Abstract
Habitat fragmentation changes thermal conditions in remnant patches, and thermal conditions strongly influence organism morphology, distribution, and activity patterns. However, few studies explore temperature as a mechanism driving ecological responses to fragmentation. Here we offer a conceptual framework that integrates thermal biology into fragmentation research to better understand individual, species, community, and ecosystem‐level responses to fragmentation. Specifically, the framework addresses how fragmentation changes temperature and how the effects of those temperature changes spread through the ecosystem, from organism response via thermal sensitivity, to changes in species distribution and activity patterns, to shifts in community structure following species' responses, and ultimately to changes in ecosystem functions. We place a strong emphasis on future research directions by outlining “Critical gaps” for each step of the framework. Empirical efforts to apply and test this framework promise new understanding of fragmentation's ecological consequences and new strategies for conservation in an increasingly fragmented and warmer world.
Keywords: Climate change, conservation, edge effect, extinction, habitat fragmentation, spatial, temperature, thermal biology, thermal safety margin, warming tolerance
Introduction
Habitat fragmentation is one of the greatest contributors to biodiversity loss worldwide (CBD 2010) and increasing rates of fragmentation underscore the importance of understanding the full spectrum of its ecological consequences (Haddad et al. 2015). Fragmentation can be defined as the division of once‐continuous habitat into smaller and more isolated patches separated by a matrix of human‐transformed land cover (Haddad et al. 2015). Most fragmentation results in habitat patches surrounded by a matrix of low biomass and low structural complexity, such as pasture, cropland or concrete, with thermal conditions unlike those of the remaining patches (Murcia 1995).
Studies on the effects of fragmentation in terrestrial systems abound, and many highlight the mechanisms underlying ecological changes following fragmentation, including altered dispersal dynamics (Templeton et al. 2011; Damschen et al. 2014) and changes in metapopulation and metacommunity processes (Jamoneau et al. 2012; De la Sancha et al. 2014). However, few studies explore temperature as a mechanism driving changes in fragmented landscapes, despite the understanding that fragmentation changes thermal conditions in remnant patches (Murcia 1995; Chen et al. 1999; Laurance 2004; Table 1), and that thermal conditions can strongly influence organism morphology, distribution, activity patterns and evolutionary trajectory (Huey 1982; Angilletta 2009; Kingsolver 2009; Puurtinen et al. 2015).
Table 1.
Findings from studies measuring differences in mean, maximum and minimum air temperature and diurnal temperature range at patch edge vs. interior sites within remnant patches in fragmented forests
| References | Forest type | Location | Edge age (YRS.) | Edge penetration distance (M) | Air temperature increases at edge | |||
|---|---|---|---|---|---|---|---|---|
| Mean (°C) | Max (°C) | Min (°C) | Range (°C) | |||||
| Kapos (1989) | Tropical lowland rainforest with pasture matrix | Brazilian Amazon | 1 month | 20–60 | 1.4–2.8 | NR | NR | NR |
| Williams‐Linera (1990) | Tropical pre‐montane forest with pasture matrix | Panama | 0.8–5 | 2.5–15 | NM | 1.5–3.1 M | 0–1.0 M | NR |
| Matlack (1993) | Oak‐Chestnut forest with field matrix | Pennsylvania & Delaware, USA | 1–5 | 24 |
0–5.3
3.1–5.8 M |
NR | NR | NR |
| Chen et al. (1993) | Old‐growth Douglas Fir forest with mixed‐conifer matrix (< 2 m tall) | Washington, USA | 10–15 | 250 |
2.7–3.6
7.2–9.1 |
3.5 | NR | 3–5
7–10 |
| Chen et al. (1995) | Old‐growth Douglas Fir forest with mixed‐conifer matrix (< 2 m tall) | Washington, USA | 10–15 | 180–240 | 4.4–5.4 | NR | NR |
1.3–7.8 1.7–15.5
1.7–15.5
|
| Didham & Lawton (1999) | Tropical lowland rainforest with pasture matrix | Brazilian Amazon | 10–12 | 100–184 | 2.6–3.7 | NR | NR | NR |
| Yan et al. (2007) | Evergreen broadleaved forests | Jinyun Mountains, China | NR | 15–25 | NR | 1.5–2 | NR |
2–6
5–9 |
| Sato et al. (2014) | Alpine Eucalyptus forest with grassy ski‐run matrix | New South Wales, Australia | NR | 2 | 3.6 | 10.8 | −0.5 | 11.3 |
For example Kapos (1989) found mean air temperatures average 1.4–2.8 °C higher at patch edges than interiors. Temperatures were measured using different methods across studies, so patterns are only broadly comparable. Soil temperatures (bold italicised) and air temperatures in the matrix (‘M’) were included when possible. ‘Edge penetration distance’ is the distance into a patch at which temperature differences between edge and interior sites are no longer noticeable. NR = ‘not reported by author’.
Indeed, one of the most immediate and consistently documented effects of forest fragmentation is an increase in direct and diffuse solar radiation at newly created edges, often leading to hotter, drier and more variable microclimate conditions within remnant patches (Murcia 1995; Chen et al. 1999; Laurance 2004). While fragmentation has important effects on a suite of microclimate variables, including light intensity, humidity, wind speed and air pressure (Murcia 1995; Chen et al. 1999; Laurance 2004), here we focus on the ecological consequences of temperature increases.
Specifically, we offer a conceptual framework that integrates thermal biology into fragmentation research to better understand the role of temperature in species, community and ecosystem‐level responses to fragmentation. Our framework addresses how fragmentation changes local and regional temperatures and how the effects of those temperature changes spread through the fabric of the ecosystem, from organism response via thermal sensitivity, to changes in species distribution and activity patterns, to shifts in community structure following species' responses, and ultimately to changes in ecosystem functions (Fig. 1). We focus on forest ecosystems because changes in thermal conditions are well documented and relatively consistent (Table 1). In section 2.1 we explore how the concepts might extend to non‐forest ecosystems.
Figure 1.
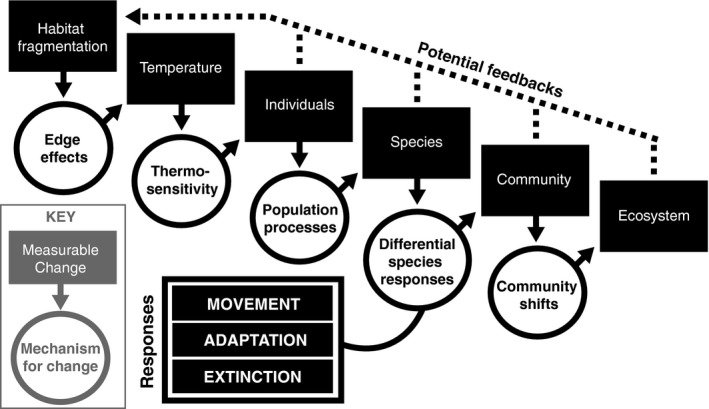
The cascade of effects that follow a thermal disturbance, such as fragmentation. Fragmentation leads to increased temperatures in remnant habitat patches, which will affect individuals, and ultimately species, according to their thermosensitivity. Broadly, species can respond to thermal stress by moving in space, adapting to new conditions or going extinct. Different species will respond differently to the same temperature increase, and both symmetries and asymmetries in species' responses can lead to changes in community structure and dynamics. Shifts at the species and community level can even alter ecosystem functions in fragmented landscapes. We expect the intensity of the thermal disturbance and the thermal sensitivity of the organisms and interactions involved determine how far the effects cascade through the ecosystem. This framework offers a new tool to examine temperature as a mechanism driving ecological changes in fragmented landscapes.
We also focus on diurnal species because they are more likely to encounter stressfully high body temperatures during their active period than are nocturnal species (Kearney et al. 2009). Night‐time temperatures are slightly lower at patch edges than at patch interiors, as areas near the edge lose higher amounts of long‐wave radiation back to the atmosphere at night (Chen et al. 1995). Sites at the interior of tropical forest fragments, for example were 0.2 ± 0.1 °C warmer than in the pasture matrix at night, regardless of weather conditions (Daily & Ehrlich 1996). Thus, it is not likely that the responses of nocturnal species to fragmentation are heavily driven by thermal biology.
The effects of warming on organisms have come to the forefront of ecological research in the last several decades because of climate change projections (Huey et al. 2009; Kearney et al. 2009; Somero 2010; Gerick et al. 2014; Dohet et al. 2015). However, climate change is not the only factor increasing temperatures in landscapes throughout the world. Ground surface temperature is strongly dependent on vegetation cover (Hong et al. 1995), and fragmentation replaces continuous vegetation cover with a mosaic of matrix and remnant habitat. These changes in vegetation cover can alter the circulation of heat, moisture and momentum in the landscape (Hong et al. 1995), and fragmentation has been linked to warmer, drier climates across the globe, including Australia (McAlpine et al. 2007), the Amazon Basin (Cochrane & Laurance 2008) and West Africa (Garcia‐Carreras & Parker 2011).
A recent trend in climate change research is to use species' thermal biology to predict their response to climate warming (Buckley 2008, Gvozdík 2012; Tattersall et al. 2012; Tunney et al. 2014). Through the integration of thermal biology, our understanding of the effects of climate change has seen several noteworthy advancements: (1) scientists can better predict range shifts because of an understanding of thermal preferences (Kearney & Porter 2009; Valladares et al. 2014); (2) scientists can better explain declines in body size because of temperature‐size relationships (Sheridan & Bickford 2011); and (3) scientists can better understand the impacts of changes in air temperature on organisms in the landscape with the use of operative temperature, as opposed to air temperature alone (Sears et al. 2011; Hovick et al. 2014; Kearney et al. 2014). The integration of thermal biology into climate change research has led to huge developments in our understanding of extinction risk under global warming scenarios, and we predict that integrating thermal biology into fragmentation research can lead to equally large advancements in our understanding of fragmentation's effects.
Fragmentation produces its own signature warming, set apart from climate change by the short time scale and spatial arrangement within which temperature increases occur. The differences between climate change and fragmentation‐driven warming can influence extinction predictions and, thus, warrant a unique framework that addresses temperature as a mechanism driving ecological changes in fragmented landscapes. We advocate for empirical efforts to apply and test the fragmentation‐temperature framework, to improve our understanding of fragmentation's ecological consequences by explicitly considering the impacts of temperature increases.
Fragmentation‐temperature framework
The fragmentation‐temperature framework links thermal biology and fragmentation research through a shared interest in understanding the effects of temperature change on organisms. The framework is structured using classic scales of ecological organisation: individual, species, community and ecosystem. We outline ‘Critical gaps’ for each scale in the hopes of guiding future research. The first step of the framework details patterns in fragmentation's effects on thermal conditions. The framework then reviews the relationships between temperature changes and organismal responses starting with the smallest scale, individuals, and ending with the largest scale, ecosystems.
The effects of fragmentation on temperature
Tree canopies intercept both incoming solar and outgoing long‐wave radiation, creating cooler and more uniform thermal environments (Murcia 1995). When overstory vegetation is removed, such as during the fragmentation process, both incoming and outgoing radiation are increased, leading to hotter, drier and more variable thermal conditions in remnant patches (Chen et al. 1999). Studies show daytime air temperatures average 2–5 °C higher at patch edges than patch interiors, daytime soil temperatures average 7–9 °C higher at patch edges than patch interiors, and maximum air temperatures at edges can average up to 10 °C higher than patch interiors (Table 1). These thermal changes hold relatively constant across forest types and latitudes (Table 1) and rival the temperature increases expected from decades of climate change (IPCC 2014), although they occur over rapid time scales. Unlike mean and maximum temperatures, minimum temperatures (Ewers & Banks‐Leite 2013, Table 1) and night‐time temperatures (Daily & Ehrlich 1996) rarely differ across edge, interior and matrix areas.
In contrast to warming from climate change, warming in fragmented landscapes has a fixed, spatial arrangement, such that increased temperatures at forest edges create a thermal gradient that declines exponentially from edge to interior (Williams‐Linera 1990; Didham & Lawton 1999; Saunders et al. 1999). Temperature gradients can extend variable distances inside a remnant patch, depending on forest structure at the edge (‘Edge penetration distance’, Table 1). However, the gradient itself can reverse over the course of the day, with temperatures declining towards the interior during the day but increasing towards the interior at night (Chen et al. 1995). Consequently, the temporal range near an edge is also much higher than the natural variation within continuous habitat (Table 1).
Ultimately, organisms near patch edges must cope with higher average temperatures, greater temperature maximums and larger thermal variability throughout the day (Table 1). For small patches or those with high edge‐to‐interior ratios, these thermal changes may penetrate the entirety of the patch (Didham & Lawton 1999). However, the severity of thermal changes depends on the season, weather conditions and orientation of the patch, which control the amount of exposure to solar radiation (Chen et al. 1993, 1995; Didham & Lawton 1999). For example the steepest thermal gradients occur on warm, sunny days and at edges receiving direct radiation (Chen et al. 1993).
The structure and thermal conditions of the matrix also influence thermal conditions within patches. For example, edge penetration distances for microclimate variables were two to five times greater at open edges than at closed edges in Amazonian forest patches (Didham & Lawton 1999). We suspect that the intensity of thermal disturbance following fragmentation influences how far the effects cascade through the ecosystem (Fig. 1). Although even modest temperature increases can generate a cascade of changing biotic interactions that affect the structural properties of the ecosystem (2.5 °C, Barton & Schmitz 2009).
Differences in thermal conditions between patch and matrix habitat are largely the result of differences in structural complexity and biomass between the two habitat types (Murcia 1995). Thus, it is possible that fragmentation will not significantly alter thermal conditions or create thermal gradients in remnant patches when the contrast between patch and matrix habitat is low (e.g. grasslands, savannas, wetlands, arid and semi‐arid systems). In marine ecosystems, such as fragmented sea grass meadows, patch edges experience greater exposure to cooler, open‐ocean currents (Fonesca & Fisher 1986), likely leading to reversed thermal gradients at patch edges and reversed spatial patterns in ecological responses. More research is needed to determine the impacts of fragmentation on thermal conditions in non‐forest ecosystems to apply this framework. Consequently, the scope of this paper is largely limited to the fragmentation of forest ecosystems.
Fragmentation not only alters microclimates within remnant forest patches, it changes the state of the atmosphere locally and can influence circulation regionally (Dirmeyer & Shukla 1994). In continuous forest, differences in surface air temperatures are minimal because the canopy shields ground temperatures from high levels of solar radiation and buffers temperature flux, leading to a thermally stable surface condition (Hong et al. 1995). In fragmented forests, however, the mosaic of matrix and forest cover leads to anomalies in surface temperatures in the landscape. When these anomalies are large, it can induce novel air circulations (Hong et al. 1995). A notable example in fragmented landscapes is a heat‐induced airflow known as the ‘vegetation breeze’ (Cochrane & Laurance 2008).
The vegetation breeze stems from the juxtaposition of deforested and forested areas and is driven by the temperature differences between them (Cochrane & Laurance 2008). The air above forests tends to be cool and moist, whereas the air above clearings tends to be hot and dry. As a consequence, the air above clearings heats up and rises, reducing local air pressure and drawing moist air from the surrounding forest patches into the clearing. As the rising air gets cooler, the moisture it carries condenses into convective clouds that produce rainfall in the clearing. The air is then recycled – as hot, dry air – back into the forest patches (Cochrane & Laurance 2008).
The net effect of the vegetation breeze is that forest clearings receive more rainfall and forest patches receive less. In West Africa, for example rainfall in warmer cropland areas was 46 times higher than cooler forest areas, and rainfall in forests decreased by more than 50% following land conversion to cropland (Garcia‐Carreras & Parker 2011). In addition, forest patches become increasingly desiccated, as moist air is actively drawn from the patches into clearings and hot, dry air is fed back in. Field observations and heat‐flux simulations suggest that desiccating conditions can penetrate up to 100–200 m into fragments from adjoining clearings (Didham & Lawton 1999; Laurance et al. 2011).
Temperature increases resulting from the fragmentation process can therefore lead to increased desiccation of forest patches, which may threaten patch persistence. In Brazilian Amazonia, where nearly 20 000 km of new forest edges are created each year, studies found sharply elevated rates of tree mortality, damage and canopy‐gap formation at patch edges, at least partially related to higher desiccation rates (Laurance 2004; Cochrane & Laurance 2008; Laurance et al. 2011).
A prediction stemming from the vegetation breeze is that patches will erode over time, as degradation at patch edges perpetuates further degradation through increased exposure to cleared areas. Instead of maintaining their size, we expect forest patches to decline in area over time, leading to increased distance between patches and, in essence, further fragmentation. However, this shrinking‐patch effect has never been described in fragmented landscapes, and it is possible that compensatory changes prohibit such shrinking from occurring. Studies in tropical forest fragments show that vines, secondary vegetation and lateral branch growth typically ‘seal’ patch edges within 5–10 years, making the patch less permeable to microclimate changes (Matlack 1993; Didham & Lawton 1999; Laurance et al. 2011). In forest types that regenerate more slowly, however, microclimate changes may persist at patch edges for longer (Table 1).
Critical gaps
A recent synthesis found that 20% of the world's remaining forests lie within 100 m of an edge and 70% lie within 1 km of an edge (Haddad et al. 2015), suggesting that a large portion of the world's remaining forests experience thermal conditions unlike those of continuous forest. We need more studies targeting thermal conditions in fragmented landscapes to better apply the concepts presented in this framework and more effectively tease apart temperature's role in species' responses to fragmentation.
Specifically, future studies should focus on thermal conditions before and after fragmentation, between edge and interior areas, over time as matrix and edge structures change, and in habitats other than forests, where patterns may differ. Our understanding of fragmentation‐driven warming will grow even more rapidly by incorporating innovative technologies into thermal studies, like infrared imagery, devices tracking field body temperatures, high‐resolution thermal sensors and biophysical models.
The effects of temperature on individuals
In the second step of the fragmentation‐temperature framework, we explore the relationship between thermal conditions and fitness and present new ways to estimate the impacts of fragmentation on individuals.
Organisms regulate body temperature to stay within a preferred thermal range and away from thermal limitations, which can be quantified with a thermal performance curve. A thermal performance curve measures the relationship between body temperature and a physiological performance, such as metabolic rate, digestive efficiency or sprint speed (Huey 1982; Huey & Kingsolver 1989; Angilletta et al. 2002; Fig. 2). From a thermal performance curve, we can determine the temperature at which the organism's performance is maximised (T OPT) and the range of body temperatures over which the organism can perform relatively well (performance breadth) (Huey 1982; Huey & Kingsolver 1989; Fig. 2). Thermal performance curves also provide the critical thermal limits (CT MAX and CT MIN) beyond which the organism can no longer perform essential functions, such as the righting response or locomotion, and thus loses the ability to forage or escape from predators (Huey 1982; Sato et al. 2014; Fig. 2).
Figure 2.
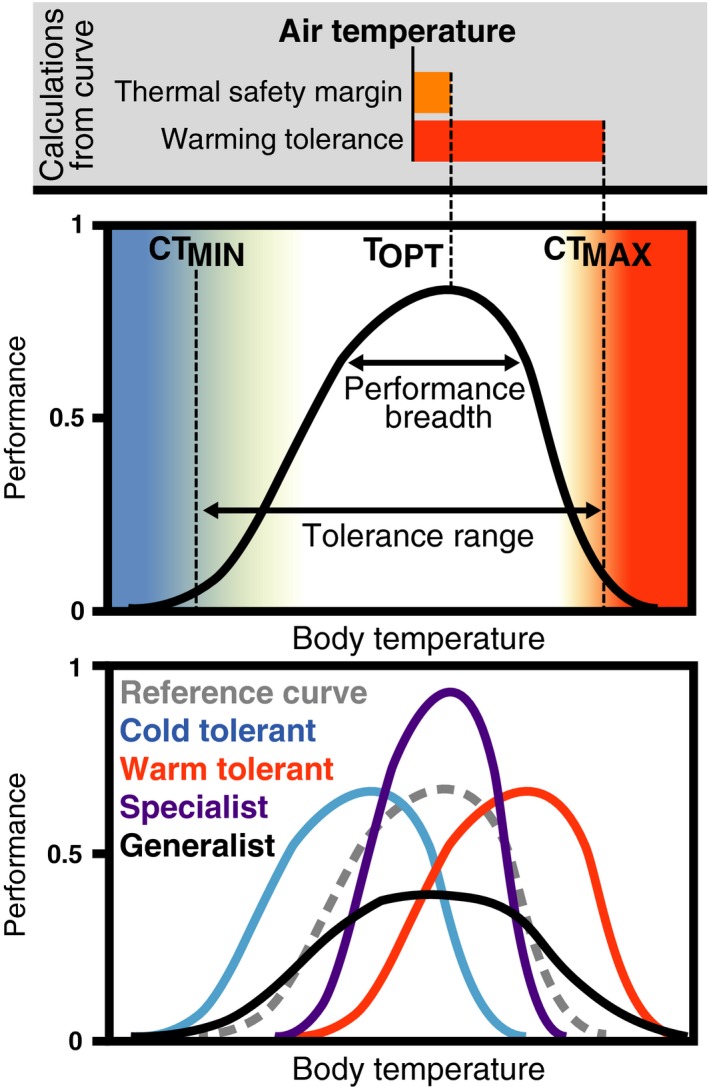
A thermal performance curve describes the relationship between an organism's body temperature and its physiological performance. Middle panel: anatomy of a hypothetical thermal performance curve, including the organism's critical thermal minimum (CTMIN), critical thermal maximum (CTMAX), optimal body temperature (TOPT), performance breadth and tolerance range. Top panel: we can compare environmental temperature data (here we use air temperature) with the thermal performance curve to estimate the impacts of warming on physiological performance. Two indications of vulnerability to warming include (1) how much air temperature differs from the organism's optimal temperature (thermal safety margin) and (2) how much air temperature differs from the organism's thermal maximum (warming tolerance). Bottom panel: thermal performance curves vary with life history. These curves represent cold‐tolerant species (blue), warm‐tolerant species (red), thermal specialist species (purple), and thermal generalist species (black) and a generic reference curve (dashed grey) for comparison.
Researchers typically develop thermal performance curves for ectothermic species, but the same nonlinear relationship between body temperature and physiological performance exists in endotherms (Angilletta et al. 2010; Huey et al. 2012). Ectotherms and endotherms differ in that body temperature in ectotherms mirrors environmental temperatures, allowing us to predict changes in performance using changes in environmental conditions (Kingsolver 2009).
Thermal performance curves prove vital for this framework because researchers can use them to calculate changes in physiological performance in fragmented landscapes, given known changes in thermal conditions. It is important to note that thermal performance curves are based on body temperature and not air temperature. However, air temperature is easier to measure than the body temperature of individuals, so researchers developed ‘operative temperature’ models to gain access to thermal performance curves for making predictions (Porter & Gates 1969). Operative temperature is the temperature experienced by an organism, which is calculated from air temperature using a host of environmental and biophysical variables (Porter & Gates 1969; Kearney et al. 2014). Here, we use air temperature as a proxy for body temperature in ectotherms to demonstrate concepts and calculations. Researchers applying the framework should use operative temperature or field body temperature data when possible to provide superior estimates of physiological performance at sites in fragmented landscapes.
To offer an example, air temperatures in recently fragmented forests average 2–5 °C higher at patch edges than interiors (Table 1), which implies that ectotherms at newly created edges experience body temperatures approximately 2–5 °C higher than before fragmentation. We can estimate the impacts of these higher temperatures on ectotherm fitness by measuring the degrees between new air temperatures and T OPT (thermal safety margin) and the degrees between new air temperatures and CT MAX (warming tolerance) (Deutsch et al. 2008; Kingsolver 2009; Gerick et al. 2014) (Fig. 2). Because thermal conditions vary throughout the day and across seasons, researchers often calculate thermal safety margins and warming tolerance during peak activity time or during the warmest months to more accurately measure thermal stress. For example, to measure the effects of climate warming on sprint speed in diurnal lizards, Huey et al. (2009) focused on air temperature records between 10 : 00 and 14 : 00 in the summer season, whereas Gerick et al. (2014) used maximum summer air temperatures to measure thermal safety margins.
We predict that individuals with small thermal safety margins will exhibit lower fitness at patch edges and in small patches because thermal conditions following fragmentation are more likely to exceed the organism's thermal optimum, resulting in reduced physiological performance. For example researchers often use the thermal performance curve for sprint speed as a proxy for the relationship between body temperature and predation success (Huey 1982). Crested anoles (Anolis cristatellus) sprint at > 90 % of their maximal speed from sunrise to sunset in the current thermal conditions of their forest habitat (Huey et al. 2009). Huey et al. (2009) showed that a 3 °C increase in air temperature – a conservative estimate for temperature increase following fragmentation – would result in elevated body temperatures that exceed the species' T OPT, reducing sprint speed in A. cristatellus individuals to 40–80% of maximal during peak activity time.
We also predict that individuals with low warming tolerance will experience lower fitness at patch edges and in small patches because thermal conditions following fragmentation are more likely to approach the organism's critical thermal limit, increasing its risk of physiological shutdown. For example Huey et al. (2009) showed that a 3 °C increase in air temperature would push summer body temperatures in Puerto Rican ground‐dwelling geckos (Sphaerodactylus) to only 3.5 °C below the species' CT MAX, resulting in severe thermal stress in individuals. The thermal extremes that often occur at patch edges (10 °C higher than interiors, Sato et al. 2014) and in cleared areas (13 °C higher than in forest patches, Currylow et al. 2012; 20 °C higher than in forest patches, Boggs & McNulty 2010) will be especially problematic to individuals with low warming tolerance because of the high risk of overheating.
However, temperature increases following fragmentation may create more favourable thermal conditions for some individuals, allowing them to forage earlier and longer in remnant patches than in continuous forest (Saunders et al. 1991). For species with high thermal optima, for example warming may confer a considerable advantage in terms of time spent within optimal limits (Kearney et al. 2009). In section 2.3, we identify some species for whom a positive response to warming is likely. Similarly, in the absence of thermal stress, higher temperatures should yield faster biochemical reactions, which could improve performance at the organismal level (‘hotter is better’ theory, Angilletta 2009).
For individuals that can thermoregulate effectively, changes in air temperature may not extend to changes in body temperature through simple shifts in thermoregulatory behaviour. For example a common method for ectothermic organisms to thermoregulate is to ‘shuttle’ between sun and shade or hot and cold microclimates (Huey 1974). Shuttling refers to the movement between hot and cold states to maintain an optimal temperature (Dreisig 1984). When environmental temperatures increase, individuals can adjust their shuttling behaviour to reduce risk of overheating, such as increasing the amount of time spent in thermal refugia (Sinervo et al. 2010; Kearney 2013) or shifting activity time to cooler parts of the day (Logan et al. 2006; Huey et al. 2012). However, activity restrictions can limit foraging time, constraining metabolic functions like reproduction (Sinervo et al. 2010). Thermoregulation in hotter forest patches therefore necessitates that individuals renegotiate the trade‐off between time spent foraging and time spent cooling down to avoid reductions in physiological performance.
Shuttling behaviour also becomes more challenging following fragmentation because increased temperatures are coupled with declining availability of thermal refugia. Most fragmentation reduces structural complexity (Murcia 1995); thus, shade and cool microclimates become harder to find, and individuals must increase movement within a patch and/or disperse to larger patches to meet their thermoregulatory needs. These actions require increased energetic output and often increase predation risk (Huey 1974; Angilletta et al. 2002), making thermoregulation through shuttling behaviour a longer, more energetically costly, and riskier process in fragmented landscapes.
These challenges can be exacerbated by a commonly observed phenomenon in remnant patches known as the ‘crowding effect,’ whereby population densities in remnant patches increase following habitat removal, as surviving individuals in the matrix crowd into the remaining habitat (Debinski & Holt 2000; Grez et al. 2004). When population densities increase, there will likely be higher competition for limited thermal refugia, leading to increased antagonistic interactions and higher competition for places to cool off.
Critical gaps
Ultimately, fragmentation will impact individuals according to their thermosensitivity and their capacity to buffer the impacts of warming through behaviour, morphology and physiology (Kearney et al. 2009). We can predict thermally driven reductions or enhancement in fitness by calculating thermal safety margins and warming tolerance before and after fragmentation, in different patch sizes and shapes, and between edge and interior areas.
Thermal performance curves exist for a wide range of ectothermic species (Angilletta 2006), and thermal safety margins and warming tolerance can be easily calculated using estimated or known increases in environmental temperatures. However, more reliable projections of the impacts of fragmentation on performance can be developed by combining temperature data with information on the species' thermal physiology, ability to alter thermoregulatory behaviour, and activity patterns (Huey et al. 2009). For many organisms in remnant patches, the negative consequences of warming will not result from increased exposure to lethal temperatures, but will instead result from sub‐lethal effects, such as energetic imbalances and reductions in activity time (Gunderson & Leal 2016). Thermal constraints on activity are considered as a mechanistic link between temperature increases and population processes (Kearney 2013; Gunderson & Leal 2016). Thus, studies targeting increases or reductions in activity time following fragmentation will provide new insight into population dynamics and persistence in remnant patches.
We suggest studies employ quantitative approaches, such as calculating body temperatures given behavioural scenarios, including (1) individuals sitting passively on the surface in matrix (full sun) conditions, (2) individuals sitting passively on the surface in forest interior (deep shade) conditions and (3) individuals at the patch edge actively shuttling between forest and matrix habitat to thermoregulate (see Kearney et al. 2009 for details). Such models can shed new light onto the thermoregulatory value of different types of matrix habitat, patch configurations and stage of matrix regrowth.
The relationship between body temperature and fitness is well established, but this knowledge has rarely been applied to individuals in fragmented landscapes. We need studies targeting temperature‐driven reductions and enhancements in physiological performance in remnant patches and the extent to which thermal performance curves can predict such outcomes. Few studies approach behavioural and physiological responses to fragmentation from a thermal biology perspective (Currylow et al. 2012; Stangler et al. 2014), but we think this line of research could yield important new insight into fragmentation's effects.
The effects of temperature on species
When populations of individuals experience thermal stress, then the species as a whole is more prone to local extinction. The third step of the fragmentation‐temperature framework (Fig. 1) uses the same calculations presented in the second step of the framework to identify species at greatest risk of extinction following fragmentation (Table 2).
Table 2.
Predictions for species at high and low risk of thermally driven extinction following fragmentation, based on three thermal traits (Thermal safety margin, Warming tolerance and Performance breadth)
| Thermal trait | Low risk | High risk | Extinction mechanism | How to calculate |
|---|---|---|---|---|
| Thermal safety margin |
Large thermal safety margin (High T OPT) |
Small thermal safety margin (Low T OPT) |
Species with large thermal safety margins have a lower probability of new temperatures exceeding their optimum (and thus reducing performance) following an increase in temperature. | Measure the degrees between environmental temperatures and T OPT |
| Warming tolerance |
Large warming tolerance (High CT MAX) |
Small warming tolerance (Low CT MAX) |
Species with large warming tolerance have a lower probability of experiencing severe heat stress (and thus experiencing physiological shutdown) following an increase in temperature. | Measure the degrees between environmental temperatures and CT MAX |
| Performance breadth | Low thermal specialisation (wide performance breadth) | High thermal specialisation (narrow performance breadth) | Every degree of temperature change will have a disproportionately larger impact on performance in species with narrow performance breadths (thermal specialists) than on performance in species with wide performance breadths (thermal generalists). | Measure the range of body temperatures that individuals maintain in the field or in the lab, when offered a thermal gradient |
The fourth column briefly explains the extinction mechanism related to the thermal trait, and the last column provides details on how to calculate the trait using the species' thermal performance curve and environmental temperature data. These predictions are derived from the climate change and thermal biology literature (for a full review, see Kearney et al. 2009; Somero 2010), and should be modified to address the unique temporal and spatial considerations of fragmentation‐driven warming.
Fragmentation consistently reduces species richness (Haddad et al. 2015) but within this overarching pattern, species responses to fragmentation are notoriously idiosyncratic (Bregman et al. 2014). Predictions of species responses have been improved by explicitly considering life history traits, such as functional role (Bregman et al. 2014), dietary niche (Kennedy et al. 2010; Yong et al. 2011) and trophic level (Davies et al. 2000; Murphy & Romanuk 2012). We expect that including the thermal traits of species, such as thermal safety margin, warming tolerance and performance breadth, will further improve our ability to predict and interpret their responses to fragmentation (Table 2). Thermal performance curves are developed for species by averaging the performance curves of intraspecific individuals. The fragmentation‐temperature framework uses averaged performance curves for species‐level measures of thermosensitivity.
Broadly, we predict that species that can tolerate a large amount of temperature change (wide performance breadth) and species with thermal safety margins and warming tolerance > 2–5 °C are more likely to persist in fragmented landscapes (Table 2). For example Saunders et al. (1991) hypothesise that ants of the Iridomyrmex genus will benefit from fragmentation because Iridomyrmex species only forage at high temperatures, so elevated temperatures at patch edges following fragmentation allow the species to forage for a longer period of time. In contrast, temperature‐driven reductions in foraging time are considered a major factor in the extinction of Carnaby's short‐billed black cockatoos (Calyptorhynchus latirostris) following fragmentation (Saunders 1982).
Species with thermal safety margins and warming tolerance < 2–5 °C are less likely to persist in small and edge‐dominant patches following fragmentation. Tropical ectotherms, for example exhibit particularly small thermal safety margins and narrow performance breadths (Deutsch et al. 2008; Huey et al. 2009), which is alarming given that deforestation has increased significantly in the tropics over the past half century (Haddad et al. 2015). In the Brazilian Atlantic Forest, for example, the proportion of forest farther than 1 km from the forest edge has decreased from 90% (historical) to < 9% (today) (Haddad et al. 2015). These high rates of deforestation, coupled with the sensitivity of tropical organisms to warming, raise new alarm regarding the future of ecosystems in some of our most biodiverse regions of the planet.
However, extinction is not the only response available to species experiencing thermal stress in remnant patches. Broadly, species facing higher temperatures have two possible compensatory responses: (1) given enough time and dispersal, populations can move to more favourable thermal environments in the landscape or (2) species can adjust to new conditions through behavioural plasticity, physiological plasticity or adaptation (Sinervo et al. 2010). These responses can help mitigate thermal stress in hotter environments, and recent reviews suggest that species' capacity to buffer the impacts of warming through movement and adaptation can alter extinction trajectories (Kearney et al. 2009; Huey et al. 2012, Valladares et al. 2014; Buckley et al. 2015). Here we discuss how species might (A) move and (B) adapt to mitigate the impacts of elevated temperatures in remnant patches.
A. Movement: The thermal gradient present in remnant patches suggests that the distribution of species in remnant patches may be predictable if the thermosensitivities of the focal species are known. We expect individuals will disperse along the thermal gradient, spanning from patch edge to interior, to find areas with optimal thermal conditions. Because average temperatures are consistently higher at patch edges (Table 1), we expect different species to require different distances from the forest edge, depending on their thermal needs and according to their dispersal ability (Saunders et al. 1991) (Fig. 3). In a network of boreal lakes, increased air temperatures produced steep thermal gradients at the water surface (Tunney et al. 2014). In response, temperature‐sensitive lake trout (Salvelinus namaycush) shifted their habitat use to colder, offshore waters, where thermal conditions remained unchanged (Tunney et al. 2014).
Figure 3.
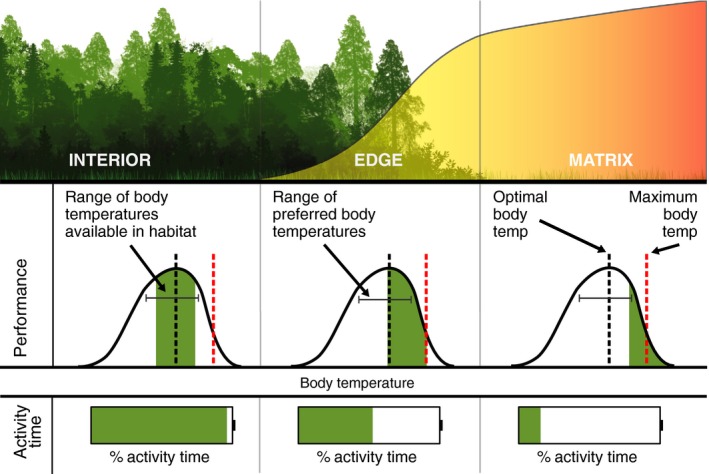
The spatial relationships between thermal conditions and performance along the edge of a remnant forest patch. Top row: illustration of a classic thermal gradient extending from a cleared matrix across the patch edge and into the forest interior. Middle row: hypothetical thermal performance curves for the same individual at three locations along the thermal gradient (patch interior, patch edge and matrix). The range of body temperatures experienced by the individual at that location (green shaded area under the curve), the individual's preferred body temperatures (bracketed grey line) and the individual's optimal and maximum body temperatures (black and red dashed lines respectively) are labelled. Bottom row: battery icons represent the relative amount of time the individual has available for activity (e.g. foraging and mating) at that location, based on the thermal conditions available. Activity time is reduced when the body temperatures experienced exceed the organism's optimal temperature.
Temperature‐sensitive species (Table 2) should only occupy areas far from patch edges and are likely to contract their distribution towards patch interiors, where thermal conditions are perhaps unchanged. For small and edge‐dominant patches, temperature increases may penetrate the entire patch (Didham & Lawton 1999), forcing thermally sensitive species to disperse to larger patches in search of cooler conditions. This increased emigration of species from small to large patches could spark a diversity of ecological cascades, and such movement may have important consequences for how individuals, species, communities and ecosystems are affected by fragmentation.
Studies consistently document the replacement of forest specialist species with generalist species in small and edge‐dominant patches (Lövei et al. 2006), but thermosensitivity provides a new mechanism underlying this pattern and further emphasises the need to disentangle the drivers of biodiversity changes in remnant patches. Many drivers of change in fragments will be co‐linear with temperature increases, including desiccation and resultant vegetation changes, so identifying which of these is the ‘true’ driver will take some disentangling.
B. Adaptation: Because of the immediacy with which temperature increases occur and constraints arising from the genetic architecture of thermal tolerance, many species cannot evolve rapidly enough to adapt to hotter thermal conditions following fragmentation (Sinervo et al. 2010; Somero 2010). However, studies suggest that plastic responses, such as shifts in thermoregulatory behaviour and physiology, can mitigate thermal stress in hotter conditions (Kearney et al. 2009; Huey et al. 2012; Valladares et al. 2014) and might serve as an important mechanism underlying species persistence in remnant patches.
Terrestrial ectotherms often respond to warming by shifting activity patterns to reduce risk of overheating, such as increasing the amount of time spent in thermal refugia (Sinervo et al. 2010; Kearney 2013), shifting activity time to cooler parts of the day (Logan et al. 2006; Huey et al. 2012) or shifting seasonal activities, like reproduction, to cooler parts of the year (Kearney et al. 2009). Warming‐driven shifts in activity time are considered a way for ectotherms to buffer the impacts of warming (Huey et al. 2009), but such shifts are more commonly linked to population declines than population persistence. For spiny lizards (Sceloporus), for example, heat‐driven restrictions in foraging time during the breeding season led to local extinction events due to reductions in energy gain and ultimately lower fecundity (Sinervo et al. 2010). While studies have documented shifts in activity time in animals in remnant patches (Norris et al. 2010), such studies are rare and much needed.
The extent to which plastic responses (e.g. shifts in distribution or thermoregulatory behaviour) help or hinder species persistence in fragmented landscapes remains an important and relatively unexplored question. Researchers should target changes in the spatial arrangement, physiology and thermoregulatory behaviour of species in remnant patches to gain new insight into the potential for plastic responses to buffer species from the impacts of fragmentation or climate change‐driven warming. Studies suggest that changes in thermoregulatory behaviour can alter extinction outcomes in warming scenarios (Kearney et al. 2009; Huey et al. 2012; Valladares et al. 2014), and we think recently fragmented landscapes offer experiments primed to test these types of thermal questions.
Critical gaps
Evidence from the climate change literature suggests that thermal traits, including thermal safety margins, performance breadth, and warming tolerance, provide useful predictors of extinction risk in changing thermal environments (Deutsch et al. 2008; Somero 2010) (Table 2). We need a barrage of studies testing the predictive power of thermal traits for species' responses to fragmentation to determine the value of this approach in the context of fragmented landscapes. Future studies should compare the time spent in thermally stressful conditions before and after fragmentation, between edge and interior habitat, and over time as the matrix, vegetation density and edge structure change (Fig. 3 presents an example). If predictions derived from thermal traits prove effective indicators of extinction risk, we can then offer a new, easy‐to‐use tool to guide biodiversity conservation in fragmented landscapes.
The effects of temperature on community dynamics
No organism is an ecological island (Huey et al. 2012), and species' responses to warming can indirectly lead to shifts in community dynamics through direct changes in biotic interactions (Gilman et al. 2010). In step four of the framework, we explore how the thermal response of one species (movement, adaptation or extinction) may cascade through the ecological community, altering interactions between competitors, predators, parasites or mutualists in remnant patches (Peck et al. 2009; Gilbert et al. 2014; Dohet et al. 2015).
For example, Tunney et al. (2014) show that when lake trout shifted their habitat use to combat warming, they developed a stronger dependence on deep‐water resources, specifically phytoplankton production and cold‐water prey. This shift lengthened the trophic pathways to lake trout and, ultimately, resulted in an increase in the food chain length of the entire ecosystem (Tunney et al. 2014), likely leading to further ecological cascades. The effects of the trout's shift on its warm‐tolerant competitors and on the abundance of its prey are not yet known (Tunney et al. 2014), but it is possible that a variety of biotic interactions have been altered.
Differences in thermosensitivity can also shift ecological communities via species loss (Peck et al. 2009), often through the replacement of cold‐adapted or thermally specialised species by thermal generalist species taking advantage of warmer temperatures (Dohet et al. 2015). Extinctions from warming are hypothesised to shift communities towards dominance of smaller, more active species, which typically have higher thermal tolerance and are more capable of making behavioural and physiological shifts (Peck et al. 2009).
These results suggest that if details about taxa thermosensitivity and changes in thermal conditions are known, changes in community dynamics following fragmentation may be predictable. In many cases, however, we do not know enough about individual sets of interactions between species or how temperature regulates the strength and direction of those interactions (Gilbert et al. 2014). In the hopes of making a generalisable statement, we predict that temperature‐sensitive species (Table 2) will shift their habitat use to cooler, forest interior areas and develop a greater reliance on forest interior resources. We expect shifts in the behaviour and habitat use of temperature‐sensitive species to have broader impacts on community dynamics in remnant patches, such as lengthening or shortening trophic pathways and restructuring food web architecture.
Critical gaps
There has been a recent surge of interest in the relationship between temperature increases and biotic interactions (Gilman et al. 2010; Gilbert et al. 2014; Dohet et al. 2015), but few studies have applied these finding to interactions in fragmented landscapes (Stangler et al. 2014). We need studies targeting temperature‐driven shifts in biotic interactions that consider the spatial structure of thermal conditions in remnant patches when developing hypotheses and predictions. Furthermore, we need studies tracking subsequent changes in community structure and dynamics in remnant patches that emerge from shifts in biotic interactions. The relationship between temperature increases and altered biotic interactions in fragmented landscapes is relatively untested (Stangler et al. 2014) and presents a great opportunity for gaining new understanding of fragmentation's ecological consequences.
This relationship also presents an opportunity for insight into the overall impact strength of fragmentation events. We know very little about the connection between the severity of thermal disturbance following fragmentation and how far the effects of that fragmentation event cascade through the ecosystem, but we expect better understanding of impact strength will be a key advancement of this framework.
The effects of temperature on ecosystem function
Temperature indirectly affects ecosystem functions, such as food web stability, biomass accumulation and carbon cycling, by directly changing species' activity and distribution patterns, biotic interactions and community dynamics (Fagan et al. 1999; Chown & Gaston 2008; Traill et al. 2010; Nelson et al. 2013). In the final step of the fragmentation‐temperature framework, we explore how warming‐driven shifts at the species and community level can lead to changes in ecosystem function within remnant patches or across fragmented landscapes. We illustrate this concept using two important ecosystem functions: decomposition and pollination.
Litter decomposition helps maintain the carbon cycle, and soil temperature exerts strong effects on litter composition rates due to the temperature sensitivities of soil biota (Chen et al. 1999; Laurance et al. 2002; Crockatt & Bebber 2015). Studies consistently show decomposition rates are lower at patch edges than patch interiors due to the hotter, drier conditions at patch edges (Laurance et al. 2002; Crockatt & Bebber 2015) and Chen et al. (1995) propose that we should see gradients in decomposition rate from the patch edge to interior driven by thermal conditions (e.g. Crockatt & Bebber 2015).
Klein (1989) found lower rates of dung decomposition in Amazonian forest fragments compared to continuous forest, which he links to high rates of heat‐driven mortality in dung and carrion beetles at patch edges. Klein (1989) proposes that extreme temperatures at forest‐clearing boundaries desiccate dung beetle larvae in the soil at patch edges and overheat adults venturing into cleared areas. This increased mortality of beetles has been implicated in a compositional shift in the carrion‐feeding guild, with heat‐tolerant ants becoming more dominant and heat‐sensitive carrion beetles less dominant in small fragments and cleared areas (Klein 1989; Fagan et al. 1999).
Much like decomposition, pollination often relies on the temperature‐sensitive behaviour and activity patterns of animals. For example, orchids rely entirely on euglossine bees for pollination, and foraging behaviour in euglossine bees is thermally driven, limited by low air temperatures in the morning and by overheating in the late afternoon (Armbruster & Berg 1994; Traill et al. 2010). Studies suggest that bees have less time available for pollen‐collecting behaviour in remnant patches following fragmentation, leading to lower food intake for the bees and eventually lower fecundity in orchids (Traill et al. 2010). Perhaps because of restricted activity time, studies also show that many euglossine species disappear entirely from remnant patches following fragmentation, further jeopardising the future of orchids and other bee‐pollinated plant species (Laurance et al. 2002).
Higher temperatures often result in general ecosystem instability, as temperature accelerates biological rates and can lead to cyclic or erratic dynamics (Laurance et al. 2002; Nelson et al. 2013). This phenomenon has been observed at patch edges in the form of hyperdynamism, whereby edges experience faster rates of ecological processes, including population and species turnover (Laurance et al. 2002). Hyperdynamism makes patch edges intrinsically less stable than patch interiors, and therefore ecosystem functions that rely on ecological processes at patch edges (e.g. soil respiration and seed production) are also less stable (Laurance et al. 2002). For example small mammal abundances fluctuate more widely in forest fragments than in continuous forest, particularly in the first few years following fragmentation (Malcolm 1991 cited in Laurance et al. 2002). These fluctuations in abundance can lead to more dramatic fluctuations in broader forest functioning, as small mammals perform critical roles in forest ecosystems including predation on insect herbivores and seed dispersal.
Critical gaps
The impacts of fragmentation on ecosystem functions are not well understood (Peh et al. 2014), and we need more studies directly addressing temperature's role in altered ecosystem functions in fragmented landscapes to better predict the trajectories of ecosystems following fragmentation. We predict the magnitude and direction of fragmentation's effects on ecosystem functions will depend on both the severity of temperature change at patch edges and the thermosensitivity of the species on which the key functions depend. We expect ecosystem functions that rely on thermally sensitive species (Table 2) will consistently respond negatively to fragmentation, whereas functions influenced by a wider range of biotic and abiotic factors should exhibit more variable responses (Peh et al. 2014).
Fragmentation‐temperature framework: untested connections
The fragmentation‐temperature framework provides a skeleton for measuring temperature's influences on processes across ecological scales, ranging from individuals to ecosystems, but there are untested connections that emerge from this framework that must be addressed for a more comprehensive understanding of fragmentation's impacts. Here, we present two untested connections: (1) the relationship between thermal biology and patch isolation and (2) confounding factors in determining temperature's role in ecological responses.
Thermal isolation
Patch isolation can lead to extinction in fragmented landscapes (Ricketts 2001; Prugh et al. 2008; Haddad et al. 2015), and increasing evidence suggests that the thermal conditions available and species' thermosensitivity play a role in determining patch isolation, particularly in ectotherms (Currylow et al. 2012; Munguia‐Vega et al. 2013).
For lizards in a Eucalyptus forest fragmented within a pine plantation, for example, mode of thermoregulation was considered a potential driver of isolation patterns (Mortelliti et al. 2015). The eastern three‐toed earless skink (Hemiergis talbingoensis) gains heat by direct contact with warm substrate (thigmothermy) and had increased colonisation rates of eucalypt patches embedded within the plantation. The southern rainbow skink (Carlia tetradactyla) gains heat by direct solar radiation (heliothermy) and instead had decreased colonisation rates of eucalypt patches. The authors suggest these differences in colonisation rates may stem from thermal biology, such that the high levels of shade associated with pine plantations facilitated dispersal in the thigmothermic species but acted as a barrier to the heliothermic species, which could no longer meet its thermoregulatory needs during dispersal (Mortelliti et al. 2015).
From a thermal perspective, the challenge of surviving dispersal in fragmented landscapes is multifaceted. First, the extreme surface temperatures in cleared areas means species have an intrinsically higher risk of exceeding their CT MAX. In the Central Hardwoods Region of the U.S., for example, monthly average temperatures in cleared areas can be 13 °C warmer than in forest patches and reach over 40 °C (Currylow et al. 2012). These high matrix temperatures require that eastern box turtles (Terrapene carolina carolina) travelling through the region maintain significantly higher body temperatures when in the clearings than when in the forest (Currylow et al. 2012).
In addition, matrix habitat often lacks thermal refugia, which limits opportunities to thermoregulate during dispersal. Likely resulting from thermal stress in cleared areas, box turtles in the Central Hardwoods maintained generally smaller home ranges, concentrated their movement along forest edges, and ventured only short distances into forest clearings. The long‐term implications of these changes in movement behaviour are not yet known, but Munguia‐Vega et al. (2013) found that, for Urosaurus nigricaudus lizards, the inability to disperse through or use a thermally hostile matrix could result in frequent, time‐delayed extinctions in remnant patches.
Patch isolation is typically measured using the distance between patches, presence of movement corridors and resistance of the matrix to the interpatch movement of individuals (Ricketts 2001). We believe that thermal conditions and thermosensitivity can be easily integrated into matrix resistance to identify potential dispersal barriers. The greater the distance between patches, and the smaller the differential between the thermal conditions available and the species' CT MAX, the more significant the barrier to dispersal becomes (i.e. the greater the ‘thermal isolation’). Thermal isolation can be easily integrated into metapopulation, metacommunity and connectivity models via thermally driven changes in colonisation and network flow rates.
Confounding factors
An important question emerging from this framework is how important is warming relative to other fragmentation impacts, such as resource loss, in determining species responses to fragmentation? Similarly, changes in thermal conditions are often confounded with other abiotic edge effects, such as increased light levels, greater dessication and evapotranspiration rates, and decreased soil moisture and humidity (Murcia 1995; Chen et al. 1999; Didham & Lawton 1999; Laurance 2004). How do we begin to disentangle the effects of changes in thermal conditions from other abiotic changes?
At this stage, we can only point to this gap in our understanding of temperature's role in ecological responses to fragmentation. Forest‐interior species have multiple adaptations to their forest environment, and expanding our knowledge of thermal biology may not be useful if it is primarily resource availability, nesting sites, cover from predators, or low light levels that restrict species to continuous forest stands. Manipulative experiments altering thermal conditions, while holding other abiotic and biotic variables constant, will be fundamental to teasing apart temperature's relative influence.
We expect that warming is a significant effect of fragmentation for diurnal, thermally sensitive (Table 2), ectothermic species in forest ecosystems, as well as for community dynamics and ecosystem functions dependent on such species. Thermal generalist, nocturnal and endothermic species are presumably less affected by fragmentation‐driven warming. We also expect the fragmentation‐temperature framework is less relevant to species in ecosystems where fragmentation does not result in higher temperatures and greater thermal variability. However, much more research is needed to begin to address this untested connection.
Conclusion: treating temperature as a mechanism in fragmentation research
The field of thermal biology has progressed rapidly over the last 50 years, and topics once restricted to thermal biology – including thermal performance curves – are finding new life in climate change research and new applications to ecological problems (Angilletta 2009). The integration of thermal biology into climate change research has led to important advancements in our understanding of extinction risk under global warming scenarios, and we expect that integrating thermal biology into fragmentation research can lead to equally large advancements in our understanding of fragmentation's ecological consequences.
Temperature increases that result from the fragmentation process and increases that result from global climate warming differ in important ways, primarily the spatial arrangement and time frame over which the temperature increases occur. Fragmentation‐driven temperature increases occur instantly, as opposed to gradually, limiting opportunities for genetic adaptation and relying more heavily on behavioural and phenotypic plasticity (Sinervo et al. 2010; Somero 2010). Increases are also concentrated at patch edges, likely leading to species' responses that vary along fine‐scale thermal gradients. These warming patterns remain an underexplored mechanism affecting individuals, species, communities and ecosystems in fragmented landscapes.
The research methods used to study the effects of temperature in fragmented landscapes require the same tools used in climate change and thermal biology research. These include high‐resolution temperature sensors, infrared imagery, field body temperature sensors, laboratory experiments measuring and manipulating thermosensitivity, as well as maps and models of thermal conditions. In particular, we suggest researchers apply these research methods to the long‐term, large‐scale fragmentation experiments that exist throughout the world (Debinski & Holt 2000; Haddad et al. 2015), many of which offer opportunities to study the effects of temperature in controlled, replicated, before‐after experimental setups. Fragmentation experiments can further thermal biology research, which is historically laboratory based, because they provide an opportunity to test laboratory findings in a field system. Fragmentation experiments can also further climate change research because temperature increases following fragmentation provide opportunities to test models and predictions regarding species responses to warming.
Understanding the connection between fragmentation‐driven temperature increases and ecological responses requires more than just additional experiments. It requires the development of new theory to deal with temperature as a mechanism driving change in fragmented landscapes. Here, we presented two untested connections (thermal isolation and confounding factors), but countless connections remain to be explored, including defining climate space in fragmented vs. continuous forests for niche modelling (Kearney & Porter 2009) and developing quantitative models of restrictions in activity time in remnant patches that build upon those that exist for climate change (Kearney 2013; Gunderson & Leal 2016).
Scientific understanding advances most rapidly when theories and findings from multiple disciplines are integrated, and fragmentation research has succeeded in incorporating a variety of ecological subfields, including invasion, disease, community and ecosystem ecology. However, thermal biology has remained outside the scope of fragmentation research, even though fragmentation changes temperature and thermal biology examines the effects of temperature change on biological and ecological processes.
Temperature is unique in its pervasiveness, influencing all levels of biological processes, from genes to interactions to energy flow. As Angilletta (2009, pg.1) explains, ‘Unlike many other variables that concern biologists, temperature is not just a property of life; it is a property of matter. Nothing escapes its control’. We need to combine our understanding of the effects of fragmentation on temperature with our understanding of the effects of temperature on organisms to gain a more comprehensive understanding of the mechanisms behind biodiversity loss in remnant patches and to provide new strategies for habitat conservation in an increasingly fragmented and heated world.
Statement of authorship
KTT developed the initial framework. All authors contributed substantially to the ideas in this manuscript and to its writing. We are grateful for support from the National Science Foundation (DEB‐0841892 and DEB‐1350872).
Acknowledgements
Many thanks to members of the Davies and Melbourne labs for fun and productive discussions on these topics, to Scott Holman and Sara Fall at the Graduate Writing Center for commenting on countless earlier drafts, and to the four anonymous reviewers and three editors (Dr. Marcel Holyoak, Dr. Peter Thrall and Dr. Mary O'Connor) that provided invaluable feedback throughout this process. KTT was supported by the University of Colorado's Dissertation Completion Fellowship during the writing and revising process, which was extremely helpful.
References
- Angilletta, M.J. (2006). Estimating and comparing thermal performance curves. J. Therm. Biol, 31, 541–545. [Google Scholar]
- Angilletta, M.J. (2009). Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press, Oxford, UK. [Google Scholar]
- Angilletta, M.J. , Niewiarowski, P.H. & Navas, C.A. (2002). The evolution of thermal physiology in ectotherms. J. Therm. Biol, 27, 249–268. [Google Scholar]
- Angilletta, M.J. , Cooper, B.S. , Schuler, M.S. & Boyles, J.G. (2010). The evolution of thermal physiology in endotherms. Front Biosci., 2, 861–881. [DOI] [PubMed] [Google Scholar]
- Armbruster, W.S. & Berg, E.E. (1994). Thermal ecology of male euglossine bees in a tropical wet forest ‐ fragrance foraging in relation to operative temperature. Biotropica, 26, 50–60. [Google Scholar]
- Barton, B.T. & Schmitz, O.J. (2009). Experimental warming transforms multiple predator effects in a grassland food web. Ecol. Lett., 12, 1317–1325. [DOI] [PubMed] [Google Scholar]
- Boggs, J.L. & McNulty, S.G. (2010). Changes in canopy cover alter surface air and forest floor temperature in a high‐elevation red spruce (Picea rubens Sarg.) forest. Proceedings from the Conference on the Ecology and Management of High‐Elevation Forests in the Central and Southern Appalachian Mountains: 13–21. Snowshoe Mountain Resort, WV.
- Bregman, T.P. , Sekercioglu, C.H. & Tobias, J.A. (2014). Global patterns and predictors of bird species responses to forest fragmentation: implications for ecosystem function and conservation. Biol. Conserv., 169, 372–383. [Google Scholar]
- Buckley, L.B. , Ehrenberger, J.C. & Angilletta, M.J. (2015). Thermoregulatory behaviour limits local adaptation of thermal niches and confers sensitivity to climate change. Funct. Ecol., 29, 1038–1047. [Google Scholar]
- Chen, J. , Franklin, J.F. & Spies, T.A. (1993). Contrasting microclimates among clearcut, edge, and interior of old‐growth Douglas‐fir forest. Agric. For. Meteorol., 63, 219–223. [Google Scholar]
- Chen, J. , Franklin, J.F. & Spies, T.A. (1995). Growing‐season microclimatic gradients from clearcut edges into old‐growth douglas‐fir forests. Ecol. Appl., 5, 74–86. [Google Scholar]
- Chen, J. , Saunders, S.C. , Crow, T.R. , Naiman, R.J. , Brosofske, K.D. , Mroz, G.D. et al (1999). Microclimate in forest ecosystem and landscape ecology ‐ Variations in local climate can be used to monitor and compare the effects of different management regimes. Bioscience, 49, 288–297. [Google Scholar]
- Chown, S.L. & Gaston, K.J. (2008). Macrophysiology in a changing world. Proc. R. Soc. B, 275, 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane, M.A. & Laurance, W.F. (2008). Synergisms among fire, land use, and climate change in the Amazon. Ambio, 37, 522–527. [DOI] [PubMed] [Google Scholar]
- Crockatt, M.E. & Bebber, D.P. (2015). Edge effects on moisture reduce wood decomposition rate in a temperate forest. Glob. Change Biol., 21, 698–707. [DOI] [PubMed] [Google Scholar]
- Currylow, A.F. , MacGowan, B.J. & Williams, R.N. (2012). Short‐term forest management effects on a long‐lived ectotherm. PLoS ONE, 7, e40473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily, G.C. & Ehrlich, P.R. (1996). Nocturnality and species survival. PNAS, 93, 11709–11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschen, E.I. , Baker, D.V. , Bohrer, G. , Nathan, R. , Orrock, J.L. , Turner, J.R. et al (2014). How fragmentation and corridors affect wind dynamics and seed dispersal in open habitats. PNAS, 111, 3484–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, K.F. , Margules, C.R. & Lawrence, J.F. (2000). Which traits of species predict population declines in experimental forest fragments? Ecology, 81, 1450–1461. [Google Scholar]
- De la Sancha, N.U. , Higgins, C.L. , Presley, S.J. & Strauss, R.E. (2014). Metacommunity structure in a highly fragmented forest: has deforestation in the Atlantic Forest altered historic biogeographic patterns? Diversity Distrib., 20, 1058–1070. [Google Scholar]
- Debinski, D.M. & Holt, R.D. (2000). A survey and overview of habitat fragmentation experiments. Conserv. Biol., 14, 342–355. [Google Scholar]
- Deutsch, C.A. , Tewksbury, J.J. , Huey, R.B. , Sheldon, K.S. , Ghalambor, C.K. , Haak, D.C. et al (2008). Impacts of climate warming on terrestrial ectotherms across latitude. PNAS, 105, 6668–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didham, R.K. & Lawton, J.H. (1999). Edge Structure Determines the Magnitude of Changes in Microclimate and Vegetation Structure in Tropical Forest Fragments. Biotropica, 31, 17–30. [Google Scholar]
- Dirmeyer, P.A. & Shukla, J. (1994). Albedo as a modulator of climate response to tropical deforestation. J. Geophys. Res., 99, 20863–20877. [Google Scholar]
- Dohet, A. , Hlúbiková, D. , Wetzel, C.E. , L'Hoste, L. , Iffly, J.F. , Hoffmann, L. et al (2015). Influence of thermal regime and land use on benthic invertebrate communities inhabiting headwater streams exposed to contrasted shading. Sci. Total Environ., 505, 1112–1126. [DOI] [PubMed] [Google Scholar]
- Dreisig, H. (1984). Control of body temperature in shuttling ectotherms. J. Therm. Biol, 9, 229–233. [Google Scholar]
- Fagan, W.F. , Cantrell, R.S. & Cosner, C. (1999). How habitat edges change species interactions. Am. Nat., 153, 165–182. [DOI] [PubMed] [Google Scholar]
- Fonesca, M.S. & Fisher, J.S. (1986). A comparison of canopy friction and sediment movement between four species of seagrass with reference to their ecology and restoration. Mar. Ecol. Prog. Ser., 29, 15–22. [Google Scholar]
- Garcia‐Carreras, L. & Parker, D.J. (2011). How does local tropical deforestation affect rainfall? Geophys. Res. Lett., 38, 1–7. [Google Scholar]
- Gerick, A.A. , Munshaw, R.G. , Palen, W.J. , Combes, S.A. & O'Regan, S.M. (2014). Thermal physiology and species distribution models reveal climate vulnerability of temperate amphibians. J. Biogeogr., 41, 713–723. [Google Scholar]
- Gilbert, B. , Tunney, T.D. , Mccann, K.S. , Delong, J.P. , Vasseur, D.A. , Savage, V. et al (2014). A bioenergetic framework for the temperature dependence of trophic interactions. Ecol. Lett., 17, 902–914. [DOI] [PubMed] [Google Scholar]
- Gilman, S.E. , Urban, M.C. , Tewksbury, J. , Gilchrist, G.W. & Holt, R.D. (2010). A framework for community interactions under climate change. TREE, 25, 325–331. [DOI] [PubMed] [Google Scholar]
- Grez, A. , Zaviezo, T. , Tischendorf, L. & Fahrig, L. (2004). A transient, positive effect of habitat fragmentation on insect population densities. Oecologia, 141, 444–451. [DOI] [PubMed] [Google Scholar]
- Gunderson, A.R. & Leal, M. (2016). A conceptual framework for understanding thermal contraints on ectotherm activity with implication for prediction responses to global change. Ecol. Lett., 19, 111–120. [DOI] [PubMed] [Google Scholar]
- Gvozdík, L. (2012). Plasticity of preferred body temperatures as means of coping with climate change? Biol. Lett., 8, 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad, N.M. , Brudvig, L.A. , Clobert, J. , Davies, K.F. , Gonzalez, A. , Holt, R.D. et al (2015). Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci. Adv., 1, e1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, X. , Leach, M.J. & Raman, S. (1995). Role of vegetation in generation of mesoscale circulation. Atmos. Environ., 29, 2163–2176. [Google Scholar]
- Hovick, T.J. , Elmore, R.D. , Allred, B.W. , Fuhlendorf, S.D. & Dahlgren, D.K. (2014). Landscapes as a moderator of thermal extremes: a case study from an imperiled grouse. Ecosphere, 5, 35. [Google Scholar]
- Huey, R.B. (1974). Behavioral thermoregulation in lizards: importance of associated costs. Science, 184, 1001–1003. [DOI] [PubMed] [Google Scholar]
- Huey, R.B. (1982). Temperature, physiology, and the ecology of reptiles. Biol. Reptilia, 12, 25–91. [Google Scholar]
- Huey, R. & Kingsolver, J.G. (1989). Evolution of thermal sensitivity of ectotherm performance. TREE, 4, 131–135. [DOI] [PubMed] [Google Scholar]
- Huey, R.B. , Deutsch, C.A. , Tewksbury, J.J. , Vitt, L.J. , Hertz, P.E. , Alvarez Pérez, H.J. et al (2009). Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B, 276, 1939–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey, R.B. , Kearney, M.R. , Krockenberger, A. , Holtum, J.A.M. , Jess, M. & Williams, S.E. (2012). Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B, 367, 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC . (2014). Climate Change 2014: Synthesis Report In: Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Core Writing Team , Pachauri R.K. & Meyer L.A.). IPCC, Geneva, Switzerland, pp. 10–13. [Google Scholar]
- Jamoneau, A. , Chabrerie, O. , Closset‐Kopp, D. & Decocq, G. (2012). Fragmentation alters beta‐diversity patterns of habitat specialists within forest metacommunities. Ecography, 35, 124–133. [Google Scholar]
- Kapos, V. (1989). Effects of isolation on the water status of forest patches in the Brazilian Amazon. J. Trop. Ecol., 5, 173–185. [Google Scholar]
- Kearney, M. (2013). Activity restriction and the mechanistic basis for extinctions under climate warming. Ecol. Lett., 16, 1470–1479. [DOI] [PubMed] [Google Scholar]
- Kearney, M. & Porter, W. (2009). Mechanistic niche modelling: combining physiological and spatial data to predict species' ranges. Ecol. Lett., 12, 334–350. [DOI] [PubMed] [Google Scholar]
- Kearney, M. , Shine, R. & Porter, W.P. (2009). The potential for behavioral thermoregulation to buffer “cold‐blooded” animals against climate warming. PNAS, 106, 3835–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney, M.R. , Isaac, A.P. & Porter, W.P. (2014). microclim: global estimates of hourly microclimate based on long‐term monthly climate averages. Sci. Data, 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, C.M. , Marra, P.P. , Fagan, W.F. & Neel, M.C. (2010). Landscape matrix and species traits mediate responses of Neotropical resident birds to forest fragmentation in Jamaica. Ecol. Monogr., 80, 651–669. [Google Scholar]
- Kingsolver, J.G. (2009). The well‐temperatured biologist. (American Society of Naturalists Presidential Address). Am. Nat., 174, 755–768. [DOI] [PubMed] [Google Scholar]
- Klein, B.C. (1989). Effects of forest fragmentation on dung and carrion beetle communities in Central Amazonia. Ecology, 70, 1715–1725. [Google Scholar]
- Laurance, W.F. (2004). Forest‐climate interactions in fragmented tropical landscapes. Phil. Trans. R. Soc. Lond. B, 359, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance, W.F. , Lovejoy, T.E. , Vasconcelos, H.L. , Bruna, E.M. , Didham, R.K. , Stouffer, P.C. et al (2002). Ecosystem decay of Amazonian forest fragments: a 22‐year investigation. Conserv. Biol., 16, 605–618. [Google Scholar]
- Laurance, W.F. , Camargo, J.L.C. , Luizão, R.C.C. , Laurance, S.G. , Pimm, S.L. , Bruna, E.M. et al (2011). The fate of Amazonian forest fragments: a 32‐year investigation. Biol. Conserv., 144, 56–67. [Google Scholar]
- Logan, J.D. , Wolesensky, W. & Joern, A. (2006). Temperature‐dependent phenology and predation in arthropod systems. Ecol. Model., 196, 471–482. [Google Scholar]
- Lövei, G.L. , Magura, T. , Tóthmérész, B. & Ködöböcz, V. (2006). The influence of matrix and edges on species richness patterns of ground beetles (Coleoptera: Carabidae) in habitat islands. Global Ecol. Biogeogr., 15, 283–289. [Google Scholar]
- Malcolm, J.R. (1991). The small mammals of Amazonian forest fragments: Pattern and process. Ph.D. thesis, University of Florida, Gainesville.
- Matlack, G.R. (1993). Microenvironment variation within and among forest edge sites in the eastern United States. Biol. Conserv., 66, 185–194. [Google Scholar]
- McAlpine, C.A. , Syktus, J. , Deo, R.C. , Lawrence, P.J. , McGowan, H.A. , Watterson, I.G. et al (2007). Modeling the impact of historical land cover change on Australia's regional climate. Geophys. Res. Lett., 34, 1–6. [Google Scholar]
- Mortelliti, A. , Michael, D.R. & Lindenmayer, D.B. (2015). Contrasting effects of pine plantations on two skinks: results from a large‐scale “natural experiment” in Australia. Anim. Conserv., 18, 433–441. [Google Scholar]
- Munguia‐Vega, A. , Rodriguez‐Estrella, R. , Shaw, W.W. & Culver, M. (2013). Localized extinction of an arboreal desert lizard caused by habitat fragmentation. Biol. Conserv., 157, 11–20. [Google Scholar]
- Murcia, C. (1995). Edge effects in fragmented forests: implications for conservation. TREE, 10, 58–62. [DOI] [PubMed] [Google Scholar]
- Murphy, G.E. & Romanuk, T.N. (2012). A meta‐analysis of community response predictability to anthropogenic disturbances. Am. Nat., 180, 316–327. [DOI] [PubMed] [Google Scholar]
- Nelson, W.A. , Bjørnstad, O.N. & Yamanaka, T. (2013). Recurrent insect outbreaks caused by temperature‐driven changes in system stability. Science, 341, 796–799. [DOI] [PubMed] [Google Scholar]
- Norris, D. , Michalski, F. & Peres, C.A. (2010). Habitat patch size modulates terrestrial mammal activity patterns in Amazonian forest fragments. J. Mammal., 91, 551–560. [Google Scholar]
- Peck, L.S. , Clark, M.S. , Morley, S.A. , Massey, A. & Rossetti, H. (2009). Animal temperature limits and ecological relevance: effects of size, activity and rates of change. Funct. Ecol., 23, 248–256. [Google Scholar]
- Peh, K.S.‐H. , Lin, Y. , Luke, S.H. , Foster, W.A. & Turner, E.C. (2014). Forest Fragmentation and Ecosystem Function In: Global Forest Fragmentation (ed Kettle C.J. & Koh L.P.). CABI, Wallingford, UK, pp. 96–114. [Google Scholar]
- Porter, W. & Gates, D. (1969). Thermodynamic equilibria of animals with environment. Ecol. Monogr., 39, 227–244. [Google Scholar]
- Prugh, L.R. , Hodges, K.E. , Sinclair, A.R.E. & Brashares, J.S. (2008). Effect of habitat area and isolation on fragmented animal populations. PNAS, 105, 20770–20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puurtinen, M. , Elo, M. , Jalasvuori, M. , Kahilainen, A. , Ketolo, T. , Kotiaho, J.S. et al (2015). Temperature‐dependent mutation robustness can explain fast molecular evolution as warm temperature, affecting speciation rate and global patterns of species diversity. Ecography, 38, 1–9. [Google Scholar]
- Ricketts, T.H. (2001). The matrix matters: effective isolation in fragmented landscapes. Am. Nat., 158, 87–99. [DOI] [PubMed] [Google Scholar]
- Sato, C.F. , Wood, J.T. , Schroder, M. , Green, K. , Osborne, W.S. , Michael, D.R. et al (2014). An experiment to test key hypotheses of the drivers of reptile distribution in subalpine ski resorts. J. Appl. Ecol., 51, 13–22. [Google Scholar]
- Saunders, D.A. (1982). The breeding behaviour and biology of the short‐billed form of the white‐tailed black cockatoo Calyptorhynchus funereus . The Ibis, 124, 422–455. [Google Scholar]
- Saunders, D.A. , Hobbs, R.J. & Margules, C.R. (1991). Biological consequences of ecosystem fragmentation: a review. Conserv. Biol., 5, 18–32. [Google Scholar]
- Saunders, S.C. , Chen, J. , Drummer, T.D. & Crow, T.R. (1999). Modeling temperature gradients across edges over time in a managed landscape. For. Ecol. Manage., 117, 17–31. [Google Scholar]
- Sears, M.W. , Raskin, E. & Angilletta, M.J. (2011). The world is not flat: defining relevant thermal landscapes in the context of climate change. Integr. Comp. Biol., 51, 666–675. [DOI] [PubMed] [Google Scholar]
- Secretariat of the Convention on Biological Diversity (CBD) . (2010). Global Biodiversity Outlook 3.
- Sheridan, J.A. & Bickford, D. (2011). Shrinking body size as an ecological response to climate change. Nat. Clim. Chang., 1, 401–406. [Google Scholar]
- Sinervo, B. , Mendez‐de‐la‐Cruz, F. , Miles, D.B. , Heulin, B. , Bastiaans, E. , Cruz, M.V.S. et al (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science, 328, 894–899. [DOI] [PubMed] [Google Scholar]
- Somero, G.N. (2010). The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine “winners” and “losers”. J. Exp. Biol., 213, 912–920. [DOI] [PubMed] [Google Scholar]
- Stangler, E.S. , Hanson, P.E. & Steffan‐Dewenter, I. (2014). Interactive effects of habitat fragmentation and microclimate on trap‐nesting Hymenoptera and their trophic interactions in small secondary rainforest remnants. Biodivers. Conserv., 24, 563–577. [Google Scholar]
- Tattersall, G.J. , Sinclair, B.J. , Withers, P.C. , Fields, P.A. , Seebacher, F. , Cooper, C.E. et al (2012). Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr. Physiol., 2, 2151–2202. [DOI] [PubMed] [Google Scholar]
- Templeton, A.R. , Brazeal, H. & Neuwald, J.L. (2011). The transition from isolated patches to a metapopulation in the eastern collared lizard in response to prescribed fires. Ecology, 92, 1736–1747. [DOI] [PubMed] [Google Scholar]
- Traill, L.W. , Lim, M.L.M. , Sodhi, N.S. & Bradshaw, C.J.A. (2010). Mechanisms driving change: altered species interactions and ecosystem function through global warming. J. Anim. Ecol., 79, 937–947. [DOI] [PubMed] [Google Scholar]
- Tunney, T.D. , McCann, K.S. , Lester, N.P. & Shuter, B.J. (2014). Effects of differential habitat warming on complex communities. PNAS, 111, 8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares, F. , Matesanz, S. , Guilhaumon, F. , Araújo, M.B. , Balaguer, L. , Benito‐Garzón, M. et al (2014). The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett., 17, 1351–1364. [DOI] [PubMed] [Google Scholar]
- Williams‐Linera, G. (1990). Vegetation structure and environmental conditions of forest edges in Panama. J. Ecol., 78, 356–373. [Google Scholar]
- Yan, M. , Zhong, Z. & Liu, J. (2007). Habitat fragmentation impacts on biodiversity of evergreen broadleaved forests in Jinyun Mountains. China. Front. Biol. China, 2, 62–68. [Google Scholar]
- Yong, D.L. , Qie, L. , Sodhi, N.S. , Koh, L.P. , Peh, K.S.H. , Lee, T.M. et al (2011). Do insectivorous bird communities decline on land‐bridge forest islands in in Peninsular Malaysia? J. Trop. Ecol., 27, 1–14. [Google Scholar]


