We report the cumulative frequency, including previous studies,1,2 showing varicella-zoster virus (VZV) antigen in 73/104 (70%) giant cell arteritis (GCA)–positive temporal arteries (TAs), in 58/100 (58%) GCA-negative TAs (patients who manifest clinical and laboratory features of GCA whose TA biopsies are pathologically negative for GCA) and in 11/61 (18%) normal TAs acquired at autopsy. The location of VZV antigen in specific arterial layers and pathologic findings in GCA-negative arteries helps to explain disease pathogenesis.
Methods.
De-identified formalin-fixed, paraffin-embedded TA biopsies were obtained from participants >50 years of age. Of 204 TAs, 104 were GCA-positive and 100 were GCA-negative. Age and sex were available for 96 GCA-positive TAs, 36 (37%) of which were in men and 60 (63%) in women, age 51–89 years. Of 100 GCA-negative TAs, age and sex data were available for 95 TAs, 40 (42%) of which were in men and 55 (58%) in women, age 50–89 years. Sixty-one control TAs were removed postmortem from age-matched participants. All TAs were examined immunohistochemically as described.1 Location of viral antigen in adventitia, media, or intima was documented. VZV antigen-positive sections from 58 GCA-positive TAs, 58 GCA-negative TAs, and 11 normal TAs were analyzed for VZV DNA as described.1
Sections 5 μm adjacent to those containing VZV antigen were stained with hematoxylin & eosin. If a GCA-negative TA section appeared to contain inflammatory cells, it was destained and immunostained with rabbit anti-CD45 as described.2
Results.
Immunohistochemical analysis revealed VZV antigen in 73/104 (70%) GCA-positive and 58/100 (58%) GCA-negative TAs compared to 11/61 (18%) normal TAs. VZV antigen was 3.89-fold more likely to be present in GCA-positive TAs than in normal TAs (95% confidence interval [CI] 2.3819, 7.2384, p < 0.0001) and 3.22 times more likely to be present in GCA-negative TAs than in normal TAs (95% CI 1.9391, 6.0303, p < 0.0001).
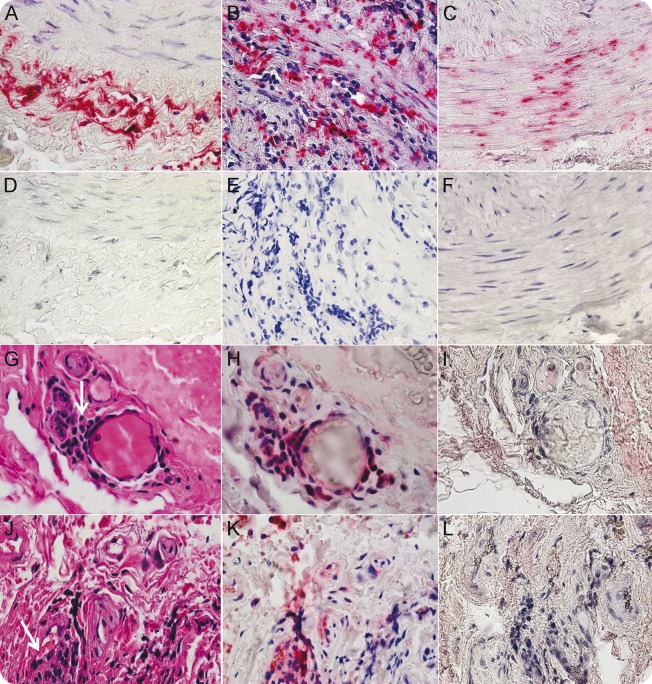
All TAs contained viral antigen in multiple arterial layers (figure, A–F). In GCA-positive and GCA-negative TAs, viral antigen was seen in the adventitia (86% and 95%, respectively), media (67% and 53%, respectively), and intima (52% and 45%, respectively); in normal TAs, viral antigen was seen in the adventitia (91%) and equally in media (82%) and intima (82%).
Figure. Varicella-zoster virus (VZV) antigen in giant cell arteritis (GCA)–positive and GCA-negative temporal arteries (TAs) and inflammation adjacent to VZV antigen in GCA-negative TAs.
VZV antigen in TAs of GCA-positive and GCA-negative patients (clinical and laboratory features of GCA whose TA biopsies were pathologically negative for GCA) was detected immunohistochemically using mouse anti-VZV gE immunoglobulin (Ig) G1 antibody (Santa Cruz Biotechnology, Dallas, TX; catalog no. SC-56995). After immunostaining with primary and biotinylated secondary antibodies, slides were treated with prediluted streptavidin-alkaline phosphatase (BD Biosciences, San Diego, CA) for 1 hour. The color reaction was developed under a light microscope using the fresh fuchsin substrate system (Dako, Carpinteria, CA) with levamisole (Dako; 24 μg/mL). VZV antigen is shown in the adventitia of a positive control VZV-infected cadaveric cerebral artery 14 days after infection in vitro (A), in the adventitia and media of a GCA-positive TA (B), and in the media and intima of a GCA-negative TA (C). No staining was seen when mouse isotype IgG1 was substituted for primary mouse anti-VZV gE IgG1 antibody (D–F). In GCA-negative TAs, hematoxylin & eosin (H&E) staining of TA sections adjacent to those containing VZV antigen revealed inflammatory cells (G, J, arrows) identified as CD45-positive (H, K, pink) after destaining H&E sections and immunostaining with rabbit anti-CD45 antibody (Abcam, Cambridge, MA; catalog no. AB10558). No staining was seen when normal rabbit serum was substituted for rabbit anti-CD45 antibody (I, L). 600× magnification.
Of 58 GCA-positive, VZV antigen–positive TAs examined, all contained cellular DNA and 23 (40%) contained VZV DNA. Of 58 GCA-negative, VZV antigen–positive TAs examined, 51 contained cellular DNA, 9 (18%) of which contained VZV DNA. Nine of 11 normal VZV antigen-positive TAs contained cellular DNA, of which 3 (33%) contained VZV DNA.
Adventitial inflammation was seen adjacent to viral antigen in 26 (52%) of 58 GCA-negative participants whose TAs contained VZV antigen (figure, G–L). No inflammation was seen in normal TAs containing VZV antigen.
Discussion.
Detection of VZV in 73/104 GCA-positive TAs (70%) and 58/100 GCA-negative TAs (58%) compared to 11/61 normal TAs (18%) was highly statistically significant (p < 0.0001). Despite formalin fixation, VZV DNA was detected by PCR in many VZV antigen–positive sections. Detection of VZV antigen without inflammation in normal TAs indicates that VZV reactivates subclinically in some people over age 50 years. The greater frequency of VZV in the adventitia than in media and intima in all groups most likely reflects transaxonal transport of virus along afferent nerve fibers that innervate the TA after reactivation from ganglia.
VZV antigen and inflammation was usually patchy and detected in only some, but not all sections. Our exhaustive, research-focused evaluation is not practical for routine diagnostic work-up of TA biopsies. Immunohistochemical evaluation is worthwhile, but a negative result should cite possible shortfalls: specifically, a negative biopsy does not rule out GCA or VZV reactivation.
Detection of adventitial inflammation adjacent to VZV antigen in 52% of GCA-negative TAs for the first time connects the presence of VZV with pathology in GCA-negative TAs. Inflammation restricted to the adventitia may represent a milder form of GCA3 and has also been associated with ischemic optic neuropathy (ION).4,5 Interestingly, we initially detected VZV in a TA from a patient with ION whose biopsy revealed both adventitial and intimal inflammation but not classic GCA.6 Overall, finding VZV mostly in the adventitia of GCA-positive TAs and VZV with inflammation in the adventitia of GCA-negative TAs indicates that inflammation follows VZV reactivation from ganglia and transaxonal transport to arterial adventitia. The tempo and evolution of GCA (transmural inflammation and necrosis with giant or epithelioid cells) after virus infection of the adventitia and adventitial inflammation remains to be determined.
Acknowledgments
Acknowledgment: The authors thank their colleagues who shared biopsy specimens for evaluation, Marina Hoffman for editorial review, and Cathy Allen for word processing and formatting.
Footnotes
Author contributions: Dr. Gilden: drafted and revised the manuscript for content; designed and supervised the study; collected, analyzed, and interpreted data. T. White: collected, analyzed, and interpreted data; revised the manuscript for content. N. Khmeleva: collected, analyzed, and interpreted data. Dr. Boyer: analyzed and interpreted data. Dr. Nagel: drafted and revised the manuscript for content, designed and supervised the study, collected data, analyzed and interpreted data.
Study funding: Supported in part by NIH grants AG032958 to D.G. and M.A.N. and NS094758 to M.A.N.
Disclosure: D. Gilden is a senior associate editor for Journal of Neurovirology, is on the editorial board for Journal of Virology, Neurology®, and Journal of the Neurological Sciences and received research support from NIH. T.M. White, N. Khmeleva, and P.J. Boyer report no disclosures. M.A. Nagel received research support from NIH. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology 2015;84:1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel MA, White T, Khmeleva N, et al. Analysis of varicella-zoster virus in temporal arteries biopsy positive and negative for giant cell arteritis. JAMA Neurol 2015;72:1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor-Gjevre R, Vo M, Shukla D, Resch L. Temporal artery biopsy for giant cell arteritis. J Rheumatol 2005;32:1279–1282. [PubMed] [Google Scholar]
- 4.Nagel MA, Bennett JL, Khmeleva N, et al. Multifocal VZV vasculopathy with temporal artery infection mimics giant cell arteritis. Neurology 2013;80:2017–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breuer GS, Nesher R, Reinus K, Nesher G. Association between histological features in temporal artery biopsies and clinical features of patients with giant cell arteritis. Isr Med Assoc J 2013:15:271–274. [PubMed] [Google Scholar]
- 6.Salazar R, Russman AN, Nagel MA, et al. Varicella zoster virus ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol 2011;68:517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]