Abstract
Inflammatory monocytes and resident tissue macrophages are key regulators of tissue repair, regeneration, and fibrosis. Following tissue injury, monocytes and macrophages undergo marked phenotypic and functional changes to play critical roles during the initiation, maintenance, and resolution phases of tissue repair. Disturbances in macrophage function can lead to aberrant repair, with uncontrolled inflammatory mediator and growth factor production, deficient generation of anti-inflammatory macrophages, or failed communication between macrophages and epithelial cells, endothelial cells, fibroblasts, and stem or tissue progenitor cells all contributing to a state of persistent injury, which may lead to the development of pathological fibrosis. In this review, we discuss the mechanisms that instruct macrophages to adopt pro-inflammatory, pro-wound healing, pro-fibrotic, anti-inflammatory, anti-fibrotic, pro-resolving, and tissue regenerating phenotypes following injury, and highlight how some of these mechanisms and macrophage activation states could be exploited therapeutically.
Introduction
Tissue repair and regeneration are critical biological processes that are fundamental to the survival of all living organisms (Das et al., 2015). When tissues are injured during infection or following toxic or mechanical injury, an inflammatory response is induced in response to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) released by dead and dying cells and invading organisms, respectively (Zhang et al., 2010). These molecular triggers induce a complex inflammatory response that is characterized by the recruitment, proliferation, and activation of a variety of hematopoietic and non-hematopoietic cells including neutrophils, macrophages, innate lymphoid cells (ILCs), NK cells, B cells, T cells, fibroblasts, epithelial cells, endothelial cells, and stem cells that together make up the cellular response that orchestrates tissue repair (Wynn, 2008). When the wound healing response is well organized and controlled, the inflammatory response resolves quickly and normal tissue architecture is restored. However, if the wound healing response is chronic or becomes dysregulated it can lead to the development of pathological fibrosis or scarring, impairing normal tissue function and ultimately leading to organ failure and death (Wynn and Ramalingam, 2012). Therefore, wound-healing responses must be tightly regulated. Although many cell types are involved in tissue repair, because of their highly flexible programming (Mosser and Edwards, 2008), macrophages have been shown to exhibit critical regulatory activity at all stages of repair and fibrosis (Wynn and Barron, 2010). Consequently, because they represent potentially important therapeutic targets, there has been great deal of interest over the past few years in deciphering the contributions of the different macrophage populations that control the initiation, maintenance, and resolution of wound healing responses in different organ systems.
The distinct tissue macrophage populations that take up residence in many tissues of the body are mostly derived from the yolk sac during embryogenesis, with fetal liver and hematopoietic stem cells contributing macrophages to some but not all tissues at later time points (Epelman et al., 2014a; Epelman et al., 2014b; Gomez Perdiguero et al., 2015). These tissue macrophages play critical roles during development and also provide important trophic signals that support neighboring parenchymal tissues (Wynn et al., 2013). Thus, they are critically involved in normal tissue homeostasis. However, following tissue injury, large numbers of inflammatory monocytes, macrophage precursors, are recruited from the bone marrow via chemokine gradients and various adhesion molecules, with these recruited cells often exceeding the resident tissue macrophage population by many-fold (Davies et al., 2013; Galli et al., 2011). The recruited and resident macrophage populations proliferate and also undergo marked phenotypic and functional changes in response to growth factors and cytokines released in the local tissue microenvironment (Jenkins et al., 2011; Jenkins et al., 2013), with many recent studies identifying specialized and critically timed roles for different monocyte and macrophage activation states in tissue repair, regeneration, and fibrosis (Lech and Anders, 2013; Munoz-Canoves and Serrano, 2015; Murray and Wynn, 2011; Novak et al., 2014; Sica and Mantovani, 2012).
Although early research investigating the contribution of macrophages to wound repair focused on their role as scavenger cells that phagocytize cellular debris, invading organisms, neutrophils, and other apoptotic cells following tissue injury (Peiser et al., 2002), it is now clear that monocytes and macrophages exhibit much more complex roles, not only in tissue repair but also in the mechanisms of fibrosis and tissue regeneration (Wynn et al., 2013). Macrophages are an important source of chemokines, matrix metalloproteinases, and other inflammatory mediators that drive the initial cellular response following injury (Wynn and Barron, 2010). Indeed, if macrophages are depleted early after injury, the inflammatory response is often greatly diminished (Duffield et al., 2005). However, their removal can also result in decreased wound debridement and lead to less efficient repair and regeneration (Zhang et al., 2012). After the early inflammatory phase subsides, the predominant macrophage population assumes a wound healing phenotype that is characterized by the production of numerous growth factors including PDGF, TGF-β1, IGF-1, and VEGF-α, that promote cellular proliferation and blood vessel development (Berse et al., 1992; Chujo et al., 2009; Rappolee et al., 1988; Shimokado et al., 1985) (Willenborg et al., 2012). They also produce soluble mediators that stimulate local and recruited tissue fibroblasts to differentiate into myofibroblasts that facilitate wound contraction and closure as well as the synthesis of extracellular matrix components (Murray and Wynn, 2011). The proliferation and expansion of neighboring parenchymal and stromal cells are also regulated by macrophages, and if the injury is severe, macrophages may activate additional stem cell and local progenitor cell populations that participate in repair. Thereafter, monocytes and/or macrophages exhibiting a mostly anti-inflammatory phenotype become the dominant population (Ramachandran et al., 2015). These macrophages respond to interleukin-10 (IL-10) and other inhibitory mediators, secrete a variety of anti-inflammatory mediators like IL-10 and TGF-β1, and express cell surface receptors like PD-L1 and PD-L2 that play major roles in suppressing the immune system and quieting the inflammation that if not controlled effectively, can lead to collateral cell death and ultimately delay the repair process (Khalil et al., 1989; Said et al., 2010; Shouval et al., 2014; Zigmond et al., 2014). Thus, the different stages of tissue repair must be carefully regulated, with monocyte and macrophages of different phenotypes playing unique and critical roles at each stage. Indeed, disturbances at any point in the process can lead to aberrant repair, with uncontrolled inflammatory mediator and growth factor production, or deficiencies in the generation of inhibitory macrophages all contributing to the development of chronic wounds, which can ultimately contribute to the formation of pathological fibrosis (Figure 1).
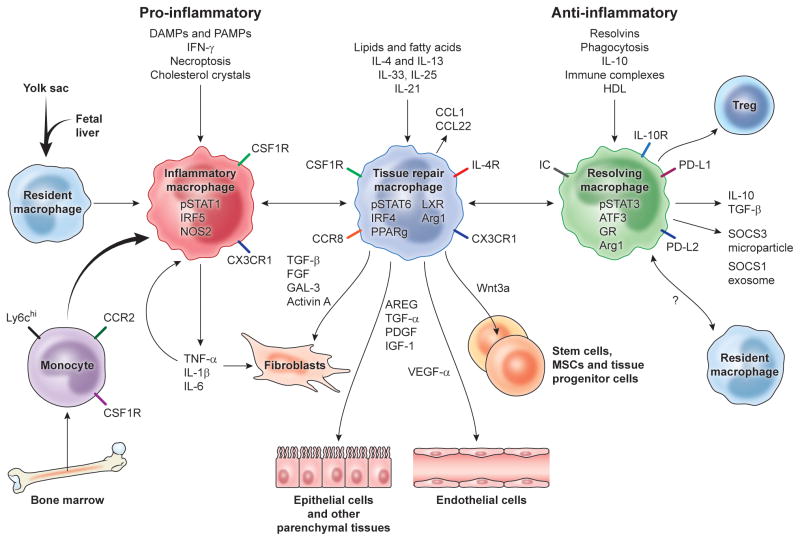
Fig. 1. Mechanisms driving major macrophage activation phenotypes in tissue repair, regeneration, and fibrosis.
In many tissues the resident tissue macrophage population is derived from the yolk sac and fetal liver during development but are complimented by inflammatory monocytes recruited from the bone marrow following injury. The recruited and resident macrophages undergo marked phenotypic and functional changes in response to DAMPs, PAMPs, growth factors, cytokines, and other mediators released in the local tissue microenvironment, with the dominant phenotypic variants depicted here regulating inflammation, tissue repair, regeneration, and resolution. Macrophages produce a variety of factors that stimulate the proliferation, differentiation, and activation of fibroblasts, epithelial cells, endothelial cells, and stem and progenitor cells that facilitate tissue repair. During the later stages of the repair process, they assume a regulatory pro-resolving phenotype that ensures the tissue damaging inflammatory response is suppressed and normal tissue architecture is restored. If the process is not controlled effectively, persistent inflammation and/or maladaptive repair processes can lead to tissue destructive fibrosis. In some cases, the recruited monocytes seed the tissues and adopt a resident macrophage phenotype, however the mechanisms that restore tissue homeostasis are still under debate. Damage associated molecular patterns (DAMPs), PAMPs (pathogen associated molecular patterns), Regulatory T cells (Treg), interferon-regulatory factor 5 (IRF5), nitric oxide synthase 2 (NOS2), Liver X receptor (LXR), Amphiregulin (AREG), Arginase-1 (Arg1), interferon regulatory factor 4 (IRF4), peroxisome proliferator-activated receptor gamma (PPARγ), fibroblast growth factor (FGF), galectin-3 (GAL-3), transforming growth factor (TGF), Immune complex (IC), glucocorticoid receptor (GR), transcription factor ATF3, silencers of cytokine signaling (SOCS).
Research over the past few years has focused on identifying and characterizing the various macrophage populations that regulate the different stages of tissue repair (Duffield et al., 2013). Specifically, the role of resident tissue macrophages versus recruited monocytes has become an important area of research, as there is accumulating evidence that different monocyte and macrophage populations play distinct and non-redundant roles in tissue repair, fibrosis, and regeneration (Gundra et al., 2014; Vannella et al., 2014). The mechanisms that instruct macrophages to adopt pro-inflammatory, pro-wound healing, pro-fibrotic, anti-inflammatory, anti-fibrotic, pro-resolving, and tissue regenerating properties in various organ systems has also been the subject of intensive research (Kluth, 2007; Mitchell et al., 2002). It also remains unclear whether an individual macrophage (local or recruited) is capable of adopting all of these attributes at different times in response to signals found in the local tissue microenvironment or whether there are truly distinct functional subsets of monocytes and macrophages that are hard-wired to regulate these different and often opposing activities. This review focuses on recent findings that have advanced our understanding of the role of monocytes and resident tissue macrophages in wound repair, tissue regeneration, and fibrosis.
Evidence that distinct macrophage phenotypes regulate tissue injury and repair
Using a transgenic CD11b-DTR mouse to selectively deplete CD11bhi macrophages at different stages in a reversible model of liver injury induced by carbon tetrachloride, Duffield and colleagues have shown that if macrophages are depleted after CCL4 chemokine exposure is terminated, the liver is less capable of restoring normal tissue architecture because matrix degradation is substantially impaired. In contrast, if CD11bhi macrophages are depleted during ongoing CCL4 exposure, liver injury is reduced. These studies are important because they suggest functionally distinct CD11b+ macrophages regulate the injury and recovery phases of tissue repair (Duffield et al., 2005). Recent studies have suggested that yolk sac-derived resident tissue macrophages and monocytes recruited from the bone marrow play distinct roles during the different stages of repair in some organs. For example, although genetic fate mapping studies have confirmed the majority of macrophages in the adult heart are derived from yolk sac and fetal progenitors, CCR2+ monocyte-derived cells are the dominant macrophages driving the early inflammatory response in cardiac tissues following injury (Epelman et al., 2014a). In contrast, embryonic-derived resident cardiac macrophages are the critical cells promoting recovery (Lavine et al., 2014). The latter studies show that resident tissue macrophages induce cardiomyocyte proliferation and blood vessel development following injury. Consequently, when monocyte recruitment to the adult heart is suppressed following injury, the embryonic population of macrophages is largely preserved, resulting in reduced inflammation and accelerated repair. Thus, recruited bone marrow derived monocytes exhibit tissue destructive activity while embryonic-derived resident tissue populations facilitate the resolution of inflammation and instruct tissue repair in the heart.
Distinct pro-inflammatory and wound healing macrophage phenotypes have also been observed in models of spinal cord injury and repair, with the functionally distinct macrophage populations recruited to the site of tissue injury by unique chemokine gradients. In the spinal cord, a pro-inflammatory Ly6chiCX3CR1lo monocyte population homes to sites of tissue injury in a CCR2 and CCL2 dependent manner, while the reparative Ly6cloCx3Cr1hi population travels along a distinct path guided by VCAM-1, VLA-4 and CD73, adhesion proteins and endothelial cell surface enzymes involved in leukocyte extravasation (Shechter et al., 2013). These authors have found that the route of monocyte and macrophage entry to the central nervous system also provides additional instructional signals to shape the unique functional activities of the recruited cells.
Similar studies conducted in other tissues identified regenerating islet-derived 3 beta (Reg3β) as an essential regulator of macrophage trafficking to cardiac tissues following injury (Lorchner et al., 2015). Using a mass spectrometry secretome approach, these authors have found that damaged cardiomyocytes release large amounts of Reg3β in response to the cytokine Oncostatin M produced by inflammatory monocytes and neutrophils. Reg3β recruits the reparative macrophage subset that facilitates the removal of neutrophils that would otherwise trigger extensive matrix degradation, delayed collagen deposition, and cardiac rupture. Thus, a critical feed-forward loop between injured cardiomyocytes, inflammatory monocytes, and reparative macrophages facilitates tissue healing. Korf-Klingebiel et al. have carried out a similar bioinformatics secretome analysis in humans and identified a macrophage-derived growth factor encoded by an open reading frame on chromosome 19 (C19orf10) that induces cardiac myocyte survival and angiogenesis following acute myocardial infarction (MI) (Korf-Klingebiel et al., 2015). The study has shown this growth factor is also critical for tissue repair following acute myocardial infarction (MI) and is likely produced by the same reparative macrophage population described by (Lorchner et al).
There is also evidence in some tissues that a single population of monocytes can both be pro-inflammatory and pro-repair, suggesting that in situ conversion rather than recruitment of the pro-reparative Ly6C- subset is critical in some settings. For example, Activin-A, a protein that instructs oligodendrocyte differentiation during central nervous system (CNS) remyelination, has been recently identified as an important macrophage-derived reparative mediator. Following CNS demyelination, microglia and peripherally derived inflammatory macrophages switch from a pro-inflammatory or classically activated M(IFN-γ) phenotype to a pro-repair or alternatively-activated M(IL-4)-like phenotype as repair commences, and intra-lesional M(IL-4) cell depletion substantially delayed oligodendrocyte differentiation (Miron et al., 2013). In this case, both the microglia and recruited macrophages switch to the reparative Activin-A producing phenotype, suggesting that recruited and resident populations may both participate in repair in some tissues. Similar findings have also been reported in models of liver injury, with IL-4, IL-10, and phagocytosis playing critical roles in the conversion of inflammatory monocytes into cells exhibiting a reparative phenotype (Dal-Secco et al., 2015; Ramachandran et al., 2012).
An M(IL-4) population of macrophages is also thought to play a critical role in wound repair following nematode invasion (Chen et al., 2012a). Using a helminth infection model in which parasitic nematode larvae cause significant hemorrhaging and inflammation, Chen and colleagues have found that IL-17 serves as a major pathogenic inflammatory mediator during parasite migration through the lung, with subsequent IL-4R signaling in macrophages driving insulin-like growth factor 1 (IGF-1) and IL-10 production, which together contribute to the rapid resolution of the IL-17-induced damage. Here again, macrophage conversion from a pro-inflammatory to anti-inflammatory phenotype has been hypothesized to promote lung healing following acute pathogen-induced injury, which is consistent with studies that identified critical but divergent roles for type-1 and type-2 cytokine responses in tissue repair (Sandler et al., 2003).
Subsequent studies by Vannella and colleagues have identified distinct roles for resident and recruited alternatively activated macrophages (M(IL-4)) in the pathogenesis of schistosomiasis, a disease characterized by chronic granulomatous inflammation and development of hepatic fibrosis (Vannella et al., 2014). In these studies, IL-4Rαflox/delta mice were crossed with Lyz2-cre mice to generate mice with a conditional deletion of the IL-4 receptor in tissue macrophages. Because LysM is expressed at high amounts in mature tissue macrophages but is expressed at much lower amounts in recruited monocytes, the conditional mutant provides a tool to dissect the contributions of resident versus recruited M(IL-4)-like cells. In these studies, Lyz2hiF4/80+CD11b+ mature tissue macrophages have been identified as the critical M(IL-4) population suppressing inflammation in the liver, while LyzloF4/80+CD11b+ monocytes expressing high amounts of arginase-1 were largely responsible for the inhibition of fibrosis during chronic infection. These studies are of interest because they suggest unique roles for different populations of IL-4 and/or IL-13-activated inflammatory monocytes and resident tissue macrophages in the resolution of inflammation, tissue repair, and fibrosis.
Together, the preceding studies, which encompass various organ systems and experimental models, nicely illustrate the distinct and often opposing roles of inflammatory monocytes and resident tissue macrophages in tissue repair. They also demonstrate how the timely conversion of monocytes and macrophages from a pro-inflammatory to a reparative phenotype plays a decisive role in wound healing and tissue regenerative responses.
Pro-inflammatory macrophages exacerbate tissue injury
Interest in elucidating the signaling pathways and distinct macrophage populations that sustain tissue damaging inflammatory responses has grown substantially over the past few years, as a better understanding of these mechanisms will likely guide the development of therapeutics for inflammatory and fibrotic diseases (Han et al., 2013; Xu et al., 2012; Xu et al., 2015). For example, an exciting recent study by Jay and colleagues has shown that macrophages expressing triggering receptor expressed on myeloid cells 2 (TREM2) are required for the development of Alzheimer’s disease (AD) (Jay et al., 2015). Inflammatory Ly6c+ macrophages express TREM2 at high amounts, and when TREM2 activity is genetically deleted, the Ly6c+ population is virtually eliminated, resulting in reduced inflammation and ameliorated amyloid and tau pathologies. Thus, TREM2 has been identified as an important instructional signal in inflammatory macrophage development, suggesting it may represent a therapeutic target in neurodegenerative diseases like AD.
Therapeutic strategies that reduce the accumulation of tissue destructive infiltrating blood monocytes may also hold promise for diseases associated with severe or persistent inflammation. For example, although axonal repair following traumatic spinal cord injury is dependent upon the rapid development of reparative macrophages (Shechter et al., 2013), sustained recruitment of inflammatory blood derived macrophages can facilitate extensive secondary axonal dieback and delay the reparative process substantially. Using a radiation chimera model to distinguish bone marrow-derived cells from microglia, Evans and colleagues have determined that the vast majority of accumulated cells in spinal cord injury are derived from the blood, and CX3CR1+ macrophages but not CCR2+ monocytes were tightly associated with axonal dieback (Evans et al., 2014). Thus, therapeutic targeting of the CX3CR1+ subset may accelerate repair and reduce secondary axonal injury following traumatic spinal cord injury. Studies in the liver, however, identified a critical pro-injury role for CCR2+ monocytes, suggesting that blocking CCR2 may prove beneficial in the treatment of pathological inflammation in the liver (Mitchell et al., 2009). Factors that prevent accumulating tissue monocytes from converting from a pro-inflammatory to reparative phenotype can also impair healing. Two such factors are TNF-α, which blocks phagocytosis-mediated conversion of inflammatory macrophages to the reparative M(IL-4)-like phenotype, and iron, which accumulates in local injury-associated macrophages and supports TNF-α production (Kroner et al., 2014). Thus, iron accumulation and sustained TNF production by inflammatory macrophages can further delay tissue repair following injury.
Repair of vascular tissues is also impacted by mechanisms that maintain inflammatory macrophage numbers or prevent their conversion to a reparative anti-inflammatory phenotype. As observed for TNF-α in models of spinal cord injury, secretion of the inflammatory cytokine IL-1β by macrophages has been shown to be a major driver in the pathogenesis of atherosclerosis. Investigating a murine model of atherosclerosis, Warnatsch and colleagues have found that cholesterol crystals act both as priming and danger signals for IL-1β production (Warnatsch et al., 2015). Cholesterol crystals trigger neutrophils to release extracellular traps (NETs), which prime local macrophages to transcribe immature IL-1β, with cholesterol crystals serving a second role as a danger signal that activates inflammasomes, which process immature IL-1β for secretion. Thus, cholesterol and other sterile inflammatory signals contribute to the sustained activation of inflammatory macrophages, which disrupt normal tissue homeostasis and impede vascular repair. Sustained IL-1β production through NLRP3 inflammasome activation in macrophages has also been shown to be a major driver of persistent inflammation and fibrosis in other tissues as well, including the liver during chronic hepatitis C virus infection (Negash et al., 2013). Thus, therapeutic strategies that suppress NLRP3, IL-1β, and TNF-α activity may contribute to tissue repair following infection, injury, or sterile inflammation by reducing the negative impacts of sustained pro-inflammatory cytokine production by macrophages.
Macrophage necroptosis, a form of programmed necrosis characterized by the death of inflammatory cells has been recently identified as a key signal maintaining microbial induced type 1 inflammation. Here, programmed “suicide” of infected Kupffer cells triggers significant monocyte recruitment and anti-microbial type 1 immunity (Bleriot et al., 2015). However, at the same time, Kupffer cell death promotes a type 2 response involving the hepatocyte-derived alarmin IL-33 and basophil-derived IL-4, which quickly converts the monocyte-derived macrophages to an M(IL-4)-like phenotype that restores liver homeostasis and replenishes the depleted Kupffer cell population. Resident tissue macrophages and Kupffer cells have also been shown to play a key role in the liver following acetaminophen-induced acute liver failure (AALF) in mice and humans. During AALF, a marked increase in inflammatory macrophages is observed in areas of hepatic necrosis while circulating monocytes are generally reduced, with the lowest amounts observed in patients developing adverse outcomes (Antoniades et al., 2012). The resident tissue macrophages are thought to quickly convert to a pro-resolution tissue repair phenotype during AALF, so expanding their numbers through local proliferation or recruitment from the monocyte pool has been hypothesized to be a critical determinant controlling survival following severe liver injury. In support of this conclusion, recent studies of AALF and partial hepatectomy in mice showed that by expanding the number of resident tissue macrophages and recruited monocytes exhibiting a reparative anti-inflammatory phenotype, colony-stimulating factor 1-Fc treatment holds promise as a therapeutic strategy following acute liver injury (Stutchfield et al., 2015).
Regulation and function of pro-fibrotic macrophages
Although approaches that either reduce the numbers of inflammatory macrophages exhibiting an M(IFN-γ) skew phenotype or increase the numbers of reparative anti-inflammatory M(IL-4)-like macrophages have been shown to accelerate the repair of many tissues, persistent activation or sustained recruitment of the M(IL-4)-like cells has also been hypothesized to contribute to the development of pathological fibrosis (Wynn and Ramalingam, 2012). Nevertheless, to date, most mechanistic studies of fibrosis have focused on the role of inflammatory macrophages, with many fewer studies actually investigating the specific contributions of M(IL-4)-like macrophages in repair and fibrosis. Using several elegant in vivo strategies to globally deplete monocytes and macrophages, Gibbons and colleagues have concluded that macrophages are critically required for the development of bleomycin induced pulmonary fibrosis (Gibbons et al., 2011). Although the reduction in fibrosis in macrophage-depleted mice is associated with decreases in the expression of several markers of alternative macrophage activation including Ym1 and Arginase-1, the depletion strategies used were not specific to the M(IL-4) subset as circulating Ly6Chi inflammatory monocytes were also depleted. Therefore it is difficult to conclude definitively whether fibrosis is dependent on M(IL-4) cells, although adoptive transfer studies suggest this was likely the case.
In models of idiopathic pulmonary fibrosis, the pro-fibrotic function of macrophages has also been attributed to their production and activation of the pro-fibrotic cytokine TGF-β1 (Murray et al., 2011). Fibrosis is typically associated with impaired angiogenesis and sustained development of local tissue hypoxia, with hypoxia-inducible factor-1a (HIF-1a), a transcription factor that functions as a master regulator of oxygen homeostasis directly implicated in TGF-β1-driven fibrogenesis (Ueno et al., 2011). Indeed, silencing of HIF-1a expression markedly decreases TGFβ1 production in alveolar macrophages and attenuates the development of bleomycin-induced fibrosis, confirming a critical role for TGF-β1-producing macrophages in the development of fibrosis in response to bleomycin.
Nevertheless, some studies have suggested that fibrosis can also develop in a TGF-β1-independent manner (Kaviratne et al., 2004), with the type 2 cytokine IL-13 playing a dominant role in many settings (Wynn, 2007). However, the contribution of macrophages to the development and maintenance of IL-13-dependent fibrosis is less clear as macrophages are not thought to be a major source of IL-13 (Wynn, 2004). To investigate the role of macrophages in a model of IL-13 driven fibrosis, Borthwick and colleagues have employed CD11b-DTR mice and depleted CD11b+ monocytes and macrophages at different time points in three models of type-2 cytokine-driven disease (Borthwick et al., 2015). Here, monocyte and macrophage but not dendritic cell depletion during the maintenance and resolution phases caused a profound decrease in inflammation, fibrosis, and type 2 gene expression in the tissues but not in secondary lymphoid organs, suggesting that macrophages are critical to the maintenance of type 2 inflammation in the injured lung. In support of this conclusion, local tissue macrophages were identified as a critical source of the CD4+ T helper-2 (Th2) cell recruiting chemokines CCL1 and CCL22. Related studies have also identified the chemokine receptor CCR8 as a major mediator of macrophage recruitment to injured liver, with CCL1-directed migration controlling the recruitment of classical inflammatory monocytes that promote TGF-β1-dependent fibrosis induced by CCL4 or bile duct ligation (Heymann et al., 2012). Thus, in addition to producing important pro-fibrotic mediators like TGF-β1 (Figure 2), monocytes and macrophages can also promote fibrosis indirectly by orchestrating local inflammatory reactions that maintain fibrotic responses or by blocking the emergence of pro-resolution pathways (Borthwick et al., 2015; Ehling et al., 2014; Mitchell et al., 2009).
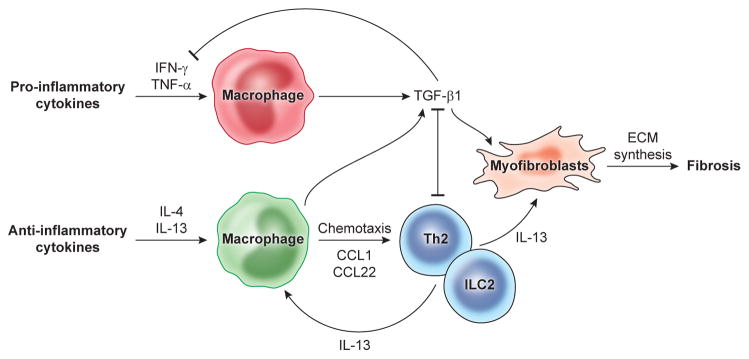
Fig. 2. Cytokine and macrophage-mediated mechanisms of fibrosis.
Inflammatory responses that develop following tissue injury are associated with the production of a variety of inflammatory mediators like IFN-γ and TNF-α that promote classical macrophage activation. TGF-β1 is then produced by macrophages as a regulatory feedback mechanism to facilitate the resolution of the pro-inflammatory response. TGF-β1 also triggers fibroblast activation and development of ECM-producing myofibroblasts that facilitate repair and drive fibrosis. The anti-inflammatory cytokine IL-13, produced by a variety of cell types including type 2 innate lymphoid cells, eosinophils, basophils, and CD4+ Th2 cells, also serves as a major driver of tissue repair and fibrosis by inducing TGF-β1, by directly targeting myofibroblast function, and by promoting the development of restorative M(IL-4)-like macrophages that facilitate the recruitment of IL-13-producing leukocytes to sites of tissue injury.
In addition to producing pro-fibrotic mediators and facilitating the recruitment of inflammatory cells, macrophages have also been shown to directly enhance the survival and activation of myofibroblasts, the key extracellular matrix (ECM) producing cells in all organs. Pradere and colleagues showed that hepatic macrophages enhance myofibroblast survival by stimulating nuclear factor kappa B (NF-κb) activity in fibroblasts, which is critical for the development of liver fibrosis (Pradere et al., 2013). In vivo depletion studies have suggested that mononuclear phagocytes are critical to the activation of myofibroblasts, although further details on the source and phenotype of the pro-fibrotic macrophage population are unclear. They determined that pro-fibrotic macrophage function is highly dependent on TNF-α and IL-1β-induced survival but not activation of hepatic stellate cells in vitro and in vivo. In contrast, macrophage-derived galectin-3, a beta-galactoside-biding lectin that is markedly upregulated in progressive renal fibrosis was critical for the conversion of fibroblasts to the pro-fibrotic phenotype (Henderson et al., 2008). Thus, macrophages exhibiting a pro-fibrotic phenotype participate in the activation and expansion of ECM-producing myofibroblasts via multiple mechanisms.
Macrophages are also important producers of matrix metalloproteinases (MMPs), enzymes that degrade all kinds of ECM proteins, with some MMPs serving as essential drivers of fibrosis. For example, Mmp12 is a macrophage-secreted elastase that is highly induced by IL-13 in the lung and liver during the development of IL-13-dependent fibrosis. Mmp12−/− mice have been shown to develop reduced pulmonary and hepatic fibrosis in response to S. mansoni eggs, with the decreased fibrotic response associated with increased expression of the ECM-degrading MMPs: Mmp2, Mmp9, and Mmp13 (Madala et al., 2010). These findings suggest that IL-13 promotes fibrosis, at least in part, by increasing macrophage metalloelastase activity, which in turn reduces the activity of matrix degrading metalloproteinases. Mmp12 activity is also increased in CCL4 and thioacetamide-induced liver fibrosis, but in these models Mmp12 activity had either no effect or led to slightly decreased fibrosis (Pellicoro et al., 2012). Thus, macrophage-derived Mmp12 exhibits contrasting roles in models of IL-13 and TGF-β1-driven fibrosis.
Finally, Knipper and colleagues have investigated a model of skin repair and showed that collagen fibril assembly following injury is also highly dependent on M(IL-4) macrophages (Knipper et al., 2015). In the absence of IL-4Rα or the M(IL-4)-associated gene Retnla (Relm-alpha), induction of lysyl hydroxylase 2 (LH2), an enzyme that directs persistent pro-fibrotic collagen cross-links, is greatly diminished in injured skin. Thus, in addition to producing several important wound healing and pro-fibrotic mediators, M(IL-4) macrophages also regulate the 3-dimensional structure and size of collagen fibrils during repair.
Protective roles for anti-inflammatory and anti-fibrotic macrophages
Numerous studies have also focused on identifying and characterizing the mechanisms that drive macrophages to exhibit anti-inflammatory and anti-fibrotic activity, as these phenotypes are thought to be critical to the resolution of most wound healing responses. The immunoregulatory cytokine IL-10 is produced by a variety of cell types including Th2 cells, regulatory T (Treg) cells, and macrophages and is known to function as a critical anti-inflammatory mediator (Saraiva and O’Garra, 2010). However, the relative importance of IL-10 secretion versus IL-10 signaling in macrophages has been previously unclear. Two recent papers have addressed this question in models of mucosal healing by performing adoptive transfer studies and generating mice with genetic deletions of IL-10 or the IL-10 receptor alpha chain in macrophages. Shouval and colleagues showed that IL-10R signaling in innate immune cells is critical for the maintenance of anti-inflammatory activity in the intestine, with IL-10R signaling in anti-inflammatory macrophages preventing the development of colitis (Shouval et al., 2014). They have shown that development of early onset inflammatory bowel disease (IBD) in IL-10R-deficient patients is also associated with major defects in the generation and function of anti-inflammatory macrophages. Using mice harboring IL-10 or IL-10 receptor mutations in resident intestinal chemokine receptor CX3CR1-expressing macrophages, Zigmond et al. obtained complimentary findings that showed macrophage-derived IL-10 is dispensable for gut homeostasis and maintenance of colonic Treg cells (Zigmond et al., 2014). In contrast, loss of IL-10 receptor expression was shown to impair the conditioning of monocyte-derived macrophages and resulted in spontaneous development of colitis. Together, these studies identify IL-10 receptor signaling in CX3CR1hi intestinal macrophages as the critical factor controlling intestinal inflammation. A similar role for IL-10R signaling in macrophages has been recently generated in a model of corneal lymphangiogenesis. In these studies, development of lymphangiogenesis is exacerbated in both Il10−/− and Stat3fl/fl lysozyme M cre expressing mice, suggesting that IL-10 and Stat3-mediated signaling in myeloid cells is critical to the prevention of disease (Hos et al., 2015). Together, these studies suggest an ongoing dialogue between IL-10 responsive anti-inflammatory macrophages and other IL-10 producing cells like Treg cells and Th2 cells is critical to the maintenance of immune homeostasis in mucosal tissues.
Although the resolution of inflammation is often associated with the expansion of IL-10 induced anti-inflammatory macrophages, other mechanisms have also been shown to trigger anti-inflammatory macrophage function. For example, De Nardo and colleagues have investigated the mechanisms by which high-density lipoprotein (HDL) protects against atherosclerosis and identified the transcriptional regulator ATF3 as an HDL-inducible target gene in macrophages that down regulates Toll-like receptor-induced pro-inflammatory cytokine production (De Nardo et al., 2014). The transcription factor Nr4a1 has also recently shown to suppress autocrine norepinephrine production in macrophages, which is known to regulate the severity of experimental autoimmune encephalitis (EAE), a mouse model of multiple sclerosis (Shaked et al., 2015). Lack of Nr4a1 in myeloid cells leads to enhanced norepinephrine production, accelerated infiltration of leukocytes into the CNS, and disease exacerbation in vivo. The metabolic controller, orphan nuclear receptor estrogen-related receptor alpha (NR3B1), has been also identified as an important negative regulator of TLR-induced inflammation (Yuk et al., 2015). NR3B1 regulates anti-inflammatory macrophage function by inducing Tnfaip3 transcription and controlling metabolic reprogramming in macrophages. IL-4 and IL-13 have also been shown to induce miR-142-5p and downregulate miR-130a-3p in macrophages, which sustains Stat6 activation and pro-fibrotic gene expression (Su et al., 2015). Finally, N-3 polyunsaturated fatty acids, which exhibit substantial anti-inflammatory in vivo, confer important cardio protective effects by inhibiting macrophage-mediated activation of cardiac fibroblasts. Thus, a variety of mechanisms, besides IL-10, are involved in the development of macrophages with pro-wound healing and anti-inflammatory activity.
The effector mechanisms by which anti-inflammatory macrophages regulate tissue-damaging inflammation have also been a topic of intensive research. For example, a recent study by Westphalen and colleagues has identified a specialized population of sessile alveolar macrophages (AMs) that protects the lung from tissue damaging inflammation by directly communicating immunosuppressive signals to neighboring epithelium (Westphalen et al., 2014). These AMs are attached to the alveolar wall and form connexin 43 (Cx43)-containing gap junction channels with the epithelium. Following LPS-induced inflammation they directly transmit immunosuppressive signals through synchronized Ca2+ waves using the epithelium as the conducting pathway. Bourdonnay and colleagues have also identified trans-cellular delivery of vesicular suppressor of cytokine signaling (SOCS) proteins as another unique form of intercommunication between AMs and epithelial cells, a mechanism that also plays important roles in the resolution of inflammation in the lung (Bourdonnay et al., 2015). They have shown that AMs secrete SOCS1 and -3 exosomes and microparticles, respectively, which are taken up by alveolar epithelial cells leading to the suppression of Stat activation (Figure 3). Delivery of SOCS proteins is actively inhibited in response to cigarette smoke, suggesting that it is a modifiable response during the initiation and resolution of inflammatory responses. In addition to communicating anti-inflammatory signals directly to epithelial cells, macrophages also regulate communication in the epidermis. Knuever and colleagues have shown that myeloid cell-restricted insulin and IGF-1 receptor deficiency protects mice from skin inflammation by decreasing pro-inflammatory cytokine production from epidermal cells (Knuever et al., 2015). Monocyte and macrophage-derived IGF-1 has also been identified as a critical factor in skeletal muscle repair (Tonkin et al., 2015). Finally, anti-inflammatory macrophages also regulate the development and maintenance of IL-10 and TGF-β1 producing Treg cells, which contribute to the resolution of tissue-damaging inflammatory responses in multiple tissues (Soroosh et al., 2013).
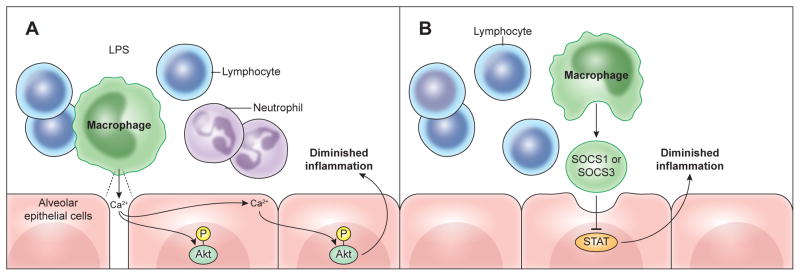
Fig. 3. Anti-inflammatory signaling by macrophages.
Alveolar macrophages transmit key signals to neighboring cells that help facilitate the resolution of inflammation in the lung. A) After LPS inhalation, a specialized population of sessile alveolar macrophages attached to the alveolar wall form gap junction channels with the epithelium, which suppress inflammation by transmitting Ca2+ waves to epithelial cells that result in the phosphorylation of the pro-survival kinase Akt. B) Alveolar macrophages can also dampen inflammation by secreting SOCS1 or SOCS3 proteins in vesicles that are taken up by alveolar epithelial cells. Delivery of SOCS subsequently inhibits epithelial STAT activation, leading to the suppression of pro-inflammatory mediator production.
Although macrophages activated by type-2 cytokines are often linked with tissue repair because they can antagonize the function of pro-inflammatory M(IFN-γ) macrophages that exacerbate tissue damage (Campbell et al., 2013; Kratochvill et al., 2015), recent studies have suggested that they can also exhibit potent anti-fibrotic activity, particularly when the tissue repair response becomes chronic. Indeed, mechanistic studies investigating the role of M(IL-4)-skewed macrophages in chronic models of fibrosis and cancer have suggested they slow the progression of fibrosis and augment cancer progression and metastasis by suppressing local CD4+ T cells responses and reducing ECM production by myofibroblasts (Ostuni et al., 2015; Pesce et al., 2009). In tumors and granulomas, M(IL-4)-skewed macrophages are tightly associated with other inflammatory cells and actively compete with neighboring T cells and myofibroblasts for the amino acids L-arginine and L-ornithine, which become depleted in areas of hypoxia, yet they are critically required for the maintenance of local T cell proliferation and myofibroblast activation (Hesse et al., 2000; Pesce et al., 2009). Thus, nutrient competition between local tissue macrophages and neighboring immune cells has been identified as an additional potent immunosuppressive mechanism employed by regulatory macrophages (Murray et al., 2015). Interestingly, the cytokines IL-6, IL-10, and IL-21 have all been found to enhance IL-4 receptor expression on macrophages, and contribute to the development of anti-inflammatory and anti-fibrotic macrophage function following stimulation with IL-4 or IL-13 (Lang et al., 2002; Mauer et al., 2014; Pesce et al., 2006). Consequently, a variety of cytokines, signaling pathways, and mechanisms collaborate to drive the recruitment, differentiation, and expansion of macrophages that control the resolution of chronic inflammatory and fibrotic responses.
Therapeutic manipulation of macrophage function in repair and fibrosis
Because macrophages serve as both drivers and regulators of disease, therapeutic strategies that reduce the number or function of pro-inflammatory or pro-fibrotic macrophages or boost the activity of anti-inflammatory, anti-fibrotic, pro-resolving, or pro-wound healing macrophages are actively being investigated in several pre-clinical and clinical studies. One relatively straightforward way to manipulate macrophage function is to regulate their numbers by targeting CSF1 and CSF-1R signaling, as CSF1 is critically required for the differentiation of myeloid progenitors into heterogeneous populations of monocytes and macrophages (Hume and MacDonald, 2012). It also regulates their migration, proliferation, function, and survival. Thus, overexpressing or inhibiting CSF1 signaling can substantially alter macrophage density and function. Consequently, CSF-1 protein, antibodies against the ligand and receptor, and inhibitors of CSF-1R kinase activity are all being tested in various disease models and more recently clinical trials have also been initiated. MacDonald and colleagues have found that anti-CSF-1R Ab treatment primarily depletes the maturation and replacement of resident monocytes and tissue macrophages but does not affect the numbers of pro-inflammatory monocytes (MacDonald et al., 2010). Consequently, they have hypothesized it might have little impact on the maintenance of inflammatory disease. Nevertheless, anti-CSF-1R therapy has been recently shown to reduce cutaneous and pulmonary chronic graft-versus-host disease (Alexander et al., 2014). Stutchfield and colleagues also have shown that CSF1-Fc therapy could be used to expand the numbers of protective tissue macrophages in models of acute liver injury and partial hepatectomy (Stutchfield et al., 2015). Therapeutic strategies that target important monocyte and macrophage recruiting chemokine or chemokine receptors have also emerged as possible therapeutic targets (Baeck et al., 2012; Baeck et al., 2014; Chen et al., 2015b; Wehr et al., 2014). Serum amyloid P, a member of the pentraxin family, has been shown to inhibit the accumulation of pro-fibrotic macrophages in the lungs of mice following bleomycin exposure, suggesting it might be developed as a therapeutic strategy for IPF (Murray et al., 2011). Alendronate inhalation into the lungs has also been shown to ameliorate emphysema in mice by inducing apoptosis of alveolar macrophages (Ueno et al., 2015). Adoptive transfer and transplantation techniques employing bone marrow-derived and pulmonary macrophages are also being investigated as strategies to increase the number of restorative macrophages (Suzuki et al., 2014; Thomas et al., 2011). Thus, several distinct therapeutic approaches that lead to changes in the number of macrophages are being investigated.
Another way to enhance the therapeutic potential of macrophages is to specifically target or alter their function. For example, Cao and colleagues have shown that macrophages modified ex vivo by IL-10 and TGF-β stimulation could be used to ameliorate inflammation, pathological structural changes, and functional decline during kidney injury (Cao et al., 2010). Zheng et al. generated a similar protective population by polarizing isolated macrophages with IL-4 and IL-13 and then adoptively transferring the stimulated cells into mice with pancreatic and renal injuries (Zheng et al., 2011). Indeed, pancreatic beta cell renewal following bile duct injury is tightly regulated by M(IL-4) macrophages expressing TGF-β1 (Xiao et al., 2014). IL-25, which promotes type-2 cytokine production and M(IL-4) macrophage development, has also been shown to protect mice from kidney disease (Cao et al., 2011). Lentiviral-mediated delivery of IL-10 to macrophages also represents a promising strategy to induce and sustain macrophage polarization towards a restorative anti-inflammatory phenotype in vivo (Boehler et al., 2014).
Altering key macrophage transcription factors that stabilize or induce particular phenotypes, as Jay et al. did in a model of Alzheimer’s disease, also holds promise for inflammatory and fibrotic diseases (Jay et al., 2015). The transcription factor IRF5 has also been implicated in the polarization of macrophages towards an inflammatory phenotype that can impair wound repair and promote persistent inflammation. Thus, reducing IRF5 activity could be used to increase the number of anti-inflammatory and pro-wound healing macrophages, as was demonstrated recently in models of obesity and insulin resistance (Dalmas et al., 2015). Rapamycin, which blocks mTOR signaling in macrophages and myofibroblasts, has also been shown to ameliorate kidney fibrosis in mice (Chen et al., 2012b). MicroRNAs may also be used to target key transcription factors in macrophages, transforming them from highly activated tissue destructive macrophages into cells resembling a normal quiescent phenotype (Ponomarev et al., 2011). Finally, by mimicking the anti-inflammatory effects of apoptotic cell, phosphatidlyserine-presenting liposomes have also been used to induce reparative IL-10 and TGFβ1-producing cardiac macrophages (Harel-Adar et al., 2011).
Macrophages in Tissue Regeneration
Although macrophages play key regulatory roles during tissue injury and repair, the resident tissue macrophage populations that reside in nearly all tissues of the body also control normal tissue homeostasis and organ regeneration (Wynn et al., 2013). In fact, a recent cell depletion study reveals that macrophages are critically required for full limb regeneration in adult salamanders, but surprisingly, wound closure following limb amputation is much less dependent on macrophages (Godwin et al., 2013). Macrophages were however critical to the clearance of senescent cells (Yun et al., 2015). Therefore, elucidating the mechanisms by which macrophages create a regeneration-permissive environment may reveal strategies for the regeneration of injured organs in adult mammals.
Aurora and colleagues have recently employed a similar macrophage depletion strategy in mice and determined that macrophages provide critical signals that drive angiogenesis and tissue regeneration following myocardial infarction (MI) in neonatal hearts, which are capable of complete regeneration, but only during the earliest days of gestation (Aurora et al., 2014). In the absence of macrophages, however, neonates lose the ability to regenerate myocardia and form fibrotic scars similar to those seen in older animals following an infarct. Interestingly, the beneficial effects of mesenchymal stem cells (MSCs) in adult MI also appear to be dependent on macrophages (Ben-Mordechai et al., 2013), with MSC’s primarily serving a regulatory role by shifting local macrophages from a pro-inflammatory M(IFN-γ) phenotype to a tissue regenerative phenotype similar to M(IL-4) macrophages (Cho et al., 2014). Multi-potent adult progenitor cells have also been shown to exhibit similar M(IL-4) polarizing activity, leading to a reduction in macrophage mediated axonal dieback in spinal cord injury (Busch et al., 2011). M(IL-4) cells, in turn, establish an anti-inflammatory environment that is more accommodating to the survival and growth of both mesenchymal stem cells and progenitor populations in injured tissues, suggesting a mutually beneficial feed-back loop exists between anti-inflammatory macrophages and stem cell populations that drive tissue regeneration (Freytes et al., 2013; Mounier et al., 2013). Therefore, differences in the number of MSCs and other progenitor cells in neonates and adults and/or alterations in the dialogue between stem cells and macrophages can have major impacts on tissue regeneration following injury and in aging.
A complex dialogue between macrophages and several other cell types is also required for the regeneration of peripheral nerves. Similar to neonatal hearts, the peripheral nervous system displays remarkable regenerative ability in that it can fully repair a completely severed nerve. An exciting study by Cattin and colleagues show that blood vessels play a critical role in nerve regeneration by serving as guides or tracks for the regenerative nerve cells to grow along (Cattin et al., 2015). The multi-cellular process is initiated by injury induced hypoxia, which is sensed by local tissue macrophages that then secrete VEGF-α to induce a polarized vasculature that relieves the hypoxia, but at the same time it creates a path for proliferating Schwann cells to migrate across to reconnect the nerve. Thus, VEGF-α production by macrophages was identified as an indispensable mechanism in nerve regeneration.
In addition to stimulating blood vessel development (Ehling et al., 2014), monocytes and macrophages produce a variety of additional mediators that regulate the renewal and function of local tissue progenitor cells that are critical to tissue regeneration. For example, London and colleagues have identified a population of monocyte-derived macrophages producing IL-10 that is required for progenitor cell renewal and neuroprotection in the injured adult murine retina (London et al., 2011). IL-10 also plays a central role in switching muscle macrophages from a pro-inflammatory to reparative phenotype that promotes muscle regeneration (Deng et al., 2012). Nevertheless, studies with Il4rαf/fLyz2cre mice has questioned the overall importance of anti-inflammatory M(IL-4)-like macrophages in muscle regeneration (Goh et al., 2013; Heredia et al., 2013). However, recent studies have also questioned whether the Lyz2-cre mouse is an adequate tool to dissect the roles of M(IL-4)-like monocytes/macrophages in vivo (Vannella et al., 2014). Wnt signaling in macrophages has also been identified as a critical pathway driving parenchymal regeneration in models of liver injury. Following hepatocyte cell death, macrophage engulfment of hepatocyte debris induces Wnt3a, which leads to canonical Wnt signaling in nearby hepatic progenitor cells that facilitates their specification to hepatocytes (Boulter et al., 2012). Regenerative macrophages also facilitate skeletal muscle regeneration by directly targeting myogenic precursor cells (MPCs). Saclier and colleagues demonstrated in vitro that through the differential secretion of cytokines and growth factors, anti-inflammatory macrophages strongly promote MPC differentiation by increasing their commitment into differentiated myocytes and mature myotubes (Saclier et al., 2013). Pro-inflammatory macrophages, in contrast, inhibit myogenic precursor fusion. Thus pro-inflammatory and anti-inflammatory macrophages have direct effects on stem cell fate, leading to substantial impacts on tissue regeneration.
Although effective wound repair and tissue regeneration is often associated with the preferential expansion of local tissue macrophages exhibiting an anti-inflammatory phenotype, when the injury is locally severe or chronic, additional inflammatory monocytes may also be required to restore normal tissue architecture. For example, Chen and colleagues have shown that CCL2 is released from neighboring hair follicles when hairs are plucked, leading to the rapid recruitment of TNF-α secreting inflammatory macrophages, which accumulate near the plucked hair and provide key signals to local stem cells that facilitate the regeneration of new hair follicles (Chen et al., 2015a). In this case, recruitment of pro-inflammatory macrophages is critical to the initiation of the stem cell response that facilitated new hair growth. Nevertheless, the rapid conversion of these pro-inflammatory TNF-α producing macrophages to an anti-inflammatory IL-10 and TGF-β1 producing phenotype appears to be critical to the long-term survival of stem and progenitor cell populations in most tissues (Freytes et al., 2013; Heredia et al., 2013; Lemos et al., 2015). Thus, to facilitate effective organ regeneration and prevent fibrosis, the monocyte and macrophage response must be finely tuned.
Future Directions
As illustrated in this review, monocytes and macrophages are recruited and activated by several distinct mechanisms and assume many functional characteristics that are critical to tissue injury and repair. Although pro-inflammatory and anti-inflammatory macrophages are the two most frequently investigated phenotypes in studies of wound repair, fibrosis and tissue regeneration, macrophages exhibiting pro-wound healing, pro-fibrotic, anti-fibrotic, pro-resolving, and tissue regenerating characteristics are also commonly mentioned in the literature. As highlighted here, there is evidence that these macrophage activation states are not always mutually exclusive. Nevertheless, it remains unclear if some functional subsets represent distinct populations or whether they are simply subtle variants of pro-inflammatory and anti-inflammatory macrophages. Thus, in future work, it will be important to include as many distinguishing characteristics about the macrophages being studied as possible (Murray et al., 2014), including cell surface markers and gene expression analyses that reveal transcriptional and epigenetic profiles, as this will increase our ability to compare findings between research groups and expand our understanding of the unique contributions of the different macrophage populations and activation states during tissue injury and repair in multiple organ systems. A more uniform macrophage nomenclature will also become increasingly important as the number of clinical trials that are based on manipulating monocyte and macrophage function will likely increase substantially over the next few years.
Acknowledgments
TAW and KMV are supported by the intramural research program at NIAID/NIH. We would like to sincerely thank Ethan Tyler and Alan Hoofring, NIH Medical Arts, for help with figures. Both authors would also like to acknowledge our colleagues at the NIH and abroad for many fruitful discussions on this topic, including Peter Murray, John Pesce, Mark Wilson, Satish Madala, Rafael Prado, Trey Gieseck, David Cantu, Kayla Knilans, Robert Thompson, Lee Borthwick, Luke Barron, Kevin Hart, and Thiru Ramalingam.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander KA, Flynn R, Lineburg KE, Kuns RD, Teal BE, Olver SD, Lor M, Raffelt NC, Koyama M, Leveque L, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–4280. doi: 10.1172/JCI75935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–746. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- Baeck C, Wei X, Bartneck M, Fech V, Heymann F, Gassler N, Hittatiya K, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology. 2014;59:1060–1072. doi: 10.1002/hep.26783. [DOI] [PubMed] [Google Scholar]
- Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I, Blum G, Epstein FH, Silman Z, Cohen S, Leor J. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–1901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–158. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Boehler RM, Kuo R, Shin S, Goodman AG, Pilecki MA, Gower RM, Leonard JN, Shea LD. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol Bioeng. 2014;111:1210–1221. doi: 10.1002/bit.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick LA, Barron L, Hart KM, Vannella KM, Thompson RW, Oland S, Cheever A, Sciurba J, Ramalingam TR, Fisher AJ, Wynn TA. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdonnay E, Zaslona Z, Penke LR, Speth JM, Schneider DJ, Przybranowski S, Swanson JA, Mancuso P, Freeman CM, Curtis JL, Peters-Golden M. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med. 2015;212:729–742. doi: 10.1084/jem.20141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Hamilton JA, Horn KP, Cuascut FX, Cutrone R, Lehman N, Deans RJ, Ting AE, Mays RW, Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci. 2011;31:944–953. doi: 10.1523/JNEUROSCI.3566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ. Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol. 2013;133:2461–2470. doi: 10.1038/jid.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Wang C, Zheng D, Wang Y, Lee VW, Wang YM, Zheng G, Tan TK, Yu D, Alexander SI, et al. IL-25 induces M2 macrophages and reduces renal injury in proteinuric kidney disease. J Am Soc Nephrol. 2011;22:1229–1239. doi: 10.1681/ASN.2010070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell. 2015a;161:277–290. doi: 10.1016/j.cell.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012a;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen H, Wang C, Peng Y, Sun L, Liu H, Liu F. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One. 2012b;7:e33626. doi: 10.1371/journal.pone.0033626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, Li X. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest. 2015b;125:2399–2412. doi: 10.1172/JCI80467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujo S, Shirasaki F, Kondo-Miyazaki M, Ikawa Y, Takehara K. Role of connective tissue growth factor and its interaction with basic fibroblast growth factor and macrophage chemoattractant protein-1 in skin fibrosis. J Cell Physiol. 2009;220:189–195. doi: 10.1002/jcp.21750. [DOI] [PubMed] [Google Scholar]
- Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B, Ransohoff RM, Charo IF, Jenne CN, Kubes P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212:447–456. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmas E, Toubal A, Alzaid F, Blazek K, Eames HL, Lebozec K, Pini M, Hainault I, Montastier E, Denis RG, et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat Med. 2015;21:610–618. doi: 10.1038/nm.3829. [DOI] [PubMed] [Google Scholar]
- Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, Xu D, Zimmer S, Lahrmann C, Schildberg FA, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Mockel D, Baeck C, Hittatiya K, Eulberg D, Luedde T, et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960–1971. doi: 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014a;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014b;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, Huang AY, Silver J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol. 2014;254:109–120. doi: 10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184:569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, Chawla A. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, de Bruijn M, Rodewald HR, Geissmann F. The Origin of Tissue-Resident Macrophages: When an Erythro-myeloid Progenitor Is an Erythro-myeloid Progenitor. Immunity. 2015;43:1023–1024. doi: 10.1016/j.immuni.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, Wiens KE, Tang MS, Basu-Roy U, Mansukhani A, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123:e110–122. doi: 10.1182/blood-2013-08-520619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108:1827–1832. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M, Cheever AW, Jankovic D, Wynn TA. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157:945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann F, Hammerich L, Storch D, Bartneck M, Huss S, Russeler V, Gassler N, Lira SA, Luedde T, Trautwein C, Tacke F. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology. 2012;55:898–909. doi: 10.1002/hep.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hos D, Bucher F, Regenfuss B, Dreisow ML, Bock F, Heindl LM, Eming SA, Cursiefen C. IL-10 Indirectly Regulates Corneal Lymphangiogenesis and Resolution of Inflammation via Macrophages. Am J Pathol. 2015 doi: 10.1016/j.ajpath.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J Exp Med. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170:727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth DC. Pro-resolution properties of macrophages in renal injury. Kidney Int. 2007;72:234–236. doi: 10.1038/sj.ki.5002332. [DOI] [PubMed] [Google Scholar]
- Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maass T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg ME, et al. Interleukin-4 Receptor alpha Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity. 2015;43:803–816. doi: 10.1016/j.immuni.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuever J, Willenborg S, Ding X, Akyuz MD, Partridge L, Niessen CM, Bruning JC, Eming SA. Myeloid Cell-Restricted Insulin/IGF-1 Receptor Deficiency Protects against Skin Inflammation. J Immunol. 2015;195:5296–5308. doi: 10.4049/jimmunol.1501237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf-Klingebiel M, Reboll MR, Klede S, Brod T, Pich A, Polten F, Napp LC, Bauersachs J, Ganser A, Brinkmann E, et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat Med. 2015;21:140–149. doi: 10.1038/nm.3778. [DOI] [PubMed] [Google Scholar]
- Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, McEvoy J, Roussel MF, Dyer MA, Qualls JE, Murray PJ. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12:1902–1914. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011;208:23–39. doi: 10.1084/jem.20101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorchner H, Poling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, et al. Myocardial healing requires Reg3beta-dependent accumulation of macrophages in the ischemic heart. Nat Med. 2015;21:353–362. doi: 10.1038/nm.3816. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- Madala SK, Pesce JT, Ramalingam TR, Wilson MS, Minnicozzi S, Cheever AW, Thompson RW, Mentink-Kane MM, Wynn TA. Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis. J Immunol. 2010;184:3955–3963. doi: 10.4049/jimmunol.0903008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Renia L, Pol S, Mallet V, Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174:1766–1775. doi: 10.2353/ajpath.2009.080632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, Sanz N, Ferry A, Sakamoto K, Foretz M, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Munoz-Canoves P, Serrano AL. Macrophages decide between regeneration and fibrosis in muscle. Trends Endocrinol Metab. 2015;26:449–450. doi: 10.1016/j.tem.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43:154–162. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Rathmell J, Pearce E. SnapShot: Immunometabolism. Cell Metab. 2015;22:190–190. e191. doi: 10.1016/j.cmet.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, Gale M., Jr IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol. 2014;232:344–355. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, Hartland SN, Vernon M, Duffield JS, Benyon RC, et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
- Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, Jang MK, Guenther ND, Mederacke I, Friedman R, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Iredale JP, Fallowfield JA. Resolution of liver fibrosis: basic mechanisms and clinical relevance. Semin Liver Dis. 2015;35:119–131. doi: 10.1055/s-0035-1550057. [DOI] [PubMed] [Google Scholar]
- Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988;241:708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Shaked I, Hanna RN, Shaked H, Chodaczek G, Nowyhed HN, Tweet G, Tacke R, Basat AB, Mikulski Z, Togher S, et al. Transcription factor Nr4a1 couples sympathetic and inflammatory cues in CNS-recruited macrophages to limit neuroinflammation. Nat Immunol. 2015;16:1228–1234. doi: 10.1038/ni.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokado K, Raines EW, Madtes DK, Barrett TB, Benditt EP, Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985;43:277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, Mascanfroni ID, Al Adham Z, Lavoie S, Ibourk M, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutchfield BM, Antoine DJ, Mackinnon AC, Gow DJ, Bain CC, Hawley CA, Hughes MJ, Francis B, Wojtacha D, Man TY, et al. CSF1 Restores Innate Immunity After Liver Injury in Mice and Serum Levels Indicate Outcomes of Patients With Acute Liver Failure. Gastroenterology. 2015;149:1896–1909. e1814. doi: 10.1053/j.gastro.2015.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Su S, Zhao Q, He C, Huang D, Liu J, Chen F, Chen J, Liao JY, Cui X, Zeng Y, et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015;6:8523. doi: 10.1038/ncomms9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Arumugam P, Sakagami T, Lachmann N, Chalk C, Sallese A, Abe S, Trapnell C, Carey B, Moritz T, et al. Pulmonary macrophage transplantation therapy. Nature. 2014;514:450–454. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, Van Deemter M, Hume DA, Iredale JP, Forbes SJ. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musaro A, Rosenthal N. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol Ther. 2015;23:1189–1200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Maeno T, Nishimura S, Ogata F, Masubuchi H, Hara K, Yamaguchi K, Aoki F, Suga T, Nagai R, Kurabayashi M. Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat Commun. 2015;6:6332. doi: 10.1038/ncomms7332. [DOI] [PubMed] [Google Scholar]
- Ueno M, Maeno T, Nomura M, Aoyagi-Ikeda K, Matsui H, Hara K, Tanaka T, Iso T, Suga T, Kurabayashi M. Hypoxia-inducible factor-1alpha mediates TGF-beta-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L740–752. doi: 10.1152/ajplung.00146.2010. [DOI] [PubMed] [Google Scholar]
- Vannella KM, Barron L, Borthwick LA, Kindrachuk KN, Narasimhan PB, Hart KM, Thompson RW, White S, Cheever AW, Ramalingam TR, Wynn TA. Incomplete deletion of IL-4Ralpha by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog. 2014;10:e1004372. doi: 10.1371/journal.ppat.1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr A, Baeck C, Ulmer F, Gassler N, Hittatiya K, Luedde T, Neumann UP, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS One. 2014;9:e112327. doi: 10.1371/journal.pone.0112327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, Song Z, El-Gohary Y, Prasadan K, Shiota C, Gittes GK. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci U S A. 2014;111:E1211–1220. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, Outtz H, Kitajewski J, Shi C, Weber S, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13:642–650. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chi F, Guo T, Punj V, Lee WN, French SW, Tsukamoto H. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125:1579–1590. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk JM, Kim TS, Kim SY, Lee HM, Han J, Dufour CR, Kim JK, Jin HS, Yang CS, Park KS, et al. Orphan Nuclear Receptor ERRalpha Controls Macrophage Metabolic Signaling and A20 Expression to Negatively Regulate TLR-Induced Inflammation. Immunity. 2015;43:80–91. doi: 10.1016/j.immuni.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Yun MH, Davaapil H, Brockes JP. Recurrent turnover of senescent cells during regeneration of a complex structure. Elife. 2015;4 doi: 10.7554/eLife.05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, et al. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Wang Y, Cao Q, Lee VW, Zheng G, Sun Y, Tan TK, Wang Y, Alexander SI, Harris DC. Transfused macrophages ameliorate pancreatic and renal injury in murine diabetes mellitus. Nephron Exp Nephrol. 2011;118:e87–99. doi: 10.1159/000321034. [DOI] [PubMed] [Google Scholar]
- Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, Brenner O, Krauthgamer R, Varol C, Muller W, Jung S. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]