Abstract
Background:
Inoperable chronic thromboembolic pulmonary hypertension (CTEPH) is a severe clinical syndrome characterized by right cardiac failure and possibly subsequent liver dysfunction. However, whether serum markers of liver dysfunction can predict prognosis in inoperable CTEPH patients has not been determined. Our study aimed to evaluate the potential role of liver function markers (such as serum levels of transaminase, bilirubin, and gamma-glutamyl transpeptidase [GGT]) combined with 6-min walk test in the prediction of prognosis in patients with inoperable CTEPH.
Methods:
From June 2005 to May 2013, 77 consecutive patients with inoperable CTEPH without confounding co-morbidities were recruited for this prospective cohort study. Baseline clinical characteristics and 6-min walk distance (6MWD) results were collected. Serum biomarkers of liver function, including levels of aspartate aminotransferase, alanine aminotransferase, GGT, uric acid, and serum bilirubin, were also determined at enrollment. All-cause mortality was recorded during the follow-up period.
Results:
During the follow-up, 22 patients (29%) died. Cox regression analyses demonstrated that increased serum concentration of total bilirubin (hazard ratio [HR] = 7.755, P < 0.001), elevated N-terminal of the prohormone brain natriuretic peptide (HR = 1.001, P = 0.001), decreased 6MWD (HR = 0.990, P < 0.001), increased central venous pressure (HR = 1.074, P = 0.040), and higher pulmonary vascular resistance (HR = 1.001, P = 0.018) were associated with an increased risk of mortality. Serum concentrations of total bilirubin (HR = 4.755, P = 0.007) and 6MWD (HR = 0.994, P = 0.017) were independent prognostic predictors for CTEPH patients. Patients with hyperbilirubinemia (≥23.7 μmol/L) had markedly worse survival than those with normobilirubinemia.
Conclusion:
Elevated serum bilirubin and decreased 6MWD are potential predictors for poor prognosis in inoperable CTEPH.
Keywords: Chronic Thromboembolic Pulmonary Hypertension, Heart Failure, Liver Function, Prognosis
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is a clinical syndrome characterized by obstruction of the pulmonary arteries with organized pulmonary thromboembolic and subsequent elevated blood pressure in the arteries.[1] Although pulmonary endarterectomy (PEA) has been considered the treatment of choice and potential cure for CTEPH,[2] approximately 20–40% of CTEPH patients are considered inoperable due to the predominant distal pulmonary arteriopathy or severe co-morbidities,[3,4] contributing to most deaths related to this disease. Indeed, 1- and 3-year survival rates among inoperable CTEPH patients were reported as only 82% and 70%, respectively.[5] Despite the development of novel treatments, risk stratification for patients with CTEPH would also be beneficial for determining appropriate treatment strategies and timing. Although some markers have been explored as prognostic predictors in pulmonary hypertension patients, including hemodynamic parameters through right heart catheterization, noninvasive markers of right ventricle size and function, and measurements of exercise capacity using the 6-min walk test (6MWT) and cardiopulmonary exercise testing,[6,7,8,9] whether these prognostic markers can be applied to CTEPH remains unclear.[10,11,12,13]
Liver dysfunction is a severe consequence of heart failure due to hepatic congestion. Passive hepatic congestion due to increased central venous pressure (CVP) in patients with right heart failure may cause elevations of both direct and indirect serum bilirubin and gamma-glutamyl transpeptidase (GGT).[14,15,16,17] Indeed, serum levels of transaminase, GGT, and bilirubin have been indicated as prognostic markers for decompensated heart failure.[14,18,19] However, to the best of our knowledge, few studies have evaluated the potential role of liver function markers (such as serum levels of transaminase, bilirubin, and GGT) in the prediction of prognosis in patients with inoperable CTEPH. In the present study, the prognostic value of serum levels of transaminase, GGT, and total bilirubin and 6-min walk distance (6MWD) were evaluated in inoperable CTEPH patients during a long-term follow-up.
METHODS
Patient population and study design
This study was designed as a prospective cohort study, and the study protocol was approved by the Ethics Committee of Chao-Yang Hospital before its performance. Between June 2005 and May 2013, 126 consecutive CTEPH cases were confirmed to be inoperable. All patients underwent detailed clinical examinations, including one of followings: Ventilation/perfusion lung scan, computed tomography angiography, and pulmonary angiography. Right heart catheterizations were performed to confirm the diagnosis. The diagnostic criteria for inoperable CTEPH were as follows: (1) Mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest and pulmonary arterial wedge pressure (PAWP) <15 mmHg, (2) ventilation-perfusion lung scanning, computed tomographic angiography or pulmonary angiography showing chronic thromboemboli in the pulmonary artery, and (3) exclusion of other types of pulmonary hypertension. The determination of inoperability was made by two experienced PEA surgeons and one physician. Patients who had predominant distal pulmonary arteriopathy, imbalance between severity of pulmonary hypertension and morphologic lesion, pulmonary vascular resistance (PVR) >1500 dyn·s·cm−5, age, and severe co-morbidities for PEA were seen as “inoperable CTEPH.”[3] Patients were excluded if they met any of the following criteria: History of chronic liver and biliary tract disease, malignancies, and taking chronic hepatotoxic medications or alcohol abuse. The patient inclusion process is shown in Figure 1. Finally, 77 patients were included in the study, and all provided written informed consent.
Figure 1.
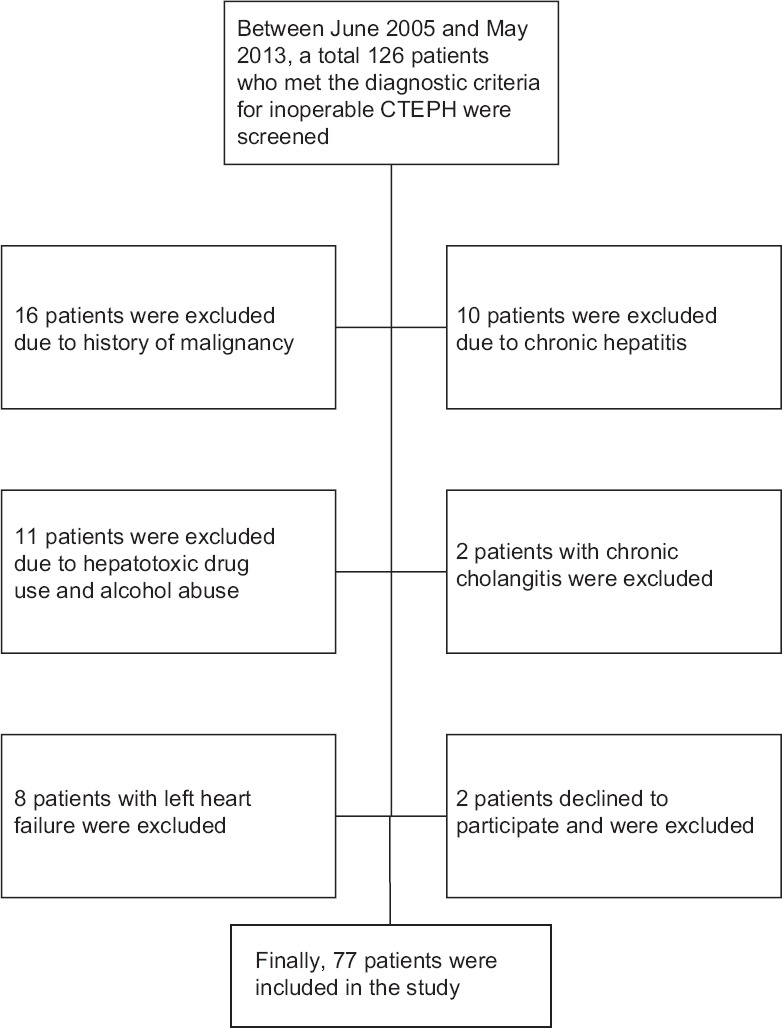
Flowchart of patient enrollment.
Right heart catheterization examination
Right heart catheterizations were done in the Interventional Radiology Department in our hospital using a Philips monitoring system (Shenzhen Goldway Industrial Inc., China). A Swan-Ganz catheter was inserted via the jugular vein to assess the right chambers and pulmonary artery pressures (mPAP, systolic PAP [sPAP], and diastolic PAP) and to obtain oximetry samples. For excluding postcapillary pulmonary hypertension, the PAWP was determined. Cardiac output (CO) was determined by thermodilution and measured 3 times to obtain a mean value. PVR was calculated based on CO, mPAP, and PAWP.
Clinical and biomarker examination
Baseline clinical information including gender, age, height, weight, blood pressure, and respiratory rate was collected. Venous blood samples were collected at the time of initial diagnostic evaluation. All blood samples were drawn from a peripheral vein after an overnight fast. For measurements of the serum concentration of N-terminal of the prohormone brain natriuretic peptide (NT-proBNP), samples were immediately placed on ice and centrifuged at 4°C. NT-proBNP was measured using a chemiluminescence immunoassay by Roche 411 in our core laboratory. Serum bilirubin (including total bilirubin, direct bilirubin, and indirect bilirubin), alanine aminotransferase (ALT), aspartate aminotransferase (AST), GGT, and uric acid (UA) were measured in the core laboratory. The normal ranges were as follows: NT-proBNP 0–142 pg/ml, direct bilirubin 0–6.8 µmol/L, indirect bilirubin 3.4–17.1 µmol/L, ALT 10–40 U/L, AST 10–42 U/L, GGT 5–85 U/L, and UA 150–410 µmol/L.
Treatment and follow-up of the enrolled patients
Treatment for CTEPH consisted of routine anticoagulation (with the aim of maintaining an international normalized ratio of 2.0–3.0), diuretics, digitalis, and oxygen. Also, 7 patients were treated with sildenafil at the beginning of treatment, and only 3 of these patients continued using sildenafil during the follow-up period. The primary outcome was all-cause mortality, and all patients were followed up until May 31, 2013.
Statistical analysis
Statistical analyses were performed with SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Survival time was defined as the time from baseline until May 31, 2013. Data are presented as counts and percentages, mean ± standard deviation (SD), or median and interquartile range (IQR: 25th and 75th percentiles) according to their types and distributions. The modified Kolmogorov–Smirnov test was used to test normal distribution of continuous variables. Comparisons of parameters between two groups were performed with the independent samples t-test, Mann–Whitney U-test, or Fisher's exact test as appropriate. Categorical variables were compared with a Fisher exact test in the case of a 2 × 2 contingency table. Before performing survival analysis, a receiver operating characteristic (ROC) curve was appointed to determine the bilirubin level that provided the best combination of sensitivity and specificity for predicting the endpoint. The prognostic values of the variables were tested in Cox proportional hazards regression analyses and compared by Wald's test, with the results presented as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). The candidate variables were first tested in single variable models and were included in the multiple variable model if P < 0.05. Survival curves were derived using the Kaplan–Meier method. A P < 0.05 was considered statistically significant in all analyses.
RESULTS
Baseline characteristics of the enrolled patients
The baseline clinical and hemodynamic data of the enrolled patients are shown in Table 1. Mean follow-up time was 32 ± 20 months. The mean age of the included patients was 57 ± 12 years; 41 (53%) patients were female, and 40 (52%) patients were in the World Health Organization (WHO) functional class III–IV. During the mean follow-up period of 32 months, 22 patients (29%) died. Among these, 21 patients died of right heart failure, and 1 died of hemoptysis. The serum total bilirubin, direct bilirubin, GGT, and NT-proBNP levels of the included patients were above normal levels, whereas the AST, ALT, and UA levels were generally within the normal ranges. Compared with the normal population, our patients were likely to have shorter 6MWD and higher CVP. sPAP, mPAP, and PVR were also remarkably increased in our patients. As shown in Table 2, compared with survivors of inoperable CTEPH patients, nonsurvivors were more likely to have a higher serum concentration of total bilirubin (32.5 ± 18.7 µmol/L vs. 18.1 ± 11.9 µmol/L, P < 0.05), direct bilirubin (13.0 µmol/L [IQR, 6.2–15.6] vs. 6.8 µmol/L [IQR, 2.24–7.4], P < 0.05), indirect bilirubin (19.0 ± 9.3 µmol/L vs. 11.4 ± 5.7 µmol/L, P < 0.05), and higher CVP (11.8 ± 3.0 mmHg vs. 6.9 ± 3.3 mmHg, P = 0.000). The 6MWD of nonsurvivors was significantly shorter than that of survivors (241 ± 73 m vs. 340 ± 104 m, P = 0.000). ROC analysis [Figure 2] identified a total bilirubin cut-off of 23.7 µmol/L, which had the best combination of sensitivity (77%) and specificity (78%) for predicting mortality. According to this cut-off value, we divided the patients into a high-bilirubin group and a low-bilirubin group [Table 3]. More importantly, patients with high-bilirubin (≥23.7 µmol/L) were associated with worse survival than those with low-bilirubin (<23.7 µmol/L) [Figure 3]. Patients with high serum bilirubin had a shorter 6MWD (250 ± 82 m vs. 344 ± 103 m, P = 0.001), an increased level of GGT (97.3 U/L [IQR,44.5–131.0] vs. 67.4 U/L [IQR, 34.5–79.3, P = 0.013]), a higher CVP (10.9 ± 3.7 mmHg vs. 6.7 ± 3.1 mmHg, P < 0.05), and elevated PVR (16.35 ± 7.61 woods units vs. 13.08 ± 5.23 woods units, P < 0.05).
Table 1.
Characteristics of the enrolled patients in this study
| Characteristics | Results (n = 77) |
|---|---|
| Females | 41 (53) |
| Age (years) | 57 ± 12 |
| WHO functional class | |
| WHO FC I–II | 37 (48) |
| WHO FC III–IV | 40 (52) |
| Treatment | |
| Diuretics | 77 |
| Anticoagulants | 77 |
| Sildenafil | 7 |
| Biomarker at baseline | |
| Total bilirubin (µmol/L) | 22.2 ± 15.4 |
| Direct bilirubin (µmol/L) | 8.6 (2.3, 10.4) |
| Indirect bilirubin (µmol/L) | 13.5 ± 7.6 |
| GGT (U/L) | 78.7 (37.0, 94.5) |
| ALT (U/L) | 27.4 (18.0, 32.5) |
| AST (U/L) | 29.5 (19.0, 35.3) |
| Uric acid (µmol/L) | 360 ± 139 |
| NT-proBNP (pg/ml) | 2189 (672, 3627) |
| Hemodynamic and exercise capacity | |
| 6MWD (m) | 321 ± 103 |
| CVP (mmHg) | 8.5 ± 5.3 |
| sPAP (mmHg) | 87 ± 17 |
| mPAP (mmHg) | 50 ± 10 |
| PVR (wood units) | 14.4 ± 6.4 |
Data are shown as n (%), mean ± SD or median (IQR). WHO FC: World Health Organization functional class; ALT: Alanine transaminase; AST: Aspartate transaminase; GGT: Gamma-glutamyl transpeptidase; 6WMD: 6-min walk distance; CVP: Central venous pressure; sPAP: Systolic pulmonary artery pressure; mPAP: Medium pulmonary artery pressure; and PVR: Pulmonary vascular resistance; IQR: Interquartile range; SD: Standard deviation.
Table 2.
Comparisons between survivors and nonsurvivors in this study
| Parameters | Survivors (n = 55) | Nonsurvivors (n = 22) | Statistical values | P |
|---|---|---|---|---|
| Females | 31 (56) | 10 (45) | 0.453 | |
| Age (years) | 57 ± 11 | 57 ± 16 | −0.028* | 0.977 |
| WHO Functional Class | ||||
| WHO FC I–II | 28 (52) | 9 (41) | 0.460 | |
| WHO FC III–IV | 27 (48) | 13 (59) | ||
| Biomarker | ||||
| Total bilirubin (mmol/L) | 18.1 ± 11.9 | 32.5 ± 18.7 | −4.030* | 0.000 |
| Direct bilirubin (mmol/L) | 6.8 (2.2, 7.4) | 13.0 (6.2, 15.6) | 307.5† | 0.001 |
| Indirect bilirubin (mmol/L) | 11.4 ± 5.7 | 19.0 ± 9.3 | −3.416* | 0.002 |
| ALT (U/L) | 27.6 (17.0, 30.0) | 27.0 (18.0, 35.5) | 582.5† | 0.800 |
| AST (U/L) | 29.1 (18.0, 34.0) | 30.6 (20.8, 37.8) | 499.5† | 0.234 |
| GGT (U/L) | 78 (36, 96) | 80 (42, 96) | 544.0† | 0.491 |
| Uric Acid (mmol/L) | 367 ± 142 | 349 ± 136 | 0.505* | 0.615 |
| NT-proBNP (pg/ml) | 1905 (643, 3293) | 2898 (1061, 4433) | 452.0† | 0.084 |
| Hemodynamic and exercise capacity | ||||
| 6MWD (m) | 355 ± 95 | 237 ± 72 | 4.753* | 0.000 |
| CVP (mmHg) | 7.5 ± 4.4 | 10.9 ± 6.6 | −2.196* | 0.036 |
| SPAP (mmHg) | 86 ± 16 | 88 ± 21 | −0.541* | 0.590 |
| mPAP (mmHg) | 51 ± 12 | 49 ± 9 | −0.718* | 0.475 |
| PVR (woods units) | 13.72 ± 4.88 | 15.93 ± 9.32 | −1.068* | 0.296 |
Data are shown as n (%), mean ± SD or median (IQR). P values were calculated by independent samples t-test, Mann–Whitney U-test, or Fisher’s exact test. *t values; †U values. GGT: Gamma-glutamyl transpeptidase; ALT: Alanine transaminase; AST: Aspartate transaminase; GGT: Gamma-glutamyl transpeptidase; NT-proBNP: N-terminal pro-peptide of brain natriuretic peptide; 6WMD: 6-min walk distance; CVP: Central venous pressure; sPAP: Systolic pulmonary artery pressure; mPAP: Medium pulmonary artery pressure; PVR: Pulmonary vascular resistance; IQR: Interquartile range; SD: Standard deviation.
Figure 2.

ROC analysis of total bilirubin as a prognostic predictor in inoperable chronic thromboembolic pulmonary hypertension patients. ROC demonstrating that a cut-off for total bilirubin level of 23.7 μmol/L results in a sensitivity of 77% and a specificity of 78% for predicting mortality in inoperable chronic thromboembolic pulmonary hypertension patients. ROC: Receiver operating characteristic curve; AUC: Area under the curve.
Table 3.
Comparisons between high-bilirubin and low-bilirubin patients in this study
| Parameters | High-bilirubin (n = 29) | Low-bilirubin (n = 48) | Statistical values | P |
|---|---|---|---|---|
| Female | 14 (47) | 27 (56) | 0.346 | |
| Age (years) | 55 ± 14 | 58 ± 11 | −0.257* | 0.798 |
| WHO Functional Class | ||||
| WHO FC I–II | 11 (38) | 26 (54) | 0.239 | |
| WHO FC III–IV | 18 (62) | 22 (46) | ||
| Biomarker | ||||
| ALT (U/L) | 26.4 (16.0, 34.0) | 28.0 (18.3, 30.0) | 689.5† | 0.945 |
| AST (U/L) | 31.6 (21.0, 39.0) | 28.3 (17.3, 33.0) | 540.0† | 0.101 |
| GGT (U/L) | 97.3 (44.5, 131.0) | 67.4 (34.5, 79.3) | 460.0† | 0.013 |
| Uric acid (mmol/L) | 377 ± 180 | 354 ± 115 | –0.974* | 0.335 |
| NT-proBNP (pg/ml) | 2872 (730, 4665) | 1776 (649, 2761) | 521.5† | 0.067 |
| Hemodynamic and exercise capacity | ||||
| 6MWD | 262 ± 98 | 358 ± 90 | 4.410* | 0.000 |
| CVP (mmHg) | 10.8 ± 6.0 | 7.1 ± 4.4 | −2.815* | 0.000 |
| sPAP (mmHg) | 91 ± 17 | 84 ± 17 | −1.322* | 0.190 |
| mPAP (mmHg) | 52 ± 11 | 49 ± 10 | −1.326* | 0.189 |
| PVR (woods units) | 16.35 ± 7.61 | 13.08 ± 5.23 | −2.073* | 0.042 |
Data are shown as n (%), mean ± SD or median (IQR). P values were calculated by independent samples t-test, Mann–Whitney U-test or Fisher’s exact test. Hyperbilirubinemia means a serum concentration of total bilirubin ≥23.7 μmol/L, and normal bilirubinemia means a serum concentration of total bilirubin <23.7 μmol/L. *t values; †U values. WHO FC: World Health Organization functional class; ALT: Alanine transaminase; AST: Aspartate transaminase; GGT: Gamma-glutamyl transpeptidase; NT-proBNP: N-terminal pro-peptide of brain natriuretic peptide; 6WMD: 6-min walk distance; CVP: Central venous pressure; sPAP: Systolic pulmonary artery pressure; mPAP: Medium pulmonary artery pressure; PVR: Pulmonary vascular resistance; IQR: Interquartile range; SD: Standard deviation.
Figure 3.
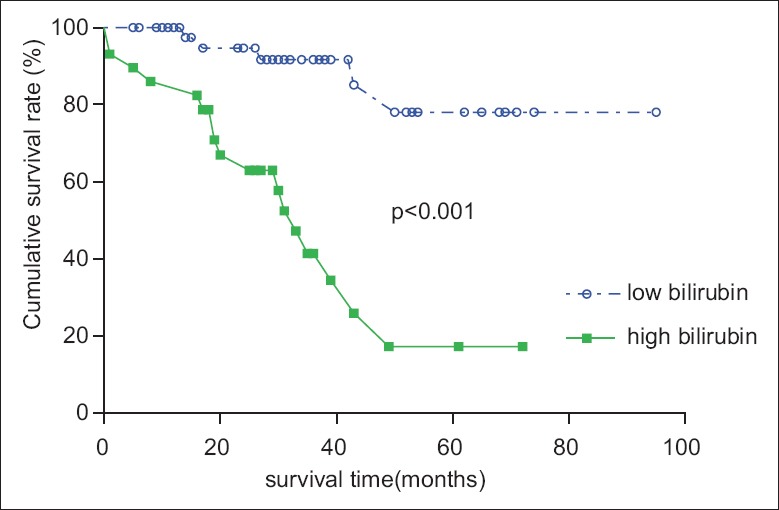
Kaplan–Meier estimation of survival in patients with or without hyperbilirubinemia. Kaplan–Meier cumulative survival curves showing cumulative rates of survival for 77 inoperable chronic thromboembolic pulmonary hypertension patients, stratified by the identified total bilirubin cut-off value. Survival in patients with a total bilirubin ≥23.7 μmol/L differed significantly from that in patients with a total bilirubin <23.7 μmol/L.
Predictors of mortality outcome of inoperable chronic thromboembolic pulmonary hypertension
Cox proportional hazard analyses were performed to determine the predictors of mortality risk by including variables of biomarkers of total bilirubin, ALT, AST, GGT, UA, and NT-proBNP, as well as the hemodynamic and exercises capacity indicators of 6MWD, WHO functional class, CVP, sPAP, mPAP, and PVR. As shown in Table 4, the univariable analyses showed that increased serum concentration of total bilirubin (HR = 7.755, P = 0.000), elevated NT-proBNP (HR = 1.001, P = 0.001), decreased 6MWD (HR = 0.990, P = 0.000), increased CVP (HR = 1.074, P = 0.040), and higher PVR (HR = 1.001, P = 0.018) were associated with an increased risk of mortality. After adjusting for the interaction of related factors [Table 5], we found that serum concentrations of total bilirubin (HR = 4.755, P = 0.007) and 6MWD (HR = 0.994, P = 0.017) were independent predictors of mortality for inoperable CTEPH patients.
Table 4.
Single variable HRs for death in patients
| Parameters | P | HR | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Female | 0.341 | 0.657 | 0.277 | 1.561 | |
| Age | 0.743 | 0.994 | 0.960 | 1.030 | |
| Total bilirubin | 0.000* | 7.755 | 2.826 | 21.295 | |
| ALT | 0.513 | 0.988 | 0.952 | 1.025 | |
| AST | 0.469 | 1.008 | 0.987 | 1.029 | |
| GGT | 0.851 | 1.000 | 0.995 | 1.006 | |
| Uric acid | 0.964 | 1.000 | 0.997 | 1.003 | |
| NT-proBNP | 0.001* | 1.001 | 1.0001 | 1.002 | |
| 6MWD | 0.000* | 0.990 | 0.986 | 0.955 | |
| WHO FC | 0.809 | 0.898 | 0.375 | 2.151 | |
| CVP | 0.040* | 1.074 | 1.003 | 1.151 | |
| sPAP | 0.224 | 1.014 | 0.992 | 1.036 | |
| mPAP | 0.132 | 1.032 | 0.990 | 1.076 | |
| PVR | 0.018* | 1.001 | 1.000 | 1.002 | |
*Potential prognostic predictors for in CTEPH. HR: Hazard ratio; CI: Confidence interval; ALT: Alanine transaminase; AST: Aspartate transaminase; GGT: Gamma-glutamyl transpeptidase; NT-proBNP: N-terminal pro-peptide of brain natriuretic peptide; 6MWD: 6-min walk distance; WHO FC: World Health Organization functional class; CVP: Central venous pressure; sPAP: Systolic pulmonary artery pressure; mPAP: Medium pulmonary artery pressure; PVR: Pulmonary vascular pressure; IQR: Interquartile range; SD: Standard deviation; CTEPH: Chronic thromboembolic pulmonary hypertension.
Table 5.
Multiple variable model HRs for death in patients
| Parameters | P | HR | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Total bilirubin | 0.007* | 4.755 | 1.541 | 14.676 | |
| 6MWD | 0.017* | 0.994 | 0.988 | 0.999 | |
All of the parameters related to prognosis in single variable analysis were input into the multivariate analysis. *Independent prognostic predictor for in CTEPH. HR: Hazard ratios; CI: Confidence interval; 6MWD: 6-min walk distance; CTEPH: Chronic thromboembolic pulmonary hypertension.
DISCUSSION
In this study, we found that the levels of serum total bilirubin and performance on the 6MWT were correlated with the severity of disease in patients with inoperable CTEPH. In addition, the total bilirubin level was a strong predictor of poor prognosis for patients with inoperable CTEPH, even after adjustment of a variety of clinical, biochemical, and hemodynamic variables. Our findings may be helpful for the identification of high-risk patients. Moreover, to our knowledge, this was the first study to investigate the relationship between liver function and the prognosis of CTEPH patients. A previous study showed that serum total bilirubin is an independent risk factor for death in patients with pulmonary arterial hypertension (PAH) and suggested that serum bilirubin should be monitored in the management of patients with PAH.[20] The results of our study are consistent with those of this previous study. However, the study population in our study was different from that in the previous study. Moreover, the sample size of their study was relatively small and did not include some markers such as 6MWD and PVR, which had been proved to be important prognostic predictors for mortality.
The primary cause underlying higher level of serum total bilirubin in CTEPH patients is congestive hepatopathy caused by right heart failure, and the congestive hepatopathy during this pathologic process, from our point of view, could be explained by the follows: Increased hepatic venous pressure, decreased hepatic blood flow, and decreased arterial oxygen saturation.[21] Elevated CVP caused by right heart failure is transmitted through the hepatic veins and into the small hepatic venules. The effect of this transmitted pressure is passive congestion of the liver with subsequent elevation of hepatic venous pressure, which can further impair the delivery of oxygen and nutrients to hepatocytes, leading to sinusoidal fenestrae enlargement.[15] In our study, we found that the CVP of the high-bilirubin group was higher than that of the low-bilirubin group, which further confirmed the mechanism of congestive hepatopathy in CTEPH patients.
Liver function test (LFT) abnormalities related to congestive hepatopathy are more characteristic of a cholestatic pattern (i.e., increased alkaline phosphatase, GGT, total bilirubin, and hypoalbuminemia).[22,23] This was also found in the Candesartan in Heart Failure – Assessment of Reduction in Mortality and Morbidity program, which showed that elevated total bilirubin is the strongest LFT predictor for the adverse outcome of cardiovascular death and that bilirubin is superior to transaminases in sensitivity to hemodynamic abnormality in patients.[24] Previous studies proved that GGT and ALP were also closely related to severity and adverse outcome of chronic heart failure,[17,23] which was not significant in our study. Differences of inclusion patients and severity of the disease may be the reasons of this phenomenon. In our study, we found that total bilirubin was significantly higher in the severe CTEPH group, whereas no differences in serum transaminases between the surviving and nonsurviving patients were detected, which is consistent with this characteristic of congestive hepatopathy. Compared with that of other organs such as kidney, the liver's complex dual blood supply makes it relatively resistant to hepatocyte necrosis from hemodynamic perturbations, and striking transaminase elevations could only be expected in cases of marked hypotension or hypoperfusion, which was confirmed in CTEPH patients in our study.[25]
It is well known that bilirubin could be formed when red blood cells die, and their hemoglobin is broken down within the macrophages into heme and globins. In the liver, bilirubin is conjugated with uridine diphosphate-glucuronate, making it water-soluble diglucuronide. Prehepatic (include hemolysis and hematoma resorption), intrahepatic (include alcohol abusing, infectious hepatitis, drug reactions, and autoimmune disorders), and posthepatic (mainly include disorders of biliary tract) conditions all cause hyperbilirubinemia.[26] In our study, we applied exclusion criteria to avoid these confounding factors. The medical history of each patient had been recorded, and anyone who had a history of alcohol abuse, infectious hepatitis, or autoimmune liver disease was excluded. Abdomen ultrasound examinations were performed to exclude active biliary tract disease and chronic liver disease. Urine samples were tested to screen for hemolysis and hematoma resorption.
The present study demonstrated that the 6MWD is also an independent factor that predicts survival of inoperable CTEPH patients. Patients with a longer 6MWD may have better survival than those with a shorter 6MWD. This finding is consistent with the results of a study by Miyamoto et al. in PAH, which also showed that a poor 6MWD is an independent predictor of mortality.[27] Another study also demonstrated that the 6MWD can reflect the clinical and hemodynamic severity of disease in patients of CTEPH before and after PEA.[28] However, the 6MWD varies with the experience of the examiner and the compliance of the examinee. Also, some patients with severe disease cannot finish this test. Such limitations may explain why performance on the 6MWT showed only a weak association with mortality among CTEPH patients in our study.
There are some limitations in our study that should be considered when interpreting the results. First, some related biomarkers that were investigated as prognostic markers in former studies were not evaluated in our study, including levels of alkaline phosphates, albumin, high-density lipoprotein, and heart type fatty acid-binding protein. Second, although mortality was the only endpoint in our study, some patients were hospitalized due to exacerbation or lung transplantation during the follow-up. Therefore, further research is needed to investigate the factors related to disease deterioration. Third, our study found that indirect bilirubin levels were higher than direct bilirubin levels in our patients, which could be explained by hemolysis occurring in the process. However, the precise cause deserves further investigation. Although anticoagulation therapy had been applied for all of the patients, modern PAH target treatments are not routinely administered due to the availability of targeted medications. The relationship between treatments and changes in bilirubin levels requires further investigation.
In conclusion, an elevated serum bilirubin level and a poor 6MWD are powerful prognostic predictors for mortality in inoperable CTEPH patients. Measurements of serum bilirubin and exercise capacity may help to identify high-risk patients and predict their outcome.
Financial support and sponsorship
This study was supported by National Natural Science Foundation of China (No. 81570049 and No. 81300044); the Fund of China Key Research Projects of the 12th National Five-year Development Plan (No. 2011BAI11B17); the Beijing Youth Star of Science and Technology Program (No. 2007B037); the National Department of Public Benefit Research Foundation by the Ministry of Health P. R. China (No. 201302008); and the Beijing Natural Science Foundation (No. 7152062).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Dr. Lan Zhao and Christopher J Rhodes for their valuable assistance in the statistical analyses, grammar correction, and efforts in the preparation of this manuscript.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, et al. Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–63. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 2.Piazza G, Goldhaber SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2011;364:351–60. doi: 10.1056/NEJMra0910203. [DOI] [PubMed] [Google Scholar]
- 3.Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–10. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Mayer E. Surgical and post-operative treatment of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2010;19:64–7. doi: 10.1183/09059180.00007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1122–7. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 6.Foris V, Kovacs G, Tscherner M, Olschewski A, Olschewski H. Biomarkers in pulmonary hypertension: What do we know? Chest. 2013;144:274–83. doi: 10.1378/chest.12-1246. [DOI] [PubMed] [Google Scholar]
- 7.Cracowski JL, Leuchte HH. The potential of biomarkers in pulmonary arterial hypertension. Am J Cardiol. 2012;110(6 Suppl):32S–8S. doi: 10.1016/j.amjcard.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Gomberg-Maitland M. Prognostication in pulmonary arterial hypertension. Heart Fail Clin. 2012;8:373–83. doi: 10.1016/j.hfc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, et al. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2011;107:628–32. doi: 10.1016/j.amjcard.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Deboeck G, Scoditti C, Huez S, Vachiéry JL, Lamotte M, Sharples L, et al. Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur Respir J. 2012;40:1410–9. doi: 10.1183/09031936.00217911. [DOI] [PubMed] [Google Scholar]
- 11.Zhai Z, Murphy K, Tighe H, Wang C, Wilkins MR, Gibbs JS, et al. Differences in ventilatory inefficiency between pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Chest. 2011;140:1284–91. doi: 10.1378/chest.10-3357. [DOI] [PubMed] [Google Scholar]
- 12.Surie S, Reesink HJ, van der Plas MN, Hardziyenka M, Kloek JJ, Zwinderman AH, et al. Plasma brain natriuretic peptide as a biomarker for haemodynamic outcome and mortality following pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Interact Cardiovasc Thorac Surg. 2012;15:973–8. doi: 10.1093/icvts/ivs415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golpe R, Castro-Añón O, Pérez-de-Llano LA, González-Juanatey C, Muñiz-Fernández C, Testa-Fernández A, et al. Prognostic significance of six-minute walk test in non-group 1 pulmonary hypertension. Heart Lung. 2014;43:72–6. doi: 10.1016/j.hrtlng.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140:111–20. doi: 10.1067/mhj.2000.107177. [DOI] [PubMed] [Google Scholar]
- 15.Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis. 2002;6:947–67. doi: 10.1016/s1089-3261(02)00056-9. viii-ix. [DOI] [PubMed] [Google Scholar]
- 16.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: Clinical presentation and pathogenesis. Am J Med. 2000;109:109–13. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 17.Poelzl G, Ess M, Von der Heidt A, Rudnicki M, Frick M, Ulmer H. Concomitant renal and hepatic dysfunctions in chronic heart failure: Clinical implications and prognostic significance. Eur J Intern Med. 2013;24:177–82. doi: 10.1016/j.ejim.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Batin P, Wickens M, McEntegart D, Fullwood L, Cowley AJ. The importance of abnormalities of liver function tests in predicting mortality in chronic heart failure. Eur Heart J. 1995;16:1613–8. doi: 10.1093/oxfordjournals.eurheartj.a060785. [DOI] [PubMed] [Google Scholar]
- 19.Shinagawa H, Inomata T, Koitabashi T, Nakano H, Takeuchi I, Naruke T, et al. Prognostic significance of increased serum bilirubin levels coincident with cardiac decompensation in chronic heart failure. Circ J. 2008;72:364–9. doi: 10.1253/circj.72.364. [DOI] [PubMed] [Google Scholar]
- 20.Takeda Y, Takeda Y, Tomimoto S, Tani T, Narita H, Kimura G. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med. 2010;10:22. doi: 10.1186/1471-2466-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, et al. Cardiohepatic interactions in heart failure: An overview and clinical implications. J Am Coll Cardiol. 2013;61:2397–405. doi: 10.1016/j.jacc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 22.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol. 2002;90:1405–9. doi: 10.1016/s0002-9149(02)02886-2. [DOI] [PubMed] [Google Scholar]
- 23.Poelzl G, Ess M, Mussner-Seeber C, Pachinger O, Frick M, Ulmer H. Liver dysfunction in chronic heart failure: Prevalence, characteristics and prognostic significance. Eur J Clin Invest. 2012;42:153–63. doi: 10.1111/j.1365-2362.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 24.Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, et al. Liver function abnormalities and outcome in patients with chronic heart failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009;11:170–7. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers RP, Cerini R, Sayegh R, Moreau R, Degott C, Lebrec D, et al. Cardiac hepatopathy: Clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. 2003;37:393–400. doi: 10.1053/jhep.2003.50062. [DOI] [PubMed] [Google Scholar]
- 26.Roche SP, Kobos R. Jaundice in the adult patient. Am Fam Physician. 2004;69:299–304. [PubMed] [Google Scholar]
- 27.Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–92. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 28.Reesink HJ, van der Plas MN, Verhey NE, van Steenwijk RP, Kloek JJ, Bresser P. Six-minute walk distance as parameter of functional outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;133:510–6. doi: 10.1016/j.jtcvs.2006.10.020. [DOI] [PubMed] [Google Scholar]


