Abstract
Critically ill cirrhotic patients have high mortality rates, particularly when they present with acute kidney injury (AKI) on admission. The Kidney Disease: Improving Global Outcomes (KDIGO) group aimed to standardize the definition of AKI and recently published a new AKI classification. However, the efficacy of the KDIGO classification for predicting outcomes of critically ill cirrhotic patients is unclear. We prospectively enrolled 242 cirrhotic patients from a 10-bed specialized hepatogastroenterology intensive care unit (ICU) in a 2000-bed tertiary-care referral hospital. Demographic parameters and clinical variables on day 1 of admission were prospectively recorded. The overall in-hospital mortality rate was 62.8%. Liver diseases were usually attributed to hepatitis B viral infection (26.9%). The major cause of ICU admission was upper gastrointestinal bleeding (38.0%). Our result showed that the KDIGO classification had better discriminatory power than RIFLE and AKIN criteria in predicting in-hospital mortality. Cumulative survival rates at the 6-month after hospital discharge differed significantly between patients with and without AKI on ICU admission day. In summary, we identified that the outcome prediction performance of KDIGO classification is superior to that of AKIN or RIFLE classification in critically ill cirrhotic patients.
Acute kidney injury (AKI) is a common and serious complication in critically ill cirrhotic patients. The pathophysiological factors associated with AKI are also attributed to the dysfunction of other organs, indicating that AKI is often part of a multiple organ failure syndrome1,2. In the literature, the occurrence of AKI signifies a lower chance of survival in cirrhotic patients3,4,5,6,7,8.
To unify the definition of AKI, the Kidney Disease: Improving Global Outcomes (KDIGO) group classification was proposed based on the Risk, Injury, Failure, Loss of Kidney Function, and End-stage Kidney Disease (RIFLE) and the Acute Kidney Injury Network (AKIN) classifications in 20129. An elevation of the serum creatinine (SCr) level exceeding 0.3 mg/dl within 48 h, or an increase in SCr to 1.5 times the baseline value, which is known or presumed to have occurred within 7 days before, or a urine volume of <0.5 ml·kg−1·h−1 for 6 h were defined as AKI. Like the AKIN criteria, KDIGO also classifies patients starting renal replacement therapy (RRT) as having stage 3 AKI, and it removes the threshold of a 0.5 mg/dl increment for SCr > 4 mg/dl in the criteria of stage 3 AKI. To date, the new AKI classification has been assessed in several investigations10,11,12,13,14. However, its application to critically ill cirrhotic patients and its prediction accuracy compared with the previous two classifications have not been thoroughly evaluated.
The objective of this prospective study was to determine the association between hospital mortality/short-term prognosis and the KDIGO classification in this homogeneous critically ill cirrhotic patient group. The AKIN and RIFLE classifications were also applied for comparison (Table 1).
Table 1. The definitions and classification for AKI.
| SCr criteria | UO Criteria | |
|---|---|---|
| (A) RIFLE | ||
| Definition | SCr changes over 1–7 days, sustained for more than 24 hrs | UO < 0.5 ml/kg/h x 6 hrs |
| Risk | Increase in SCr ≥ 1.5 x baseline or decrease in GFR ≥ 25% | UO < 0.5 ml/kg/h x 6 hrs |
| Injury | Increase in SCr ≥ 2.0 x baseline or decrease in GFR ≥ 50% | UO < 0.5 ml/kg/h x 12 hrs |
| Failure | Increase in SCr ≥ 3.0 x baseline or an absolute serum creatinine ≥4.0 mg/dl with an acute rise of at least 0.5 mg/dl or or decrease in GFR ≥ 75% | UO < 0.5 ml/kg/h x 24 hrs or anuria x 12hrs |
| Loss | Complete loss of kidney function > 4 wks | |
| ESRD | End-stage renal disease (>3 mo) | |
| (B) AKIN | ||
| Definition | Acute SCr changes occur within a 48 hrs period during hospitalization | Same as RIFLE |
| Stage 1 | Increased in SCr of ≥0.3 mg/dl or increase to 1.5–1.9 x baseline | |
| Stage 2 | Increase in SCr to 2.0–2.9 x baseline | |
| Stage 3 | Increase in SCr ≥ 3.0 x baseline or SCr ≥ 4.0 mg/dl with an acute rise of at least 0.5 mg/dl or Initiation of RRT | |
| (C) KDIGO | ||
| Definition | SCr changes ≥ 1.5 x baseline to have occurred within the prior 7 days or a 0.3 mg/d lincrease in SCr must occur within a 48 hrs period | Same as RIFLE |
| Stage 1 | Increased in SCr ≥ 1.5 x baseline or of 0.3 mg/dl | |
| Stage 2 | Increase in SCr ≥ 2.0 x baseline | |
| Stage 3 | Increase in SCr ≥ 3.0 x baseline or increase in serum creatinine to ≥ 4.0 mg/dl or initiation of RRT | |
*Abbreviations: SCr, serum creatinine; UO, urine output; hrs, hours; wks, weeks; ESRD, end-stage renal disease; RRT, renal replacement therapy; RIFLE, risk of renal failure, injury to kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; AKIN, acute kidney injury network; KDIGO, kidney disease improving global outcomes.
Results
Patient characteristics
Between September 2012 and August 2014, 242 cirrhotic patients were enrolled at the specialized hepatogastroenterology ICU in our institution. The mean age of the patients was 58 years; 183 of them were male (75.7%) and 59 were female (24.3%). The overall in-hospital mortality rate was 62.8% (152 of 242), and the 6-month mortality was 77.7% (188 of 242). Table 2 lists the patient demographic data and the clinical characteristics of both survivors and nonsurvivors. Table 3 lists the causes of cirrhosis and the primary reasons for ICU admission. Liver diseases were usually attributed to hepatitis B viral infection (26.9%). The most frequent reason for ICU admission was upper gastrointestinal bleeding (38.0%).
Table 2. Patients’ demographic data and clinical characteristics.
| All patients (n = 242) | Survivors (n = 90) | Non-survivors (n = 152) | p-value | |
|---|---|---|---|---|
| Age (years) | 58 ± 14 | 58 ± 14 | 58 ± 14 | NS (0.775) |
| Gender (M/F) | 183/59 | 72/18 | 111/41 | NS (0.278) |
| Body weight (kg) | 65.2 ± 12.7 | 65.3 ± 12.6 | 65.1 ± 12.8 | NS (0.919) |
| Length of ICU stay (days) | 8 ± 8 | 6 ± 6 | 9 ± 9 | 0.002 |
| Length of hospital stay (days) | 26 ± 24 | 29 ± 28 | 24 ± 22 | NS (0.093) |
| MAP, ICU admission (mmHg) | 72.3 ± 15.6 | 78.8 ± 14 | 68 ± 15 | <0.001 |
| Glasgow coma scale, ICU admission | 9 ± 5 | 11 ± 5 | 8 ± 5 | <0.001 |
| Serum Creatinine, ICU first day (mg/dl) | 2.6 ± 2.1 | 1.6 ± 1.3 | 3.2 ± 2.1 | <0.001 |
| Leukocytes, ICU first day (10^3/dl) | 13.0 ± 7.9 | 10.8 ± 6.3 | 14.4 ± 8.5 | <0.001 |
| Hemoglobin, ICU first day (g/dl) | 9.1 ± 2.1 | 9.1 ± 2.0 | 9.1 ± 2.2 | NS (0.940) |
| Platelets, ICU first day (x10^9/l) | 95 ± 74 | 90 ± 55 | 98 ± 83 | NS (0.422) |
| Albumin, ICU first day (g/dl) | 2.5 ± 0.6 | 2.6 ± 0.6 | 2.4 ± 0.5 | <0.001 |
| Sodium, ICU first day (mmol/l) | 137 ± 10 | 138 ± 8 | 137 ± 10 | NS (0.292) |
| Bilirubin, ICU first day (mg/dl) | 10.2 ± 11.1 | 4.7 ± 6.5 | 13.9 ± 11.9 | <0.001 |
| Prothrombin time INR, ICU first day | 2.3 ± 1.5 | 1.7 ± 0.5 | 2.7 ± 1.8 | <0.001 |
| AST, ICU first day | 179 ± 501 | 95 ± 176 | 228 ± 613 | 0.021 |
| ALT, ICU first day (units/l) | 518 ± 1962 | 212 ± 412 | 704 ± 2450 | 0.013 |
| DM (yes/no) | 72/170 | 32/58 | 40/112 | NS (0.147) |
| Hepatic encephalopathy (yes/no) | 151/91 | 47/43 | 104/48 | 0.014 |
| Previous ascites (yes/no) | 186/56 | 68/22 | 118/34 | NS (0.753) |
| Previous SBP (yes/no) | 53/189 | 13/77 | 40/112 | 0.036 |
| Previous EV bleeding (yes/no) | 134/108 | 58/32 | 76/76 | 0.033 |
| Previous peptic ulcer bleeding (yes/no) | 85/157 | 29/61 | 56/96 | NS (0.489) |
| Previous hepatoma (yes/no) | 74/168 | 27/63 | 47/105 | NS (1.000) |
| Previous renal failure (yes/no) | 63/179 | 18/72 | 45/107 | NS (0.129) |
| Respiratory failure, ICU first day (yes/no) | 151/91 | 49/41 | 102/50 | NS (0.055) |
| Sepsis, ICU admission (yes/no) | 116/71 | 32/24 | 84/47 | NS (0.412) |
| Child-Pugh points | 11 ± 2 | 10 ± 2 | 12 ± 2 | <0.001 |
| MELD score | 28 ± 12 | 19 ± 7 | 33 ± 10 | <0.001 |
| APACHE II | 25 ± 9 | 20 ± 7 | 28 ± 9 | <0.001 |
| APACHE III | 105 ± 40 | 78 ± 31 | 121 ± 35 | <0.001 |
| SOFA | 11 ± 5 | 7 ± 3 | 13 ± 4 | <0.001 |
Abbreviation: M, male; F, female; ICU, intensive care unit; MAP, mean arterial pressure; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DM, diabetes mellitus; SBP, spontaneous bacterial peritonitis; EV, esophageal varices; MELD, model for end-stage liver disease; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organfailure assessment.
Values in bold are statistically significant (P-value < 0.05).
There were significant differences in length of ICU stay, mean arterial pressure, Glasgow coma scale, serum creatinine, albumin, bilirubin, AST, ALT, prothrombin time INR, leukocyte count, presence of hepatic encephalopathy, previous SBP, previous EV bleeding, liver and ICU prognostic scores.
Table 3. Causes of cirrhosis and reasons of ICU admission according to hospital survival.
| All patients (n = 242) | Survivors (n = 90) | Non-survivors (n = 152) | p-value | |
|---|---|---|---|---|
| Causes of cirrhosis | ||||
| Alcoholic | 54 (22.3%) | 24 (26.7%) | 30 (19.7%) | NS (0.263) |
| Hepatitis B | 65 (26.9%) | 19 (21.1%) | 46 (30.3%) | NS (0.135) |
| Hepatitis C | 52 (21.5%) | 21 (23.3%) | 31 (20.4%) | NS (0.629) |
| Alcoholic + Hepatitis B | 30 (12.4%) | 8 (8.9%) | 22 (14.5%) | NS (0.231) |
| Alcoholic + Hepatitis C | 7 (2.9%) | 4 (4.4%) | 3 (2.0%) | NS (0.429) |
| Hepatitis B + Hepatitis C | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Alcoholic + Hepatitis B + Hepatitis C | 6 (2.5%) | 1 (1.1%) | 5 (3.3%) | NS (0.416) |
| Other causesa | 28 (11.6%) | 13 (14.4%) | 15 (9.9%) | NS (0.303) |
| Primary ICU admission | ||||
| Severe UGI bleeding | 92 (38.0%) | 46 (51.1%) | 46(30.3%) | 0.002 |
| Hepatic encephalopathy | 60 (24.8%) | 26 (28.9%) | 34 (22.4%) | NS (0.283) |
| Respiratory failure | 28 (11.6%) | 6 (6.7%) | 22 (14.5%) | NS (0.095) |
| Severe sepsis | 47 (19.4%) | 7 (7.8%) | 40 (26.3%) | <0.001 |
| HCC rupture | 10 (4.1%) | 4 (4.4%) | 6 (3.9%) | NS (1.000) |
| Acute pancreatitis | 2 (0.8%) | 0 (0%) | 2 (1.3%) | NS (0.531) |
| Acute renal failure | 6 (2.5%) | 1 (1.1%) | 5 (3.3%) | NS (0.416) |
Abbreviation: HCC, hepaocellular carcinoma; ICU, intensive care unit; UGI, upper gastrointestinal;
a “Other causes” includes primary biliary cirrhosis, autoimmune hepatitis, andother unknown causes.
Values in bold are statistically significant (P-value < 0.05).
Cause of cirrhosis: none of the causes was independently associated with in-hospital mortality.
Primary ICU admission reason: sever UGI bleeding and severe sepsis were independently associated with in-hospital mortality.
Comparison of AKI incidence according to the AKIN, RIFLE, and KDIGO classifications
The AKI severities determined by using the AKIN, RIFLE, and KDIGO classifications are listed in Table 4. The incidence rate of AKI was highest when using the KDIGO classification (67%). The KDIGO criteria identified 11 more patients with AKI than did the AKIN criteria; 1 of them was in stage I, and 10 were in stage III. The KDIGO criteria also identified 5 more patients with AKI than did the RIFLE criteria; 3 of them were in stage I and 2 were in stage III. When the overall patients were divided into the survivor and nonsurvivor groups, the AKI incidence of the survivor group determined by all the three classifications were almost the same and every single survivor was almost classified into the same AKI stage. However, in the nonsurvivor group, the KDIGO classification identified more patients with AKI than the other two classifications as mentioned above. The correlations between the scores of the AKIN, RIFLE, and KDIGO classifications on the first day of ICU admission are also listed in Table 5. The KDIGO classification showed positive correlations with the AKIN and RIFLE classifications in terms of the likelihood of in-hospital mortality (r > 0.25, p < 0.01). Concerning the correlation among the three criteria, the KDIGO criteria correlated better with the RIFLE criteria (0.988) than with the AKIN criteria (0.903). The correlation between the KDIGO and RIFLE criteria was also better than that between the RIFLE and AKIN criteria (0.900) (Table 5).
Table 4. Survivors and non-survivors in different scoring system.
| All patients (n = 242) | Survivors (n = 90) | Non-survivors (n = 152) | p-value | |
|---|---|---|---|---|
| AKIN | <0.001 | |||
| No AKI | 90 (37%) | 55 (61%) | 35 (23%) | <0.001 |
| AKI | 152 (63%) | 35 (39%) | 117 (77%) | <0.001 |
| Stage I | 15 (6%) | 7 (8%) | 8 (5%) | NS (0.286) |
| Stage II | 40 (17%) | 13 (14%) | 27 (18%) | NS (0.436) |
| Stage III | 97 (40%) | 15 (17%) | 82 (54%) | <0.001 |
| RIFLE | <0.001 | |||
| No AKI | 84 (35%) | 58 (64%) | 26 (18%) | <0.001 |
| AKI | 158 (65%) | 32 (36%) | 126 (82%) | <0.001 |
| Risk | 13 (5%) | 5 (6%) | 8 (5%) | NS (1.000) |
| Injury | 40 (17%) | 12 (13%) | 28 (18%) | NS (0.284) |
| Failure | 105 (43%) | 15 (17%) | 90 (59%) | <0.001 |
| KDIGO | <0.001 | |||
| No AKI | 79 (33%) | 57 (63%) | 22 (14%) | <0.001 |
| AKI | 163 (67%) | 33 (37%) | 130 (86%) | <0.001 |
| Stage I | 16 (7%) | 6 (7%) | 10 (7%) | NS (1.000) |
| Stage II | 40 (17%) | 12 (13%) | 28 (18%) | NS (0.284) |
| Stage III | 107 (44%) | 15 (17%) | 92 (61%) | <0.001 |
Abbreviation: AKI, acute kidney injury; RIFLE, risk of renal failure, injury to kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; AKIN, acute kidney injury network; KDIGO, kidney disease improving global outcomes; NS, not significant
Values in bold are statistically significant (P-value < 0.05).
Table 5. Correlation between scoring systems on the first day of ICU admission (Spearman rank correlation coefficients: r).
| Scores | AKIN | RIFLE |
|---|---|---|
| RIFLE | 0.903** | – |
| KDIGO | 0.903** | 0.988** |
Abbreviation: RIFLE, risk of renal failure, injury to kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; AKIN, acute kidney injury network; KDIGO, kidney disease improving global outcomes
**p < 0.01.
Comparison of calibration and discrimination for predicting in-hospital mortality according to the AKIN, RIFLE, and KDIGO classifications
The in-hospital mortality rates of AKI patients defined by the AKIN, RIFLE, and KDIGO classifications were 77% (117 of 152), 80% (126 of 158), and 80% (130 of 163), respectively. The in-hospital mortality rate was significantly higher for patients with AKI than those without AKI, regardless of the applied criteria: AKIN (77% vs. 39%, p < 0.001), RIFLE (80% vs. 31%, p < 0.001), and KDIGO (80% vs. 28%, p < 0.001) (Table 4). Table 6 shows the results of goodness-of-fit as measured by the Hosmer–Lemeshow χ2 statistic, denoting the predicted mortality risk and the predictive accuracy of the AKIN, RIFLE, and KDIGO classifications. A comparison between the discriminatory values of the three AKI classifications is also shown in Table 6. The AUROC analysis verified that the KDIGO classification had the best discriminatory power for predicting in-hospital mortality.
Table 6. Comparison of calibration and discrimination of the AKI scores in predicting hospital mortality.
| Calibration |
Discrimination |
|||||
|---|---|---|---|---|---|---|
| Hosmer-Lemeshow χ2 | df | p | AUROC ± SE | 95% CI | p | |
| AKIN | 1.739 | 2 | 0.419 | 0.741 ± 0.033 | 0.675–0.806 | <0.001 |
| RIFLE | 2.302 | 2 | 0.316 | 0.774 ± 0.032 | 0.712–0.837 | <0.001 |
| KDIGO | 2.473 | 2 | 0.290 | 0.781 ± 0.032 | 0.719–0.843 | <0.001 |
Abbreviation: RIFLE, risk of renal failure, injury to kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; AKIN, acute kidney injury network; KDIGO, kidney disease improving global outcomes; df, degree of freedom; AUROC, areas under the receiver operating characteristic curve; SE, standard error; CI, confidence intervals
Values in bold are statistically significant (P-value < 0.05).
Short-term prognosis of AKI and non-AKI patients defined according to the AKIN, RIFLE, and KDIGO classifications
The number of patients and the in-hospital mortality rate calculated according to the stratification data of the three AKI criteria are listed in Table 7. A progressive and significant increase in the mortality rate was observed to correlate with the increasing AKI stage defined according to the three AKI criteria. The increase of odds ratio between the increasing AKI stages is greatest in the KDIGO classification.
Table 7. Odds Ratio of AKI stages in predicting hospital mortality according to AKIN, RIFLE, and KDIGO criteria.
| Score | n | Hospital mortality (%) | Beta coefficient | Standard error | Odds rations (95% CI) | p |
|---|---|---|---|---|---|---|
| AKIN | ||||||
| No AKI | 90 | 39 | – | – | 1 (reference) | – |
| AKIN-1 | 15 | 53 | 0.097 | 0.950 | 1.101 (0.171–7.094) | 0.919 |
| AKIN-2 | 40 | 68 | 1.275 | 0.438 | 3.580 (1.518–8.442) | 0.004 |
| AKIN-3 | 97 | 85 | 2.188 | 0.386 | 8.922 (4.189–19.002) | <0.001 |
| Constant | – | – | −0.502 | 0.264 | 0.605 | 0.057 |
| RIFLE | ||||||
| No AKI | 84 | 31 | – | – | 1 (reference) | – |
| RIFLE-R | 13 | 62 | 1.312 | 0.618 | 3.112 (1.105–12.470) | 0.044 |
| RIFLE-I | 40 | 70 | 1.689 | 0.420 | 5.413 (2.377–12.327) | <0.001 |
| RIFLE-F | 105 | 86 | 2.644 | 0.367 | 14.075 (6.852–28.911) | <0.001 |
| Constant | – | – | −0.842 | 0.239 | 0.431 | <0.001 |
| KDIGO | ||||||
| No AKI | 79 | 28 | – | – | 1 (reference) | – |
| KDIGO-1 | 16 | 63 | 1.153 | 0.592 | 3.167 (0.992–10.111) | 0.042 |
| KDIGO-2 | 40 | 70 | 1.712 | 0.422 | 5.542 (2.422–12.677) | <0.001 |
| KDIGO-3 | 107 | 86 | 2.679 | 0.370 | 14.567 (7.057–30.070) | <0.001 |
| Constant | – | – | −0.865 | 0.243 | 0.421 | <0.001 |
Abbreviation: AKI, acute kidney injury, RIFLE, risk of renal failure, injury to kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; AKIN, acute kidney injury network; KDIGO, kidney disease improving global outcomes.
Values in bold are statistically significant (P-value < 0.05).
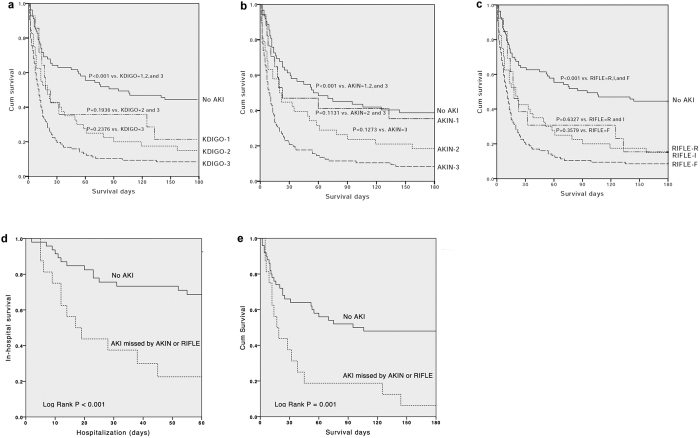
Figure 1 shows that the 180-day cumulative survival rates differed significantly for patients with AKI and those without AKI defined by the three AKI criteria on the first day of ICU admission (p < 0.05).
Figure 1. Survival Functions.
Kaplan-Meier survival analysis for 242 critically ill cirrhotic patients according to the 3 AKI classifications on the first day of ICU admission. (a) Based on the KDIGO classification, the 180-day cumulative survival rates differed significantly for patients without AKI versus patients with KDIGO stages 1 to 3 (p < 0.001). The comparisons between patients with KDIGO stage 1 and those with KDIGO stages 2 to 3, and between patients with KDIGO stages 2 and those with KDIGO stage 3 have been depicted on the figure. (b) Based on the AKIN classification, the 180-day cumulative survival rates differed significantly for patients without AKI versus patients with AKIN stages 1 to 3, p < 0.001). The comparisons between patients with AKIN stage 1 and those with AKIN stages 2 to 3, and between patients with AKIN stages 2 and those with AKIN stage 3 have been depicted on the figure. (c) Based on the RIFLE classification, the 180-day cumulative survival rates differed significantly for patient without AKI versus patients with RIFLE-R, RIFLE-I, and RIFLE-F (p < 0.001). The comparisons between patients with RIFLE-R and those with RIFLE-I to F, and between patients with RIFLE-I and those with RIFLE-F have been depicted on the figure. (d) Patients with AKI diagnosed according to KDIGO but missed by AKIN or RIFLE classification had significantly lower hospital survival rate than patients without AKI (Log Rank P < 0.001). (e) Patients with AKI diagnosed according to KDIGO but missed by AKIN or RIFLE classification had significantly lower 180-day cumulative survival rate than patients without AKI (Log Rank P = 0.001). * Abbreviation: AKI, acute kidney injury; RIFLE, risk of renal failure, injury to kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; AKIN, acute kidney injury network; KDIGO, kidney disease improving global outcomes.
Discussion
This study included 242 cirrhotic patients with critical illnesses. The overall in-hospital mortality rate was 62.8%, which is well in keeping with that obtained in previous studies5,15,16. This investigation showed that the severity of AKI on the ICU admission day was associated with a significantly graded risk of death in critically ill cirrhotic patients, irrespective of which classification was used (Table 4). The analytical results also showed that the KDIGO classification was an excellent scoring system for predicting the outcome for critically ill cirrhotic patients (Table 6).
Several studies had compared the prediction accuracy of the KDIGO, AKIN, and RIFLE classifications. Most of the studies were performed retrospectively10,11,12,13. Luo X et al. had conducted a prospective investigation and showed that the KDIGO and AKIN classification had similar ability to predict mortality in critically ill patients, whereas the prediction accuracy of the RIFLE classification was inferior14. Our study focused on critically ill cirrhotic patients and a key aspect of this investigation is the definition of baseline SCr. For general population of patients without a previous SCr value before hospitalization, an alternative estimated baseline SCr value back-calculated by the Modification of Diet in Renal Disease (MDRD) formula have been widely adopted12,14. However, it is well known that creatinine-based equations, such as MDRD and Cockcroft-Gault formulas, are inaccurate in the estimation of glomerular filtration rate (GFR) in cirrhotic patients17,18. In this study, we used the last SCr value within the previous 3 months before hospitalization as the baseline SCr. For patients without an available SCr value before hospitalization, we followed the recommendations of the International Club of Ascites and used the first SCr value measured during hospitalization as the baseline SCr18,19. For taking account the specific feature of patients with cirrhosis, our study design might more precisely compare the prediction performance of the 3 AKI classifications in these patients.
The KDIGO classification reconciles the AKI definition of the RIFLE and AKIN classifications. In this investigation, the AKI incidence determined by using the KDIGO classification was higher than that obtained according to the RIFLE or AKIN classification. Compared with AKIN and RIFLE, the KDIGO classification identified 5% (11 of 242) and 2% (5 of 242) more patients fulfilling the AKI criteria (Table 4). Among the patients with AKI diagnosed according to KDIGO but missed with the AKIN or RIFLE classification, 82% (9 of 11) and 60% (3 of 5) of them died and accounted for 6% (9 of 152) and 2% (3 of 152) of the overall mortality, respectively. This AKI subgroup was also correlated to significant lower hospital and 180-day cumulative survival rates (Fig. 1). Tsien et al. observed that regardless of AKI episodes reversed or not, cirrhotic patients with AKI were more vulnerable to further renal dysfunction and to poor survival compared with those without AKI20. The greater sensitivity of the KDIGO classification might allow AKI episodes to be recognized earlier and make potential interventions possible.
In patients with chronic liver disease, the absolute level and relative change of SCr concentration are significantly lower than that in the general population18,21,22,23,24. Many studies have reported that even a minor fluctuation in SCr level appears to be strongly associated with adverse outcomes13,25,26. The percentage increase in SCr is even more obscure in patients with previous renal dysfunction27, which is particularly relevant among cirrhotic patients because of a high proportion of patients with preexisting impaired renal function on hospital admission19,28. The lack of capturing of small changes of SCr in RIFLE and considering the preadmission renal function in AKIN may explain their discriminative inferiority to the KDIGO classification (Table 6). Moreover, our data showed a progressive stepwise and significantly elevated in-hospital and 180-day mortality associated with increasing KDIGO stages (Table 7 and Fig. 1). On the basis of the study results, we strongly believe that the KDIGO classification is of great importance for standardizing the definition of AKI as well as facilitating advances in clinical practice and research.
Despite the encouraging results obtained in our study, several potential study limitations should also be considered. First, this study was conducted on patients from only one academic tertiary-care medical center, which limits the generalization of our findings. Our results may be unsuitable for direct extrapolation to other hospitals with different patient populations. Second, in our study, given that hepatitis B viral infection (27%) was the leading cause of liver cirrhosis, the use of our classification system may not be appropriate for patients in North America and in Europe where liver diseases are mostly attributed to hepatitis C viral infection and alcoholism. Third, sequential measurement of these scoring systems (e.g., daily or weekly) may reflect the dynamic aspects of clinical diseases, thus providing superior information on mortality risk. Fourth, the prognostic instruments were tested on patients already admitted to the specialized hepatogastroenterology ICU, rather than being used as a preadmission screening test, which may have skewed the measured results. Finally, the predictive accuracy of logistic regression models has its own limitations.
Conclusion
This study showed the grave prognosis in critically ill cirrhotic patient with AKI. The analytical data demonstrated that the KDIGO classification is a better tool with superior prediction performance for short-term prognosis than the AKIN or RIFLE classification. We confirmed that the KDIGO classification is a great scoring system for risk stratification, and is capable of providing a more sensitive and standardized method for early AKI detection in critically ill cirrhotic patients.
Materials and Methods
Ethics statement
This clinical study was conducted in full compliance with the ethical principles of the Declaration of Helsinki and was consistent with Good Clinical Practice guidelines and the applicable local regulatory requirements. The local institutional review board of Chang Gung Memorial Hospital approved our study protocol (approval no. 98-3658A3). Patients who met the inclusion criteria were invited to participate in this study on their first day of admission to the intensive care unit (ICU). Trained physicians evaluated the patients’ mental status during the screening and proceeded to perform informed consent procedures. Written informed consent was obtained from all mentally competent patients or from the next-of-kin of compromised patients before their participation.
Patient information and data collection
This study was conducted from September 2012 to August 2014, in a 10-bed specialized ICU (hepatogastroenterology ICU) at a 2000-bed tertiary-care referral hospital in Taiwan. In this study, we included 242 consecutive patients with hepatic cirrhosis requiring intensive monitoring and/or treatment unavailable elsewhere. The following patients were excluded: pediatric patients (age 18 years or below), patients or their next of kin who declined to be enrolled in the study, patients who stayed in the hospital for <24 h, patients who had a previous end-stage renal disease and were undergoing regular RRT, and patients who had undergone liver transplantation. For patients who were readmitted, we only recorded the clinical condition at the first admission to avoid double weighing the same patient.
Prospective data were collected, including demographic data, reason for admission to the ICU, immediate diagnosis, severity of the illness, serum and urine biochemical analysis, urine microscopy, urine output, duration of ICU and hospital stay, and treatment outcome. The Child–Pugh points, model for end-stage liver disease (MELD), Sequential Organ Failure Assessment (SOFA), Acute Physiology and Chronic Health Evaluation (APACHE) II and III scores determined on the first day of ICU admission, and related clinical data were also recorded. The primary study outcome was in-hospital mortality rate. Follow-up examinations were performed at 6 months after hospital discharge of the patients, by analyzing the chart records.
Definitions
Cirrhosis was diagnosed on the basis of past medical history, the results of liver histology, or a combination of physical signs and symptoms and findings from biochemical analysis and ultrasonography. The severity of the liver disease on admission to the ICU was determined by using the Child–Pugh points and the MELD scoring system. The severity of the illness can also be assessed by using the APACHE II, APACHE III, and SOFA scores. The worst physiological and biochemical values determined on the first day of ICU admission were recorded.
The occurrence of AKI was determined by using the RIFLE, AKIN, and KDIGO classifications. We used the last SCr value within the previous 3 months before hospitalization as the baseline SCr in the RIFLE and KDIGO criteria. In patients without a previous SCr value, we used the first SCr value measured during hospitalization as the baseline SCr. For the AKIN criteria, the lowest SCr within 48 h before ICU admission was used as the baseline SCr. Both of the SCr and urine output criteria were considered in the three AKI classifications, and the criteria resulting in the worst possible classification were used. We applied a simple model for mortality, as follows: non-AKI (0 points); RIFLE-R, KDIGO stage 1, and AKIN stage 1 (1 point); RIFLE-I, KDIGO stage 2, and AKIN stage 2 (2 points); RIFLE-F, KDIGO stage 3, and AKIN stage 3 (3 points) on day 1 of ICU admission29,30.
Respiratory failure was defined as a respiratory rate of ≤5/min or ≥50/min, and/or the requirement for mechanical ventilation for ≥3 days, and/or fraction of inspired oxygen (FiO2) of >0.4, and/or a positive end-expiratory pressure of >5 cm H2O31,32,33. Sepsis was defined as systemic inflammatory response syndrome (SIRS) plus suspected or proven infection. According to the guidelines of the American College of Chest Physician/Society of Critical Care Medicine Consensus Conference, SIRS was defined as patients with more than one of the following clinical findings: body temperature, >38 °C or <36 °C; heart rate, >90 beats/min; hyperventilation evidenced by a respiratory rate of >20 cycles/min or a PaCO2 of <32 mm Hg; and a white blood cell count of >12,000 or <4000 cells/μL34.
Statistical analysis
Continuous variables were summarized with means and standard derivations unless otherwise stated. Primary analysis compared hospital survivors with nonsurvivors. All variables were tested for normal distribution with the Kolmogorov–Smirnov test. Student’s t-test was applied to compare the means of continuous variables and normally distributed data; otherwise, the Mann–Whitney U-test was employed. This study used the χ2 test for trend to assess the categorical data associated with the RIFLE, AKIN, and KDIGO classifications. Correlations of paired-group variables were assessed by using linear regression and Pearson analysis.
Calibration was assessed by using the Hosmer–Lemeshow goodness-of-fit test (C-statistic) to compare the number of observed and predicted deaths in risk groups for the entire range of death probabilities. Discrimination was examined by using the area under the receiver operating characteristic curve (AUROC). To compare the areas under the two resulting AUROC curves, we used a nonparametric approach. Cumulative survival curves as a function of time were plotted by using the Kaplan–Meier approach and were compared by using the log rank test. All statistical tests were two-tailed, and a value of p < 0.05 was considered statistically significant. Data were analyzed with the Statistical Package for the Social Sciences software, version 19.0 for Windows (SPSS Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Pan, H.-C. et al. Acute Kidney Injury Classification for Critically Ill Cirrhotic Patients: A Comparison of the KDIGO, AKIN, and RIFLE Classifications. Sci. Rep. 6, 23022; doi: 10.1038/srep23022 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Science Council of Taiwan (NSC 99-2314-B-182A-009-MY3). The authors thank the staff of the MICU-6 of CGMH. We also express our sincere gratitude to all members of the Taiwan Consortium for Acute Kidney Injury and Renal Diseases (CAKs). A full list of consortium members appears in the Supplementary Information
Footnotes
Author Contributions Y.C.C. contributed to the conception, design, and interpretation of data. H.C.P. and Y.S.C. contributed to data collecting and manuscript drafting. M.H.T. and Y.C.C. provided patient information, participated in the design and coordination, and helped draft the manuscript. C.C.J., P.C.F., C.H.C., M.Y.C., Y.C.T., C.C.H., J.T.F. and C.W.Y. provided intellectual content for the work and were involved in editing and revising the manuscript. All authors discussed, contributed to, and approved the final manuscript version.
References
- Uchino S. et al. Acute renal failure in critically ill patients. JAMA: the journal of the American Medical Association 294, 813 (2005). [DOI] [PubMed] [Google Scholar]
- Ostermann M. & Chang R. W. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 35, 1837–1843 (2007). [DOI] [PubMed] [Google Scholar]
- Garcia‐Tsao G., Parikh C. R. & Viola A. Acute kidney injury in cirrhosis. Hepatology 48, 2064–2077 (2008). [DOI] [PubMed] [Google Scholar]
- Ginès P. & Schrier R. W. Renal failure in cirrhosis. New England Journal of Medicine 361, 1279–1290 (2009). [DOI] [PubMed] [Google Scholar]
- Cholongitas E. et al. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. Journal of gastroenterology and hepatology 24, 1639–1647 (2009). [DOI] [PubMed] [Google Scholar]
- Wong F. et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 60, 702–709 (2011). [DOI] [PubMed] [Google Scholar]
- Pan H. C. et al. Risk models and scoring systems for predicting the prognosis in critically ill cirrhotic patients with acute kidney injury: a prospective validation study. PLoS One 7, 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack A. J. et al. Predicting the development of acute kidney injury in liver cirrhosis – an analysis of glomerular filtration rate, proteinuria and kidney injury biomarkers. Alimentary pharmacology & therapeutics 37, 989–997, doi: 10.1111/apt.12299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120, 7 (2012). [DOI] [PubMed] [Google Scholar]
- Bastin A. J. et al. Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 28, 389–396 (2013). [DOI] [PubMed] [Google Scholar]
- Zeng X., McMahon G. M., Brunelli S. M., Bates D. W. & Waikar S. S. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 9, 12–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Uchino S., Takinami M. & Bellomo R. Validation of the Kidney Disease Improving Global Outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol 9, 848–854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M. N., Nakazone M. A. & Maia L. N. Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc 29, 299–307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Critical care (London, England) 18, R144, doi: 10.1186/cc13977 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H. C. et al. Scoring systems for 6-month mortality in critically ill cirrhotic patients: a prospective analysis of chronic liver failure-sequential organ failure assessment score (CLIF-SOFA). Aliment Pharmacol Ther 11, 12953 (2014). [DOI] [PubMed] [Google Scholar]
- Cárdenas A. et al. Hyponatremia influences the outcome of patients with acute-on-chronic liver failure: an analysis of the CANONIC study. Critical care 18, 700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoz C. et al. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the model for end-stage liver disease score. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 16, 1169–1177, doi: 10.1002/lt.22128 (2010). [DOI] [PubMed] [Google Scholar]
- Angeli P. et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 62, 968–974 (2015). [DOI] [PubMed] [Google Scholar]
- Fagundes C. et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol 59, 474–481 (2013). [DOI] [PubMed] [Google Scholar]
- Tsien C. D., Rabie R. & Wong F. Acute kidney injury in decompensated cirrhosis. Gut 62, 131–137 (2013). [DOI] [PubMed] [Google Scholar]
- Spencer K. Analytical reviews in clinical biochemistry: the estimation of creatinine. Ann Clin Biochem 23, 1–25 (1986). [DOI] [PubMed] [Google Scholar]
- Caregaro L. et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med 154, 201–205 (1994). [PubMed] [Google Scholar]
- Sherman D. S., Fish D. N. & Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis 41, 269–278 (2003). [DOI] [PubMed] [Google Scholar]
- Slack A., Yeoman A. & Wendon J. Renal dysfunction in chronic liver disease. Crit Care 14, 9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow G. M., Burdick E., Honour M., Bonventre J. V. & Bates D. W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16, 3365–3370 (2005). [DOI] [PubMed] [Google Scholar]
- Newsome B. B. et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 168, 609–616 (2008). [DOI] [PubMed] [Google Scholar]
- Waikar S. S. & Bonventre J. V. Creatinine kinetics and the definition of acute kidney injury. Journal of the American Society of Nephrology 20, 672–679 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín–Llahí M. et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 140, 488-496. e484 (2011). [DOI] [PubMed] [Google Scholar]
- Jenq C. C. et al. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med 33, 1921–1930 (2007). [DOI] [PubMed] [Google Scholar]
- Lin C. Y. et al. Evaluation of outcome scoring systems for patients on extracorporeal membrane oxygenation. Ann Thorac Surg 84, 1256–1262 (2007). [DOI] [PubMed] [Google Scholar]
- Tran D. D. et al. Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Crit Care Med 18, 474–479 (1990). [DOI] [PubMed] [Google Scholar]
- Knaus W. A. et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100, 1619–1636 (1991). [DOI] [PubMed] [Google Scholar]
- Tsai M. H. et al. Organ system failure scoring system can predict hospital mortality in critically ill cirrhotic patients. Journal of clinical gastroenterology 37, 251 (2003). [DOI] [PubMed] [Google Scholar]
- Bone R. C. et al. American-college of chest physicians society of critical care medicine consensus conference-definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical care medicine 20, 864–874 (1992). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.