INTRODUCTION
Autoimmunity is defined as, a condition characterized by a specific humoral or cell-mediated immune response against the constituents of the body's own tissues (autoantigens). In numerous autoimmune diseases, such an immune response is well recognized that causes damage to the self-constituents of body tissues by the products of the immune system. Graves’ hyperthyroidism occurs after the loss of tolerance to the thyroid stimulating hormone receptor (TSHR) and the generation of thyroid stimulatory antibodies that mimic the action of thyroid-stimulating hormone (TSH).[1]
Increased attention has been paid to the prevention and treatment of autoimmune diseases by rebuilding immune tolerance. Indeed, using a specific autoantigen protein by different routes to induce the immune tolerance to attenuate host immunity was adopted extensively in the studies to prevent and treat autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis.[2,3] However, studies on tolerance induction against Graves’ disease are seldomly reported. It is worth mentioning that the immune tolerance against Graves’ disease was successfully induced in female BALB/c mice within 24 h after birth using a specific TSHR antigen (Ad-TSHR289) in a high dose either by intraperitoneal (i.p.) or intramuscular (i.m.) injection at our laboratory in 2011.[4] On this basis, we intended to further improve the method of neonatal tolerance induction by investigating the effects on other doses and time of antigen pretreatment.
METHODS
Animals
BALB/c mice were purchased from the Laboratory Animal Center of Xi’an Jiaotong University Health Science Center and placed in a pathogen-free animal colony. For the generation of newborn mice, one adult male and three females were bred in the each cage. As soon as the female mice became pregnant, they were separated and caged individually. Suckling mice weaned after 3 weeks of age. Neonatal mice (3 days) were divided into model group, normal control, tolerance groups, and tolerance controls. Mice in tolerance groups or tolerance controls were divided into low-dose, medium-dose, and high-dose groups, according to different doses pretreated with Ad-TSHR289 or Ad-lacz. All experimental procedures were carried out according to the guidelines of the Institutional Animal Ethics Committee.
Construction of thyroid stimulating hormone receptor adenoviruses
Construction of replication-deficient recombinant Ad-TSHR289 was performed as previously described.[4] Ad-TSHR289 and control adenovirus, expressing galactosidase (Ad-Lacz), were propagated in HEK293 cells and purified by ion-exchange chromatographic column. The concentration of viral particles was determined by measuring the absorbance at 260 nm. Both Ad-TSHR289 and Ad-Lacz in this study were obtained from the same preparation, aliquoted and stored at −80°C.
Immunization protocol
Tolerance groups were injected peritoneally with three different doses of Ad-TSHR289 at 3 days after birth: 5 × 106 particles (low-dose), 5 × 107 particles (medium-dose), and 5 × 108 particles (high-dose) per injection (in 100 μl PBS), while control groups were injected with equivalent Ad-lacz in the same way. Model group and normal control were not pretreated with recombinant adenovirus in neonates. All the mice, except normal control treated with Ad-lacz in adults, were injected intramuscularly after 6–7 weeks with Ad-TSHR289 in 50 μl PBS to induce Graves’ disease, totally three times at 3-week intervals. Eight weeks after the last immunization, animals were euthanized to obtain blood for thyroid stimulating hormone receptor antibody (TRAb), total thyroxine (TT4), TSH, and thyroid tissues were removed for histological examination.
Thyroid stimulating hormone receptor antibodies measured by thyroid stimulating hormone binding inhibition
TSHR antibodies were measured by the TSH-binding inhibition assays (TBI) with a commercial kit according to the manufacturer's protocol (Medipan GmbH, Germany). Briefly, duplicate serum aliquots (50 μl unless indicated otherwise) were incubated with detergent-solubilized TSHR, 125I-labeled bovine TSH was added, and the TSHR-ab complexes were precipitated out with polyethylene glycol. TRAb values were expressed in percentages. Values greater than mean ± 2 standard deviation (SD) of normal control were considered positive.
Thyroid function tests
TT4 was measured using 50 μl undiluted serum with a commercially available radioimmunoassay kit (Union Medical and Pharmaceutical Technology (Tianjin) Ltd., China). TT4 values were expressed in nmol/L. The normal range was defined as mean ± 2 SD of normal control for TT4. Serum TSH level was determined by a heterologous disequilibrium double-Ab precipitation radioimmunoassay (Union Medical and Pharmaceutical Technology (Tianjin) Ltd.). The upper and lower limit of TSH was expressed as mean ± 2SD of normal control.
Thyroid gland histology
Thyroid tissues were removed and fixed with 10% formalin. Tissues were embedded in paraffin and 5 μm thick section was prepared and stained with hematoxylin and eosin for microscopic examination.
Statistical analysis
The results were expressed as the mean ± SD. The statistical significance of the difference between the magnitude of response in different groups was determined by one-way analysis of variance (ANOVA) for normally distributed or by nonparametric tests. Fisher's exact test was used to determine the significant difference between the numbers of mice in a group positive or negative for a particular parameter. Values of P < 0.05 were considered statistically significant. Statistical analysis was calculated using SPSS 15.0 (IBM Corporation, Armonk, NY, USA).
RESULTS
Thyroid stimulating hormone receptor antibodies in mice immunized with Ad-thyroid stimulating hormone receptor 289 in adults
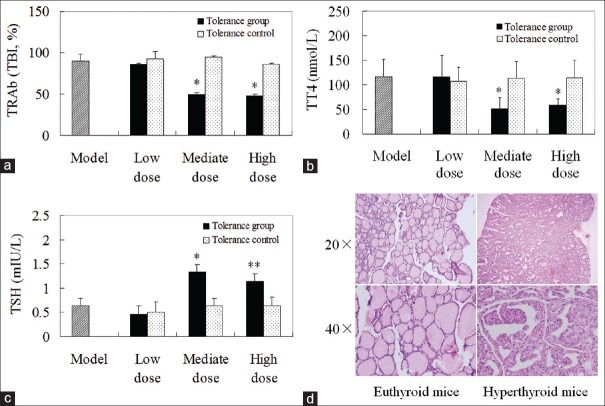
We tested the mouse serum for TSHR antibodies by TBI assay. Eight weeks after the third immunization, nearly all mice immunized with Ad-TSHR289 in adults, developed higher-leveled antibodies against TSHR. The TRAb level was lower in three doses in the tolerance groups than in the respective controls or model group. However, statistical significance was only observed between high-dose or medium-dose tolerance groups and their control or model group. There was no difference in the Ab-levels among the mice pretreated with different doses of Ad-lacz. The TRAb titers in medium-dose or high-dose tolerance groups were significantly less than those in low-dose group [Figure 1a].
Figure 1.
The thyroid function and thyroid histology. (a) Thyroid stimulating hormone receptor antibody levels in mice immunized with Ad-thyroid stimulating hormone receptor 289 as adults. (b) Total thyroxine levels in mice immunized with Ad-thyroid stimulating hormone receptor 289 as adults. (c) Thyroid stimulating hormone levels in mice immunized with Ad-thyroid stimulating hormone receptor 289 as adults. (d) H and E-stained paraffin block sections of the thyroid glands from normal and hyperthyroid mice. *P < 0.05 versus corresponding tolerance control, model group, low-dose tolerance group; **P < 0.01 versus corresponding tolerance control, model group, low-dose tolerance group.
Thyroid function in mice immunized with Ad-thyroid stimulating hormone receptor 289 in adults
We assessed thyroid function by determining serum TT4 and TSH levels in the mice immunized with Ad-TSHR289 in adults. Most of the mice with positive TRAb showed elevated TT4 level in various degrees except for medium-dose and high-dose tolerance groups. There was no obvious difference in TT4 level between low-dose tolerance group and its control. TT4 level was significantly lower in the medium-dose and high-dose tolerance groups than those in the corresponding tolerance control or model group. Furthermore, TT4 levels in the medium-dose or high-dose tolerance groups were significantly reduced compared to the low-dose tolerance group [Figure 1b]. In TSH level assay, reduction in TSH concentration was observed in the mice of elevated TT4. There was no significant difference among three tolerance controls. The TSH levels were increased in the medium-dose or high-dose tolerance groups versus the corresponding tolerance controls or model group. Compared to low-dose tolerance group, medium-dose or high-dose tolerance group showed significantly increased TSH level [Figure 1c]. The incidence of hyperthyroidism was slight, but no significant decrease in the medium-dose or high-dose tolerance groups than in the corresponding tolerance control or model groups [Table 1].
Table 1.
Total number of mice in each group and the number of positive TRAb or hyperthyroidism (n)
| Items | Model group | Tolerance groups | Control groups | ||||
|---|---|---|---|---|---|---|---|
| Low-dose | Medium-dose | High-dose | Low-dose | Medium-dose | High-dose | ||
| Total number for each group | 8 | 10 | 8 | 8 | 8 | 8 | 8 |
| Positive number of TRAb | 8 | 9 | 6 | 6 | 8 | 8 | 8 |
| Number of hyperthyroidism | 5 | 7 | 1 | 1 | 6 | 6 | 5 |
TRAb: Thyroid stimulating hormone receptor antibody.
Thyroid histology
Mice with elevated TT4 levels had diffused goiters with hypertrophy and hypercellularity of thyroid epithelial cells as previously reported.[4] No lymphocytic infiltration was observed [Figure 1d].
DISCUSSION
Previous studies reported that pretreatment of newborn mice with a high-dose (1 × 109 particles) antigen results in immunological unresponsiveness in our lab.[4] The present study showed that day 3 intraperitoneal injection of 5 × 107 or 5 × 108 particles of Ad-TSHR289 partially blocked the development of TSH receptor antibodies and Graves’ hyperthyroidism. These findings suggest that the day 3 exposure to a certain level of TSHR289 antigen induces the insufficient neonatal tolerance.
The concentration of the antigen presented to the immune system and the age of the hosts are two critical factors in the process of establishment of neonatal tolerance. Three different doses were compared in this study to determine the influence of Ad-TSHR289 dosage on immune tolerance induction. Pretreatment with higher-level TSHR A-subunit in neonatal mice can partially reduce the development of Graves’ hyperthyroidism. Conversely, mice injected with low-dose antigen developed high-titer TRAb. Nevertheless, the tolerance induction is more effective in previous work rather than the current study. One possible explanation is the induction of neonatal tolerance, critically dependent on the level of the antigen-expression. In addition, neonatal periods have been thought of as a window in ontogeny during which the developing immune system is particularly susceptible to toleration.[5] However, the time of tolerance induction is a narrow window. One-day old mice were extremely susceptible to tolerance induction, while Ad-TSHR289 may be less tolerogenic when administered to mice that were 3 days of age. The exact mechanisms of tolerance window are still unclear; the further investigation needs to be performed in the subsequent study.
Our study further confirmed that immunotolerance against Graves’ disease could be induced in neonatal mice with higher-dose Ad-TSHR289 by intraperitoneal pathway. The higher dose of antigen and narrow time window were the two key factors of neonatal tolerance induction. This immune tolerance model may provide a basis for new approaches toward the prevention of Graves’ disease.
Financial support and sponsorship
This study was supported by the grants from National Natural Science Foundation of China (No. 81170729 and No. 81200574).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Professors Basil Rapoport, Sandra M. McLachlan, and Chen CR (University of California, Los Angeles), for providing us the plasmid expressing human TSHR289 (psv2-neo-ECE-TSHR289).
Footnotes
Edited by: Xiu-Yuan Hao
REFERENCES
- 1.McLachlan SM, Nagayama Y, Rapoport B. Insight into Graves’ hyperthyroidism from animal models. Endocr Rev. 2005;26:800–32. doi: 10.1210/er.2004-0023. [DOI] [PubMed] [Google Scholar]
- 2.Xie J, Lin YK, Wang K, Che B, Li JQ, Xu X, et al. Induced immune tolerance of autoantigen loaded immature dendritic cells in homogenic lupus mice. Genet Mol Res. 2014;13:1251–62. doi: 10.4238/2014.February.27.10. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida Y, Tsuji T, Watanabe S, Matsushima A, Matsushima Y, Banno R, et al. Efficacy of combination treatment with fingolimod (FTY720) plus pathogenic autoantigen in a glucose-6-phosphate isomerase peptide (GPI325-339)-induced arthritis mouse model. Biol Pharm Bull. 2013;36:1739–46. doi: 10.1248/bpb.b13-00297. [DOI] [PubMed] [Google Scholar]
- 4.Wu L, Xun L, Yang J, Xu L, Tian Z, Gao S, et al. Induction of murine neonatal tolerance against Graves’ disease using recombinant adenovirus expressing the TSH receptor A-subunit. Endocrinology. 2011;152:1165–71. doi: 10.1210/en.2010-0737. [DOI] [PubMed] [Google Scholar]
- 5.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]