Abstract
Three genes of the prion protein gene family are expressed in gonads. Comparative analyses of their expression patterns in mice and goats revealed constant expression of PRNP and SPRN in both species and in both male and female gonads, but with a weaker expression of SPRN. By contrast, expression of PRND was found to be sex‐dimorphic, in agreement with its role in spermatogenesis. More importantly, our study revealed that PRND seems to be a key marker of foetal Leydig cells specifically in goats, suggesting a yet unknown role for its encoded protein Doppel during gonadal differentiation in nonrodent mammals.
Keywords: Doppel, foetal Leydig, goat, mouse, prion, Shadoo
Abbreviations
- CNS
central nervous system
- dpc
days post coïtum
- Dpl
Doppel
- dpp
days post partum
- ECL
enhanced chemiluminescence
- GPI
glycophosphatidylinositol
- IHC
immuno‐histo‐chemistry
- mpp
months post partum
- PBS
phosphate saline buffer
- qRT‐PCR
quantitative RT‐PCR
- Sho
Shadoo
- TSE
transmissible spongiform encephalopathy
The prion gene family is mainly composed of three genes; Prnp, encoding the prion protein (PrP), Prnd, encoding Doppel (Dpl), both located on the same genomic locus on mouse chromosome 2 and Sprn, encoding Shadoo (Sho), located on mouse chromosome 7. These proteins are glycophosphatidylinositol (GPI)‐anchored glycoproteins and they shared some structural homology one with each other. The N‐terminal regions of PrP and Sho are composed of basic repeat regions and of an hydrophobic domain, whereas the C‐terminal regions of PrP and Dpl contain alpha helices 1. PrP plays a pivotal role in transmissible spongiform encephalopathy (TSE), a fatal neurodegenerative disorder affecting animals and humans 2, 3, 4. PrP is almost ubiquitously expressed with higher amount of expression occurring in the central nervous system (CNS). In mice and rams, PrP was found to be expressed in germ cells 5, 6, 7 but the genetic ablation of its gene in mice 8, 9, cattle 10 and goats 11 does not induce a fertility‐associated phenotype and/or major neuronal disorders 12. Thus, the PrP biological function remains elusive even if various roles have been proposed 13, 14.These observations suggested a biological redundancy between PrP and another PrP‐like protein in mammals.
Sho is expressed in the CNS and both Sho and PrP share neuro‐protective properties 15. Using Sprn reporter mice, a recent study describes expression of Sprn in the male and female gonads suggesting an involvement of Sho in reproduction 16. The Sprn mRNA knockdown in Prnp 0/0 embryos produces early embryonic lethality, suggesting that Sho and PrP could play a role in early developmental stages 17, 18. However, the Sprn ablation (Sprn 0/0) or the double‐knockout of Sprn and Prnp (Sprn 0/0/Prnp 0/0) in mice resulted in no drastic developmental phenotype 19.
Dpl is mainly expressed in the testis of adult mammals. Its ectopic expression induces some neuro‐degeneration in the CNS only in the absence of PrP 20, 21, 22, 23, suggesting a biological link between Dpl and PrP. Prnd ablation in mice (Prnd 0/0) resulted in male infertility characterized by the sperm's inability to perform the acrosome reaction and by an elevated level of oxidative DNA damage 24, 25. The double‐knockout of Prnd and Prnp (Prnd 0/0/Prnp 0/0) only mimicked the effect of the Prnd single inactivation 25. Immunohistochemical studies of Dpl were performed in gonads of various species, such as humans, rodents, boars and bovidae. The cellular localization of Dpl depends on the maturation stage of the gonads, on the studied species and the antibodies 12. For instance, in rodents and sheep, Dpl was only detected in germinal and somatic cells in mature testis, whereas in humans, boars and bovine, DPL seems to be present during most of the developing stages of the germ cells and in the Sertoli cells of foetal and mature gonads 26, 27, 28, 29. In goats and bovine, DPL was detected both in immature testis and in young female follicles 28, 30. Nevertheless, these different observations suggested a role of Dpl in early and/or mature sex differentiation 12.
To get deeper into the potential role of the prion protein gene family during gonad development, we report the comparative expression profiles of the three members of the prion protein gene family and the comparative localizations of their encoded proteins during ovary and testis development in two different species: goats and mice. These data suggest that Prnd may exert a yet unknown specific role in goat foetal Leydig cells.
Materials and methods
Animals and tissue samples
Procedures for handling goats were conducted in compliance with the guidelines for Care and Use of Agricultural Animals in Agricultural Research and Teaching (authorization no. 78–34). All goat foetuses and young goats were obtained from pregnant females, following hormonal treatment as previously described 31.
For mice, animal experiments were carried out in strict accordance with the recommendations in the guidelines of the Code for methods and Welfare Considerations in Behavioral Research with Animals (Directive 86/609EC). Experiments were approved by the Local ethics committee of Jouy‐en‐Josas on the Ethics of Animals Experiments of the author's institution, INRA (Permit Number RTA06‐091). All transgenic animal manipulations were performed according to the recommendations of the Haut Conseil des Biotechnologies (Permit number 6461). All mouse foetuses and pups were obtained from pregnant FVB/N, FVB/N Prnp 0/0 [quoted in 17] and Prnd 0/0 females 24.
Day 0 post coïtum corresponds to the day of mating. The genetic sex of all foetuses was determined by PCR amplification of SRY and ZFY/ZFX genes, on liver genomic DNA 31. For each goat sample, one gonad was frozen in liquid nitrogen for molecular analysis; the other one was fixed for immuno‐histological studies. For mice, samples of a same sex were pooled before molecular analysis. Two or 3 gonads were fixed for immuno‐histological studies at each developmental stage. Table 1 summarized the number of individuals used at each developmental stage in mice and goats.
Table 1.
Number of animals used at each developmental stage for gene expression studies with real‐time PCR
| Species | Stages | Sex | Tissues | Number of individual | Number of independent RT |
|---|---|---|---|---|---|
| Mouse | 12.5 dpc | Female | 2 Gonads + Mesonephros | 9 | 2 |
| Male | 2 Gonads + Mesonephros | 7 | 2 | ||
| 13.5 dpc | Female | 2 Gonads + Mesonephros | 6 | 2 | |
| Male | 2 Gonads + Mesonephros | 12 | 2 | ||
| 14.5 dpc | Female | 2 Gonads | 4 | 2 | |
| Male | 2 Gonads | 6 | 2 | ||
| 18.5 dpc | Female | 2 Gonads | 6 | 2 | |
| Male | 2 Gonads | 9 | 2 | ||
| 5 dpp | Female | 2 Gonads | 11 | 2 | |
| Male | 1 Gonad | 4 | 2 | ||
| 25 dpp | Female | 1 Gonad | 5 | 2 | |
| Male | 1/2 Gonad | 4 | 2 | ||
| 50 dpp | Female | 1 Gonad | 3 | 3 | |
| Male | 1/2 Gonad | 3 | 3 | ||
| 4 mpp | Female | 1 Gonad | 3 | 3 | |
| Male | 1/2 Gonad | 3 | 3 | ||
| 6 mpp | Female | 1 Gonad | 2 | 2 | |
| Male | 1/2 Gonad | 3 | 3 | ||
| 6–8 mpp | Female | 1 Gonad | 3 | 3 | |
| Male | 1/2 Gonad | 3 | 3 | ||
| 10–12 mpp | Female | 1 Gonad | 3 | 3 | |
| Male | 1/2 Gonad | 3 | 3 | ||
| Goat | 30 dpc | Female | 1 Gonads + Mesonephros | 1 | 2 |
| Male | 1 Gonads + Mesonephros | 2 | 4 | ||
| 32 dpc | Female | 1 Gonads + Mesonephros | 1 | 2 | |
| Male | 1 Gonads + Mesonephros | 2 | 4 | ||
| 34 dpc | Female | 1 Gonads + Mesonephros | 2 | 3 | |
| Male | 1 Gonads + Mesonephros | 2 | 4 | ||
| 36 dpc | Female | 1 Gonads + Mesonephros | 3 | 3 | |
| Male | 1 Gonads + Mesonephros | 2 | 4 | ||
| 41 dpc | Female | 1 Gonad | 4 | 2 | |
| Male | 1 Gonad | 4 | 4 | ||
| 45 dpc | Female | 1 Gonad | 9 | 3 | |
| Male | 1 Gonad | 4 | 4 | ||
| 50 dpc | Female | 1 Gonad | 4 | 3 | |
| Male | 1 Gonad | 3 | 4 | ||
| 60 dpc | Female | 1 Gonad | 2 | 3 | |
| Male | 1 Gonad | 2 | 4 | ||
| 70 dpc | Female | 1 Gonad | 2 | 3 | |
| Male | 1 Gonad | 2 | 4 | ||
| 90 dpc | Female | 1 Gonad | 2 | 3 | |
| Male | Piece of gonad | 2 | 4 | ||
| 130 dpc | Female | 1 Gonad | 1 | 3 | |
| Male | Piece of gonad | 1 | 2 | ||
| 5 dpp | Female | Piece of gonad | 1 | 3 | |
| Male | Piece of gonad | 1 | 3 | ||
| 1 mpp | Female | Piece of gonad | 1 | 3 | |
| Male | Piece of gonad | 1 | 4 | ||
| 3 mpp | Female | Piece of gonad | 1 | 3 | |
| Male | Piece of gonad | 1 | 4 | ||
| 7 mpp | Female | Piece of gonad | 1 | 3 | |
| Male | Piece of gonad | 1 | 4 | ||
| 4 ypp | Male | Piece of gonad | 1a | 3 |
Three different pieces of the same testis was used to realize three independent RT.
PCR primers
PCR primers were designed using primer express Software for Real‐Time PCR 3.0 (Applied Biosystems, ThermoFisher Scientific, Courtaboeuf, France) analysis of Prnp, Prnd and Sprn expression in mice and goats (Table 2). Mice and goats gene sequences were obtained from GenBank. Primer efficiencies and specificities were evaluated on genomic DNA. The chosen sets of primers share similar efficiencies (not below 90%).
Table 2.
Primers used in the present study
| Gene | Gene ID | Ref seq | Forward primer | Reverse primer | E‐efficiency | Amplicon size (bp) |
|---|---|---|---|---|---|---|
| Mice primers | ||||||
| Prnp | ENSMUSG00000079037 | NM_011170 | TTTTCTCCTCCCCTCCTGTCA | ACCACGAGAATGCGAAGGAA | 1.896 | 100 |
| Prnd | ENSMUSG00000027338 | NM_023043 | CGGGAGAAGCAGGATAGCAA | CTCCCCTTTCCAGCCAGAA | 1.846 | 100 |
| Sprn | ENSMUSG00000045733 | NM_183147 | GAACCGACCGAGGAGTCTACAG | GGTCTAAGGCCGAAGC | 1.89 | 125 |
| Hprtl | ENSMUSG00000025630 | NM_013556 | AAGACTTGCTCGAGATGTCATGAA | ATCCAGCAGGTCAGCAAAGAA | 1.912 | 100 |
| Ywhaz | ENSMUSG00000022285 | NM_011740 | TGGCAGCCTGCATGAAGTC | CGGGCTCCTACAACGTTTTTAT | 1.966 | 100 |
| H2afz | ENSMUSG00000037894 | NM_016750 | CAGCTGTCCAGTGTTGGTGATT | CTAATTAAGCCTCCAACTTGCTCAA | 1.938 | 100 |
| Goat primers | ||||||
| PRNP | ENSBTAG00000027937 | NM_001271626 | TGCAGGTAACACAGCCAGCTA | TTCGTATTATGCTCATTCCTTGTGA | 1.98 | 100 |
| PRND | ENSBTAG00000011010 | NM_174158 | AGTTGGCTTGTTCATCATTGCA | CCTGGCACATTCTTTTATCTGCTTA | 2.00 | 100 |
| SPRN | ENSBTAG00000047474 | NM_001080321 | AGGAATGATGGCGGCAAAA | GGAGGCACTTGTCCTGAGTGA | 1.97 | 102 |
| 3βHSD | ENSBTAG00000006769 | NM_174343 | GCACCTTGTACACTTGTGCCC | GAT GCCGTTGTTATT CAAGGC | 2.10 | 101 |
| ACTB | ENSBTAG00000026199 | NM_173979 | CAGCAAGCAGGAGTACGATGAG | AAGGGTGTAACGCAGCTAACAGT | 1.90 | 85 |
| YWHAZ | ENSBTAG00000000236 | NM_174814 | GGAGCCCGTAGGTCATCTTG | CTCGAGCCATCTGCTGTTTTT | 1.95 | 85 |
| H2AFZ | ENSBTAG00000004428 | GCGTATTACCCCTCGTCACTTG | CAGCAATTGTAGCCTTGATGAGA | 1.97 | 80 | |
Quantitative RT‐PCR
RNAs were extracted using the RNeasy Mini kit (Qiagen, Courtaboeuf, France). Super‐Script II (Invitrogen, ThermoFisher Scientific) was used to synthesize cDNA for qRT‐PCR from 1μg (mice) or 2 μg (goats) of gonad RNA (Table 1). To identify appropriate qRT‐PCR normalizing genes for foetal and postnatal gonads in mice, expression stability of seven genes (Gapdh, Actb, B2 m, Mapk1, H2afz, Ywhaz and Hprt1) was tested at each time point and the GeNorm program 32 used to select a combination of the most stable genes. The three retained genes were Ywhaz, H2afz and Hprt1 (Table 2). For goats, the previously described ACTB, YWhAZ and H2AFZ genes were used 33 (Table 2). qRT‐PCR was performed on all genes at all time points, in triplicates, using the Absolute Blue SYBR Green ROX mix (ThermoFisher Scientific) and the StepOnePlus Real‐Time PCR System (Applied Biosystems). The results were analysed by the relative standard curve method with the qbase Software 34. Data points were plotted using Excel. Statistical analyses were performed using the invivostat software 35 that combines an ANOVA approach followed by a Fisher's Least Significant Difference (LSD)‐test.
Immunostaining
Freshly dissected gonads were fixed in 4% paraformaldehyde in phosphate saline buffer (PBS) at 4 °C for 1 h or overnight (according to the size of the gonad). After washes in PBS with increasing concentrations of sucrose (0, 12%, 15% and 18%), tissue specimens were embedded in Jung Tissue Freezing Medium (Leica Biosystems, Nanterre, France) and frozen at −80 °C. Cryo‐sections (7 μm thick) were obtained and stored at −80 °C until used. The sections were air‐dried, rehydrated in PBS and permeabilized during 30 min in PBS with 0.5% triton and 1% BSA. The primary antibodies were then applied overnight at 4 °C. Table 3 describes the antibody references and concentrations 15, 28, 36, 37, 38. After several washes, the sections were incubated with secondary antibodies for 1 h at room temperature. The slides were then rinsed in PBS, mounted in Vectashield mounting medium with DAPI (Vector) and observed as above.
Table 3.
Antibodies used in the present study
| Primary antibody | Reference | Source | IHC dilution | WB dilution |
|---|---|---|---|---|
| DPL – purified boDpl67–81 | Gift of Dr Paltrinieri (Rondena et al. 28) | Rabbit | 1 : 150 | |
| PRP – Sha31 | (Feraudet et al. 36) | Mouse | 1 : 1000 | |
| Biotinylated PRP – b Sha31 | (Feraudet et al. 36) | Mouse | 1 : 50 000 | |
| Murine SHO | Gift of Dr Westaway (Watts et al. 15) | Rabbit | 1 : 500 | |
| Ovine SHO | Gift of Dr Peelman (Lampo et al. 37) | Rabbit | 1 : 50 | |
| Cyp17A | Gift of Dr Hales (Hales et al.38) | Rabbit | 1 : 200 |
| Secondary antibody | Reference | Conjugate | IHC dilution | WB dilution |
|---|---|---|---|---|
| Anti‐rabbit IgG‐Dylight 488 | 072‐03‐15‐06, KPL | Fluorescein | 1 : 200 | |
| Anti‐rabbit IgG‐Dylight 594 | 072‐09‐15‐06, KPL | Fluorescein | 1 : 200 | |
| Anti‐mouse IgG‐Cy3 | AP160C, Millipore | Cyanine 3 | 1 : 200 | |
| Anti‐rabbit IgG‐B | BA‐1000, Vector | Biotin | 1 : 200 | |
| Anti‐mouse‐IgG‐HRP strep |
Horseradish peroxydase Streptavidin |
1/20 000 | ||
| Fluorescein Streptavidin | SA‐5001, Vector | 1 : 200 | ||
| Texas Red® Streptavidin | SA‐5006, Vector | 1 : 200 | ||
| Mounting medium | Reference | |||
| Vectashield for fluorescence with DAPI | H‐1200, Vector |
Western blot analysis
Frozen tissues of adult mice and goats were homogenized in 50 nm Tris HCl, 150 mm NaCl, 0.5% sodium deoxycholate (w/v), 0.1% sodium dodecyl sulphate (w/v), 1% of a nonionic nondenaturing detergent (NP‐40), one complete EDTA free mini‐protease inhibitor tablet per 10 mL (Roche Diagnostic, Saint‐Egrève, France). Whole extracts (20 μg of total protein) were subject to 4–15% gradient SDS/PAGE and transferred to a poly(vinylidene difluoride) membrane (GE healthcare Life Sciences, Vélizy‐Villacoublay, France). The membrane was probed with anti‐biotinylated‐PrP antibody (bSha 31; Table 3). The secondary antibody used was horseradish peroxydase/streptavidin conjugated antirabbit (Table 3). Immunodetection using the enhanced chemiluminescence (ECL) method (PIERCE) was performed according to the manufacturer's instruction and the images were recorded on an image analysis station (Luminescent Image analyse Las‐1000plus Fujifilm).
Results and discussion
By contrast with mice, PRND is highly expressed in goat foetal testis
We have previously carried out an expressional study of the Prion gene family in the goat species 30 that suggested an involvement of PRND in early gonadal differentiation. The aim of the present study was to complete this observation by (i) including the recently discovered SPRN gene and (ii) establishing a comparative view of the expression of the three members of the Prion gene family throughout all gonad developmental stages, from differentiation to adulthood (Fig. 1A), in (iii) two mammalian species, mice and goats. Gonadal expression profiles of PRNP, SPRN and PRND have been precisely determined using quantitative RT‐PCR instead of classical RT‐PCR as previously used 30, at 7 and 15 developmental stages in mice and goats respectively (Fig. 1B–G), and during ageing in mice where four additional stages were studied. A stable gonadal expression of these three genes in the mouse species was observed during ageing since 50 dpp until 10–12 months of age (Fig. 2). In both species, Prnp/PRNP gene expression slightly increases during development and appears to be more intense around birth (Fig. 1B,C). During gonadal development, SPRN was found to be expressed in male and female gonads of mice and goats at all tested stages (Fig. 1B–E) but only faintly when compared to PRNP and PRND (as for example in goat testis samples, the cycle thresholds, CT, values are for PRNP and PRND between 21 and 30, but only between 31 and 36 for SPRN). Sprn/SPRN appears to be more expressed in ovaries of both species during early follicles formation (i.e. from 18.5 dpc to 5 dpp in mice and 70–90 dpc in goats); and specifically in goat ovaries before the beginning of germ cell meiosis (Fig. 1D,E). Indeed, the highest SPRN expression level is found at this premeiotic stage (i.e. 41–50 dpc) only in the goat species. The duration of this premeiotic stage, which starts after gonad commitment in one sex or the other (i.e. 12.5 dpc in mice, 36 dpc in goats), appears quite different in mice and goats, 24 h in mice instead of 2 weeks in goats. Although no profound change could be noticed in mouse ovaries during this period, goat ovaries were organized into cortical and medullar compartments where germ cells were concentrated in the cortex and estrogens were produced by the medulla part under the control of the FOXL2 gene that has been shown to be a major ovarian‐determining gene in goats, by contrast with mice 39, 40, 41.
Figure 1.
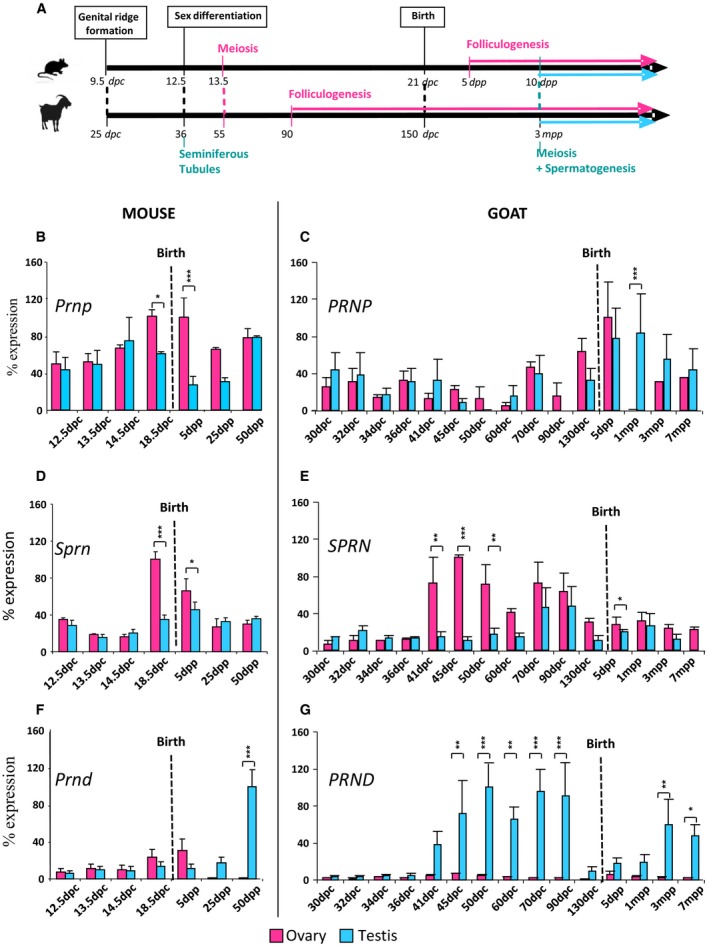
Expression of Prion family genes during gonad development. (A) Chronology of gonad differentiation in mice and goats. Expression of the Prnp/PRNP (B, C), Sprn/SPRN (D, E) and PrndPRND (F, G) genes was quantified using real‐time RT‐PCR in ovaries (pink histograms) and testes (blue histograms) in mice (B, D, F) and goats (C, E, G). From 12.5 dpc to 13.5 dpc (mice) and 30 dpc to 36 dpc (goats): gonad + mesonephros, others stages: gonad only. Prnp/PRNP, PrP protein gene; Sprn/SPRN, Sho gene; Prnd/PRND, Dpl gene; dpc, days post coïtum; dpp, days post partum; mpp, months post partum. Values are expressed in percentage according to the highest one noted 100%. Means ± SD were plotted. Planned comparisons were made on the predicted means with ‘sex’ and ‘stage’ as treatment factors (two‐way ANOVA approach, followed by a Fisher's LSD‐test). For each stage, significant differences between the two sexes are showed by stars (*P‐value ≤ 0.05; **P‐value ≤ 0.01; ***P‐value < 0.001).
Figure 2.
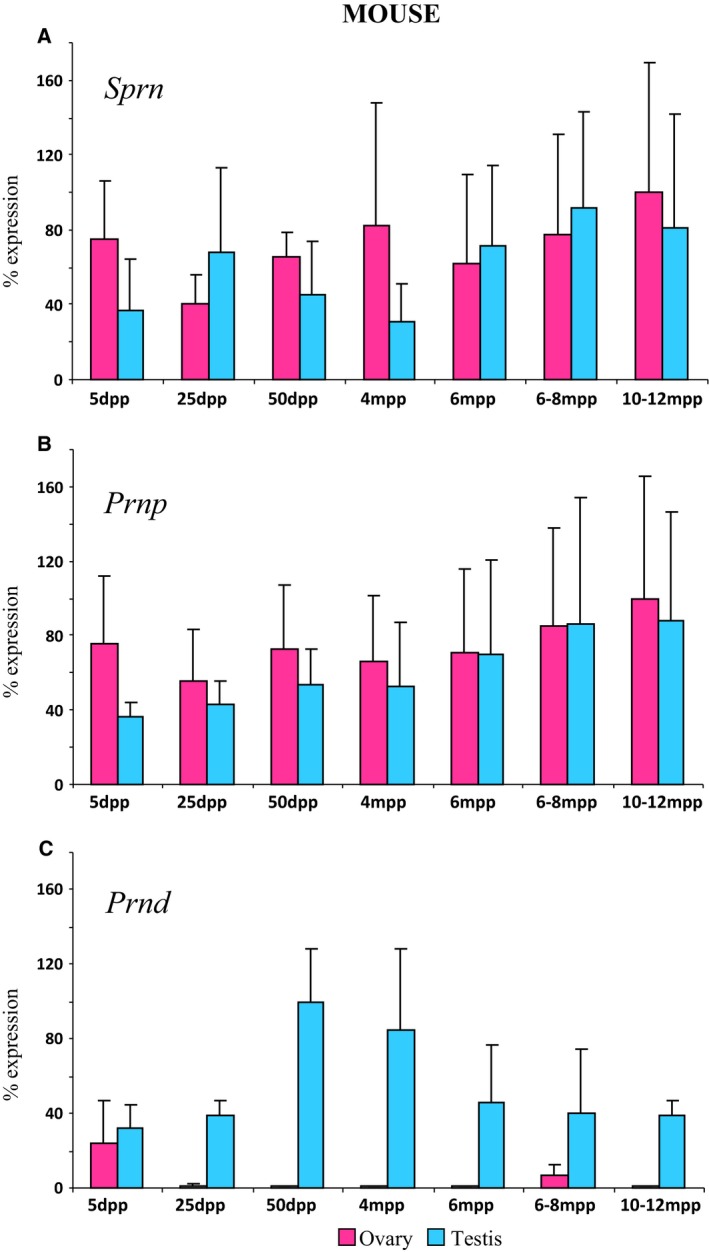
Expression of Prion family genes during postnatal gonad development in mice. Sprn (A), Prnp (B) and Prnd (C) gene expressions were quantified using real‐time RT‐PCR in postnatal ovaries (pink histograms) and testes (blue histograms) in mice. Sprn, Sho gene; Prnp, PrP protein gene; Prnd, Dpl gene; dpp, days post partum; mpp, months post partum.
Finally and as previously noticed 30, Prnd/PRND expression is found higher in testes than in ovaries at adulthood for both species, but this sex‐dimorphism appears since early testicular differentiation specifically in the goat species (Fig. 1F,G). The expression of Prnd/PRND in adult testes is well‐documented and Prnd has been shown to be required for spermiogenesis in mice 24, 25. However, the concomitant increased level of PRND expression with the start of testicular differentiation appears specific to the goat species as it was not observed in mice and it has not been described to date in any other species (Fig. 1G).
Sho and PrP cellular localizations remain difficult to ascertain
In order to gain more information on the 3 proteins encoded by the 3 Prion gene family members, we carried out IHC (Immuno‐Histo‐Chemistry) and western blot experiments using available antibodies (Table 3). Two antibodies have been tested against Sho. Each gives a specific staining by IHC that remains different (i) from each other, (ii) from one species to another and also (iii) from Sho gonadal expression already described by additive transgenesis of a Sprn‐LacZ mini‐gene in mice 16. Thus, we are unable to ascertain in what gonadal cell type Sho could be detected. The recent derivation of Sho‐knockout mice 19 might help to decipher the real expression pattern of this protein by comparative IHC analysis, but these mice were not available to us at the time of this experiment. In the same way, the PrP protein was detected by IHC and western blot at adulthood in both species and at 130 dpc in goats (Fig. 3). By discarding the strong nonspecific staining in the interstitial area of adult mouse testes, identified by using Prnp 0/0 testicular samples (Fig. 3A), the PrP protein appears to be present at all stages mainly inside the seminiferous tubules, most likely in the Sertoli cells (clearly visible at 130 dpc in goats) and in the germ cells at the end of spermiogenesis (see the staining of elongated spermatids on Fig. 3B,C). Presence of PrP in the testis was confirmed by western blot performed on adult mice and goats testes and spermatozoa (Fig. 3F). The presence of PrP on ejaculated sperm cells has already been reported in humans, cattle and mice 5, but its testicular or epididymal origin remains debated, as recently discussed 12. To our knowledge, this is the first time that PrP is clearly detected in the Sertoli cells of immature (130 dpc in goats) and mature (in adult mice) testes.
Figure 3.
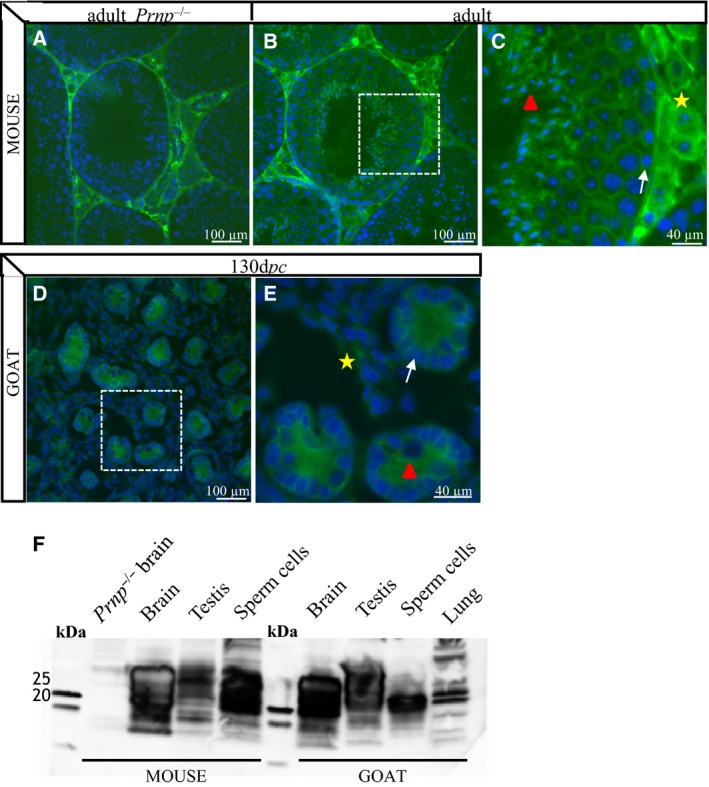
PrP immunodetection in mice and goat testes. (A): The specificity of PrP antibody (sha31) was tested on adult Prnp 0/0 testis in mice. Testicular interstitial cells showed a nonspecific fluorescence. PrP immunodetection was performed on adult testis in mice (B, C) and on 130 dpc testis in goats (D, E). The fluorescent staining is presented with a 4, 6‐diamine‐2‐phenylidole‐dihydrochloride (DAPI) blue nuclear‐specific counterstaining. (C, E) photographs correspond to an enlargement of the white rectangles depicted on (B, D) photographs. Some cells are marked as followed: Sertoli cells (white arrow, pointed on a Sertoli cell nucleus), Leydig cells (yellow star) and germinal cells (red arrowhead). dpc, days post coïtum. (F): Western blot assays of endogenous PrP (Sha31b) of adult homogenates tissues (brain, testis and lung) and sperm cells in mice and goats. Bands denoted the presence of various PrP isoforms in these tissues. No protein was detected in Prnp 0/0 brain mice used as negative control.
By contrast with mice, DPL is detected in goat Leydig cells
We have previously shown that the DPL protein could be detected in germ cells of both sexes and in foetal Leydig cells of early goats developing testes at 44 and 62 dpc 30. According to qRT‐PCR results (Fig. 1F,G) showing a high expression of PRND in goat developing testes, we checked its cellular localization throughout testis development in immature and mature testes and on spermatozoa of both species (Fig. 4). First, the specificity of anti‐DPL antibody boDpl67‐81 28 was confirmed as no signal was obtained on adult Prnd 0/0 mouse testes (Fig. 4A,B). Using this antibody, DPL is detected in the cytoplasm of some germ cells in both species; germ cells that have passed the zygotene stage of meiosis (i.e. pachytene and spermatides)(Fig. 4C,F and K,N) and its presence persists on spermatozoa with a high staining of the acrosomal vesicle (Fig. 4G,H and O,P). These results are in complete agreement with the role of DPL in spermiogenesis 24, 25.
Figure 4.
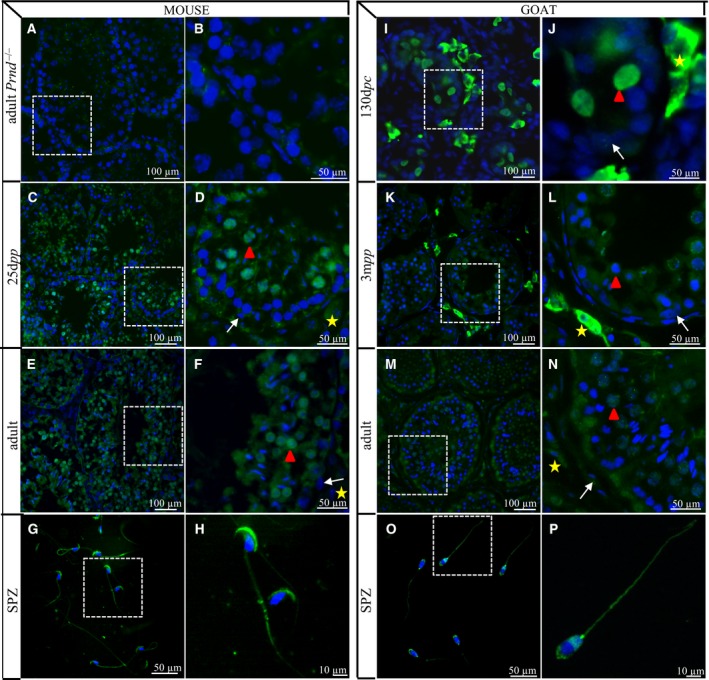
Dpl immunodetection during testis development in mice and goats. In mice, the specificity of the Dpl antibody (boDpl67–81) was tested on adult Prnd 0/0 testis (A, B). Dpl immunodetection was performed on 25 dpp (C, D), adult (E, F) testes and on spermatozoa (G, H). In goats, Dpl immunostaining was carried out 130 dpc (I, J), 3 mpp (K, L), adult (M, N) testes and on spermatozoa (O, P). The fluorescent staining is presented with a 4, 6‐diamine‐2‐phenylidole‐dihydrochloride (DAPI) blue nuclear‐specific counterstaining. The second (B, D, F, H) and fourth (J, L, N, P) columns correspond to an enlargement of the white rectangles depicted on the first (A, C, E, G) and third (I, K, M, O) columns respectively. Cells are marked as followed: Sertoli (white arrow), Leydig (yellow star) and germinal (red arrowhead). dpc, days post coïtum; dpp, days post partum; mpp, months post partum; SPZ, spermatozoa.
More interestingly, a strong staining is detected in some cells of the interstitial testicular space, specifically in the goat species and at immature stages, as the staining disappears in adult testes (compare Fig. 4I,L with M,N). In order to precisely define this DPL staining in the interstitial testicular compartment, we carried out double IHC with an anti‐CYP17 antibody detecting a Leydig cell‐specific marker corresponding to a key enzyme of steroid synthesis, the cytochrome P450 17alpha‐hydroxylase/17,20‐lyase 38. DPL and CYP17 are found colocalized in the same interstitial cells (Fig. 5A–F). DPL staining in goat testes disappears between the prepubertal 3‐month and the pubertal 7‐month stages (Fig. 4K–N), suggesting that PRND is specifically expressed in the foetal Leydig cell population. These cells disappear after birth and are replaced by adult Leydig cells at puberty 42. Finally, the PRND expression profile appear hugely similar to that of 3βHSD, another Leydig cell‐specific marker, until 1 month after birth indicating that during testis development the major part of PRND expression is Leydig specific (Fig. 5G). This observation could explain the high testicular levels of PRND transcripts detected from 41 dpc to 1 mpp specifically in goat testes (Fig. 1G) and not in the mouse testes of the corresponding stages, from 12.5 dpc to 5 dpp (Fig. 1F). During the prepubertal period, spermatogenesis starts (25 dpp in mice, 3 mpp in goats), the number of germ cells increases and more importantly differentiated meiotic germ cells expressing PRND/Prnd appear. From these prepubertal stages, the PRND/Prnd expression profiles are similar between mice and goats (Fig. 1F,G), but diverge from that of 3βHSD in goats (Fig. 5G). It indicates that around puberty PRND expression increases in postmeiotic germ cells and disappears from foetal Leydig cells because of their own disappearance.
Figure 5.
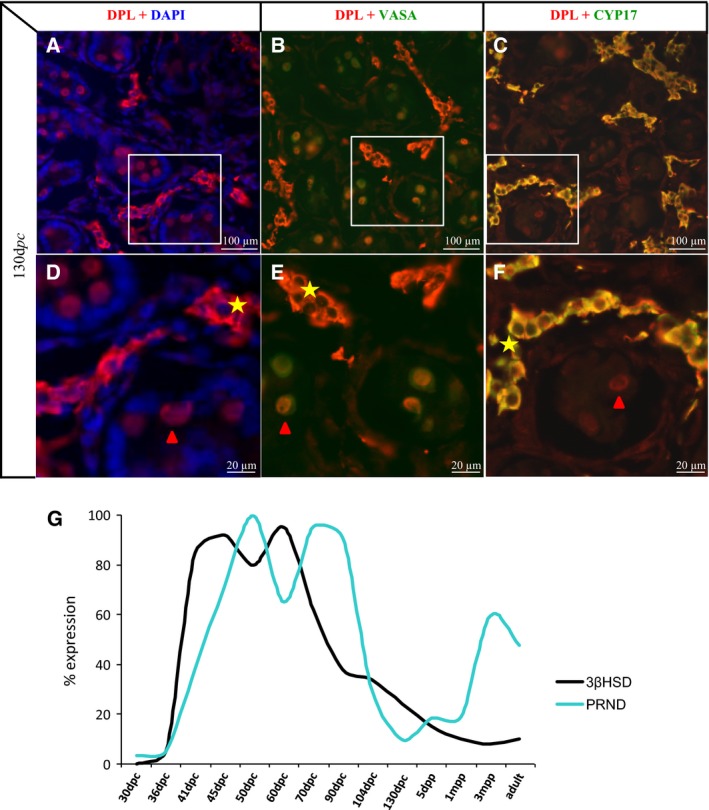
Expression of Leydig cell markers in goat testis. Dpl (A–F), VASA (B, E) and CYP17 (C, F) immunodetections were performed in a goat testis at 130 dpc. (D–F) photographs correspond to an enlargement of the white rectangles depicted on (A–C) photographs. (G): 3βHSD (black curve) and PRND (blue curve) expression was quantified using real‐time RT‐PCR during testis development in goat and represented on the same graph. At 30 and 36 dpc gonads are not dissected from mesonephros. dpc, days post coïtum; dpp, days post partum; mpp, months post partum.
In conclusion, we report the differential expression of the three members of the prion protein gene family in the developing gonads of mice and goats. Only relatively low levels of expression were detected for Sho, an observation that might relate with the lack of reproductive‐associated phenotype in mouse Sprn‐knockout mice 8, 19. By contrast and in addition to its conserved role in spermiogenesis, PRND seems to be a key marker of foetal Leydig cells in goats. This observation adds PRND to the list of genes having potentially different biological roles in mice and humans or ruminants (see 39, 43 for recent examples). The present results highlight that PRND is a key candidate gene for functional studies in goats because its involvement in foetal Leydig cells cannot be studied in the widely used mouse mammalian model. Deciphering this role may have important implications for human reproduction. In order to determine if PRND could be a crucial actor of foetal Leydig cell development, its targeting is currently under way in the goat species by using genome editing technologies, recently proven successful by us in this species 39.
Author contributions
AAB, MP, KMG, JLV and EP participated in the conception of the study, interpretation of data and in the drafting of the article. BP and JC participated in the design and realization of animal experiments. AAB, ME, MA and FMT performed experiments.
Acknowledgements
We thank all the staff of UCEA (INRA's Joint Animal Experimental Unit) for management of the goats, and Mrs Elaine Rémy for English corrections. We also thank the ANR (National Research Agency) for its financial support via the following grant, ANR‐09‐BLAN‐0015‐01, PRIFAGENE.
References
- 1. Watts JC and Westaway D (2007) The prion protein family: diversity, rivalry, and dysfunction. Biochim Biophys Acta 1772, 654–672. [DOI] [PubMed] [Google Scholar]
- 2. Aguzzi A and Polymenidou M (2004) Mammalian prion biology: one century of evolving concepts. Cell 116, 313–327. [DOI] [PubMed] [Google Scholar]
- 3. Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144. [DOI] [PubMed] [Google Scholar]
- 4. Kovacs GG and Budka H (2009) Molecular pathology of human prion diseases. Int J Mol Sci 10, 976–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaked Y, Rosenmann H, Talmor G and Gabizon R (1999) A C‐terminal‐truncated PrP isoform is present in mature sperm. J Biol Chem 274, 32153–32158. [DOI] [PubMed] [Google Scholar]
- 6. Weber P, Schuler M, Gérard C, Mark M, Metzger D and Chambon P (2003) Temporally controlled site‐specific mutagenesis in the germ cell lineage of the mouse testis. Biol Reprod 68, 553–559. [DOI] [PubMed] [Google Scholar]
- 7. Ecroyd H, Sarradin P, Dacheux JL and Gatti JL (2004) Compartmentalization of prion isoforms within the reproductive tract of the ram. Biol Reprod 71, 993–1001. [DOI] [PubMed] [Google Scholar]
- 8. Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M and Weissmann C (1992) Normal development and behaviour of mice lacking the neuronal cell‐surface PrP protein. Nature 356, 577–582. [DOI] [PubMed] [Google Scholar]
- 9. Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I and Hope J (1994) 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol 8, 121–127. [DOI] [PubMed] [Google Scholar]
- 10. Richt JA, Kasinathan P, Hamir AN, Castilla J, Sathiyaseelan T, Vargas F, Sathiyaseelan J, Wu H, Matsushita H, Koster J et al (2007) Production of cattle lacking prion protein. Nat Biotechnol 25, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu G, Chen J, Xu Y, Zhu C, Yu H, Liu S, Sha H, Xu X, Wu Y, Zhang A et al (2009) Generation of goats lacking prion protein. Mol Reprod Dev 76, 3. [DOI] [PubMed] [Google Scholar]
- 12. Allais‐Bonnet A and Pailhoux E (2014) Role of the prion protein family in the gonads. Front Cell Dev Biol 2, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I and Brentani RR (2008) Physiology of the prion protein. Physiol Rev 88, 673–728. [DOI] [PubMed] [Google Scholar]
- 14. Lopes MH and Santos TG (2012) Prion potency in stem cells biology. Prion 6, 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watts JC, Drisaldi B, Ng V, Yang J, Strome B, Horne P, Sy MS, Yoong L, Young R, Mastrangelo P et al (2007) The CNS glycoprotein Shadoo has PrP(C)‐like protective properties and displays reduced levels in prion infections. EMBO J 26, 4038–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young R, Le Guillou S, Tilly G, Passet B, Vilotte M, Castille J, Beringue V, Le Provost F, Laude H and Vilotte JL (2011) Generation of Sprn‐regulated reporter mice reveals gonadic spatial expression of the prion‐like protein Shadoo in mice. Biochem Biophys Res Commun 412, 752–756. [DOI] [PubMed] [Google Scholar]
- 17. Young R, Passet B, Vilotte M, Cribiu EP, Béringue V, Le Provost F, Laude H and Vilotte JL (2009) The prion or the related Shadoo protein is required for early mouse embryogenesis. FEBS Lett 583, 3296–3300. [DOI] [PubMed] [Google Scholar]
- 18. Passet B, Young R, Makhzami S, Vilotte M, Jaffrezic F, Halliez S, Bouet S, Marthey S, Khalifé M, Kanellopoulos‐Langevin C et al (2012) Prion protein and Shadoo are involved in overlapping embryonic pathways and trophoblastic development. PLoS ONE 7, e41959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daude N, Wohlgemuth S, Brown R, Pitstick R, Gapeshina H, Yang J, Carlson GA and Westaway D (2012) Knockout of the prion protein (PrP)‐like Sprn gene does not produce embryonic lethality in combination with PrP(C)‐deficiency. Proc Natl Acad Sci USA 109, 9035–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rossi D, Cozzio A, Flechsig E, Klein MA, Rülicke T, Aguzzi A and Weissmann C (2001) Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J 20, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore RC, Lee IY, Silverman GL, Harrison PM, Strome R, Heinrich C, Karunaratne A, Pasternak SH, Chishti MA, Liang Y et al (1999) Ataxia in prion protein (PrP)‐deficient mice is associated with upregulation of the novel PrP‐like protein doppel. J Mol Biol 292, 797–817. [DOI] [PubMed] [Google Scholar]
- 22. Anderson L, Rossi D, Linehan J, Brandner S and Weissmann C (2004) Transgene‐driven expression of the Doppel protein in Purkinje cells causes Purkinje cell degeneration and motor impairment. Proc Natl Acad Sci USA 101, 3644–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al Bersaoui R, Robert I, Lutz Y, Blanc F, Sommermeyer‐Leroux G, Shibaguchi H, Aunis D and Fuchs JP (2005) Purkinje‐cell degeneration in prion protein‐deficient mice is associated with a cerebellum‐specific Doppel protein species signature. FEBS Lett 579, 2715–2721. [DOI] [PubMed] [Google Scholar]
- 24. Behrens A, Genoud N, Naumann H, Rülicke T, Janett F, Heppner FL, Ledermann B and Aguzzi A (2002) Absence of the prion protein homologue Doppel causes male sterility. EMBO J 21, 3652–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paisley D, Banks S, Selfridge J, McLennan NF, Ritchie AM, McEwan C, Irvine DS, Saunders PT, Manson JC and Melton DW (2004) Male infertility and DNA damage in Doppel knockout and prion protein/Doppel double‐knockout mice. Am J Pathol 164, 2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peoc'h K, Serres C, Frobert Y, Martin C, Lehmann S, Chasseigneaux S, Sazdovitch V, Grassi J, Jouannet P, Launay JM et al (2002) The human “prion‐like” protein Doppel is expressed in both Sertoli cells and spermatozoa. J Biol Chem 277, 43071–43078. [DOI] [PubMed] [Google Scholar]
- 27. Espenes A, Harbitz I, Skogtvedt S, Fuglestveit R, Berg KA, Dick G, Krogenaes A and Tranulis MA (2006) Dynamic expression of the prion‐like protein Doppel in ovine testicular tissue. Int J Androl 29, 400–408. [DOI] [PubMed] [Google Scholar]
- 28. Rondena M, Ceciliani F, Comazzi S, Pocacqua V, Bazzocchi C, Luvoni C, Chigioni S and Paltrinieri S (2005) Identification of bovine doppel protein in testis, ovary and ejaculated spermatozoa. Theriogenology 63, 1195–1206. [DOI] [PubMed] [Google Scholar]
- 29. Serres C, Peoc'h K, Courtot AM, Lesaffre C, Jouannet P & Laplanche JL (2006) Spatio‐developmental distribution of the prion‐like protein doppel in Mammalian testis: a comparative analysis focusing on its presence in the acrosome of spermatids. Biol Reprod 74, 816–823. [DOI] [PubMed] [Google Scholar]
- 30. Kocer A, Gallozzi M, Renault L, Tilly G, Pinheiro I, Le Provost F, Pailhoux E and Vilotte J (2007) Goat PRND expression pattern suggests its involvement in early sex differentiation. Dev Dyn 236, 836–842. [DOI] [PubMed] [Google Scholar]
- 31. Pailhoux E, Vigier B, Vaiman D, Servel N, Chaffaux S, Cribiu E and Cotinot C (2002) Ontogenesis of female‐to‐male sex‐reversal in XX polled goats. Dev Dyn 224, 39–50. [DOI] [PubMed] [Google Scholar]
- 32. Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA & Speleman F (2002) Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montazer‐Torbati F, Kocer A, Auguste A, Renault L, Charpigny G, Pailhoux E and Pannetier M (2010) A study of goat SRY protein expression suggests putative new roles for this gene in the developing testis of a species with long‐lasting SRY expression. Dev Dyn 239, 3324–3335. [DOI] [PubMed] [Google Scholar]
- 34. Hellemans J, Mortier G, De Paepe A, Speleman F and Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real‐time quantitative PCR data. Genome Biol 8, R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark RA, Shoaib M, Hewitt KN, Stanford SC and Bate ST (2012) A comparison of InVivoStat with other statistical software packages for analysis of data generated from animal experiments. J Psychopharmacol 26, 1136–1142. [DOI] [PubMed] [Google Scholar]
- 36. Féraudet C, Morel N, Simon S, Volland H, Frobert Y, Créminon C, Vilette D, Lehmann S and Grassi J (2005) Screening of 145 anti‐PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem 280, 11247–11258. [DOI] [PubMed] [Google Scholar]
- 37. Lampo E, Van den Broeck W, Willemarck N, Van Poucke M, Casteleyn CR, De Spiegelaere W, Van Zeveren A and Peelman LJ (2011) Distribution of the Shadoo protein in the ovine brain assessed by immunohistochemistry. Res Vet Sci 90, 372–378. [DOI] [PubMed] [Google Scholar]
- 38. Hales DB, Sha LL and Payne AH (1987) Testosterone inhibits cAMP‐induced de Novo synthesis of Leydig cell cytochrome P‐450(17 alpha) by an androgen receptor‐mediated mechanism. J Biol Chem 262, 11200–11206. [PubMed] [Google Scholar]
- 39. Boulanger L, Pannetier M, Gall L, Allais‐Bonnet A, Elzaiat M, Le Bourhis D, Daniel N, Richard C, Cotinot C, Ghyselinck NB et al (2014) FOXL2 is a female sex‐determining gene in the goat. Curr Biol 24, 404–408. [DOI] [PubMed] [Google Scholar]
- 40. Pannetier M, Elzaiat M, Thépot D and Pailhoux E (2012) Telling the story of XX sex reversal in the goat: highlighting the sex‐crossroad in domestic mammals. Sex Dev 6, 33–45. [DOI] [PubMed] [Google Scholar]
- 41. Pannetier M, Fabre S, Batista F, Kocer A, Renault L, Jolivet G, Mandon‐Pépin B, Cotinot C, Veitia R and Pailhoux E (2006) FOXL2 activates P450 aromatase gene transcription: towards a better characterization of the early steps of mammalian ovarian development. J Mol Endocrinol 36, 399–413. [DOI] [PubMed] [Google Scholar]
- 42. Dong L, Jelinsky SA, Finger JN, Johnston DS, Kopf GS, Sottas CM, Hardy MP and Ge RS (2007) Gene expression during development of fetal and adult Leydig cells. Ann N Y Acad Sci 1120, 16–35. [DOI] [PubMed] [Google Scholar]
- 43. Elzaiat M, Jouneau L, Thépot D, Klopp C, Allais‐Bonnet A, Cabau C, André M, Chaffaux S, Cribiu EP, Pailhoux E et al (2014) High‐throughput sequencing analyses of XX genital ridges lacking FOXL2 reveal DMRT1 up‐regulation before SOX9 expression during the sex‐reversal process in goats. Biol Reprod 91, 153. [DOI] [PubMed] [Google Scholar]


