Abstract
Background:
Tumor necrosis factor-α (TNF-α) plays an important role in progressive contractile dysfunction in several cardiac diseases. The cytotoxic effects of TNF-α are suggested to be partly mediated by reactive oxygen species (ROS)- and mitochondria-dependent apoptosis. Glucagon-like peptide-1 (GLP-1) or its analogue exhibits protective effects on the cardiovascular system. The objective of the study was to assess the effects of exenatide, a GLP-1 analogue, on oxidative stress, and apoptosis in TNF-α-treated cardiomyocytes in vitro.
Methods:
Isolated neonatal rat cardiomyocytes were divided into three groups: Control group, with cells cultured in normal conditions without intervention; TNF-α group, with cells incubated with TNF-α (40 ng/ml) for 6, 12, or 24 h without pretreatment with exenatide; and exenatide group, with cells pretreated with exenatide (100 nmol/L) 30 mins before TNF-α (40 ng/ml) stimulation. We evaluated apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay and flow cytometry, measured ROS production and mitochondrial membrane potential (MMP) by specific the fluorescent probes, and assessed the levels of proteins by Western blotting for all the groups.
Results:
Exenatide pretreatment significantly reduced cardiomyocyte apoptosis as measured by flow cytometry and TUNEL assay at 12 h and 24 h. Also, exenatide inhibited excessive ROS production and maintained MMP. Furthermore, declined cytochrome-c release and cleaved caspase-3 expression and increased bcl-2 expression with concomitantly decreased Bax activation were observed in exenatide-pretreated cultures.
Conclusion:
These results suggested that exenatide exerts a protective effect on cardiomyocytes, preventing TNF-α-induced apoptosis; the anti-apoptotic effects may be associated with protection of mitochondrial function.
Keywords: Cell Apoptosis, Glucagon-like Peptide-1, Mitochondrial Function, Oxidative Stress, Tumor Necrosis Factor-α
INTRODUCTION
Tumor necrosis factor-α (TNF-α) contributes to the development of several cardiac diseases, including myocardial infarction (MI), coronary micro-embolization, dilated cardiomyopathy, and heart failure, via induction of cardiomyocyte apoptosis. A variety of cellular mechanisms account for TNF-α-induced apoptosis. Interestingly, TNF-α effects have been suggested to be partly mediated by oxidative stress and cardiac mitochondrial dysfunction.[1]
Exenatide, a glucagon-like peptide-1 (GLP-1) analogue, is a novel drug used for the treatment of type-2 diabetes. GLP-1 receptor (GLP-1R) is expressed in a wide range of tissues, including the heart,[2] and GLP-1 has a direct cardio-protective effect beyond glycemic regulation. It was recently reported that GLP-1 or its analogues protect against ischemia-reperfusion injury in vivo and in vitro.[3,4] Also, GLP-1R activation was shown to mitigate cardiac injury and dysfunction following MI.[5,6,7]
The present study aimed to assess whether exenatide prevents TNF-α-induced excessive oxidative stress and apoptosis in cardiomyocytes in vitro. We found that exenatide potently inhibits TNF-α-induced apoptosis by protecting mitochondrial function.
METHODS
Cell culture
Primary cultures of cardiac myocytes were prepared from the ventricles of 1- to 3-day-old Sprague-Dawley rats provided by Department of Laboratory Animal Science, Fudan University, Shanghai, China, basically as previously described.[8] Trypsinization was performed with 0.1% trypsin (Gibco, Life Technology Corporation, Grand Island, NY, USA). Cultures were enriched for myocardial cells by preplating onto 35-mm plastic culture dishes twice for 120 min to deplete the population of nonmyocardial cells. After separation, nonattached cells were resuspended in Dulbecco's modified Eagle's medium (DMEM, low glucose, Gibco, Life Technology Corporation) supplemented with 10% fetal bovine serum (FBS, Gibco, Life Technology Corporation), streptomycin (10 µg/ml), and penicillin (10 U/ml) (HyClone Laboratories, Inc., Logan, UT, USA). Then, they were counted, plated onto 6-well plates at a density of 1 × 106 cells/ml, and cultured in a humidified environment at 37°C containing 5% CO2. Isolated neonatal cardiomyocytes were divided into three groups: (1) Control group cells were cultured in normal conditions without any treatment; (2) in the TNF-α group, cells were incubated with TNF-α (40 ng/ml, PeproTech Inc., Rocky Hill, NJ, USA) for 6, 12 or 24 h but without exenatide pretreatment; and (3) exenatide group cells were pretreated with exenatide (100 nmol/L, Alpha Diagnostic International Inc., San Antonio, TX, USA) 30 mins before TNF-α (40 ng/ml) treatment for 6, 12, or 24 h. The protocols for animal experiments were approved by the Animal Care and Use Committee of Fudan University, China.
Cell apoptosis
Flow cytometry
The Annexin V Apoptosis Detection Kit FITC (eBioscience Inc., San Diego, CA, USA) was used to assess cardiomyocyte apoptosis by flow cytometry. Cardiomyocytes were gently harvested by trypsinization, washed in phosphate-buffered saline (PBS), and dually stained with Annexin V and propidium iodide (PI) according to the manufacturer's instructions. Analysis was carried out on a FACS can flow cytometer (Becton Dickson, Franklin Lakes, NJ, USA). The percentages of Annexin V-positive and PI-negative cells were measured. Dot plot analysis was performed with Cell Quest Software (Becton Dickinson).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
Cardiomyocyte apoptosis was also assessed by transferase-mediated dUTP nick end labeling (TUNEL) assay with an in situ cell death detection kit (Roche Diagnostics Ltd., Shanghai, China). Briefly, cardiomyocytes were washed after treatment, fixed with 4% formaldehyde in PBS, and permeabilized with 0.1% Triton X-100. Then, the TUNEL assay was performed according to the manufacturer's instructions. DAPI staining (Beyotime Institute of Biotechnology, China) was used as nuclear counterstain for the fluorescent quantification of DNA content. Fluorescence was visualized by fluorescent microscopy. A total of 9 high power fields (×200 magnification) in every group were randomly selected. In each field, cells with clear TUNEL nuclear staining (green fluorescence) represented TUNEL-positive cells; those with clear DAPI nuclear staining (blue fluorescence) were counted as total cells. Cardiomyocyte apoptosis was expressed as apoptotic index (AI) calculated as follows: AI = TUNEL-positive cells/total cells. The assays were performed in a blinded manner.
Western blotting analyses
Western blotting analyses were performed according to standard protocols. In brief, proteins resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were blotted onto polyvinylidene difluoride membranes (Millipore, Belford, MA, USA). Then, membranes were blocked with 5% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20, and probed with primary antibodies raised against cytochrome-c, cleaved caspase-3, Bcl-2, Bax (Cell Signaling Technology Inc., Beverly, MA, USA) and Gapdh (Sigma Aldrich, St. Louis, MO, USA). Blots were developed using horseradish peroxidase conjugated secondary antibodies (Abbkine Inc., Redlands, CA, USA) and the SuperSignal West Pico enhanced chemiluminescence detection system (Thermo Scientific, Pierce Biotechnology, Rockford, IL, USA). Immunoblots were quantitated using the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Measurement of mitochondrial membrane potential
To examine the change of mitochondrial membrane potential (MMP), rhodamine-123 (Sigma Aldrich) was used. Cardiomyocytes were washed with prewarmed PBS (37°C) and incubated with rhodamine-123 at 4 μmol/L for 20 mins at 37°C. Afterward, fluorescence imaging was carried out on a fluorescent microscope, and averagely 9 high power fields (×600 magnification) per group were analyzed for fluorescence intensity using the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Oxidative stress assessment
Reactive oxygen species (ROS) levels in cardiomyocytes as an indicator of oxidative stress were assessed by in situ production of superoxide anions with dihydroethidium (Sigma Aldrich). Cardiomyocytes were washed with preheated PBS (37°C) and incubated with 5 μmol/L of the fluorescent dye dihydroethidium dissolved in DMEM without FBS for 30 mins at 37°C. Fluorescent images were acquired by microscopy, and averagely 9 high power fields (×600 magnification) per group were analyzed for fluorescence intensity using the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analyses
Statistical analyses were carried out using SPSS (version 16.0, SPSS Inc., Chicago, IL, USA) and Stata software (version 10.0, Stata Corp., College Station, TX, USA). All experiments were performed in triplicate and repeated 3 times. Gaussian distribution data were presented as mean ± standard deviation. Categorical variables were expressed as frequencies and percentages. Groups were compared by one-way analysis of variance (ANOVA), and Bonferroni's test was performed to identify differences between groups. A P < 0.05 was considered statistically significant.
RESULTS
Exenatide reduces tumor necrosis factor-α-induced cardiomyocyte apoptosis
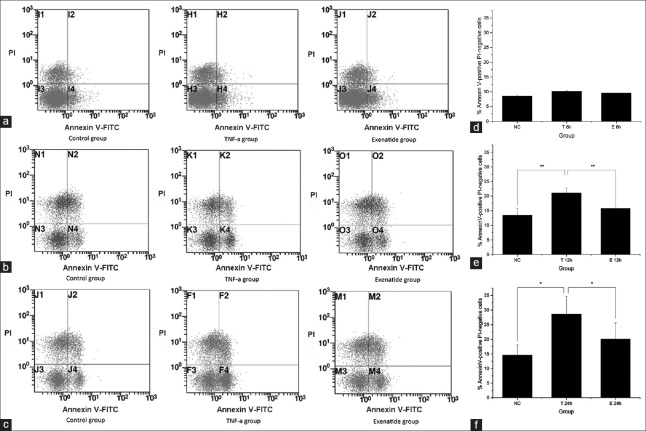
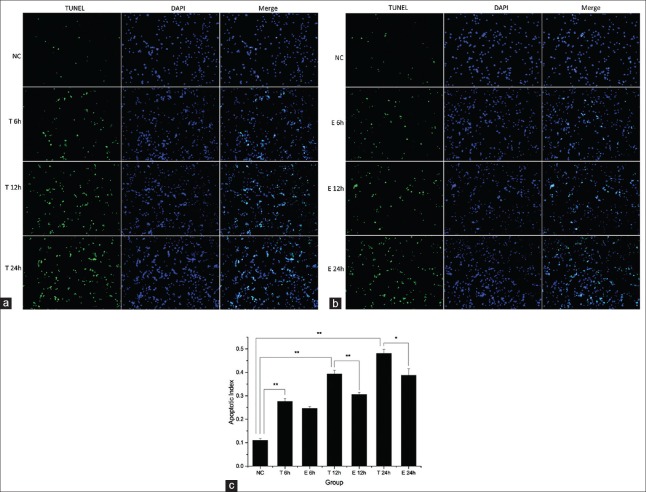
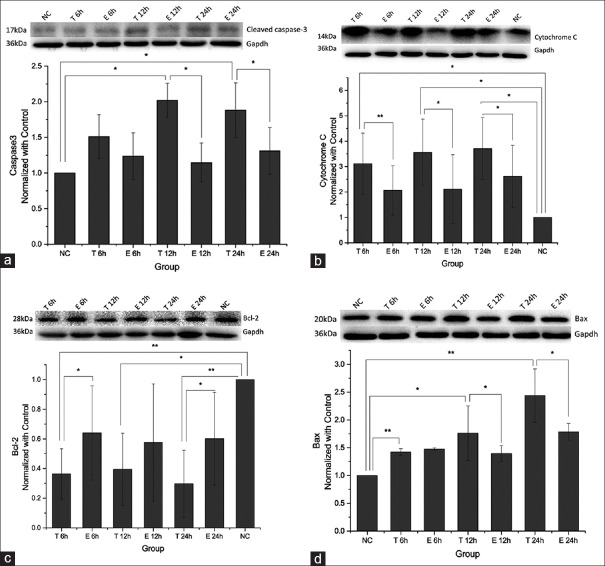
Cardiomyocyte apoptosis was measured by flow cytometry using Annexin V-FITC/PI staining and TUNEL assay. The TNF-α group showed significantly increased apoptosis rates at 12 h and 24 h; meanwhile, a marked reduction of TNF-α induced apoptosis was found in the exenatide group. However, there was no significant difference found among groups at 6 h, with 8.6 ± 0.5%, 10.2 ± 0.1%, and 9.6 ± 0.2% apoptotic cells in the Control, TNF-α, and Exenatide groups, respectively. At 12 h, 13.5 ± 2.3%, 21.1 ± 1.7%, and 15.8 ± 0.5% cells were apoptotic in the control, TNF-α, and exenatide groups, respectively (control vs. TNF-α, adjusted-P = 0.0045; TNF-α vs. exenatide, adjusted-P = 0.0138, control vs. exenatide, adjusted-P = 0.3150). A similar trend was obtained at 24 h, with 14.6 ± 5.5%, 28.6 ± 6.0%, and 20.1 ± 5.6% apoptotic cells in the control, TNF-α, and exenatide groups, respectively (control vs. TNF-α, adjusted-P = 0.0200, TNF-α vs. exenatide, adjusted-P = 0.0290, control vs. exenatide, adjusted-P = 0.2100) [Figure 1]). TUNEL assay further demonstrated that TNF-α treatment caused cardiomyocyte apoptosis that increased with time. AI values for control, 6 h, 12 h, and 24 h were 10.9 ± 1.0%, 27.6 ± 1.3%, 39.3 ± 1.5%, and 48.0 ± 1.7%, respectively. Moreover, the protective effect of exenatide was also observed in TUNEL assay. At 6 h, 27.6 ± 1.3% and 24.6 ± 0.8% apoptotic cells were obtained in the TNF-α and Exenatide groups, respectively (adjusted-P = 0.1236); AI values of 39.3 ± 1.5% and 30.6 ± 0.9% (adjusted-P = 0.0074) at 12 h, and 48.0 ± 1.7% and 38.8 ± 2.8% (adjusted-P = 0.0218) at 24 h were obtained for the TNF-α and exenatide groups, respectively [Figure 2]. To further confirm the TNF-α induced apoptosis and exenatide effect, cleaved caspase-3, which is essential for the execution of apoptosis, was evaluated by Western blotting. TNF-α stimulation induced the generation of caspase-3, which was attenuated by exenatide treatment [Figure 3a].
Figure 1.
Assessment of apoptosis by flow cytometry. (a-c) Representative dot plots of flow cytometric images at 6 h, 12 h and 24 h respectively; (d-f) The bar graphs show the percentages of Annexin V-positive and propidium iodide-negative cardiomyocytes of control, tumor necrosis factor-α and exenatide group at 6 h, 12 h and 24 h respectively. *Adjusted-P < 0.05; **adjusted-P < 0.01; NC: Control group, T: Tumor necrosis factor-α group, and E: Exenatide group.
Figure 2.
Assessment of apoptosis by transferase-mediated dUTP nick end labeling assay. (a and b) Representative fluorescence images (original magnification ×200) of cardiomyocytes of tumor necrosis factor-α group and exenatide group respectively at different time points after transferase-mediated dUTP nick end labeling assay; (c) The bar graph compares the apoptotic indexes of each group at 6 h, 12 h and 24 h. *Adjusted-P < 0.05; **adjusted-P < 0.01; NC: Control group, T: Tumor necrosis factor-α group, and E: Exenatide group.
Figure 3.
The differences of cleaved caspase-3, cytochrome-c, bcl-2 and Bax protein expressions among groups. (a-d) Representative western blot of cleaved caspase-3, cytochrome C, bcl-2 and Bax protein expressions in different groups at different time points and quantitative analyses are showed in the bar graphs. The band density values of the proteins were calculated as a ratio to Gapdh and normalized with the control group. *Adjusted-P < 0.05; **adjusted-P < 0.01; NC: Control group, T: Tumor necrosis factor-α group, and E: Exenatide group.
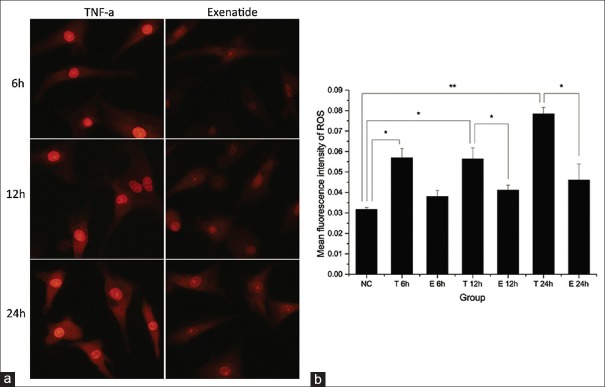
Exenatide alleviates tumor necrosis factor-α-induced reactive oxygen species production
ROS generation in cardiomyocytes was measured by the fluorescent dye dihydroethidium. Markedly increased ROS production was observed in the TNF-α groups at all the time points. Meanwhile, ROS levels in the exenatide groups were significantly lower compared with values obtained for TNF-α groups at 12 h and 24 h, and similar to control amounts. There was no significant reduction of ROS levels observed in the exenatide group at 6 h [Figure 4].
Figure 4.
Measurement of reactive oxygen species production. (a) Representative fluorescence images (original magnification ×600) of reactive oxygen species production of different groups at different time points detected by dihydroethidium; (b) the bar graph shows the mean fluorescence intensity of reactive oxygen species of each group. *Adjusted-P < 0.05; **adjusted-P < 0.01; NC: Control group, T: Tumor necrosis factor-α group, and E: Exenatide group.
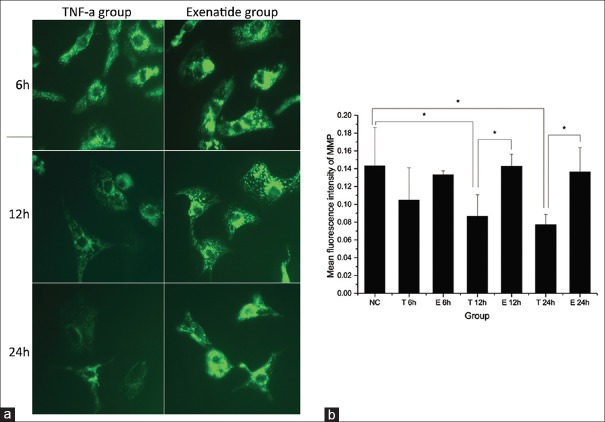
Exenatide prevents tumor necrosis factor-α-induced mitochondrial membrane potential decrease
The mean intensity of the fluorescent probe rhodamine-123 significantly decreased in the TNF-α group at 12 h and 24 h, which not at 6 h; however, cells of the exenatide group maintained a fluorescent intensity comparable to that of the control group, suggesting that exenatide prevented TNF-α-induced loss of MMP [Figure 5].
Figure 5.
Measurement of mitochondrial membrane potential. (a) Representative fluorescence images (original magnification ×600) of mitochondrial membrane potential of different groups at different time points detected by rhodamine-123; (b) the bar graph shows the mean fluorescence intensity of mitochondrial membrane potential of each group. *Adjusted-P < 0.05; NC: Control group, T: Tumor necrosis factor-α group, and E: Exenatide group.
Bcl-2 and Bax protein levels
Protein levels of bcl-2, Bax, and cytochrome-c were examined to determine the pathways involved in the exenatide-mediated anti-apoptotic effects. TNF-α stimulation caused increased Bax expression and reduced expression of bcl-2 protein, and significant differences were observed between the TNF-α and control groups [Figure 3c and d]. Cytochrome-c release to the cytoplasm, which triggers the apoptosis process, was also markedly increased in the TNF-α group. Interestingly, exenatide overtly inhibited this pro-apoptotic effect of TNF-α [Figure 3b]. Also, pretreatment with exenatide prevented the cardiomyocytes from expressing elevated Bax and reduced bcl-2 protein levels after TNF-α treatment [Figure 3c and d].
DISCUSSION
TNF-α, as one of the most contributing inflammatory cytokines, is involved in the development of heart failure, myocardial ischemia/reperfusion injury, and coronary micro-embolization.[9,10,11] The elevated TNF-α concentrations in the myocardia following ischemia, during coronary micro-embolization or other conditions, correlate with the development of contractile dysfunction; the detrimental effects may be associated with cardiac apoptosis, increased oxidative stress, and mitochondrial dysfunction.[1,11,12,13] Mariappan et al. reported that in left ventricular tissues of adult Sprague-Dawley rats, TNF-α-induces oxidative stress that alters the redox homeostasis in mitochondria, thereby causing dysfunction of mitochondria and attenuating cardiac function.[14] In the present study, the effects of TNF-α on cardiomyocytes were reconfirmed in vitro: TNF-α caused elevated cells apoptosis, increased ROS production, and decreased MMP, indicating an alteration of mitochondrial function.
TNF-α antagonists have been initially used as effective treatment of patients with chronic systemic inflammatory diseases such as Crohn's disease and rheumatoid arthritis. Furthermore, TNF-α inhibitors application in patients with severe rheumatoid arthritis reduced the risk of cardiovascular disease, such as heart failure.[15,16] Attempts have been made to introduce anti-TNF-α therapy into cardiac diseases, congestive heart failure (CHF) for instance, without chronic systemic inflammatory diseases. Unexpectedly, randomized controlled trials concluded that TNF-α antagonism does not demonstrate any clinical benefits but adversely affects the clinical status of patients with moderate to severe heart failure, worsening the prognosis of heart failure. Moreover, the occurrence of dermatological, intestinal, and ophthalmological paradoxical adverse events after use of TNF-α antagonists cannot be ignored.[15,17] The clinical implications of using anti-TNF-α therapy in patients with CHF are still not clear. Therefore, it is necessary to find other ways to counter the deleterious effects of TNF-α in cardiovascular diseases.
Native GLP-1 or GLP-1 analogues seem to engage mitochondrial signaling pathways and exhibit distinct actions in the cardiovascular system. Exendin-4, a GLP-1R agonist, reduces cardiomyocyte apoptosis after either 16 h of hypoxia/4 h of reoxygenation or hydrogen peroxide stress, and these anti-apoptosis effects are prevented by phosphatidylinositol-3 kinase- or ERK-inhibitor.[18] Chang et al. also reported that exenatide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells, which may be attributed to improved mitochondrial function.[4] Studies investigating whether exenatide can inhibit the adverse actions of TNF-α, which plays a fundamental role in MI, myocardial ischemia/reperfusion injury, and coronary micro-embolization, are scarce. Our study aimed to assess the effect of exenatide on TNF-α treated neonatal rat cardiomyocytes and unveil the possible mechanisms. By Annexin V-FITC/PI staining and the TUNEL assay, TNF-α was found to increase cardiomyocyte apoptosis. Concomitantly, TNF-α treatment led to increased ROS production, decreased MMP, abnormal levels of Bax and Bcl-2, cytochrome-c release into cytoplasm, and caspase-3 activation in cardiomyocytes. Surprisingly, pretreatment with exenatide indeed reduced TNF-α-induced cardiomyocyte apoptosis. Protection of mitochondria may be the mechanism underlying the counteraction of exenatide against TNF-α. Here, we observed that exenatide inhibits ROS excessive generation, maintains MMP, keeps Bax and Bcl-2 level balance, and reduces cytochrome-c release and caspase-3 activation.
In various cell lines, mitochondria play a key role in the regulation of cell death via ROS generation. Indeed, mitochondrial membrane permeabilization is sensitive to the redox status, and can be promoted by ROS both in vitro and in vivo.[19] Studies have demonstrated that TNF-α mediates oxidative stress by impairing the membrane permeability transition pore proteins, adenine nucleotide translocator, and voltage-dependent anion channel, thereby inducing the pore opening; this causes uncontrolled transport of substances to alter mitochondrial pH, subsequently leading to mitochondrial dysfunction.[14] It is also known that ROS overproduction stimulates lipid peroxidation in mitochondria, leading to a suppression of mitochondrial metabolism, thereby causing a change of vital mitochondrial functions such as maintenance of MMP.[20] Loss of MMP results in the collapse of mitochondria.
Bcl-2 family proteins, which regulate cytochrome-c release, are also involved in mitochondria-dependent apoptosis. They contain two distinct groups: pro- and anti-apoptotic members. Bcl-2 prevents the permeabilization of the mitochondrial membrane and release of proteins, e.g., cytochrome-c, thereby rescuing the cells from death. On the other hand, activation of Bax is characterized by oligomerization and insertion into the mitochondrial membrane, followed by increased permeabilization and release of pro-apoptotic proteins, and subsequently cell death.[20,21,22] That is, dysregulation of Bax and Bcl-2 levels disrupts mitochondrial membrane permeabilization, causing cytochrome-c release from mitochondria into the cytosol and subsequent activation of caspase cascades, the terminal step in the apoptotic process.[23] Therefore, our results indicate that exenatide attenuates the TNF-α-induced cell apoptosis by protecting the mitochondria.
Interestingly, in vivo, improved left ventricular ejection fraction and preserved left ventricular end-systolic volume were observed in a porcine model of acute MI via intracoronary infusion of alginate-encapsulated GLP-1-eluting mesenchymal stem cells.[24] Moreover, clinical trials also demonstrated the protective role of GLP-1 in cardiovascular diseases, especially ischemic heart diseases. Recently, Lønborg et al. showed improved myocardial salvage in all patients, and reduced infarct size in patients with short systemic delay (≤132 min) after 6-h continuous exenatide infusion in subjects presenting with ST-segment–elevation MI undergoing percutaneous coronary intervention.[7] Woo et al. confirmed that adjunctive exenatide treatment along with reperfusion therapy exhibits protective actions in patients presenting with ST-segment–elevation MI.[25] Infusion of exenatide improves haemodynamic function in patients with type 2 diabetes with CHF[26] and randomized controlled clinical trials are under way to evaluate the effect of exenatide on cardiovascular outcomes in type 2 diabetic patients with CHF. Therefore, the positive effect of exenatide on cardiovascular system cannot be ignored, but the appropriate clinical implication of administering it in heart diseases needs further studies to define.
In conclusion, exenatide exerts a protective effect by preventing TNF-α-induced apoptosis. The anti-apoptotic effects of exenatide may be associated with the protection of mitochondrial function. Therefore, exenatide may constitute a promising alternative for cardiac function improvement in specific heart diseases.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81200146 and 81370322), New Teacher Foundation of Ministry of Education (No. 20120071120061) and Grant of Shanghai Municipal Commission of Health and Family Planning (No. XBR2013071).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813–20. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 2.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89:8641–5. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–10. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Chang G, Zhang D, Liu J, Zhang P, Ye L, Lu K, et al. Exenatide protects against hypoxia/reoxygenation-induced apoptosis by improving mitochondrial function in H9c2 cells. Exp Biol Med (Maywood) 2014;239:414–22. doi: 10.1177/1535370214522177. [DOI] [PubMed] [Google Scholar]
- 5.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 7.Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–9. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 8.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–16. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, et al. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: Progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–6. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 10.Schulz R, Aker S, Belosjorow S, Heusch G. TNFalpha in ischemia/reperfusion injury and heart failure. Basic Res Cardiol. 2004;99:8–11. doi: 10.1007/s00395-003-0431-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZW, Qian JY, Ma JY, Chang SF, Yun H, Jin H, et al. TNF-a-induced cardiomyocyte apoptosis contributes to cardiac dysfunction after coronary microembolization in mini-pigs. J Cell Mol Med. 2014;18:1953–63. doi: 10.1111/jcmm.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, et al. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–9. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shudiefat AA, Sharma AK, Bagchi AK, Dhingra S, Singal PK. Oleic acid mitigates TNF-a-induced oxidative stress in rat cardiomyocytes. Mol Cell Biochem. 2013;372:75–82. doi: 10.1007/s11010-012-1447-z. [DOI] [PubMed] [Google Scholar]
- 14.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: Restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol. 2007;293:H2726–37. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- 15.Sinagra E, Perricone G, Romano C, Cottone M. Heart failure and anti tumor necrosis factor-alpha in systemic chronic inflammatory diseases. Eur J Intern Med. 2013;24:385–92. doi: 10.1016/j.ejim.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Peters MJ, van Sijl AM, Voskuyl AE, Sattar N, Smulders YM, Nurmohamed MT. The effects of tumor necrosis factor inhibitors on cardiovascular risk in rheumatoid arthritis. Curr Pharm Des. 2012;18:1502–11. doi: 10.2174/138161212799504786. [DOI] [PubMed] [Google Scholar]
- 17.Javed Q, Murtaza I. Therapeutic potential of tumour necrosis factor-alpha antagonists in patients with chronic heart failure. Heart Lung Circ. 2013;22:323–7. doi: 10.1016/j.hlc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Ban K, Kim KH, Cho CK, Sauvé M, Diamandis EP, Backx PH, et al. Glucagon-like peptide (GLP)-1 (9-36) amide-mediated cytoprotection is blocked by exendin (9-39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151:1520–31. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- 19.Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, et al. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem. 2011;286:6587–601. doi: 10.1074/jbc.M110.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev. 2007;39:443–55. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 22.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–9. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–44. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 24.de Jong R, van Hout GP, Houtgraaf JH, Kazemi K, Wallrapp C, Lewis A, et al. Intracoronary infusion of encapsulated glucagon-like peptide-1-eluting mesenchymal stem cells preserves left ventricular function in a porcine model of acute myocardial infarction. Circ Cardiovasc Interv. 2014;7:673–83. doi: 10.1161/CIRCINTERVENTIONS.114.001580. [DOI] [PubMed] [Google Scholar]
- 25.Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, et al. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: Results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–60. doi: 10.1161/ATVBAHA.113.301586. [DOI] [PubMed] [Google Scholar]
- 26.Nathanson D, Ullman B, Löfström U, Hedman A, Frick M, Sjöholm A, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: A double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia. 2012;55:926–35. doi: 10.1007/s00125-011-2440-x. [DOI] [PubMed] [Google Scholar]