened for LBP at the time of testing; therefore, we cannot determine if any compensatory movement patterns adopted by each group were a habitual movement pattern or a result of LBP. Finally, because only patients with dysvascular amputation were included, we are unable to generalize these findings to patients with unilateral TTA from other causes other than dysvascular disease.
5. Conclusion
This investigation demonstrated the use of segmental angular momenta to identify movement compensations in the trunk and pelvis in patients with unilateral dysvascular transtibial amputation. Coordinated compensations between the trunk and pelvis promote forward progression during locomotion, but may have long-term adverse effects from the demand placed on the musculoskeletal system to generate and arrest segmental momentum.
Research Highlights.
Examination of transtibial amputees through angular momentum of pelvis and trunk.
Inference on motion and effort used to supplement observational analyses in clinic.
Movement compensations linked to consequential muscle demand.
Acknowledgments
This project was supported by the National Institutes of Health (Grant No. K12-HD05593).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95:875–83. doi: 10.1097/00007611-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Davies B, Datta D. Mobility outcome following unilateral lower limb amputation. Prosthet Orthot Int. 2003;27:186–90. doi: 10.1080/03093640308726681. [DOI] [PubMed] [Google Scholar]
- 4.Van Velzen JM, van Bennekom CaM, Polomski W, Slootman JR, van der Woude LHV, Houdijk H. Physical capacity and walking ability after lower limb amputation: a systematic review. Clin Rehabil. 2006;20:999–1016. doi: 10.1177/0269215506070700. [DOI] [PubMed] [Google Scholar]
- 5.Silverman AK, Neptune RR. Differences in whole-body angular momentum between below-knee amputees and non-amputees across walking speeds. J Biomech. 2011;44:379–85. doi: 10.1016/j.jbiomech.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Fey NP, Silverman AK, Neptune RR. The influence of increasing steady-state walking speed on muscle activity in below-knee amputees. J Electromyogr Kinesiol. 2010;20:155–61. doi: 10.1016/j.jelekin.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Mueller MJ, Minor SD, Sahrmann Sa, Schaaf Ja, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74:299–308. doi: 10.1093/ptj/74.4.299. discussion 309–13. [DOI] [PubMed] [Google Scholar]
- 8.Ehde DM, Smith DG, Czerniecki JM, Campbell KM, Malchow DM, Robinson LR. Back pain as a secondary disability in persons with lower limb amputations. Arch Phys Med Rehabil. 2001;82:731–4. doi: 10.1053/apmr.2001.21962. [DOI] [PubMed] [Google Scholar]
- 9.Winter D, Sienko S. Biomechanics of below-knee amputee gait. J Biomech. 1988;21:361–7. doi: 10.1016/0021-9290(88)90142-x. [DOI] [PubMed] [Google Scholar]
- 10.Molina-Rueda F, Alguacil-Diego IM, Cuesta-Gómez A, Iglesias-Giménez J, Martín-Vivaldi A, Miangolarra-Page JC. Thorax, pelvis and hip pattern in the frontal plane during walking in unilateral transtibial amputees: biomechanical analysis. Brazilian J Phys Ther. 2014;18:252–8. doi: 10.1590/bjpt-rbf.2014.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S. Theories of musculoskeletal injury causation. Ergonomics. 2001;44:17–47. doi: 10.1080/00140130120716. [DOI] [PubMed] [Google Scholar]
- 12.Hendershot BD, Wolf EJ. Three-dimensional joint reaction forces and moments at the low back during over-ground walking in persons with unilateral lower-extremity amputation. Clin Biomech. 2014;29:235–42. doi: 10.1016/j.clinbiomech.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Russell Esposito E, Wilken JM. The relationship between pelvis–trunk coordination and low back pain in individuals with transfemoral amputations. Gait Posture. 2014;40:640–6. doi: 10.1016/j.gaitpost.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Hendershot BD, Wolf EJ. Mediolateral Joint Powers at the Low Back Among Persons With Unilateral Transfemoral Amputation. Arch Phys Med Rehabil. 2015;96:154–7. doi: 10.1016/j.apmr.2014.07.402. [DOI] [PubMed] [Google Scholar]
- 15.Saleh M, Murdoch G. In Defence of Gait Analysis: Observation and Measurement in Gait Assessment. J Bone Jt Surg. 1985;67:237–41. doi: 10.1302/0301-620X.67B2.3980533. [DOI] [PubMed] [Google Scholar]
- 16.Neptune RR, McGowan CP. Muscle contributions to whole-body sagittal plane angular momentum during walking. J Biomech. 2011;44:6–12. doi: 10.1016/j.jbiomech.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffney BM, Christiansen CL, Murray AM, Davidson BS. Analysis of Gait Patterns based on the Separation of Angular Momentum: Segmental Translation and Segmental Rotation. Hum Mov Sci nd. In Review. [Google Scholar]
- 18.Kasdin NJ, Paley DA. Engineering Dynamics: A Comprehensive Introduction. 1st. Scottsdale, AZ: Princeton University Press; 2011. [Google Scholar]
- 19.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–7. [PubMed] [Google Scholar]
- 20.Smith JD, Ferris AE, Heise GD, Hinrichs RN, Martin PE. Oscillation and reaction board techniques for estimating inertial properties of a below-knee prosthesis. J Vis Exp. 2014:1–16. doi: 10.3791/50977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry J, Burnfield JM. Gait Analysis: Normal and Pathologic Function. 2nd. Thorofare, NJ: SLACK Incorporated; 2010. [Google Scholar]
- 22.Miff SC, Childress DS, Gard Sa, Meier MR, Hansen AH. Temporal symmetries during gait initiation and termination in nondisabled ambulators and in people with unilateral transtibial limb loss. J Rehabil Res Dev. 2005;42:175–82. doi: 10.1682/jrrd.2004.03.0038. [DOI] [PubMed] [Google Scholar]
- 23.Silverman AK, Neptune RR. Three-dimensional knee joint contact forces during walking in unilateral transtibial amputees. J Biomech. 2014:1–7. doi: 10.1016/j.jbiomech.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Gailey R. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J Rehabil Res Dev. 2008;45:15–30. doi: 10.1682/jrrd.2006.11.0147. [DOI] [PubMed] [Google Scholar]
- 25.Friel K, Domholdt E, Smith DG. Physical and functional measures related to low back pain in individuals with lower-limb amputation: An exploratory pilot study. J Rehabil Res Dev. 2005;42:155. doi: 10.1682/jrrd.2004.08.0090. [DOI] [PubMed] [Google Scholar]
- 26.Hendershot BD, Bazrgari B, Nussbaum Ma. Persons with unilateral lower-limb amputation have altered and asymmetric trunk mechanical and neuromuscular behaviors estimated using multidirectional trunk perturbations. J Biomech. 2013;46:1907–12. doi: 10.1016/j.jbiomech.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Nolan L, Lees A. The functional demands on the intact limb during walking for active trans-femoral and trans-tibial amputees. Prosthet Orthot Int. 2000;24:117–25. doi: 10.1080/03093640008726534. [DOI] [PubMed] [Google Scholar]
- 28.Rueterbories J, Spaich EG, Larsen B, Andersen OK. Methods for gait event detection and analysis in ambulatory systems. Med Eng Phys. 2010;32:545–52. doi: 10.1016/j.medengphy.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Sabatini AM, Martelloni C, Scapellato S, Cavallo F. Assessment of walking features from foot inertial sensing. IEEE Trans Biomed Eng. 2005;52:486–94. doi: 10.1109/TBME.2004.840727. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Saito H, Koike E, Nitta K. A preliminary test of measurement of joint angles and stride length with wireless inertial sensors for wearable gait evaluation system. Comput Intell Neurosci. 2011;2011 doi: 10.1155/2011/975193. [DOI] [PMC free article] [PubMed] [Google Scholar]