Abstract
Background:
Pelvic organ prolapse (POP) is a major health problem in adult women that involves many factors. No proteomic analysis has been conducted exclusively in POP patients. This study aimed to identify the differential expression of proteins that may be involved in POP by proteomic analysis.
Methods:
Samples of the uterosacral ligament (USL) were collected from five POP patients and five non-POP patients matched according to age, parity, and menopausal status and analyzed using two-dimensional electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Quantitative real-time polymerase chain reaction (qRT-PCR) was used to verify the mRNA expression of proteins that showed differential expression in the proteomic analyses.
Results:
Proteins differentially expressed between POP and non-POP patients were detected. Eight proteins that were down-regulated in the POP group were identified by MALDI-TOF-MS. These proteins included electron transfer flavoprotein, apolipoprotein A-I, actin, transgelin, cofilin-1, cyclophilin A, myosin, and galectin-1, and their expression was verified by qRT-PCR.
Conclusion:
Using comparative proteomics, we identified eight differentially expressed proteins (including four cytoskeleton proteins and three proteins related to apoptosis) in the USL that may be involved in apoptosis associated with the tissue effects in POP pathophysiology.
Keywords: Apoptosis, Cytoskeleton, Pelvic Organ Prolapse, Proteomics, Uterosacral Ligament
INTRODUCTION
Pelvic organ prolapse (POP) is a major health problem in adult women. The mean prevalence of POP is 19.7% (range: 3.4–56.4%).[1] While not life-threatening, it often adversely affects their daily activities and quality of life. As the population is aging, it is estimated that the demand for POP care will double over the next 40 years.[2] However, the pathophysiological mechanisms of POP have not been fully elucidated.
POP is a complex disease that involves many factors including biochemical changes in pelvic connective tissue, morphological changes in anal levator muscle tissue, pelvic nerve pathological changes in the support tissues, estrogen receptor expression, and changes in specific protease-related proteins.[2] However, the development of POP cannot be explained by these factors. The underlying mechanisms of POP are poorly understood, and further exploration of the molecular biology of the disease is necessary. It is becoming increasingly evident that genetic variations affect the predisposition of women to develop pelvic floor dysfunction (PFD).[3] Genome linkage analyses provide a high level of evidence of a genetic contribution to the disease by locating specific candidate genes (LAMC-1, chromosome 9q21) associated with PFD. Several case–control studies have correlated the presence of SNPs in women with POP or stress urinary incontinence (SUI). SNPs are also present in genes coding for collagen type III, matrix metalloproteinase (MMP)-1, and MMP-9.[4] Several candidate genes and polymorphisms involved in the expression of extracellular matrix (ECM)-related proteins have been described as contributing to the pathogenesis of POP.[4] A clearer understanding of the interactions among these different genes will require further research in this field. Proteomic analyses should be conducted for POP to determine whether the genes identified with microarray analyses are translated into functional proteins.
Using proteomic analyses, the protein expression in the organism is used to detect and isolate tens of thousands of different proteins. Therefore, proteomic analyses have been widely applied to study the mechanisms of disease occurrence and drug targets to determine effective treatments. To the best of our knowledge, only one study has been conducted on the proteomic analysis of the pubocervical fascia in women with POP and SUI.[5] No proteomic analysis has been conducted exclusively in POP patients. Therefore, we conducted a study to compare the patterns of protein expression in the uterosacral ligament (USL) of women with POP with those in women with normal pelvic support. These changes may contribute to the pathophysiology of POP.
METHODS
Tissue collection
Women who underwent a hysterectomy at the Department of Gynecology of Peking Union Medical College Hospital (PUMCH) were asked to participate in the study. The study protocol was approved by the Institutional Review Board of PUMCH. Five postmenopausal women with stage 3 and above POP (according to the POP quantification [POPQ] system) who underwent a vaginal hysterectomy or laparoscopically assisted vaginal hysterectomy (LAVH) as part of a reconstructive pelvic surgery constituted the study group. Notably, women with POP also usually suffer from SUI. We decided to focus on women with POP. Patients with SUI or occult SUI were excluded according to the results of a 1-h pad test with or without a pessary.
Five matched women without POP who underwent a hysterectomy due to either cervical intraepithelial neoplasia (n = 3) or nonfunctional ovarian cysts (n = 2) served as controls. The demographic data, menopausal status, gravidity, and parity were collected from all women and matched between cases and controls. Women were excluded from the study if they had any gynecological malignancy or history of hormone treatment, previous pelvic surgery, collagen deficiency syndromes, paralysis of the pelvic floor muscles, or chronic lung disease manifested by a chronic cough. Women with endometriosis, fibroids, or other estrogen-associated diseases were also excluded from our study. The tissue biopsies were obtained from the same area in the USL (1 cm from the cervix) from each woman during the vaginal hysterectomy or LAVH. Biopsy specimens were transported to the laboratory in liquid nitrogen and stored at −80°C until analysis.
Comparative proteomics
Two-dimensional gel electrophoresis (2-DE) and mass spectrometry (MS) were performed to examine differential protein expression in patients and controls. All samples were analyzed in duplicate. The 2-DE was performed as previously described.[6]
Two-dimensional electrophoresis
Total proteins were extracted from fresh-frozen tissues. The patient and control samples were pooled into one reference sample. All samples and reference samples were labeled by different difference gel electrophoresis (DIGE) reagents [Table 1].
Table 1.
The DIGE label method for experimental and control samples
| Items | Cy3 | Cy5 | Cy2 |
|---|---|---|---|
| Gel 1 | A1 | B1 | Pool |
| Gel 2 | B2 | A2 | Pool |
| Gel 3 | A3 | B3 | Pool |
| Gel 4 | B4 | A4 | Pool |
| Gel 5 | A5 | B5 | Pool |
A: Control sample; B: Experimental sample; Pool: The pool of all control and experimental samples; DIGE: Difference gel electrophoresis.
All the labeled samples were multiplexed and resolved on one gel. Labeled proteins were dissolved in isoelectric focusing (IEF) buffer, rehydrated on an immobilized pH gradient strip for 12 h, and then separated on a two-dimensional gel. For IEF, 24 cm nonlinear strips with a pH range of 3–10 were rehydrated at 50 V for 12 h, followed by IEF at approximately 100,000 Vh. After the equilibration steps, the strips were applied to the top of 12% polyacrylamide gels and separated by sodium dodecyl polyacrylamide gel electrophoresis.[5] Gel images were scanned using a Typhoon™ Variable Mode Imager (GE Healthcare, Illinois, United States). DeCyder Differential Analysis Software (Amersham Biosciences, Uppsala, Sweden) was used to detect the protein dots and analyze the protein content for gel images. Biological variation analysis was used to match and compare the differentially expressed protein spots. Normalized protein spots in the Cy5 and Cy3 channels were compared with the internal standard (Cy2) to generate a ratio of relative amounts. Spots with an average differential rate (|AV.Ratio|) ≥1.5 and P < 0.05 were identified as differentially expressed proteins.[6]
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
The selected protein spots were in-gel digested and analyzed by matrix-assisted laser desorption/ionization time-of-flight MS (MALDI-TOF-MS). MALDI-TOF-MS was performed as previously described.[7] The samples were analyzed using a MALDI-TOF-MS (Billerica, MA, USA). The spectra were acquired in the positive delayed extraction-reflector mode. Peptides were detected in the mass range of 800–3500 Da. The mass accuracy was calibrated by matrix peaks and trypsin autolytic products. The mass tolerance was 100 ppm. The peptide mass fingerprinting data were analyzed using MASCOT (matrix science) database searching software. NCBInr was selected, and the following settings were used for peptide matching: Monoisotopic mass, one missed cleavage site, carbamidomethyl as the fixed modification, oxidation as the variable modification, and a maximum allowed mass error of 100 ppm. When the Mascot score was more than 66 points and P < 0.05, the search results were considered successful.[6]
Quantitative real-time polymerase chain reaction
To confirm the proteomic data, eight differentially expressed genes were chosen for quantitative polymerase chain reaction (PCR). Total RNA was extracted using a TRIzol RNA extraction kit (Invitrogen, USA). DNA in the RNA samples was digested by DNase I (Thermo, USA). Primers for electron transfer flavoprotein subunit alpha, mitochondrial (ETFα), actin (ACTG2), transgelin (TAGLN), cofilin-1 (CFL1), cyclophilin A (PPIA), myosin (MYL6), galectin-1 (LGALS1), apolipoprotein A-I (APOA), and control genes (β-actin) were obtained from Shanghai Sangon Company, China. The PCR primers were as follows [Table 2].
Table 2.
Primers used for RT-PCR
| Primers | Sequences (5’-3’) | Size (bp) |
|---|---|---|
| ETFα | F: GAG GAA CTG ACA CCA TTG AT | 20 |
| R: GAAATCGGGGCAACCTCAAG | 20 | |
| ACTG2 | F: GAG GCT CCC CTA AAT CCC AAG | 21 |
| R: GAATCCAGGACGATGCCTG | 19 | |
| TAGLN | F: GTG CAG TCC AAA ATC GAG AAG | 21 |
| R: CTT GCT CAG AAT CAC GCC AT | 20 | |
| CFL1 | F: GAT GCT GCC AGA TAA GGA CTG | 21 |
| R: GCA ATT CAT GCT TGA TCC CTG | 21 | |
| MYL6 | F: AAG ACC AGA CCG CAG AGT TC | 20 |
| R: TCC AGC ACC TTC ACA TTC ATC | 21 | |
| LGALS1 | F: TCG CCA GCA ACC TGA ATC TC | 20 |
| R: CGTCCTTGCTGTTGCACAC | 19 | |
| PPIA | F: CCA CCG TGT TCT TCG ACA TT | 20 |
| R: CACCACCCTGACACATAAAC | 20 | |
| APOA | F: GCT CAA AGA CAG CGG CAG | 18 |
| R: GCCTTCACCTCCTCCAG | 17 | |
| β-actin | F: GAA GTG TGA CGT GGA CAT CCG | 21 |
| R: GCC TAG AAG CAT TTG CGG TG | 20 |
ETFα: Electron transfer flavoprotein subunit alpha, mitochondrial; RT-PCR: Real-time polymerase chain reaction. F: Forward; R: Reverse.
cDNAs were synthesized from 3 μg total RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo). PCR was performed using 1 μl cDNA template in a 20-μl reaction system with TaqMan Universal PCR Master Mix. Real-time PCR was performed using Eva Green PCR Mix (Biotium, USA) according to the manufacturer, a Green PCR. The cycle threshold (Ct) of the target gene was then normalized to the geometric mean of the control genes. Finally, quantitative mRNA expression levels were calculated using the ΔΔCt method.
RESULTS
The demographic characteristics of women with and without POP are described in Table 3. There were no significant differences in age, parity, or menopausal status between the two groups.
Table 3.
Demographic characteristics of women with and without POP
| Characteristics | POP group (n = 5) | Control group (n = 5) | P |
|---|---|---|---|
| Age (years) | 67.3 ± 7.8 | 67.5 ± 8.8 | 1.00 |
| Menopausal (years) | 13.6 ± 10.8 | 15.8 ± 11.3 | 0.76 |
| BMI (kg/m2) | 24.4 ± 2.1 | 23.9 ± 3.0 | 0.76 |
| Vaginal parity | 2.6 ± 1.0 | 2.8 ± 0.8 | 0.83 |
BMI: Body mass index; POP: Pelvic organ prolapse.
High-quality proteins were extracted from the samples that could be well-separated on the two-dimensional gel. The image analysis showed that the average number of protein spots was 1111 ± 54 in DIGE. Representative 2-DE images of the proteins from the USL specimens are presented in Figure 1. Although we identified about 30 proteins by MS analysis, protein modifications were repeatedly identified. Thus, only eight different proteins remained after these repeated proteins were combined. ETF-α, apolipoprotein A-I, actin, transgelin, cofilin-1, cyclophilin A, myosin, and galectin-1, were identified in both groups using MALDI-TOF-MS, and they are listed in Table 4. We identified four proteins, namely, galectin-1, transgelin, cyclophilin A, and cofilin-1, with expression levels that differed by more than two-fold (between −2.04 and −4.06-fold) and exhibited noteworthy differences in expression between the POP patients and controls. The greatest difference was observed for galectin-1, which had an expression level of −4.06-fold in the patient group. Among the eight differentially expressed proteins in the USL, actin, transgelin, cofilin-1, and myosin are cytoskeleton proteins.
Figure 1.
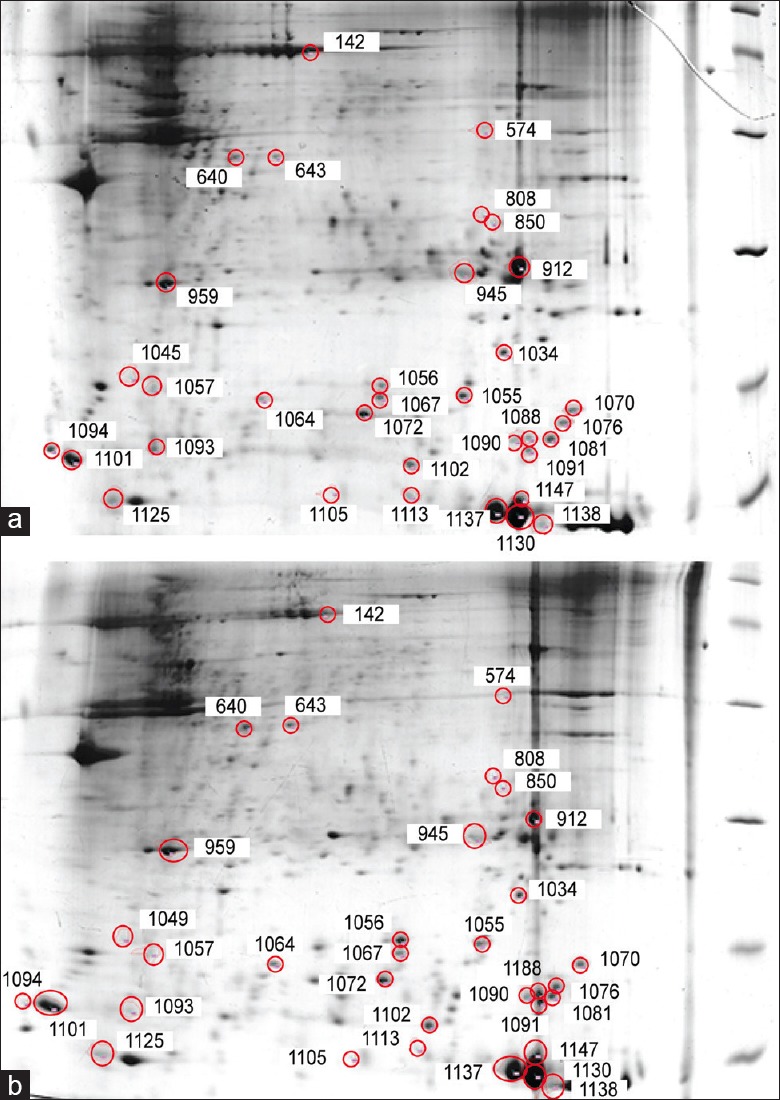
Two-dimensional electrophoresis images of the uterosacral ligament (24-cm isoelectric focusing, pH 3–10 NL, 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis). Differentially expressed proteins are indicated by red circles. (a) Control group, (b) pelvic organ prolapse group.
Table 4.
Differentially expressed proteins in USL samples obtained from women with POP and from controls
| Master no. | Av.Ratio (B/A) | NCBI index | Protein name |
|---|---|---|---|
| 850 | –1.6 | gi|2781202 | Electron transfer flavoprotein subunit alpha, mitochondrial |
| 959 | –1.7 | gi|90108664 | Apolipoprotein A-I |
| 1049 | –1.52 | gi|4501889 | Smooth muscle gamma-actin |
| 1056 | –2.34 | g i|48255905 | Transgelin |
| 1070 | –2.02 | gi|5031635 | Cofilin-1 |
| 1081 | –2.27 | gi|1633054 | Cyclophilin A |
| 1101 | –1.95 | gi|119617307 | Myosin, light polypeptide 6, alkali |
| 1125 | –4.06 | gi|42542978 | Galectin-1 |
Master no.: The number of protein spots in the computer; Av. Ratio: Abundance ratio of protein spots (POP group/control group); NCBI Index: Protein sequence number in the NCBInr; POP: Pelvic organ prolapse; USL: Uterosacral ligament.
To verify the differential expression of the eight proteins, we conducted quantitative real-time-PCR. Consistent with the 2-DE findings, a significant difference in mRNA expression was observed between the patient and control groups as shown in Table 5.
Table 5.
USL mRNA expression in women with POP and in controls
| Characteristics | POP group (n = 5) | Control group (n = 5) | P |
|---|---|---|---|
| ETFα* | 0.54 ± 0.17 | 1.31 ± 0.54 | 0.014 |
| Apolipoprotein A-I | 0.44 ± 0.16 | 1.15 ± 0.31 | <0.01 |
| Smooth muscle gamma-actin | 0.46 ± 0.18 | 1.16 ± 0.25 | <0.01 |
| Transgelin | 0.44 ± 0.23 | 1.11 ± 0.26 | <0.01 |
| Cofilin-1 | 0.49 ± 0.16 | 1.28 ± 0.26 | <0.01 |
| Cyclophilin A | 0.48 ± 0.23 | 1.47 ± 0.29 | <0.01 |
| Myosin | 0.52 ± 0.20 | 1.28 ± 0.26 | <0.01 |
| Galectin-1 | 0.55 ± 0.20 | 1.31 ± 0.21 | <0.01 |
*ETFα: Electron transfer flavoprotein subunit alpha, mitochondrial; POP: Pelvic organ prolapse; USL: Uterosacral ligament.
DISCUSSION
The USL is an important part of the pelvic support system and is the first level of support for the cervix and upper vagina.[8] The morphological changes in women with POP have been well-described.[9,10] The fractional area of smooth muscle cells has been shown to be significantly lower in patients with prolapse, whereas smooth muscle apoptosis has been shown to be significantly increased in the USL of women with POP.[10,11] In addition to alterations in the composition of the USL, Takacs et al. also demonstrated changes in the functional properties facilitated by smooth muscle regulatory proteins such as caldesmon and myosin heavy chain.[12] Therefore, studies of the USL may play a key role in exploring and explaining the mechanism and progression of POP. In our research, postmenopausal women with stage 3 and above POP (according to the POPQ system) were recruited into the POP group and matched with controls according to age, menopausal status, and parity. The exclusion criteria helped to avoid the confounding effects of estrogen-related and collagen metabolic diseases. Because women with POP usually also suffer from SUI, researchers often focus on women with the combined disease when they explore the pathophysiology of PFD. However, tissue collection is not specific, and the support tissues associated with SUI and POP are not entirely consistent. Therefore, it is more convincing to select patients with SUI or POP alone. In our research, we focused on women with POP alone and obtained the tissue from the USL, and the results were specific and convincing.
Eight proteins that were differentially expressed between the two groups were identified. Among these proteins, smooth muscle gamma-actin, transgelin, cofilin-1, and myosin are cytoskeleton proteins. POP is a disease in which loss of vaginal support results in bulging or herniation of the uterus, bladder, and/or rectum. Therefore, the cytoskeleton of pelvic support tissue may participate in the occurrence of this disease.
Among the four cytoskeleton proteins in the USL that we verified, the expression of transgelin exhibited a “noteworthy” low expression level in patients (the expression level was 2.34-fold lower in the POP group). Transgelin (SM22a) is ubiquitous in vascular and visceral smooth muscle and is an early marker of smooth muscle differentiation. It is also present in fibroblasts and some epithelial tissue. High expression levels of transgelin inhibit cell proliferation in visceral smooth muscle cells and injured arteries.[13] As a protein that affects the dynamics of the actin cytoskeleton via stabilization of actin filaments, transgelin is both directly and indirectly involved in many processes such as migration, proliferation, differentiation, and apoptosis.[14] Thus, transgelin down-regulation may participate in the occurrence and progression of POP by affecting apoptosis and smooth muscle cell proliferation.
The main function of cofilin-1 is to regulate actin cytoskeleton dynamics, which appears to be involved in many steps in the neurotoxicity processes of neurodegenerative diseases. The primary involvement of cofilin-1 dysfunction in the pathophysiology of these disorders may be related to cytoskeleton stress. Recently, cofilin-1 has also been implicated in other biological processes such as cell death by apoptosis.[15]
Myosin light chain polypeptide 6, another actin- and myosin-related protein, was also decreased in POP patients. In a study examining the cytoskeleton and apoptosis, increased phosphorylation of the myosin regulatory light chain was observed in apoptosis[16] and autophagic cell death.[17] Caspases destabilize the cytoskeleton through cleavage of a variety of cytoskeletal proteins in caspase-dependent apoptosis.[16] One of the most prominent features of DAPK-induced cell death is the effect on the cytoskeleton including the loss of matrix attachment and membrane blebbing.[17] The actin-associated protein cofilin1, a key regulator of actin filament dynamics and reorganization, and its upstream kinase LIMK1 have been identified as new DAPK interaction partners in TNF-induced apoptosis.[18] One known cytoskeletal-associated substrate of DAPK is the myosin-II light chain (MLC), which is phosphorylated by DAPK on a Ser residue.[17] Phosphorylation of this Ser residue in turn stabilizes actin stress fibers.[19]
Therefore, this complex dynamic interaction of cytoskeleton proteins in the occurrence and progression of POP may be intertwined. Apoptosis may be the common link, but the exact mechanism underlying this process remains unknown.
Galectin-1 is a lectin that induces apoptosis by interacting with either cell-surface glycoproteins or intracellular proteins.[20] In this study, galectin-1 expression was −4.06-fold lower in the patients than in the controls. We speculated that galectin-1 down-regulation may participate in apoptosis of the cells in the USL, leading to tissue degradation and loss of pelvic support.
Cyclophilin A (CypA) is the most abundant protein among the Cyps and is expressed in the cytosol. A study by Satoh showed that CypA contributes to inflammation and atherosclerosis by promoting endothelial cell (EC) apoptosis and EC expression of leukocyte adhesion molecules, increasing the proliferation of macrophages and vascular smooth muscle cells (VSMCs) and increasing pro-inflammatory signal transduction in VSMCs. CypA, as an MMP activator, initiates aortic aneurysm formation.[21] The contribution of MMP to increased collagenolysis may be related to genetic polymorphisms present at a higher frequency in women with PFD. In addition, ECM protein turnover plays a role in the development of POP and SUI. Therefore, we speculate that increased degradation of collagen due to the interplay of CypA and MMPs in apoptosis may decrease the mechanical strength of the USL, thus predisposing women to POP.
ETF is a heterodimeric complex of α- and β-subunits, known as ETF-α and ETF-β, respectively. It acts as a mobile electron carrier in the matrix of the mitochondria, linking 11 different mitochondrial FAD-containing acyl-CoA dehydrogenases involved in fatty acid β-oxidation to the ubiquinone pool of the respiratory chain. The down-regulation of ETF may affect respiratory chain function, leading to electron transfer and mitochondrial respiration dysfunction, and subsequently, apoptosis. This notion is consistent with previous work showing that oxidative stress and increased mitochondrial apoptosis may contribute to the pathological process of POP.[22]
In conclusion, we found eight differentially expressed proteins, including four cytoskeleton proteins and three proteins related to apoptosis, in the USL using comparative proteomics between POP patients and controls. Further investigations into the functions and interactions of these proteins in the USL are necessary and would be helpful to explore the etiology and determine an effective treatment for POP.
Financial support and sponsorship
This study was supported by a grant from the National Natural Science Foundation of China (No. 30901597).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: Review of prevalence and risk factors. Int Urogynecol J. 2011;22:127–35. doi: 10.1007/s00192-010-1215-0. [DOI] [PubMed] [Google Scholar]
- 2.Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol. 2009;114:1278–83. doi: 10.1097/AOG.0b013e3181c2ce96. [DOI] [PubMed] [Google Scholar]
- 3.Ward RM, Velez Edwards DR, Edwards T, Giri A, Jerome RN, Wu JM. Genetic epidemiology of pelvic organ prolapse: A systematic review. Am J Obstet Gynecol. 2014;211:326–35. doi: 10.1016/j.ajog.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campeau L, Gorbachinsky I, Badlani GH, Andersson KE. Pelvic floor disorders: Linking genetic risk factors to biochemical changes. BJU Int. 2011;108:1240–7. doi: 10.1111/j.1464-410X.2011.10385.x. [DOI] [PubMed] [Google Scholar]
- 5.Athanasiou S, Lymberopoulos E, Kanellopoulou S, Rodolakis A, Vlachos G, Antsaklis A. Proteomic analysis of pubocervical fascia in women with and without pelvic organ prolapse and urodynamic stress incontinence. Int Urogynecol J. 2010;21:1377–84. doi: 10.1007/s00192-010-1203-4. [DOI] [PubMed] [Google Scholar]
- 6.Molloy MP, Brzezinski EE, Hang J, McDowell MT, VanBogelen RA. Overcoming technical variation and biological variation in quantitative proteomics. Proteomics. 2003;3:1912–9. doi: 10.1002/pmic.200300534. [DOI] [PubMed] [Google Scholar]
- 7.Fountoulakis M. Proteomics: Current technologies and applications in neurological disorders and toxicology. Amino Acids. 2001;21:363–81. doi: 10.1007/s007260170002. [DOI] [PubMed] [Google Scholar]
- 8.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 Pt 1):1717–24. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel B, Denschlag D, Göbel H, Fittkow C, Werner M, Gitsch G, et al. Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:475–9. doi: 10.1007/s00192-005-1294-5. [DOI] [PubMed] [Google Scholar]
- 10.Ozdegirmenci O, Karslioglu Y, Dede S, Karadeniz S, Haberal A, Gunhan O, et al. Smooth muscle fraction of the round ligament in women with pelvic organ prolapse: A computer-based morphometric analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:39–43. doi: 10.1007/s00192-004-1215-z. [DOI] [PubMed] [Google Scholar]
- 11.Takacs P, Nassiri M, Gualtieri M, Candiotti K, Medina CA. Uterosacral ligament smooth muscle cell apoptosis is increased in women with uterine prolapse. Reprod Sci. 2009;16:447–52. doi: 10.1177/1933719108328611. [DOI] [PubMed] [Google Scholar]
- 12.Takacs P, Gualtieri M, Nassiri M, Candiotti K, Fornoni A, Medina CA. Differential expression of smooth muscle regulatory proteins in the uterosacral ligaments of women with uterine prolapse. Am J Obstet Gynecol. 2010;202:620.e1–5. doi: 10.1016/j.ajog.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Dong LH, Lv P, Han M. Roles of SM22α in cellular plasticity and vascular diseases. Cardiovasc Hematol Disord Drug Targets. 2012;12:119–25. doi: 10.2174/1871529x11202020119. [DOI] [PubMed] [Google Scholar]
- 14.Dvorakova M, Nenutil R, Bouchal P. Transgelins, cytoskeletal proteins implicated in different aspects of cancer development. Expert Rev Proteomics. 2014;11:149–65. doi: 10.1586/14789450.2014.860358. [DOI] [PubMed] [Google Scholar]
- 15.Schönhofen P, de Medeiros LM, Chatain CP, Bristot IJ, Klamt F. Cofilin/actin rod formation by dysregulation of cofilin-1 activity as a central initial step in neurodegeneration. Mini Rev Med Chem. 2014;14:393–400. doi: 10.2174/1389557514666140506161458. [DOI] [PubMed] [Google Scholar]
- 16.Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–36. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bialik S, Bresnick AR, Kimchi A. DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ. 2004;11:631–44. doi: 10.1038/sj.cdd.4401386. [DOI] [PubMed] [Google Scholar]
- 18.Ivanovska J, Tregubova A, Mahadevan V, Chakilam S, Gandesiri M, Benderska N, et al. Identification of DAPK as a scaffold protein for the LIMK/cofilin complex in TNF-induced apoptosis. Int J Biochem Cell Biol. 2013;45:1720–9. doi: 10.1016/j.biocel.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Kuo JC, Lin JR, Staddon JM, Hosoya H, Chen RH. Uncoordinated regulation of stress fibers and focal adhesions by DAP kinase. J Cell Sci. 2003;116(Pt 23):4777–90. doi: 10.1242/jcs.00794. [DOI] [PubMed] [Google Scholar]
- 20.Hsu DK, Yang RY, Liu FT. Galectins in apoptosis. Methods Enzymol. 2006;417:256–73. doi: 10.1016/S0076-6879(06)17018-4. [DOI] [PubMed] [Google Scholar]
- 21.Satoh K, Nigro P, Berk BC. Oxidative stress and vascular smooth muscle cell growth: A mechanistic linkage by cyclophilin A. Antioxid Redox Signal. 2010;12:675–82. doi: 10.1089/ars.2009.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim EJ, Chung N, Park SH, Lee KH, Kim SW, Kim JY, et al. Involvement of oxidative stress and mitochondrial apoptosis in the pathogenesis of pelvic organ prolapse. J Urol. 2013;189:588–94. doi: 10.1016/j.juro.2012.09.041. [DOI] [PubMed] [Google Scholar]


