Abstract
Objective:
To investigate the role of very late antigen-4 (VLA-4) on regulatory B cells (Breg) in CNS autoimmune disease.
Methods:
Experimental autoimmune encephalomyelitis (EAE) was induced in mice selectively deficient for VLA-4 on B cells (CD19cre/α4f/f) by immunization with myelin oligodendrocyte glycoprotein (MOG) peptide (p)35–55 or recombinant human (rh) MOG protein. B-cell and T-cell populations were examined by flow cytometry and immunohistochemistry. Breg were evaluated by intracellular IL-10 staining of B cells and, secondly, by coexpression of CD1d and CD5.
Results:
As previously reported, EAE was less severe in B-cell VLA-4-deficient vs control CD19cre mice when induced by rhMOG, a model that is B-cell-dependent and leads to efficient B-cell activation and antibody production. Paradoxically, B-cell VLA-4-deficient mice developed more severe clinical disease than control mice when EAE was induced with MOG p35-55, a B-cell-independent encephalitogen that does not efficiently activate B cells. Peripheral T-cell and humoral immune responses were not altered in B-cell VLA-4-deficient mice. In MOG p35-55-induced EAE, B-cell VLA-4 deficiency reduced CNS accumulation of B but not T cells. Breg were detected in the CNS of control mice with MOG p35-55-induced EAE. However, more severe EAE in B-cell VLA-4-deficient mice was associated with virtual absence of CNS Breg.
Conclusions:
Our results demonstrate that CNS accumulation of Breg is VLA-4-dependent and suggest that Breg may contribute to regulation of CNS autoimmunity in situ. These observations underscore the need to choose the appropriate encephalitogen when studying how B cells contribute to pathogenesis or regulation of CNS autoimmunity.
Very late antigen-4 (VLA-4; α4β1), the target of natalizumab, is expressed on T cells, B cells, and other peripheral blood myeloid-derived mononuclear cells, and is required for migration across the blood–brain barrier. In a previous study, we demonstrated that B-cell α4/VLA-4 expression is important in the pathogenesis of CNS autoimmunity. Selective inhibition of VLA-4 expression on B cells impeded CNS B-cell accumulation, recruitment of other leukocytes, and susceptibility to experimental autoimmune encephalomyelitis (EAE). These findings suggested that the clinical benefit of natalizumab in treatment of relapsing-remitting multiple sclerosis (MS) may, in part, be related to its ability to block B-cell trafficking into the CNS.1
Like T cells, B cells can exhibit proinflammatory or anti-inflammatory activities. In our earlier study, EAE was induced by immunization with recombinant extracellular domain of human myelin oligodendrocyte glycoprotein (MOG) protein (rhMOG), a B-cell-dependent encephalitogen that leads to proinflammatory B-cell activation and production of pathogenic MOG-specific antibodies.2 In contrast, EAE induction by encephalitogenic myelin peptides, including MOG peptide (p) 35–55, does not promote substantial B-cell activation or antibody production3; B-cell-deficient mice are completely susceptible to myelin peptide-induced EAE.4,5 Further, depletion of B cells by anti-CD20 treatment exacerbates MOG p35-55-induced EAE.3,6 Thus, B cells can have a key role in regulation of CNS autoimmunity. In this regard, it is now recognized that regulatory B cells (Breg), defined primarily by expression of the anti-inflammatory cytokine interleukin (IL)–10,6 may suppress EAE. In this investigation, we examined MOG p35-55-induced EAE in B-cell VLA-4-deficient mice and observed that its severity was greater in these mice than in control mice with normal B-cell VLA-4 expression. B-cell VLA-4 deficiency did not influence peripheral T-cell or B-cell immune modulation. Therefore, we tested the hypothesis that CNS accumulation of Breg is also VLA-4-dependent and that the greater EAE severity in B-cell VLA-4-deficient mice might reflect fewer CNS Breg. In contrast to control mice, MOG p35-55-induced EAE in B-cell VLA-4-deficient mice was associated with absence of CNS Breg. These findings demonstrate that CNS Breg accumulation is VLA-4-dependent and suggest that Breg may also participate in modulation of CNS autoimmunity in situ.
METHODS
Mice.
C57BL/6 α4flox/flox mice (referred to as α4f/f) were provided by Dr. Thalia Papayannopoulou from the University of Washington, Seattle.7 C57BL/6 CD19cre mice were purchased from the Jackson Laboratory (Bar Harbor, ME).8 CD19cre and α4f/f mice were used as controls.1 All studies were approved by the UCSF Institutional Animal Care and Use Committee and were in accordance with the US Public Health Service's Policy on Humane Care and Use of Laboratory Animals.
Antigen.
Mouse MOG p35-55 (MEVGWYRSPFSRVVHLYRNGK) was synthesized by Genemed Synthesis (San Antonio, TX). rhMOG was provided by Dr. C.C.A. Bernard, Monash University, Clayton, Australia, and was produced, purified, and refolded as previously reported.2
EAE induction and analysis.
EAE was induced in 8- to 12-week-old mice by immunization with 100 μg MOG p35-55 or rhMOG in complete Freund's adjuvant containing 200 μg Mycobacterium tuberculosis H37Ra (Difco Laboratories, Detroit, MI) on day 0. Mice received 200 ng Bordetella pertussis toxin (List Biological Laboratories, Campbell, CA) IP on days 0 and 2. Mice were observed daily for clinical EAE.2 Histology and immunohistochemistry analyses were performed as previously described.1
Cell isolation and flow cytometric analysis.
Splenic and CNS mononuclear cells were isolated after perfusion with phosphate-buffered saline as described previously.2 Anti-mouse FcRIIB/FcRIIIA monoclonal antibody (2.4G2; BD Biosciences, San Jose, CA) was used to block nonspecific staining. Live/Dead Fixable Aqua Dead Cell Stain kit was used for viable cell discrimination, and CountBright counting beads (both Thermo Fisher Scientific, Waltham, MA) for absolute cell number quantification. Antibodies to mouse CD19 PerCP-Cy5.5 (eBio1D3), CD4 APC-Cy7 (RM4-5), CD1d FITC (1B1), and CD11b PE-Cy7 (M1/70) were purchased from eBioscience (San Diego, CA). Antibodies to B220 (CD45R) FITC (RA3-6B2) and CD45 APC (30-F11) were purchased from BD Biosciences and CD5 PE-Cy7 (53-7.3) from BioLegend (San Diego, CA). Isotype- and fluorochrome-matched control antibodies were used to assess nonspecific staining for CD1d (immunoglobulin G [IgG] 2b κ FITC; BD Biosciences) and CD5 (IgG2a κ APC; BioLegend). Analysis was performed on a BD Biosciences LSRFortessa flow cytometer using FACSDiva 8.0 software (BD Biosciences) and FlowJo version 10.0.8.
Intracellular cytokine staining (ICS).
ICS for T-cell cytokines was performed as described,2 using the Cytofix/Cytoperm Plus fixation/permeabilization kit (BD Biosciences), and antibodies to CD4 PE-Cy7 (RM4-5), IL-17A PerCP-Cy5.5 (eBio17B7), and interferon-γ APC (XMG1.2) (all eBioscience). For staining of regulatory T cells (Treg), the FoxP3 staining buffer set (eBioscience) and antibodies to CD4 PerCP-Cy5.5 (RM4-5), CD25 APC (PC61.5), and FoxP3 PE (FJK-16 s) were used. Intracellular IL-10 expression was analyzed by flow cytometry as previously described,9 using the Cytofix/Cytoperm Plus fixation/permeabilization kit (BD Biosciences). The following antibodies were used: CD19 PerCP-Cy5.5 (eBio1D3), CD45 PE-Cy7 (30-F11), CD11b APC-Cy7 (M1/70), IL-10 APC (JES5-16E3) (all from eBioscience), and B220 (CD45R) FITC (RA3-6B2) (BD Biosciences). An isotype- and fluorochrome-matched control antibody (IgG2b κ APC; eBioscience) was used to assess nonspecific staining for IL-10.
Detection of anti-MOG antibodies.
Anti-MOG p35-55 and anti-rhMOG IgG were measured with a noncommercial ELISA as described,2 using MOG p35-55 or rhMOG and horseradish peroxidase–labeled goat anti-mouse IgG (Thermo Fisher Scientific).
Statistical analysis.
Prism software v6.0b (GraphPad, La Jolla, CA) was used for all statistical analyses. A value of p ≤ 0.05 was considered statistically significant.
RESULTS
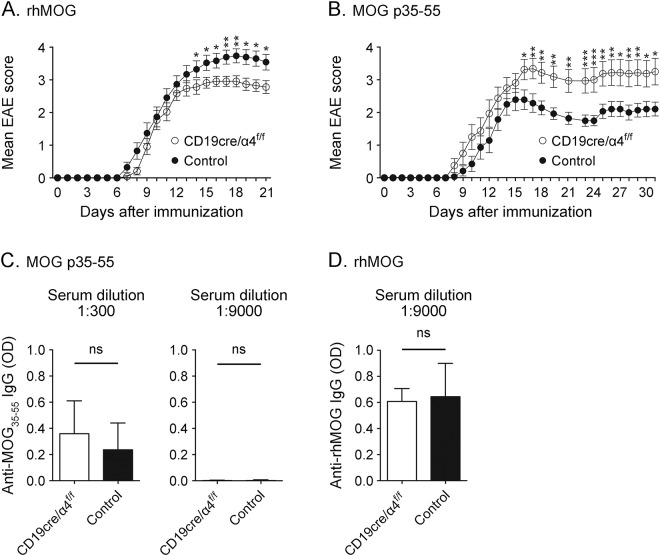
B cells in CD19cre/α4f/f mice are selectively deficient in cell surface VLA-4 expression.1 When immunized with rhMOG, these mice are less susceptible to rhMOG-induced EAE (figure 1A).1 In contrast, MOG p35-55-induced EAE is more severe in CD19cre/α4f/f mice than in control mice (figure 1B). As we observed for EAE induced by rhMOG in CD19cre/α4f/f mice,1 peripheral Th1 and Th17 responses to MOG p35-55 were not significantly affected by VLA-4 deficiency (figure e-1, A and B, at Neurology.org/nn). No detectable difference in the frequency of peripheral CD4+CD25+FoxP3+ Treg was observed in MOG p35-55-induced EAE that could explain the greater severity in CD19cre/α4f/f mice (figure e-1, C and D). Unlike MOG protein, which activates B cells and stimulates robust anti-MOG antibody titers, immunization of wild-type mice with MOG peptide does not efficiently activate B cells or elicit strong antibody responses.2,3 Similarly, we observed that immunization with MOG p35-55 elicited only a weak antibody response (figure 1C), while immunization with rhMOG induced a high titer of anti-MOG antibodies (figure 1D). However, B-cell VLA-4 deficiency did not influence antibody responses to either MOG p35-55 or rhMOG. Thus, B-cell VLA-4 deficiency did not significantly alter peripheral CD4+ T-cell or humoral responses to MOG p35-55 or to rhMOG.
Figure 1. Influence of B-cell very late antigen-4 (VLA-4) deficiency on experimental autoimmune encephalomyelitis (EAE) induced by recombinant extracellular domain of human myelin oligodendrocyte glycoprotein protein (rhMOG) or myelin oligodendrocyte glycoprotein (MOG) p35-55.
(A) CD19cre/α4f/f (n = 24 mice) and control mice (n = 23 mice) were immunized with 100 μg rhMOG. (B) CD19cre/α4f/f (n = 16 mice) and control mice (n = 14 mice) were immunized with 100 μg MOG p35-55. Cumulative data from 2 independent experiments are shown in (A) and (B). Results represent mean disease score ± standard error of the mean. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; Mann-Whitney U test. Serum immunoglobulin G (IgG) antibodies against MOG p35-55 (C) and rhMOG (D) were detected by ELISA. Values represent optical density (OD) at 450 nm. CD19cre/α4f/f or control mice (n = 3 mice/group) were immunized with 100 μg MOG p35-55 (C) or 100 μg rhMOG (D). Serum, collected at the end of the experiment, was diluted 1:300 (C, left panel) or 1:9,000 (C, right panel, D) before analysis. ns = not significant; unpaired t test with Welch correction.
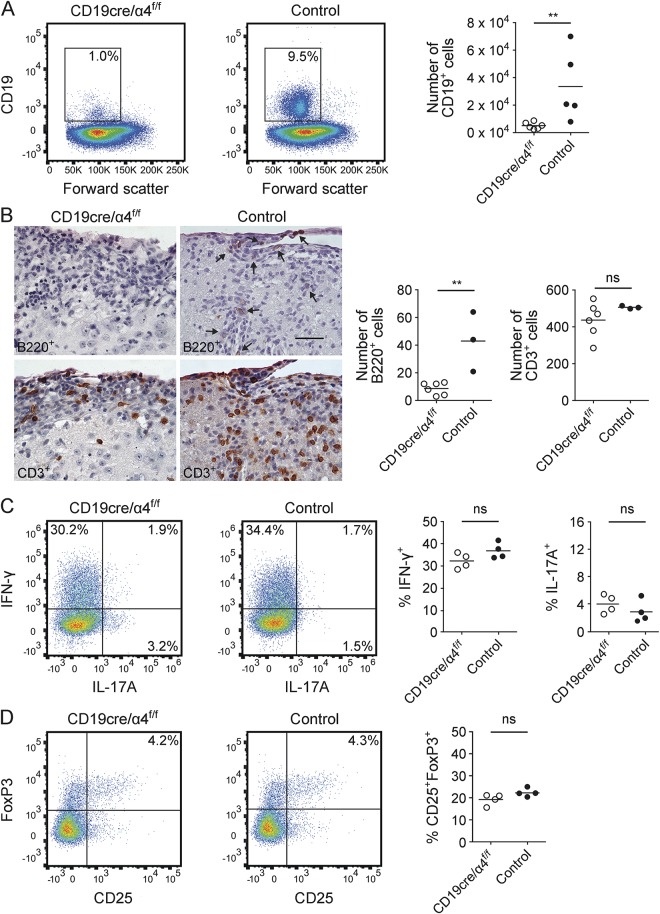
Whether the paradoxical exacerbation of MOG p35-55-induced EAE in CD19cre/α4f/f mice reflected immune changes within the CNS was also evaluated by flow cytometry, histology, and immunohistochemistry. As with rhMOG-induced EAE in CD19cre/α4f/f mice,1 there was a substantial reduction of CNS-infiltrating B cells in MOG p35-55-induced EAE, whereas there was no significant difference in accumulation of CNS T cells (figure 2, A and B). The frequencies of proinflammatory CNS Th1 and Th17 cells in CD19cre/α4f/f and control mice with MOG p35-55-induced EAE were not statistically different, although Th17 cells tended to be higher in the CD19cre/α4f/f mice (figure 2C). Similarly, there was no detectable difference in the frequency of CNS Treg between CD19cre/α4f/f and control mice with MOG p35-55-induced EAE (figure 2D). Meningeal, parenchymal, and total CNS inflammatory foci were not different between CD19cre/α4f/f and control mice (figure e-2).
Figure 2. B-cell very late antigen-4 (VLA-4) deficiency reduced CNS accumulation of B cells, but not proinflammatory or regulatory T cells (Treg), in myelin oligodendrocyte glycoprotein (MOG) p35-55-induced experimental autoimmune encephalomyelitis (EAE).
CD19cre/α4f/f or control mice were immunized with MOG p35-55. Analyses were performed 14 days after immunization. (A) CNS-infiltrating CD19+ B cells (gated on viable CD45hi lymphocytes) were collected at peak disease. Flow cytometry plots show 1 representative mouse each (n = 5–6 mice/group; pooled from 2 independent experiments). **p ≤ 0.01; Mann-Whitney U test. (B) Immunohistochemistry for T (CD3+) and B (B220+) cells in CNS infiltrates. B cells were not detected in spinal cord meningeal and inflammatory foci in CD19cre/α4f/f mice with EAE (upper left panel), whereas B cells were detected in CD19cre control mice (upper right panel, arrows). There were comparable T-cell infiltrates in inflammatory foci in corresponding adjacent serial sections (lower panels). Each panel shows a spinal nerve root. Scale bar, 30 μm, applies to the 4 panels. Semiquantitative analysis of n = 6 CD19cre/α4f/f mice and 3 control mice (right). **p ≤ 0.01; ns = not significant; unpaired t test. (C, D) CNS-infiltrating Th1 (interferon [IFN]–γ-secreting) and Th17 (interleukin [IL]–17A-secreting) cells (C) and CD25+FoxP3+ Treg (D) were measured by intracellular cytokine staining (ICS). Flow cytometry plots (gated on viable CD4+ T cells) show 1 representative mouse per group (n = 6 mice/group; pooled from 2 independent experiments). Data shown in graphs represent mean frequency (%) ± SEM. ns = not significant; unpaired t test.
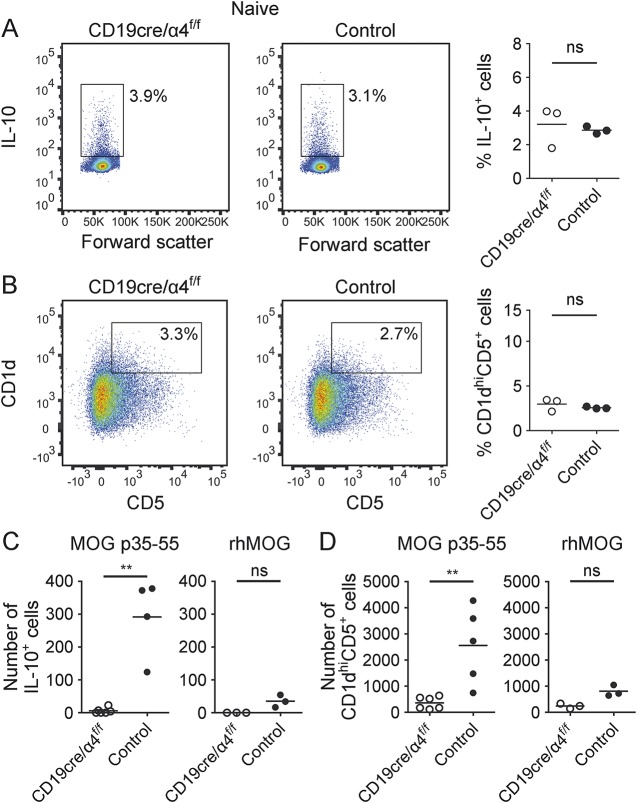
We next examined whether B-cell VLA-4 deficiency influenced CNS accumulation of Breg. Two approaches were used. First, we evaluated for the presence of IL-10-producing CD19+B220+ B cells. As Breg are also defined by coexpression of CD1d and CD5, we also examined CD19+CD1dhiCD5+ B cells (B10 cells).9 VLA-4 deficiency itself did not alter B-cell IL-10 production (figure 3A) or the numbers of CD19+CD1dhiCD5+ cells (figure 3B) in naive mice. CD19+IL-10+ B cells were detected in the CNS of control mice with MOG p35-55-induced EAE (figure 3C, left panel), which reflected 2.9% of all CNS B cells. In contrast, virtually no IL-10+ Breg were detectable in CD19cre/α4f/f mice with MOG p35-55-induced EAE (figure 3C, left panel). CNS CD19+CD1dhiCD5+ B cells were similarly affected (figure 3D, left panel). Specifically, there was an 18-fold reduction in the number of CNS CD1dhiCD5+ CNS CD19+ B cells in CD19cre/α4f/f mice with MOG p35-55-induced EAE in comparison to control mice with EAE. Of interest, while the absolute numbers of CNS Breg were reduced in CD19cre/α4f/f mice with MOG p35-55-induced EAE, the percentage of Breg among CNS B cells in CD19cre/α4f/f mice and control mice was not significantly different, indicating that although B-cell VLA-4 deficiency reduced CNS B-cell accumulation, it did not selectively influence Breg accumulation. In comparison to MOG p35-55-induced EAE, very few IL-10+ or CD1dhiCD5+ CNS Breg were detected in rhMOG-induced EAE, and those numbers were not significantly different in control and CD19cre/α4f/f mice (figure 3, C and D, right panels).
Figure 3. CNS accumulation of regulatory B cells (Breg) is very late antigen-4 (VLA-4)-dependent.
(A, B) B-cell VLA-4 expression did not influence CD19+IL-10+ (A) or CD1dhiCD5+ (B) Breg in naive mice. Spleen cells were collected from naive CD19cre/α4f/f or control mice (n = 3 mice/group). Interleukin (IL)–10-producing Breg were gated on viable CD45hiCD11b−CD19+B220+ lymphocytes and CD1dhiCD5+ Breg were gated on viable CD45hiCD19+ lymphocytes. Data are representative of 2 independent experiments. Each flow cytometry plot shows 1 representative mouse each. (C, D) Evaluation of B-cell VLA-4 expression on CNS CD19+IL-10+ (C) and CD1dhiCD5+ (D) Breg in experimental autoimmune encephalomyelitis induced by either myelin oligodendrocyte glycoprotein (MOG) p35-55 or recombinant extracellular domain of human MOG protein (rhMOG). CD19cre/α4f/f and control mice were immunized with either MOG p35-55 (n = 4–6 mice/group) or rhMOG (n = 3 mice/group). CNS tissues were collected at peak disease. Data are representative of 2 independent experiments. Data graphs represent mean percentage (%) or total number ± SEM. **p ≤ 0.01; ns = not significant; unpaired t test (A, B) or Mann-Whitney U test (C, D).
DISCUSSION
In this report, we evaluated the role of B-cell VLA-4 in EAE induced by MOG p35-55, a widely used encephalitogen that activates MOG-specific T cells, but does not efficiently activate MOG-specific B cells.2,3 Similar to B-cell-deficient mice4,5 or wild-type mice treated with anti-CD20-depleting antibodies,3,6 mice selectively deficient in B-cell VLA-4 expression developed more severe MOG p35-55-induced EAE. Activated or memory B cells may secrete proinflammatory cytokines, whereas unactivated or naive B cells more likely produce anti-inflammatory cytokines.3,10 Thus, it is thought that exacerbation of myelin peptide-induced EAE in B-cell-deficient mice or anti-CD20-treated wild-type mice may reflect a relative lack of B-cell regulation.3,5,6 Here, we observed that B-cell VLA-4 deficiency did not significantly alter peripheral or CNS T-cell responses in MOG p35-55-induced EAE. B-cell VLA-4 deficiency reduced the absolute numbers of all CNS B cells, including Breg, in MOG p35-55-induced EAE. Thus, as we have observed for B cells in general in rhMOG-induced EAE,1 CNS accumulation of Breg is also VLA-4-dependent. These observations raise the possibility that CNS Breg contribute to immune modulation in situ.
While fewer CNS Breg were detected in B-cell VLA-4-deficient mice, it is not clear whether that association alone was responsible for the exacerbation of MOG p35-55-induced EAE. The numbers of CNS infiltrating CD19+IL-10+ or CD19+CD1dhiCD5+ B cells in MOG p35-55-induced EAE were low, making it challenging to isolate sufficient numbers to evaluate how CNS Breg may influence polarization of myelin antigen–specific T cells ex vivo. Of interest, CNS Th17 cells tended to be higher in CD19cre/α4f/f mice that manifested exacerbated MOG p35-55-induced EAE, suggesting that CNS B cells may participate in CNS T-cell polarization. In 2010, by studying CD20-mediated B-cell depletion in EAE, it was first demonstrated that B cells influence M1/M2 monocyte polarization.3 Subsequent studies of CD20-mediated depletion in neuromyelitis optica (NMO)11 and MS11,12 similarly showed that B cells may influence proinflammatory cytokine production by myeloid cells. Thus, it is possible that CNS Breg could promote immune regulation directly or through interaction with T cells, infiltrating monocytes and macrophages, or resident glial cells, including microglia. Further, our finding that B-cell VLA-4-deficiency reduces CNS Breg may be relevant to the observations that natalizumab treatment can exacerbate symptoms of NMO,13 a humoral-mediated CNS autoimmune disease that involves CNS infiltration of immune cells, including B cells.14
EAE induction in B-cell VLA-4-deficient mice by MOG protein or peptide resulted in paradoxical clinical outcomes. These results are not necessarily unexpected. However, investigators may question whether MOG protein or p35-55 is the better encephalitogen for EAE studies. Although EAE induced by either MOG protein or p35-55 does not adequately reflect the pathogenesis of MS, a spontaneous disease, each one of these models has features that are advantageous for studying individual aspects of B-cell participation in CNS autoimmunity. In general, B cells and antibodies recognize conformational determinants of protein molecules and, unlike T cells, do not necessarily recognize linear peptide sequences. Thus, it may be beneficial to employ MOG protein-induced EAE for evaluation of antigen-specific B-cell and antibody responses. While proinflammatory B cells and Breg may coexist, data suggest that there is a proinflammatory B-cell bias in MS.10,15 Immunization with MOG protein activates B cells and elicits proinflammatory cytokine production. Further, in a manner similar to the benefit observed in anti-CD20 B-cell depletion of MOG protein-induced EAE, results from clinical trials indicate that anti-CD20 B-cell depletion has beneficial therapeutic effects in relapsing-remitting MS.16,17 In contrast, EAE induction with MOG p35-55, which does not efficiently activate proinflammatory MOG-specific B cells, may be advantageous when evaluating B cells in regulation of CNS autoimmunity. Our results from studying B-cell VLA-4 deficiency in EAE induced by MOG protein and MOG peptide underscore the need to consider carefully the encephalitogen that is used when studying B-cell physiology in a model of CNS autoimmunity.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Thalia Papayannopoulou (University of Washington, Seattle) for providing the α4flox/flox mice.
GLOSSARY
- Breg
regulatory B cells
- EAE
experimental autoimmune encephalomyelitis
- ICS
intracellular cytokine staining
- IgG
immunoglobulin G
- IL
interleukin
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- rhMOG
recombinant extracellular domain of human myelin oligodendrocyte glycoprotein protein
- Treg
regulatory T cells
- VLA-4
very late antigen-4
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
K. Lehmann-Horn designed research, acquired and analyzed data, performed statistical analysis, and wrote the manuscript. S.A. Sagan acquired and analyzed data, and discussed the results at all stages. R.C. Winger acquired and analyzed data and contributed to writing the manuscript. C.M. Spencer analyzed data and contributed to writing the manuscript. C.C.A.B. provided reagents. R.A. Sobel performed histology and immunohistochemistry analysis. S.S. Zamvil designed research, interpreted data, wrote the manuscript, and supervised the study.
STUDY FUNDING
K.L.-H. received research support from Deutsche Forschungsgemeinschaft and the US National Multiple Sclerosis Society. C.C.A.B. is supported by grants from the National Health and Medical Research Council of Australia/CIRM Joint Project (APP1053621), the Victoria/CIRM Joint Project (RMI-01739), the Department of Industry, Commonwealth of Australia (AISRF06680) and Eva and Les Erdi AUSiMED Fellowship in Neurological Diseases. R.A.S. received grant support from the NIH and National Multiple Sclerosis Society. S.S.Z. received research grant support from the NIH (RO1 AI073737 and RO1 NS063008), the National Multiple Sclerosis Society (RG 5180 and RG 4768), the Maisin Foundation and the Guthy Jackson Charitable Foundation.
DISCLOSURE
K. Lehmann-Horn, S.A. Sagan, R.C. Winger, and C.M. Spencer report no disclosures. C.C.A. Bernard is on the editorial board for Future Neurology, was a guest editor and editorial board member for Inflammation and Regeneration. R.A. Sobel is Editor-in-Chief for Journal of Neuropathology and Experimental Neurology, associate editor for Journal of Neuroimmunology. S.S. Zamvil served on the data safety monitoring board for BioMS, Teva Pharmaceuticals, Eli Lilly, and Com; is a member of the clinical advisory board for the Myelin Repair Foundation; is Deputy Editor for Neurology: Neuroimmunology & Neuroinflammation; has consulted for Biogen Idec, Teva Neuroscience, EMD-Serono, Genzyme, Novartis, and Roche; is on the speakers' bureau for Advanced Health Media and Biogen. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Lehmann-Horn K, Sagan SA, Bernard CC, Sobel RA, Zamvil SS. B-cell very late antigen-4 deficiency reduces leukocyte recruitment and susceptibility to central nervous system autoimmunity. Ann Neurol 2015;77:902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molnarfi N, Schulze-Topphoff U, Weber MS, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med 2013;210:2921–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber MS, Prod'homme T, Patarroyo JC, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol 2010;68:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol 1998;161:4480–4483. [PubMed] [Google Scholar]
- 5.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med 1996;184:2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 2008;118:3420–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol 2003;23:9349–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 1997;25:1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol 2010;185:2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007;178:6092–6099. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann-Horn K, Schleich E, Hertzenberg D, et al. Anti-CD20 B-cell depletion enhances monocyte reactivity in neuroimmunological disorders. J Neuroinflammation 2011;8:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R, Rezk A, Miyazaki Y, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 2015;7:310ra166. [DOI] [PubMed] [Google Scholar]
- 13.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm 2015;2:e62. 10.1212/NXI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucchinetti CF, Guo Y, Popescu BF, Fujihara K, Itoyama Y, Misu T. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol 2014;24:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-Or A, Fawaz L, Fan B, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010;67:452–461. [DOI] [PubMed] [Google Scholar]
- 16.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 17.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–1787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.