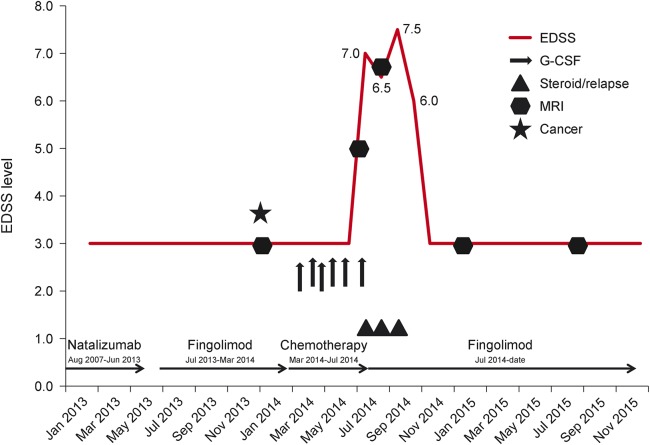
A 31-year-old woman (figure) with relapsing-remitting multiple sclerosis (MS) (first symptoms February 1994, first diagnosis August 1994, Expanded Disability Status Scale [EDSS] 3.0) was diagnosed with breast cancer in January 2014. The last relapse had occurred in August 2007. Under treatment with natalizumab from August 2007 to June 2013, the patient was clinically stable and the brain MRI showed no signs of disease activity. Because of a positive JC virus antibody test, the immunomodulatory therapy was changed to fingolimod in July 2013. The patient remained clinically stable during the switching period and thereafter. In January 2014, a brain MRI showed 2 new lesions without contrast enhancement. After diagnosis of breast cancer in January 2014 and subsequent surgery in February 2014, adjuvant chemotherapy with cyclophosphamide, doxorubicin, and docetaxel was started (March–July 2014). Fingolimod was stopped in March 2014 considering the strong immunosuppressive effect of cyclophosphamide. The patient received granulocyte colony-stimulating factor (G-CSF; pegfilgrastim) 6 mg subcutaneously at each of the 6 cycles of chemotherapy to reduce the risk of infection during the phase of chemotherapy-induced neutropenia. In July 2014, the patient experienced a numbness and tingling in her right leg, which progressed to a severe hemiparesis (EDSS 7.0) leading to hospitalization. The MRI scan of the brain revealed several new lesions including a lesion with contrast enhancement. An IV steroid pulse therapy was administered. She recovered partially (EDSS 6.5). Fingolimod was started again in August 2014. Subsequently the patient developed another relapse with a worsening of the atactic hemiparesis on the left side, resulting in an inability to walk (EDSS 7.5). Again an IV steroid pulse therapy was administered. The patient recovered partially until the end of August 2014, when she was discharged from rehabilitation. At that time, she was able to walk for 50 meters without aid (EDSS 6.0). Another brain MRI in September 2014 revealed confluent progressive lesions in the white matter with partial contrast enhancement. The patient was referred to our inpatient clinic, where again a steroid pulse therapy was administered. In the following months, the patient recovered to a clinical condition similar to that before the start of the chemotherapy (as of August 2015, EDSS 3.0). An MRI scan of the brain in September 2015 did not show new lesions or contrast enhancement compared to the preceding MRI examination in September 2014.
Figure. Treatment timeline of a 31-year-old woman with relapsing-remitting multiple sclerosis.
EDSS = Expanded Disability Status Scale; G-CSF = granulocyte colony-stimulating factor.
This case raises the suspicion that activation of the immune system by G-CSF might have contributed to a temporary flare of disease activity in this patient. Other possible reasons for the exacerbation might be the switch of the immunomodulatory therapy from natalizumab to fingolimod or the discontinuation of fingolimod. Since the observed exacerbation occurred more than 1 year after stopping natalizumab and returning disease activity is normally seen within the first 6 months after stopping, it is unlikely that the disease activity represents a rebound phenomenon after natalizumab stop. Single case reports indicated a disease exacerbation also after fingolimod withdrawal.1 However, these patients were often without any immunomodulating therapy, whereas in this case cyclophosphamide was administered. We therefore favor the third possibility that the administration of G-CSF led to a temporary increased disease activity.
The closely related hematopoietic growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF) has recently been implicated in the pathogenesis of MS.2–5 GM-CSF-deficient mice were resistant to the induction of experimental autoimmune encephalitis (EAE) and failed to sustain immune cell infiltrates in the CNS.2 Moreover, the production of GM-CSF by T cells seems to be essential for the emergence of EAE.3,4 In patients with MS, the number of GM-CSF-producing T-helper cells was found to be increased and patients with MS under treatment with immunomodulatory drugs showed a decreased level of GM-CSF-producing T cells compared to untreated patients.5 In a recent phase 1b study, blockade of GM-CSF was tested in a small group of patients with MS as a novel therapeutic approach.6 G-CSF was also already associated with increased inflammatory activity in MS in the clinical setting of a conditioning therapy before an immunoablating treatment.7 The reported case suggests a possible relationship between G-CSF treatment and a severe exacerbation of MS. We would therefore advise to critically challenge the use of G(M)-CSF in patients with MS.
Footnotes
Author contributions: Drafting the manuscript: H.R., T.D. Critical revision of the manuscript for important intellectual content: J.K., L.K., T.D.
Study funding: No targeted funding.
Disclosures: H. Rust received travel support from Bayer Healthcare, Teva, and Genzyme. J. Kuhle's institution received speaker honoraria from Swiss MS Society, Biogen, Novartis, Roche, and Genzyme; travel expenses from Merck Serono and Novartis; and he received research support from Bayer (Schweiz) AG, Genzyme, Novartis, Swiss National Research Foundation, ECTRIMS Research Fellowship Programme, University of Basel, and Swiss MS Society. L. Kappos is on the editorial board for Multiple Sclerosis Journal, Multiple Sclerosis and Related Disorders, and Journal of Neurology; received research support from Actelion, Addex, Alkermes, Almirall, Bayer Health Care, Biogen Idec, CSL Behring, Genentech, GeNeuro SA, Genzyme, Merck Serono, Mitsubishi, Novartis, Octapharma, Pfizer, Receptos, Roche, Sanofi-Aventis, Santhera, Teva, UCB, Swiss National Research Foundation, and European Union (all payments have been transferred to the research account of the University Hospital Basel); and received royalties from Neurostatus Systems AG (all payments have been transferred to the research account of the University Hospital Basel). T. Derfuss served on the scientific advisory board for Biogen Idec, Novartis Pharma, Genzyme, Merck Serono, Bayer Schering, Octapharma, GeNeuro, and Roche; received travel funding and speaker honoraria from Bayer Schering, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme; is on the editorial board for Plos One; his spouse is employed by Novartis Pharma; he has consulted for Mitsubishi Pharma and GeNeuro; he is a member of the executive board of ECTRIMS; and he received research support from Novartis Pharma, Merck Serono, Biogen Idec, Swiss National Foundation, and Swiss MS Society. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Faissner S, Hoepner R, Lukas C, et al. Tumefactive multiple sclerosis lesions in two patients after cessation of fingolimod treatment. Ther Adv Neurol Disord 2015;8:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McQualter JL, Darwiche R, Ewing C, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med 2001;194:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codarri L, Gyülvészi G, Tosevski V, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 2011;12:560–567. [DOI] [PubMed] [Google Scholar]
- 4.Cravens PD, Hussain RZ, Zacharias TE, et al. Lymph node-derived donor encephalitogenic CD4+ T cells in C57BL/6 mice adoptive transfer experimental autoimmune encephalomyelitis highly express GM-CSF and T-bet. J Neuroinflammation 2011;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann FJ, Khademi M, Aram J, et al. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat Commun 2014;5:5056. [DOI] [PubMed] [Google Scholar]
- 6.Constantinescu CS, Asher A, Fryze W, et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2015;2:e117 doi: 10.1212/NXI.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Openshaw H, Stuve O, Antel JP, et al. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology 2000;54:2147–2150. [DOI] [PubMed] [Google Scholar]