Abstract
Background:
Awake fiberoptic intubation (AFOI) is usually performed in the management of the predicted difficult airway. The aim of this study was to evaluate the feasibility of dexmedetomidine with midazolam (DM) and sufentanil with midazolam (SM) for sedation for awake fiberoptic nasotracheal intubation.
Methods:
Fifty patients with limited mouth opening scheduled for AFOI were randomly assigned to two groups (n = 25 per group) by a computer-generated randomization schedule. All subjects received midazolam 0.02 mg/kg as premedication and airway topical anesthesia with a modified “spray-as-you-go” technique. Group DM received dexmedetomidine at a loading dose of 0.5 μg/kg over 10 min followed by a continuous infusion of 0.25 μg·kg−1·h−1, whereas Group SM received sufentanil at a loading dose of 0.2 μg/kg over 10 min followed by a continuous infusion of 0.1 μg·kg−1·h−1. As necessary, since the end of the administration of the loading dose of the study drug, an additional dose of midazolam 0.5 mg at 2-min intervals was given to achieve a modified Observers’ Assessment of Alertness/Sedation of 2–3. The quality of intubation conditions and adverse events were observed.
Results:
The scores of ease of the AFOI procedure, patient's reaction during AFOI, coughing severity, tolerance after intubation, recall of the procedure and discomfort during the procedure were comparable in both groups (z = 0.572, 0.664, 1.297, 0.467, 0.895, and 0.188, respectively, P > 0.05). Hypoxic episodes similarly occurred in the two groups, but the first partial pressure of end-tidal CO2 after intubation was higher in Group SM than that in Group DM (45.2 ± 4.2 mmHg vs. 42.2 ± 4.3 mmHg, t = 2.495, P < 0.05).
Conclusions:
Both dexmedetomidine and sufentanil are effective as an adjuvant for AFOI under airway topical anesthesia combined with midazolam sedation, but respiratory depression is still a potential risk in the sufentanil regimen.
Keywords: Awake Fiberoptic Intubation, Conscious Sedation, Dexmedetomidine, Midazolam, Sufentanil
INTRODUCTION
Awake fiberoptic intubation (AFOI) is usually performed in the management of the predicted difficult airway. Adequate sedation with effective airway topical anesthesia for AFOI is paramount to improve intolerance, alleviate discomfort, and achieve successful intubation. However, it is usually difficult to achieve all the requirements for AFOI using a single drug or technique. Benzodiazepines combined with opioids are currently used for sedation for AFOI. Unfortunately, this combination of drugs has the potential to cause respiratory depression. Compared to fentanyl, sufentanil produces greater and longer-lasting analgesia and less and shorter-lasting respiratory depression,[1] but as a single agent for AFOI, it induces a high incidence of recall.[2] It may be relatively ideal of the combination of midazolam and sufentanil for AFOI. Contrary to opioids, dexmedetomidine has not been associated with respiratory depression when used alone or combined with midazolam. The effectiveness of dexmedetomidine for AFOI has been evaluated in many studies with favorable results.[2,3,4,5,6,7,8,9] In this study, our aim was to compare the feasibility of dexmedetomidine with midazolam (DM) and sufentanil with midazolam (SM) for conscious sedation for awake fiberoptic nasotracheal intubation under airway topical anesthesia with a modified “spray-as-you-go” technique in patients undergoing oromaxillofacial surgery. We hypothesized that the two regimens had at least equal efficacy in intubation conditions without respiratory depression and upper airway obstruction.
METHODS
Patients
This double-blinded, randomized comparison study was approved by the local Research Ethics Committee of Jining No. 1 People's Hospital Affiliated to Jining Medical University and written informed consent was obtained from each patient. Fifty patients with American Society of Anesthesiologists (ASA) classifications of I–II were enrolled for elective awake fiberoptic nasotracheal intubation following diagnosis maxillofacial fracture with limited mouth opening. Exclusion criteria included: Pregnant or lactating women, age <18 years or >60 years, cardiopulmonary disease, heart rate (HR) <50 beats/min, systolic blood pressure (SBP) <90 mmHg, allergy to the drugs involved in the study, inability to communicate effectively, patient refusal, history of drug or alcohol abuse, and patients on long-term opioids or sedative medication.
Study design
These patients were randomly assigned by computer-generated randomization schedule into Group DM or Group SM. Two experienced consultant anesthetists clinically managed the trial: One mainly responsible for performing the procedure of AFOI and the other mainly for observation and data collection. Study drug was prepared as 200 μg (2 ml) of dexmedetomidine in 48 ml of 0.9% saline or 80 μg (1.6 ml) of sufentanil in 48.4 ml of 0.9% saline to 50-ml syringes and administrated by a nurse anesthetist and a resident anesthetist according to group allocation and the patient's weight in kilograms. Both consultant anesthetists and patients were blinded to group assignment.
Anesthesia and awake fiberoptic intubation procedure
On arrival in the anesthetic room, standard monitoring was established including noninvasive blood pressure, pulse oximetry, electrocardiography and bispectral index (BIS). Patients were asked to breathe through each nostril to assess patency. The nostril with the greatest patency was prepared using cotton swab-stick soaked in 1% tetracaine 1 ml for 10 min. Once an intravenous cannula was inserted, midazolam 0.02 mg/kg and penehyclidine 1 mg as premedication were given intravenously and the infusion of the study drug was started. All patients received the same drug delivery mode, with which their respective drug was infused at a loading dose of 0.125 ml/kg over 10 min followed by a continuous infusion of 0.0625 ml·kg−1·h−1 via a pressure-driven syringe pump. Group DM received dexmedetomidine at a loading dose of 0.5 μg/kg followed by a continuous infusion of 0.25 μg·kg−1·h−1, while Group SM received sufentanil at a loading dose of 0.2 μg/kg followed by a continuous infusion of 0.1 μg·kg−1·h−1. Crystalloid fluids (5–10 ml·kg−1·h−1) were administered during the infusion of the study drug.
The level of sedation was assessed by the endoscopic anesthetist using modified Observers’ Assessment of Alertness/Sedation scale (OAA/S, 1 = appropriate verbal response to patient's name, 2 = lethargic response, 3 = response only after name is spoken loudly and/or repeatedly, 4 = response after mild prodding or shaking, 5 = response after painful stimuli)[10] and BIS once per minute. At the end of infusion of the loading dose, any patient with a modified OAA/S = 1 was given an additional midazolam 0.5 mg at 2-min intervals until a modified OAA/S of 2–3.
After adequate sedation was achieved, a fiberoptic scope (Olympus LF-DP 3.1 mm, Olympus, Tokyo, Japan) loaded with a 7.0-mm reinforced tracheal tube for male patients or a 6.5-mm tube for females was inserted through the prepared nostril into the hypopharynx. Airway topical anesthesia was performed with a modified “spray-as-you-go” technique using 2% lidocaine sprayed onto the airway structures via the working channel of the fiberoptic scope. A spray of 2 ml of lidocaine at 15-sec intervals was given until the tip of the fiberoptic scope can be advanced to the next airway structure, and the epiglottis, glottis, and carina were identified. Oxygen was delivered at 3 L/min via a nasal sponge placed in the other nostril. Once the position of the endoscope in the trachea was confirmed, the tracheal tube was positioned approximately 3 cm above the carina and secured.
Blood pressure, pulse, oxygen saturation, and respiratory rate were recorded every 3 min from starting the infusion of the study drug until the fiberoptic scope was introduced through the nose. Thereafter, vital signs were recorded every minute until the completion of the AFOI. Hypotension was defined as SBP <80 mmHg, diastolic blood pressure (DBP) <50 mmHg, or a SBP decrease to ≥30% below baseline. Hypertension was defined as SBP >180 mmHg, DBP >100 mmHg, or a SBP increase to ≥30% above baseline. Bradycardia was defined as HR <50 beats/min or a decrease to ≥30% below baseline. Tachycardia was defined as HR >120 beats/min or an increase to ≥30% above baseline. Respiratory depression was defined as respiratory rate <8 breaths/min or a decrease to ≥25% below baseline. Hypoxia was defined as pulse oxygen saturation (SpO2) <94% or a decrease of 10% below baseline. If the modified OAA/S score was 5, infusion of the study drug was discontinued and flumazenil 0.2 mg was intravenously administered. If a hypoxic episode occurred without an improvement via instructing to make deep breathes, infusion of the study drug was discontinued and naloxone 50 μg was intravenously administered. The patients receiving flumazenil or naloxone were excluded from the research.
Measuring variables
The primary outcome measures were the scores observed during endoscopy, intubation, and postintubation. The endoscopic anesthetist assessed the ease of placement of the fiberoptic scope and endotracheal tube on a scale of 1–3 (1 = easy, 2 = moderate, and 3 = difficult).[3] The observing anesthetist assessed patient reaction to placement of the fiberoptic scope and the tracheal tube on a 5-point scale (1 = no reaction; 2 = slight grimacing; 3 = severe grimacing; 4 = verbal objection; and 5 = defensive movement of head, hands, or feet).[11] Cough severity was rated on a 4-point scale (1 = none, 2 = slight, 3 = moderate, 4 = severe). Coughing was considered slight if no more than 2 coughs in sequence occurred, moderate if 3–5 coughs in sequence occurred and severe if more than 5 coughs in sequence occurred.[12] After intubation, the observing anesthetist assessed patient tolerance to the endotracheal tube in the trachea via slow inflation of the endotracheal tube cuff on a 3-point scale (1 = cooperative, 2 = restless/minimal resistance, 3 = severe resistance/general anesthesia required immediately).[4] The intubation time (from inserting the fiberoptic scope into the nostril to confirmation of tracheal intubation with capnography) and the number of attempts to place the fiberoptic scope and the endotracheal tube were recorded. Each patient was asked by one of the two consultant anesthetists 24 h after surgery to grade his/her recall of the procedure (1 = none, 2 = partial, 3 = full) and the discomfort during the procedure (1 = none, 2 = mild, 3 = moderate, 4 = severe).[13]
Other parameters assessed in relation to the AFOI procedure included: Airway patency on a 3-point scale (1 = patent airway, 2 = airway obstruction relieved by neck extension, 3 = airway obstruction requiring jaw retraction),[4] additional dosage of midazolam, lidocaine dosage used, the first partial pressure of end-tidal CO2 (PET CO2) after successful intubation (that is, the value of end-tidal CO2 triggered by the first respiratory cycle with establishment of respiratory circuit system), any hypoxic episode, any hypotensive or hypertensive episode and any arrhythmic episode.
Statistical analysis
According to a power calculation, 25 patients per group were at least required to demonstrate a 30% difference in the intubation sores for a power of 0.8 and a type one error of 0.05. Data are expressed as mean ± standard deviation (SD), number (proportion), or median (interquartile range [range]). Statistical analyses were calculated with Student's t-tests, repeated measures analysis of variance and Tukey–Kramer multiple comparisons tests for numerical data, Mann–Whitney tests for not normally distributed metric variables or ordinal data, and Chi-square test and Fisher's exact test for categorical data, as necessary. The SPSS 13.0 statistical software package (SPSS Inc., Chicago, IL, USA) was used for all analyses and a P < 0.05 was considered statistically significant.
RESULTS
Patients’ characteristics
No patients were excluded in this study. There were no significant differences between the groups with respect to gender, age, height, weight, body mass index, ASA, inter-incisor distance and Mallampati classification (P > 0.05) [Table 1].
Table 1.
Characteristics of patients allocated in Group DM and Group SM
| Characteristics | Group DM (n = 25) | Group SM (n = 25) | Statistical value | P |
|---|---|---|---|---|
| Males/females | 21/4 | 19/6 | 0.125* | 0.724 |
| Age, years | 37.9 ± 11.1 | 36.7 ± 11.5 | 0.375† | 0.709 |
| Weight, kg | 67.2 ± 11.2 | 65.7 ± 10.7 | 0.484† | 0.630 |
| Height, cm | 166.8 ± 7.8 | 167.7 ± 8.1 | 0.400† | 0.691 |
| BMI, kg/m2 | 24.2 ± 4.1 | 23.4 ± 3.9 | 0.707† | 0.483 |
| ASA class, I/II | 19/6 | 18/7 | 0.319‡ | 0.750 |
| Inter-incisor distance, mm | 13.5 ± 7.8 | 13.1 ± 8.2 | 0.177† | 0.860 |
| Mallampati airway class, 3/4 | 20/5 | 18/7 | 0.656‡ | 0.512 |
Data are n or mean ± SD. DM: Dexmedetomidine with midazolam; SM: Sufentanil with midazolam; BMI: Body mass index; ASA: American Society of Anesthesiologists *: χ2 value, †: t value, ‡z value.
Quality of awake fiberoptic intubation procedure
The scores of ease of AFOI, patient's reaction to the AFOI procedure, coughing severity and patient's tolerance after intubation were comparable in both groups (P > 0.05). The number of attempts and the intubation time were similar between the groups (P > 0.05). There was no difference in recall or awareness of the AFOI procedure and patient's discomfort score between the groups (P > 0.05) [Table 2].
Table 2.
Data related to the AFOI procedure in Group DM and Group SM
| Category | Group DM (n = 25) | Group SM (n = 25) | Statistical value | P |
|---|---|---|---|---|
| Ease of AFOI, 1/2/3 | 16/8/1 | 18/6/1 | 0.572* | 0.568 |
| Patient’s reaction to the AFOI procedure, 1/2/3/4/5 | 15/8/2/0/0 | 17/6/2/0/0 | 0.664* | 0.519 |
| Cough severity, 1/2/3/4 | 15/5/5/0 | 19/4/2/0 | 1.297* | 0.195 |
| Patient’s tolerance after intubation, 1/2/3 | 22/3/0 | 23/2/0 | 0.467* | 0.641 |
| Intubation time, min, mean ± SD | 4.6 ± 1.4 | 4.2 ± 1.2 | 1.085† | 0.283 |
| Numbers of attempts | 1 (1–2 [1–4]) | 1 (1–2 [1–3]) | 0.617* | 0.543 |
| Recall score, 1/2/3 | 23/2/0 | 21/3/1 | 0.895* | 0.371 |
| Discomfort score, 1/2/3/4 | 16/9/0/0 | 17/7/1/0 | 0.188* | 0.851 |
DM: Dexmedetomidine with midazolam; SM: Sufentanil with midazolam; AFOI: Awake fiberoptic intubation. *: z value, †: t value.
Data related to anesthesia
The score of the modified OAA/S was similar at the end of infusion of the loading dose between the groups (P > 0.05), but compared to Group SM, the level of sedation was deeper at immediately before endoscopy, immediately after intubation and 2 min after intubation in Group DM (z = 2.117, 2.395, and 2.380, respectively, P < 0.05). The score of airway patency, the additional dosage of midazolam, and the dosage of lidocaine used were similar in both groups (P > 0.05) [Table 3].
Table 3.
Anaesthetic data in Group DM and Group SM
| Category | Group DM (n = 25) | Group SM (n = 25) | Statistical value | P |
|---|---|---|---|---|
| Modified OAA/S; 1 (alert)/2/3/4/5 (asleep) | ||||
| End of infusion of the loading dose | 8/7/8/2/0 | 10/10/5/0/0 | 1.436* | 0.151 |
| Immediately before endoscopy | 0/11/12/2/0 | 0/18/7/0/0 | 2.117* | 0.034 |
| Immediately after intubation | 0/11/12/2/0 | 0/19/6/0/0 | 2.395* | 0.017 |
| 2 min after intubation | 0/10/13/2/0 | 0/18/7/0/0 | 2.380* | 0.017 |
| Airway patency, 1/2/3 | 24/1/0 | 22/3/0 | 1.032* | 0.302 |
| Additional dosage of midazolam, mg | 0 (0–0.5 [0–1.0]) | 0 (0–1.0 [0–2.5]) | 0.876* | 0.382 |
| Lidocaine dosage, mg, mean ± SD | 194.4 ± 55.8 | 200.8 ± 59.9 | 0.391† | 0.698 |
DM: Dexmedetomidine with midazolam; SM: Sufentanil with midazolam; OAA/S: Observers’ Assessment of Alertness/Sedation. *: z value, †: t value.
Adverse events and first partial pressure of end-tidal CO2 after intubation
The incidence of adverse events including hypertension, hypotension, tachycardia, bradycardia and hypoxia were comparable between the groups, but the first PET CO2 after intubation was higher in Group SM than that in Group DM (t = 2.495, P < 0.05) [Table 4].
Table 4.
Adverse events and first PETCO2 after intubation in Group DM and Group SM
| Category | Group DM (n = 25) | Group SM (n = 25) | Statistical value | P |
|---|---|---|---|---|
| Hypertension, n (%) | 2 (8) | 3 (12) | 0.000* | 1.000 |
| Hypotension, n (%) | 0 (0) | 0 (0) | 0.000* | 1.000 |
| Tachycardia, n (%) | 0 (0) | 1 (4) | 0.000* | 1.000 |
| Bradycardia, n (%) | 2 (0) | 0 (0) | 0.521* | 0.470 |
| Hypoxia, n (%) | 2 (8) | 3 (12) | 0.000* | 1.000 |
| First PETCO2 after intubation; mmHg | 42.2 ± 4.3 | 45.2 ± 4.2 | 2.495† | 0.016 |
PETCO2: Partial pressure of end-tidal CO2; DM: Dexmedetomidine with midazolam; SM: Sufentanil with midazolam. *: χ2 value,†: t value.
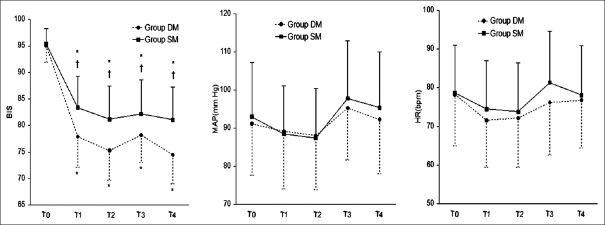
Changes in bispectral index values and hemodynamics
As shown in Figure 1, BIS, mean arterial pressure (MAP) and HR decreased continually before endoscopy and increased immediately after intubation in both groups. At the time points of the end of infusion of the loading dose (T1), immediately before endoscopy (T2), immediately after intubation (T3) and 2 min after intubation (T4), the BIS index was lower in Group DM than that in Group SM (P < 0.05), but there were no differences in MAP and HR between the two groups.
Figure 1.
Bispectral index, mean arterial pressure and heart rate measured before premedication (baseline, T0), at the end of infusion of the loading dose (T1), immediately before endoscopy (T2), immediately after intubation (T3) and 2 min after intubation (T4). DM: Dexmedetomidine with midazolam; SM: Sufentanil with midazolam; bpm: Beats/min. Points are expressed as means ± standard deviation. *P < 0.05, compared to before premedication; †P < 0.05, compared to Group DM.
DISCUSSION
This study showed that both DM regimen and SM regimen were effective as an adjuvant for AFOI under airway topical anesthesia. The two regimens were comparable in achieving desired sedation, satisfactory intubation conditions, stable hemodynamics, and low-level recall.
Optimal conditions for AFOI require sufficient comfort, adequate cooperation, stable hemodynamics and amnesia without respiratory depression. Single drug or technique currently in use is difficult to meet all the requirements for AFOI. In this study, the patients receiving DM had intubation conditions, hemodynamic profiles and recall of the AFOI procedure equal to the patients receiving SM. The additional dosage of midazolam required to achieve adequate sedation and the dosage of lidocaine consumed to perform airway topical anesthesia were similar in both groups. Nonetheless, we found while the modified OAA/S score was comparable at the end of infusion of the loading dose in both groups, the BIS scores were significantly lower in Group DM than those in Group SM, which similar with the findings of Kasuya et al.[14] and Haenggi et al.,[15] who reported that at comparable OAA/S or Ramsay scores, BIS values were significantly less with dexmedetomidine than those with propofol or midazolam. Some studies have focused on finding the correlation between the BIS and OAA/S score for dexmedetomidine[14,15] and midazolam,[16,17] and a divergence between the BIS and OAA/S scores has been reported.[14,16] A recent study found that the BIS value at which 50% patients lost consciousness was elevated from 63 when propofol used alone to 71 when propofol used together with dexmedetomidine,[18] which indicated that dexmedetomidine enhanced the hypnotic effects of propofol. The hypnotic synergism has been shown between dexmedetomidine and midazolam in two animal trials,[19,20] and to our knowledge, clinical studies have yet to verify this synergism. Therefore, since the impact of dexmedetomidine on BIS is incomprehensible, sedative scales like OAA/S may be more suitable to determine the adequacy of sedation, especially when dexmedetomidine is used with other sedatives.
Many agents have been reported to achieve conscious sedation for AFOI. Fentanyl can provide potent analgesia and blunt airway reflexes with minimal amnesic effect. Midazolam can provide sedation and amnesia without analgesic effect. Dexmedetomidine used for AFOI has become more popular because of its sedative, amnesic, analgesic, and anxiolytic effects without respiratory depression. Sufentanil has an analgesic potency 5–10 times greater than fentanyl with less and shorter-lasting respiratory depression,[2,21] which means that sufentanil may be an attractive substitute for fentanyl to provide sedation for AFOI. The loading dose (0.2 μg/kg) and the maintenance dose (0.1 μg·kg−1·h−1) of sufentanil in this study were based on previous investigations by Dhasmana et al.[22] and Conti et al.,[23] who respectively reported that fentanyl 3 μg/kg combined with midazolam 0.05 mg/kg for awake blind nasotracheal intubation provided sufficient comfort and maintained patent airway with spontaneous ventilation, and continuous infusion of sufentanil at 0.2–0.3 μg·kg−1·h−1 produced desired sedation without effects on respiration. Nevertheless, the high incidence of recall is to be expected when sufentanil used alone. Shen et al.[2] reported that the incidence of recall of airway procedure events was high to 45% with sufentanil alone. In previous studies[2,3,4,5,6,7,8,9] on administration of dexmedetomidine for AFOI, the loading dose and the maintenance dose ranged from 0.4 to 1.5 μg/kg over 10 or 15 min and 0–0.7 μg·kg−1·h−1, respectively, but dexmedetomidine 0.4 μg/kg followed by a continuous infusion of 0.7 μg·kg−1·h−1 combined with midazolam 2 mg as premedication produced less sufficiently sedation and analgesia for AFOI compared to remifentanil.[5] In this study, the loading dose and the maintenance dose of dexmedetomidine were 0.5 μg/kg and 0.25 μg·kg−1·h−1.
Recall not only affects patients on the assessment of satisfaction to the AFOI procedure but also would put them at a risk of the negative psychological state. The combination of midazolam and other sedative or analgesic agents may be an optimal sedation scheme for AFOI. The incidence of awareness of airway procedure events was reported in 40–56% of patients receiving dexmedetomidine alone,[2,4,6,7] while 16% of patients receiving DM as premedication.[3] To our knowledge, there is no study about SM for AFOI. In the present study, midazolam 0.02 mg/kg as premedication with additional doses was administrated, and the incidences of recall were comparable in both groups: 16% in Group SM which was lower than that of patients receiving sufentanil alone reported by Shen et al.,[2] and 8% in Group DM which was similar to that of Bergese et al.[3] In addition, Bergese et al.[8] described that the incidence of recall was 60.8% in patients receiving midazolam as rescue sedative combined with dexmedetomidine. The results of the above investigations remind us that midazolam causes antegrade, not retrograde, amnesia, and as premedication, helps to decrease the occurrence of recall.
Respiratory depression is the adverse event most concerned by anesthetists when the sedative and analgesic agents are selected for AFOI. While there was no indifference with regard to the incidence of respiratory depression and the score of airway patency in both groups, the first PET CO2 after intubation was higher in patients receiving SM in this study, as shown by Shen et al.,[2] who reported that the incidence of respiratory depression was high to 20% and the first PET CO2 after intubation was (47.1 ± 4.3) mmHg in patients receiving sufentanil alone. The values of the first PET CO2 after intubation in patients receiving sufentanil alone or with midazolam were approximate to but above normal values, of which the elevation indicated that the potential risk of insufficient ventilation or respiratory depression was still kept with sufentanil sedation. However, the risk of respiratory depression may be minimized by maintaining cooperation to make deep breathing during AFOI.
The “spray-as-you-go” technique is a common fashion to apply local anesthetics to the airway. The classic “spray-as-you-go” technique is that described by Sanchez et al.,[24] includes supraglottic spray 2 times at 5-min intervals, glottic spray at 3-min intervals until adequate anesthesia of the vocal cords and tracheal spray once followed by intubation after 5 min, which consumes at least 18 min. The technique was simplified with glottic spray once and tracheal spray once in several studies,[2,3,4,6] which might be not effective to blunt the gag reflex and the glottic reflex. Therefore, in order to shorten the time of endoscopy and achieve adequate airway anesthesia, the classic technique was modified with 2% lidocaine 2 ml per spray at 15-sec intervals and step by step and nonviolently advancing the tip of the fiberoptic scope to the next airway structure in the present study. The dosage of lidocaine used in our study was similar with that reported by Xue et al.,[25] significantly lower than the safe dose of 9 mg/kg.[26] The intubation time in our study was obviously shorter than that reported by Shen et al.[2] Obviously, adequate topical airway anesthesia is beneficial to facilitate AFOI.
In conclusion, the two regimens used in this study provided comparable intubating conditions, patients’ tolerance to intubation and lower recall for airway procedure events. However, it is necessary to keep responsive and cooperative in patients receiving sufentanil as an adjuvant to midazolam sedation for AFOI because respiratory depression is still a potential risk.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Bailey PL, Streisand JB, East KA, East TD, Isern S, Hansen TW, et al. Differences in magnitude and duration of opioid-induced respiratory depression and analgesia with fentanyl and sufentanil. Anesth Analg. 1990;70:8–15. doi: 10.1213/00000539-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Shen SL, Xie YH, Wang WY, Hu SF, Zhang YL. Comparison of dexmedetomidine and sufentanil for conscious sedation in patients undergoing awake fibreoptic nasotracheal intubation: A prospective, randomised and controlled clinical trial. Clin Respir J. 2014;8:100–7. doi: 10.1111/crj.12045. [DOI] [PubMed] [Google Scholar]
- 3.Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22:35–40. doi: 10.1016/j.jclinane.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Chu KS, Wang FY, Hsu HT, Lu IC, Wang HM, Tsai CJ. The effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fibreoptic nasal intubation. Eur J Anaesthesiol. 2010;27:36–40. doi: 10.1097/EJA.0b013e32832e0d2b. [DOI] [PubMed] [Google Scholar]
- 5.Cattano D, Lam NC, Ferrario L, Seitan C, Vahdat K, Wilcox DW, et al. Dexmedetomidine versus remifentanil for sedation during awake fiberoptic intubation. Anesthesiol Res Pract 2012. 2012 doi: 10.1155/2012/753107. 753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai CJ, Chu KS, Chen TI, Lu DV, Wang HM, Lu IC. A comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation. Anaesthesia. 2010;65:254–9. doi: 10.1111/j.1365-2044.2009.06226.x. [DOI] [PubMed] [Google Scholar]
- 7.Hu R, Liu JX, Jiang H. Dexmedetomidine versus remifentanil sedation during awake fiberoptic nasotracheal intubation: A double-blinded randomized controlled trial. J Anesth. 2013;27:211–7. doi: 10.1007/s00540-012-1499-y. [DOI] [PubMed] [Google Scholar]
- 8.Bergese SD, Candiotti KA, Bokesch PM, Zura A, Wisemandle W, Bekker AY Awake Study Group. A phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17:586–95. doi: 10.1097/MJT.0b013e3181d69072. [DOI] [PubMed] [Google Scholar]
- 9.Dhasmana SC. Nasotracheal fiberoptic intubation: Patient comfort, intubating conditions and hemodynamic stability during conscious sedation with different doses of dexmedetomidine. J Maxillofac Oral Surg. 2014;13:53–8. doi: 10.1007/s12663-012-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: Study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–51. [PubMed] [Google Scholar]
- 11.Puchner W, Egger P, Pühringer F, Löckinger A, Obwegeser J, Gombotz H. Evaluation of remifentanil as single drug for awake fiberoptic intubation. Acta Anaesthesiol Scand. 2002;46:350–4. doi: 10.1034/j.1399-6576.2002.460403.x. [DOI] [PubMed] [Google Scholar]
- 12.Aouad MT, Sayyid SS, Zalaket MI, Baraka AS. Intravenous lidocaine as adjuvant to sevoflurane anesthesia for endotracheal intubation in children. Anesth Analg. 2003;96:1325–7. doi: 10.1213/01.ANE.0000061586.63978.DE. [DOI] [PubMed] [Google Scholar]
- 13.Vennila R, Hall A, Ali M, Bhuiyan N, Pirotta D, Raw DA. Remifentanil as single agent to facilitate awake fibreoptic intubation in the absence of premedication. Anaesthesia. 2011;66:368–72. doi: 10.1111/j.1365-2044.2011.06687.x. [DOI] [PubMed] [Google Scholar]
- 14.Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–5. doi: 10.1213/ANE.0b013e3181c04e58. [DOI] [PubMed] [Google Scholar]
- 15.Haenggi M, Ypparila H, Hauser K, Caviezel C, Korhonen I, Takala J, et al. The effects of dexmedetomidine/remifentanil and midazolam/remifentanil on auditory-evoked potentials and electroencephalogram at light-to-moderate sedation levels in healthy subjects. Anesth Analg. 2006;103:1163–9. doi: 10.1213/01.ane.0000237394.21087.85. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim AE, Taraday JK, Kharasch ED. Bispectral index monitoring during sedation with sevoflurane, midazolam, and propofol. Anesthesiology. 2001;95:1151–9. doi: 10.1097/00000542-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Singh H, White PF. Electroencephalogram bispectral analysis predicts the depth of midazolam-induced sedation. Anesthesiology. 1996;84:64–9. doi: 10.1097/00000542-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Shao DH, Hang LH. Effects of dexmedetomidine on performance of bispectral index as an indicator of loss of consciousness during propofol administration. Swiss Med Wkly. 2013;143:w13762. doi: 10.4414/smw.2013.13762. [DOI] [PubMed] [Google Scholar]
- 19.Bol CJ, Vogelaar JP, Tang JP, Mandema JW. Quantification of pharmacodynamic interactions between dexmedetomidine and midazolam in the rat. J Pharmacol Exp Ther. 2000;294:347–55. [PubMed] [Google Scholar]
- 20.Boehm CA, Carney EL, Tallarida RJ, Wilson RP. Midazolam enhances the analgesic properties of dexmedetomidine in the rat. Vet Anaesth Analg. 2010;37:550–6. doi: 10.1111/j.1467-2995.2010.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maciejewski D. Sufentanil in anaesthesiology and intensive therapy. Anaesthesiol Intensive Ther. 2012;44:35–41. [PubMed] [Google Scholar]
- 22.Dhasmana S, Singh V, Pal US. Awake blind nasotracheal intubation in temporomandibular joint ankylosis patients under conscious sedation using fentanyl and midazolam. J Maxillofac Oral Surg. 2010;9:377–81. doi: 10.1007/s12663-010-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti G, Arcangeli A, Antonelli M, Cavaliere F, Costa R, Simeoni F, et al. Sedation with sufentanil in patients receiving pressure support ventilation has no effects on respiration: A pilot study. Can J Anaesth. 2004;51:494–9. doi: 10.1007/BF03018315. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez A, Iyer RR, Morrison DE. Preparation of the patient for awake intubation. In: Hagberg CA, editor. Benumof's Airway Management. Principles and Practice. 2nd ed. St. Louis: Mosby-Year Book Inc; 2007. pp. 263–77. [Google Scholar]
- 25.Xue FS, Liu HP, He N, Xu YC, Yang QY, Liao X, et al. Spray-as-you-go airway topical anesthesia in patients with a difficult airway: A randomized, double-blind comparison of 2% and 4% lidocaine. Anesth Analg. 2009;108:536–43. doi: 10.1213/ane.0b013e31818f1665. [DOI] [PubMed] [Google Scholar]
- 26.Williams KA, Barker GL, Harwood RJ, Woodall NM. Combined nebulization and spray-as-you-go topical local anaesthesia of the airway. Br J Anaesth. 2005;95:549–53. doi: 10.1093/bja/aei202. [DOI] [PubMed] [Google Scholar]