INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death in the developed world and associated with a high individual and socioeconomic burden.[1] It is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases. Peri-bronchiolar fibrosis was occurred in small airways in the early state of COPD, and then followed by structure changes, and finally became persistent airflow limitation.[2] Recent researches have shown that epithelial-mesenchymal transition (EMT) is one of the leading causes of fibrosis in various diseases.
EMT is a process when epithelial cells gradually transform into mesenchymal-like cells losing their epithelial functionality and characteristics. EMT is thought to be involved in the pathogenesis of several chronic lung conditions such as asthma, COPD, bronchiolitis obliterans syndrome, and lung fibrosis.[3,4] It has been confirmed that COPD is accompanied by inflammation and tissue remodeling which is characterized by emphysema, and small airway remodeling with per-bronchiolar fibrosis.
Hydrogen sulfide (H2S), the third gas transmitter, together with nitric oxide (NO) and carbon monoxide (CO), is involved in many pathophysiological processes.[5] Our previous studies have demonstrated that endogenous H2S is involved in the pathogenesis of airway obstruction in COPD, and its alteration in level may be associated with disease activity and severity.[6] However, whether H2S could attenuate the fibrosis of small airway is still unknown. Therefore, this study aimed to investigate the effect of H2S on inhibiting small airway fibrosis in bronchial epithelium.
METHODS
Materials
GYY4137 (Cayman, USA) is a H2S slowly released donor. Its formal name is (p-methoxyphenyl) morpholinophosphinodithioic acid and molecular formula is C11H16NO2·PS2C4H10NO. Transforming growth factor beta-1 (TGF-β1) was from R&D (USA). Antibodies against E-cadherin, alpha-smooth muscle actin (α-SMA) were from Abcam (USA). Antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was from Zhongshan Biotechnology (Beijing, China). IRDye 800CW-conjugated goat anti-rabbit IgG or goat anti-mouse IgG were from LI-COR Biosciences (USA). RPMI 1640 (Gibico, USA), fetal bovine serum (FBS) (Hyclone, USA), antibiotics (North China pharmaceutical Group Corporation, China) cell-lysis buffer (Applygen Technologies Inc., China), bicinchoninic acid assay (BCA) protein assay reagent (Pierce, USA) were applied in the research.
Cell culture and treatment
16-HBE, a cell line of human bronchial epithelia, was purchased from Shanghai Bogoo Biotechnology. Co., Ltd. (Shanghai, China). Cells were maintained in RPMI 1640 containing 10% FBS and antibiotics at 37°C in a humidified 5% CO2 atmosphere. After each passage, the cells grew to confluence within 1–2 days. Cells were maintained in FBS-free RPMI 1640 for 24 h before stimulation with TGF-β1. After overnight culture, cells were treated with TGF-β1 in serum-free medium as indicated. In all experiments, cells at 80–90% confluence were treated with GYY4137.
Western blotting analysis
Cells were lysated with cell-lysis buffer. The protein content was assayed by BCA protein assay reagent (Pierce Biotechnology, IL, USA). Total 30 µg protein was loaded to 10% (wt./vol.) sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to membrane. The expression of E-cadherin, α-SMA and GAPDH were detected. The primary antibodies E-cadherin (1:2000) and α-SMA (1:500) and GAPDH (1:1000) were used, followed by a 1:10,000 dilution of IRDye 800CW-conjugated goat anti-rabbit IgG or goat anti-mouse IgG for 1 h. Protein bands were visualized with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA) as previously reported.
Wound healing assay
Cells (1 × 105) were seeded onto 60-cm2 culture plates. When the cells reached 90–100% confluence, wounds were mechanically generated by scraping with a sterile pipette tip. Photomicrographs were taken at 72 h after wound generation.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). Comparisons were analyzed using one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test. A P < 0.05 was considered statistically significant. SPSS 20.0 was applied for statistical analysis (SPSS, Inc., Chicago, IL, USA).
RESULTS
Exogenous H2S inhibits 16-HBE cells morphologic changes and motility induced by transforming growth factor beta-1
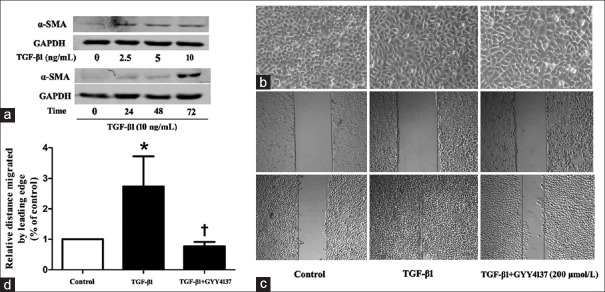
We first examined the effects of TGF-β1 on mesenchymal marker α-SMA in 16-HBE cells. Cells were pretreated with TGF-β1 (2.5, 5.0, 10.0 ng/ml) for 72 h or incubated with TGF-β1 (10 ng/ml) for various time (24, 48, 72 h). TGF-β1 resulted in dose-related and time-related increased in α-SMA expression [Figure 1a]. TGF-β1 (10 ng/ml) treatment for 72 h were used in the following experiments. We then assessed whether exogenous H2S could inhibit the morphologic changes and motility induced by TGF-β1. Untreated 16-HBE cells showed a cobblestone epithelial morphology and were tightly attached to each other. TGF-β1 treated cells showed an elongated shape, and many cells lost contact with each other and displayed spindle-shape, fibroblast-like morphologic features. While with GYY4137 treatment, cells maintained a classic cobblestone epithelial morphology [Figure 1b]. Wound healing experiments showed that TGF-β1 obviously enhanced motility of 16-HBE cells, whereas GYY4137 treatment eliminated the effects of TGF-β1 [Figure 1c and 1d].
Figure 1.
(a) Western blotting analysis of α-SMA in 16-HBE cells treated with different concentration TGF-β1 for 72 h or incubated with different time. (b) Morphologic changes observed under a light microscope in 16-HBE cells with control, TGF-β1 (10 ng/ml) or TGF-β1 (10 ng/ml) and GYY4137 (200 μmol/L) treatment for 72 h (original magnification ×100). (c) Wound healing assay observed on light microscopy in 16-HBE cells with control, TGF-β1 (10 ng/ml) or TGF-β1 (10 ng/ml) and GYY4137 (200 μmol/L) treatment for 72 h (original magnification ×50). The area between lines indicated the wound area. (d) Quantitative assay of wound healing experiment. Data are shown as mean ± standard deviation. n = 3. TGF-β1 (10 ng/ml) or TGF-β1 (10 ng/ml) and GYY4137 (200 μmol/L) treatment were for 72 h. *P < 0.05 (vs. control), †P < 0.05 (treatment vs. TGF-β1). α-SMA: Alpha-smooth muscle actin; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; TGF-β1: Transforming growth factor beta-1.
Exogenous H2S inhibits 16-HBE cells epithelial-mesenchymal transition induced by transforming growth factor beta-1
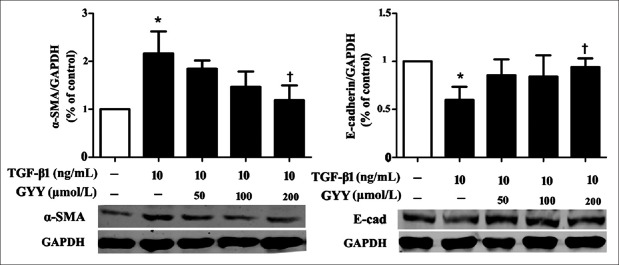
As GYY4137 treatment inhibits16-HBE cells morphologic changes and motility induced by TGF-β1. We then examined whether GYY4137 could affect the expression of EMT markers in 16-HBE cells. Cells were pretreated with GYY4137 (50, 100, 200 μmol/L) for 1 h and then incubated with TGF-β1 (10 ng/ml) for 72 h. TGF-β1 treatment significantly increased the expression of mesenchymal marker α-SMA and decreased the expression of epithelial marker E-cadherin. Compared with TGF-β1 group, GYY4137 could reverse these markers by reducing the expression of α-SMA by 45.1% (P < 0.05) and increasing the expression of E-cadherin by 57.6% (P < 0.05) [Figure 2].
Figure 2.
Dose-dependent changes in E-cadherin and α-SMA in 16-HBE cells treated with TGF-β1 (10 ng/ml) and GYY4137 (50, 100, 200 μmol/L). Cells were pretreated with GYY4137 for 1 h following treated with TGF-β1 for 72 h. Relative protein expression levels were normalized to GAPDH. Data are shown as mean ± standard deviation. n = 3.*P < 0.05 (vs. control), †P < 0.05 (treatment vs. TGF-β1). α-SMA: Alpha-smooth muscle actin; E-cad: E-cadherin; GYY: GYY4137; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; TGF-β1: Transforming growth factor beta-1.
DISCUSSION
COPD is defined as an irreversible expiratory airflow limitation, which is caused by various degrees of the following two main features: First, small airway disease, which includes airway inflammation and remodeling; and second, emphysema, which is characterized by airspace enlargement.[7,8] Arguably, airway remodeling is one of the most intractable and the most pressing problems in the disease, which leads to irreversible loss of lung function.[9] Current therapeutics could ameliorate inflammation, but there is no available therapy proven to prevent or reverse airway remodeling.
The airway epithelium is the primary target for the inhaled environmental factors and pathogens. An important function of epithelial cells is to respond to micro-environmental cues by undergoing epithelial to mesenchymal transition. It has been accepted that TGF-β signaling has played critical functional roles in lung development, injury, and repair.[10,11,12] Several studies have reported an increased expression of TGF-β1 in the airway epithelium of smokers, as well as in patients with chronic bronchitis or COPD.[13] Our results found that TGF-β1 stimulated 16-HBE transformed into mesenchymal-like cells. In addition, markers of epithelial cells such as E-cadherin are lost and markers of mesenchymal cells such as α-SMA are present. These results proved that EMT occurred in epithelial cells with TGF-β1 treatment.
H2S was believed to be a toxic environmental pollutant with no physiological significance. However, in the past few years, it has been identified as a physiologically or pathophysiologically relevant endogenous gaseous transmitter, third in line to NO and CO.[6,14] In a chronic cigarette smoke-induced COPD rat model, we found that endogenous H2S has played a protective role in anti-inflammation and bronchodilation.[6] In this study, we have further demonstrated that exogenous H2S attenuates the EMT in bronchial epithelial cells. Although H2S performs many critical functions in the lung, the role of H2S in the airway epithelium has not been well characterized. Several studies have proved that both endogenous and exogenous H2S attenuate the EMT in alveolar epithelial cells.[15] The mechanism by which H2S affects airway fate and protects against small airway fibrosis is not clear now. In rats with streptozotocin-induced diabetic kidney injury, sodium hydrosulfide inhibited albuminuria, TGF-β expression and matrix accumulation; moreover, H2S donor blocked the differentiation of quiescent renal fibroblasts to myofibroblasts by inhibiting the TGF-β1-Smad and mitogen-activated protein kinase signaling pathways.[16,17] In addition, NaHS treatment following TGF-β1 administration also resulted in decreasing human breast cancer cell invasion and decreasing EMT, which was indicated by decreasing Snail protein expression.[18] Preincubation with H2S decreased Smad2/3 phosphorylation in A549 cells stimulated by TGF-β1, and H2S-inhibited alveolar EMT was mimicked by treatment with SB505124, a Smad2/3 inhibitor, but not pinacidil, a KATP opener.[15] Until now, we still need more exploration into the role of H2S in COPD.
Our research found that H2S inhibits TGF-β1-induced cell morphological changes and EMT in 16-HBE cells. However, the underlying mechanism needs further study. A greater understanding of the H2S pathway and airway EMT will help us better harness the potent actions of H2S-antagonized airway fibrosis as a therapeutic target of COPD.
Financial support and sponsorship
This work was supported by research grants from the National Natural Sciences Foundation of China (No. 81170012 and No. 81370141).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Mannino DM, Higuchi K, Yu TC, Zhou H, Li Y, Tian H, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148:138–50. doi: 10.1378/chest.14-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–75. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: Does it exist and is it important? Thorax. 2014;69:760–5. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson DB, Saini G, Bolton CE, Steiner MC. Thorax in focus: Chronic obstructive pulmonary disease. Thorax. 2012;67:171–6. doi: 10.1136/thoraxjnl-2011-201231. [DOI] [PubMed] [Google Scholar]
- 5.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–8. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, et al. Endogenous hydrogen sulfide in patients with COPD. Chest. 2005;128:3205–11. doi: 10.1378/chest.128.5.3205. [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 8.Sturton G, Persson C, Barnes PJ. Small airways: An important but neglected target in the treatment of obstructive airway diseases. Trends Pharmacol Sci. 2008;29:340–5. doi: 10.1016/j.tips.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144:1026–32. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 10.Warburton D, Shi W, Xu B. TGF-ß-Smad3 signaling in emphysema and pulmonary fibrosis: An epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol. 2013;304:L83–5. doi: 10.1152/ajplung.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–35. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 12.Kamitani S, Yamauchi Y, Kawasaki S, Takami K, Takizawa H, Nagase T, et al. Simultaneous stimulation with TGF-ß1 and TNF-a induces epithelial mesenchymal transition in bronchial epithelial cells. Int Arch Allergy Immunol. 2011;155:119–28. doi: 10.1159/000318854. [DOI] [PubMed] [Google Scholar]
- 13.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med. 2001;163:1476–83. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Wang R. The message in the air: Hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol. 2012;184:130–8. doi: 10.1016/j.resp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Fang LP, Lin Q, Tang CS, Liu XM. Hydrogen sulfide attenuates epithelial-mesenchymal transition of human alveolar epithelial cells. Pharmacol Res. 2010;61:298–305. doi: 10.1016/j.phrs.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, et al. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant. 2011;26:2119–26. doi: 10.1093/ndt/gfq749. [DOI] [PubMed] [Google Scholar]
- 17.Kasinath BS. Hydrogen sulfide to the rescue in obstructive kidney injury. Kidney Int. 2014;85:1255–8. doi: 10.1038/ki.2013.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv M, Li Y, Ji MH, Zhuang M, Tang JH. Inhibition of invasion and epithelial-mesenchymal transition of human breast cancer cells by hydrogen sulfide through decreased phospho-p38 expression. Mol Med Rep. 2014;10:341–6. doi: 10.3892/mmr.2014.2161. [DOI] [PubMed] [Google Scholar]