Abstract
Objective:
To introduce the imaging characteristics of moyamoya disease (MMD) using high-resolution magnetic resonance imaging (HR-MRI) and to discuss the role of HR-MRI in differentiating MMD from other intracranial artery diseases, especially intracranial atherosclerotic disease (ICAD).
Data Sources:
This review was based on the data in articles published between 2005 and 2015, which were obtained from PubMed. The keywords included HR-MRI, MMD, ICAD, and intracranial artery diseases.
Study Selection:
Articles related to HR-MRI for MMD or other intracranial artery diseases were selected for review.
Results:
There are differences between the characteristic patterns of HR-MRI in MMD and ICAD. MMD is associated with inward remodeling, smaller outer diameters, concentric occlusive lesions and homogeneous signal intensity, while ICAD is more likely to be associated with outward remodeling, normal outer diameters, eccentric occlusive lesions, and heterogeneous signal intensity. Other intracranial artery diseases, such as dissection and vasculitis, also have distinctive characteristics in HR-MRI. HR-MRI may become a useful tool for the differential diagnosis of MMD in the future.
Conclusions:
HR-MRI of MMD provides a more in-depth understanding of MMD, and it is helpful in evaluating pathological changes in the vessel wall and in differentiating MMD from other intracranial artery steno-occlusive diseases, particularly ICAD.
Keywords: Atherosclerosis, High-resolution Magnetic Resonance Imaging, Intracranial Artery Steno-occlusive Disease, Intracranial Atherosclerotic Disease, Moyamoya Disease
INTRODUCTION
Moyamoya disease (MMD) is an idiopathic cerebrovascular disorder characterized by progressive stenosis or occlusion of the distal end of the internal carotid arteries (ICAs) and their proximal branches, which results in the formation of net-like collateral vessels at the base of the brain.[1] The incidence and prevalence of MMD is high in the East Asia, such as Japan and Korea.[2] It has been said that MMD is one of the leading causes of middle cerebral artery (MCA) steno-occlusion in the younger East Asian population, followed by intracranial atherosclerotic disease (ICAD).
First reported in 1957 in Japan, MMD soon spread worldwide. The diagnostic criteria for MMD, proposed by a research committee organized with the support of the Japanese Ministry of Health and Welfare in 1997, were revised in 2012.[3] The diagnostic criteria have contributed to the accurate diagnosis of this disease, but do not yet allow for a precise diagnosis, which shows that, in order to diagnose MMD, atherosclerosis and other occlusive diseases that mainly involve the distal internal carotid artery (ICA) or proximal MCA should be excluded.[3] A precise diagnosis is so important because different occlusive diseases mean different treatments. Such as, if it is MMD, extracranial-intracranial bypass surgery is the best choice, and if it is dissection, the interventional vascular therapy is the best option, while for vasculitis or atherosclerosis, we chose medicines for treatment firstly. However, the problem we often encounter clinically is how to differentiate those diseases. As a conventional angiographic evaluation focuses on the vessel lumen and not the vessel wall, it is absolutely not precise to make diagnosis because all of those occlusive diseases can present as stenostic or occlusive lesion on the conventional imaging. For example, in certain clinical cases, such as young patients with atherosclerotic risk factors whose cerebral angiographs show typical features of MMD, it is difficult to differentiate MMD from moyamoya-like arteriopathies, such as ICAD and differentiating those diseases based only on the patient's medical history, using factors such as hypertension and diabetes, will not achieve the accuracy that is required.[4]
Using high-resolution magnetic resonance imaging (HR-MRI) techniques, vessel wall imaging findings for different causes of intracranial arterial stenosis have been identified.[5,6,7] Three-dimensional HR-MRI makes it possible to directly observe the vessel wall pathology. Although the MCA is a small, deep-seated intracranial vessel, recent studies have suggested that the MCA wall and lumen can be identified and characterized using HR-MRI with good interobserver and intraobserver reproducibility. From those studies, we have obtained several significant results to differentiate MMD from moyamoya-like arteriopathies, such as ICAD. HR-MRI findings show that MMD is associated with inward remodeling, smaller outer diameters, concentric occlusive lesions, and homogeneous signal intensity in the affected MCA segments compared with ICAD.
In this review, based on previous research and our experience, we describe the characteristic of HR-MRI, focusing on the diseased vessels of MMD and MMD's differential diagnosis. In addition, we discuss current perspectives on the role of HR-MRI.
HIGH-RESOLUTION MAGNETIC RESONANCE IMAGING PROTOCOL
MRI of the intracranial arteries must be performed with high-field MRI (usually 3T or 7T) to acquire a high signal-to-noise ratio. Three-dimensional time-of-flight magnetic resonance angiography, T1-weighted, T2-weighted, proton density weighted, and contrast enhanced proton density images are required. Sample parameters of the imaging sequences include: T1-weighted (repetition time/echo time, 600/12 ms; slice thickness, 1.5 mm; 512 × 512 matrix); T2-weighted (repetition time/echo time, 3000/80 ms; slice thickness, 1.5 mm; 512 × 512 matrix); and proton density (repetition time/echo time, 1000/20 ms; field of view, 200 mm × 200 mm; matrix size, 720 × 720 matrix; slice thickness, 1 mm; interslice gap, 0.5 mm; average, 1).[8]
CHARACTERISTICS OF MOYAMOYA DISEASE ON HIGH-RESOLUTION MAGNETIC RESONANCE IMAGING
High-resolution magnetic resonance imaging has been used recently to identify intracranial vascular wall features. With several advantages over traditional MRI, HR-MRI can visualize the submillimeter arterial wall and enable a high signal-to-noise ratio and minimal scan duration. Conventional luminal images focus on the arterial stenosis ratio and the lesion site and are limited in their ability to demonstrate plaque while HR-MRI is able to directly show the characteristics of diseased vessels and plaque. Recent studies have shown that MMD is associated with inward remodeling, smaller outer diameters, concentric occlusive lesions, and homogeneous signal intensity.
Outward and inward remodeling
In studies of coronary arteries, two arterial remodeling modes were identified: Outward (positive) remodeling and inward (negative) remodeling. The remodeling ratio of MCA stenosis was defined as the ratio of the outer-wall boundary area at the lesion site to that at the reference site. Positive remodeling was defined as a remodeling ratio >1.05, and negative remodeling was defined as a remodeling ratio <0.95.[9,10] Positive remodeling was observed more frequently in symptomatic patients, whereas negative remodeling was observed more frequently in asymptomatic patients [Figure 1].[11,12,13] These histologic characteristics are observed in coronary and carotid plaques. Therefore, the intracranial arteries may share some common vascular biological features with these arteries. Recently, there have been similar reports about intracranial arteries.[12,14] One article showed that symptomatic MCA stenosis on HR-MRI had a greater remodeling ratio, a higher prevalence of positive remodeling and a lower prevalence of negative remodeling compared with asymptomatic stenosis.[12] Another recent study reported that the prevalence of an outward remodeling stenotic area in the symptomatic group was significantly higher than that observed in the asymptomatic group, and the prevalence of an inward remodeling stenotic area was significantly higher in the asymptomatic group than in the symptomatic group.[13] Both studies enrolled patients with MCA stenosis and did not clearly distinguish ICAD from MMD and other arterial stenosis diseases. While reviewing several studies focused on MMD, we observed that most patients with MMD had inward remodeling.[10,15] For example, in an article in 2014 regarding a study of 32 patients with MMD, the average remodeling index of all patients was 0.19 ± 0.11. Although it is not clear whether all patients presented with inward remodeling, the results indicate that the arterial remodeling mode in MMD is definitely inward [Figure 2].[16]
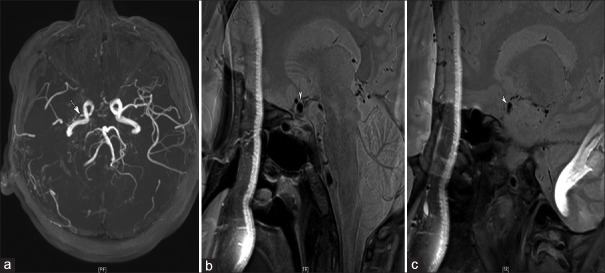
Figure 1.
An example of the remodeling of intracranial atherosclerotic disease in a 54-year-old man with asymptomatic middle cerebral artery stenosis. (a) Severe stenosis at the right M1 segment (arrow), (b) the normal outer boundary of the left M1 segment (arrow), and (c) the stenotic segment of the right M1 presenting with positive remodeling, normal outer diameters, eccentric occlusive lesions, and heterogeneous signal intensity.
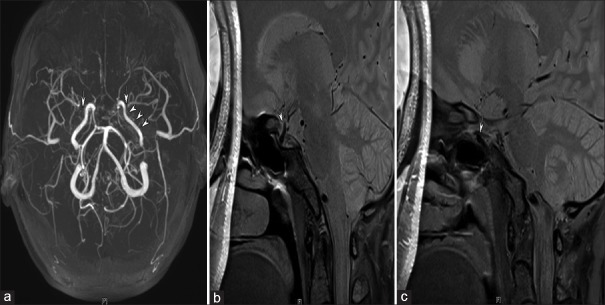
Figure 2.
An example of the remodeling of moyamoya disease in a 35-year-old woman with severe stenosis of the right internal carotid and M1 segment (a), (arrow). (b) The normal left internal carotid artery (arrow), (c) the stenotic right internal carotid artery, and (d) the stenotic right M1 segment. (c and d) Both reveal inward remodeling, smaller outer diameters, concentric occlusive lesions and homogeneous signal intensity in the internal carotid artery and M1 segments of moyamoya disease.
Outer diameter and lesion location
An earlier histopathological study of autopsy material by Takagi et al. compared the average thickness of the MCA in patients with and without MMD and determined that the outer diameter of the MCA in MMD was smaller than the other diameters.[17] Kuroda and Houkin identified the same phenomenon based on intraoperative findings, which suggests that the ICA also undergoes narrowing in MMD.[18] Conventional imaging does not allow direct visualization of the outer diameter of the narrowing diseased artery, but with the development of HR-MRI, more and more studies have demonstrated that the outer diameter of the lesion in the affected artery is smaller than the unaffected arteries in patients with MMD. As we explained above, the arterial remodeling mode in MMD is definitely inward remodeling, as inward remodeling is defined as a remodeling ratio of <0.95, patients with MMD have smaller outer diameters of the affected arteries.
Similar progression may occur in the unaffected segments of the MCA and ICA without arterial stenosis. A recent study demonstrated that the outer diameters of the terminal portion of the ICA and the proximal portion of the M1 segment in MMD patients are significantly smaller than those in normal control and atherosclerotic disease patients, even in unaffected segments [Figure 3]. As stenotic lesions of the extracranial ICA are observed in some patients with MMD, a narrowing of the outer diameters of the ICA and M1 segment may occur due to flow reduction. In that study, the authors excluded patients with cervical ICA occlusion or severe stenosis and analyzed the relationship between the inner and outer diameters in each case. Surprisingly, the final analysis showed that the narrowing of the outer diameters of the ICA and MCA in MMD was unrelated to the progression of luminal stenosis and occlusion found in digital subtraction angiography (DSA) images, indicating that the narrowing of the outer diameters of the ICA and M1 segment in MMD patients may show even mild stenosis on a DSA image. This goes against our hypothesis that the marked narrowing of the outer vascular diameter may precede internal vascular stenotic progression. As the pathological findings of the ICA and MCA of MMD patients indicate concentric intimal fibrous thickening, such intimal lesions were often multilayered with newly formed elastic lamina between the layers. They are different from those in atherosclerosis patients, as the pathologic findings in patients with atherosclerosis include intimal thickening, plaque development, and resultant stenosis of the lumen. Thus, the authors suggested that their findings may indicate that not only decreased flow but also constriction of the tortuous internal elastic lamina could cause the narrowing of the outer diameters in MMD, and both factors may play important role in MMD progression.[10]
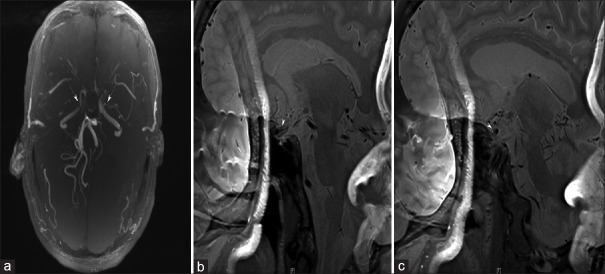
Figure 3.
High-resolution magnetic resonance imaging of a 31-year-old woman with moyamoya disease. (a-c) The left internal carotid artery is smaller than the right internal carotid artery without the stenotic segment, except in the terminal portion.
In MMD, the occlusive lesion is always located in the anterior circulation, the posterior circulation is occasionally involved, and the vertebral artery and basilar arteries (BAs) are rarely involved.[10] Kim et al. recently reported on a study of 12 MMD patients and 20 ICAD patients, which found that the stenotic portion in the MMD group existed in the proximal and middle MCA in all patients, while the stenotic portion in the ICAD group showed random involvement of the proximal, middle, and distal MCA segments.[4]
Eccentric and concentric narrowing
Two narrowing styles were identified in early studies. Generally, if the stenotic portion of the vessel wall was shown to be evenly thick in a circumferential manner on a cross-sectional image of the vessel, the wall abnormality was considered to be concentric; if not, it was considered to be eccentric stenosis.[4] We focused on atherosclerotic stenosis of the coronary arteries to find the mechanism of atherosclerosis. A previous study on carotid and coronary arteries has shown that atherosclerotic plaque is a focal lesion, and cross-sectional images of arteries with plaque showed eccentric narrowing of the stenotic portion.[15] Similarly, several recent studies of atherosclerotic MCA stenosis obtained the same results using HR-MRI.[4,6,19] However, MMD has a different narrowing style than ICAD, and a published article has demonstrated that MMD is characterized by diffuse concentric vessel walls.[13] Yuan et al. reported in 2014 that the rate of concentric stenosis was 91.4% in MMD patients, and the rate of eccentric stenosis was 36.9% in ICAD patients, based on a study of 21 MMD patients and 44 ICAD patients.[15] In the same year, Ryoo et al. reported that of 32 MMD patients, 90.6% showed concentric enhancement on the distal ICAs and MCAs, while all 12 ICAD patients in the study displayed focal eccentric enhancement.[16] The different pathogeneses of arterial narrowing in MMD and atherosclerotic disease may account for different HR-MRI features of the stenotic portions. There is increasing evidence that MMD is primarily a proliferative disease of the intima and that intimal hyperplasia from the proliferation of smooth muscle cells or endothelium can cause stenosis.[20,21] Therefore, diffuse concentric enhancement within vessels could represent hyperproliferation of the vessel wall components, whereas focal eccentric enhancement in patients with ICAD could represent focal atherosclerotic plaque.[16]
We found that almost all of the studies published to date showed that the affected arteries of some MMD patients without any atherosclerotic risks could present as eccentric stenosis, although that accounts for a very small percentage of cases [Figure 4]. A previous histopathological study of MMD reported that the intima of the major arteries showed eccentrically laminated thickening.[22] Therefore, it is possible that some early cases of MMD may present as eccentric stenosis that develops into concentric narrowing.[4] If those patients are under long-term monitoring and follow-up, new information may be revealed that could guide future research.
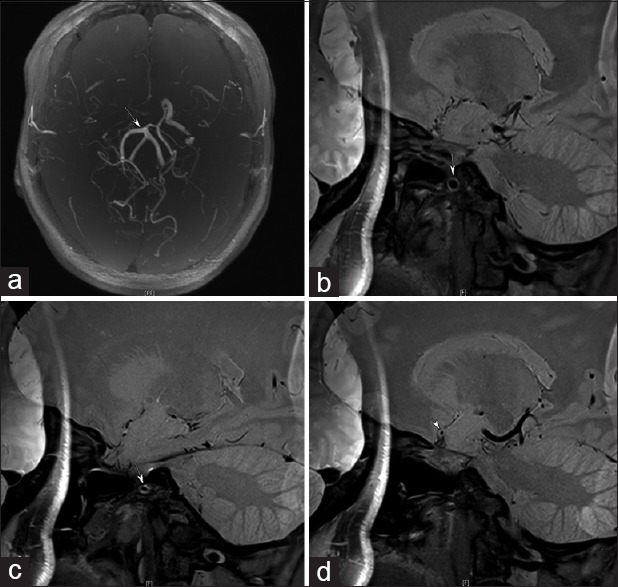
Figure 4.
A patient with moyamoya disease presents occlusion at the proximal portion of the middle cerebral arteries bilaterally (a). (b and c) the affected M1 segment presenting as eccentric stenosis (arrows).
Wall signal intensities
Many histopathological studies of atherosclerotic plaque have been conducted over the years, and it is quite clear from these studies that atherosclerotic arterial walls are characterized by a fibrous capsule, lipid deposition, inflammation, calcification, necrotic cores, and other abnormalities.[23,24] Previous studies demonstrated that different wall signal intensities of stenotic portions shown on MRIs could reflect histopathological differences. Thus, most recent studies using HR-MRI for ICAD have focused on evaluating plaque vulnerability through those wall signal intensities, as there is definitively a difference between the effects of asymptomatic artery stenosis and symptomatic artery stenosis on plaque vulnerability. Published studies have already demonstrated that plaque vulnerability is related to the remodeling pattern, the cross-sectional area of the plaque burden, intraplaque hemorrhage (IPH), and contrast enhancement all of which can be seen with HR-MRI.
For example, IPH on a carotid MRI is considered a risk factor for ischemic stroke.[17] Ryu et al. reported that T1- and T2-hyperintense foci were more frequently observed within the plaque of symptomatic stenoses than within the plaque of asymptomatic stenosis.[14] It was the first report to raise the possibility that a hyperintense lesion in plaque may represent a vulnerable lesion. It is well known that strong contrast enhancement of the plaque means an increase in the vascular supply to the plaque and increased endothelial permeability, which promotes entry of the contrast agent into the extravascular space. Enhancement of the carotid plaque on a carotid wall MRI may be a predictable marker of clinical symptoms although there is still some debate on this.[25,26]
The wall signal intensities of stenotic portions on HR-MRI for MMD mainly represent homogeneous signal intensity. The main histopathological finding of the ICA and MCA in MMD are intimal thickening with increased smooth muscle cells or endothelium[22,27] and diffuse concentric homogeneous signal intensity within vessels, which could represent hyperproliferation of the vessel wall components. In several previous studies of MMD involving HR-MRI, severely narrowed or occluded MCA branches and extensive collateralization were described, but no wall thickening or enhancement were identified. It is more likely that MMD is characterized by homogeneous signal intensity, and there is no difference in the wall signal intensities between symptomatic and asymptomatic patients.
DIFFERENTIAL DIAGNOSIS
Differences between moyamoya disease and intracranial atherosclerotic disease
Both MMD and ICAD are leading causes of MCA steno-occlusion in Asians. Although the diseases have differing vascular wall pathologies, it is not easy to differentiate MMD from ICAD in certain situations, especially in young patients with atherosclerotic risk factors. Five characteristic patterns of HR-MRI in MMD have been described above and are very useful for the differential diagnosis between MMD and ICAD. First, MMD definitely has inward remodeling, with a remodeling ratio of less than 0.95, while both inward remodeling and outward remodeling are possible for ICAD. Positive remodeling was observed more frequently in symptomatic patients, whereas negative remodeling was observed more frequently in asymptomatic patients. Second, the outer diameters of not only the stenotic lesion in the affected artery but also of the unaffected segments, such as the terminal portion of the ICA and the proximal portion of the M1 segment, are smaller than those of unaffected arteries in patients with MMD, while the outer diameter of the stenotic lesion in the affected artery of patients with ICAD is normal or sometimes even larger than normal. Third, in MMD, the occlusive lesion is always located in the anterior circulation, the posterior circulation is occasionally involved, and the vertebral artery and BAs are rarely involved. In ICAD, the stenotic portion shows the random involvement of the proximal, middle and distal MCA segments, and the vertebral artery and BAs are always involved. Finally, previous studies of MMD involving HR-MRI demonstrated that MMD is characterized by diffuse concentric vessel walls. In contrast, cross-sectional images of arteries with atherosclerotic plaque show eccentric narrowing of the stenotic portion. In addition, stenotic MCAs in ICAD patients mainly show eccentric vessel wall involvement with heterogeneous signal intensity, whereas MMD cases typically show concentric vessel wall involvement with relatively homogeneous signal intensities. Thus, there are difference between the characteristic patterns of HR-MRI in MMD and ICAD, and HR-MRI may become a useful tool for the differential diagnosis of MMD in the future.
Differences among intracranial artery diseases
There are different characteristic patterns of HR-MRI in MMD and ICAD. However, it is also important to recognize the characteristic patterns of other intracranial artery diseases, such as dissection, and vasculitis, as those diseases mainly involve distal ICA or proximal MCA occlusion and should be excluded before the confirmed diagnosis of MMD. A helpful article was published in 2015 in Stroke.[8] To investigate the etiologies of MCA steno-occlusive diseases in young adult patients with few atherosclerotic risk factors, the authors enrolled 95 young adult patients with a mean age of 40 years, and patients whose diagnosis was made with certainty were excluded. Patients were ultimately categorized as HR-athero (atherosclerotic disease), HR-MMD (MMD), HR-dissection, or HR-vasculitis.
The authors made presumptive diagnoses based on HR-MRI and previous studies, as follows: (1) Atherosclerotic disease based on HR-MRI findings (HR-athero): An HR-MRI showing eccentric, irregular wall thickening; gadolinium enhancement of plaque may be present, reflecting plaque instability; (2) MMD based on HR-MRI findings (HR-MMD): An HR-MRI showing extensive development of basal collateral vessels and concentric narrowing of the vessel lumen without plaque or eccentric wall thickening; (3) dissection based on HR-MRI findings (HR-dissection): An HR-MRI showing a dissecting flap or eccentric wall thickening associated with T1 bright wall components, representing an intramural hematoma; and (4) vasculitis based on HR-MRI findings (HR-vasculitis): An HR-MRI showing smooth circumferential concentric wall thickening with diffuse gadolinium enhancement of the inflamed wall. The pattern of stenosis was classified into focal (≤1/3 of the M1 segment), segmental (<2/3 of the M1 segment), multifocal, or total involvement.[8]
The final results showed that, of the 95 patients, there were 26 in the HR-athero group, 29 in the HR-MMD group, 22 in the HR-dissection group, and 18 in the HR-vasculitis group. In addition, the authors compared cerebral angiography and HR-MRI findings. Of the patients, 56 (11 of 26 HR-athero, 18 of 29 HR-MMD, 15 of 22 HR-dissection, and 12 of 18 HR-vasculitis) underwent additional conventional angiography. It is surprising that the presumptive diagnoses based on the cerebral angiography and the HR-MRI were identical in all but 13 cases. Among the 18 HR-MMD patients, 4 had presumptive diagnoses of atherosclerosis or MMD by cerebral angiography based on the suspicious development of moyamoya vessels. Among the 15 HR-dissection patients, 6 had a presumptive diagnosis of atherosclerosis by cerebral angiography. Among the 12 HR-vasculitis patients, 2 had suspected MMD, and one had presumed atherosclerosis, from conventional angiography. The authors demonstrated that young adult patients with fewer risk factors could develop intracranial artery stenosis caused by atherosclerosis, and HR-MRI can help make a clear differential diagnosis of intracranial artery steno-occlusive diseases with different pathological changes.
PERSPECTIVE
Definitively, HR-MRI has obvious advantages in the assessment of steno-occlusive lesions compared to conventional imaging technologies. Yamada et al. conducted a recent investigation of 46 patients suspected of having MMD.[28] The patients were examined by high-resolution turbo MR angiography with the ZFI technique, MRI, and conventional angiography. The author reported that high-resolution turbo MR angiography with reduced scan time is highly accurate in the assessment of both steno-occlusive lesions and collateral vessels in MMD, thus providing a highly accurate (98%) diagnosis and assessment of MMD. However, there are many limitations in the studies of MMD involving HR-MRI. The most significant one is that HR-MRI is difficult to correlate with pathology, as it is very difficult to obtain arterial specimens from living patients. Recently, gene tests have become available, and the identification of susceptibility genes may further assist in the diagnosis of MMD. Further studies on MMD patients may be required that used both HR-MRI and gene tests. Intracranial disease was observed to be a dynamic process with both progression and regression over long-term follow-up. Further research should focus on monitoring the progression of artery stenosis to determine the extent to which lesions undergo remodeling. It is well known that the MCA wall is composed of the intima, media, and adventitia. Takagi et al. who studied the histopathology of living tissue in the MCA (M4 segment) of patients with and without MMD, suggested that the average intimal thickness of the MCA in the MMD group was significantly greater than in the control group, but the average media thickness in the MMD group was less than that in the control group.[24] The change of attenuated media is greater than the thickening of the intima in MMD, based on a comparison of the two sets of data. Yuan's et al. study showed that the total thickness of the arterial wall remained the same in the progression of arterial progressive stenosis.[15] Therefore, the author deduced that the thickness of the adventitia may increase but not decrease. Further research is needed in this field.
CONCLUSIONS
In this review, we demonstrate that MMD is associated with inward remodeling, smaller outer diameters, concentric occlusive lesions, and homogeneous signal intensity in the affected MCA segments compared with ICAD. HR-MRI of MMD provides us with a more in-depth understanding of MMD and is helpful in evaluating pathological changes in the vessel wall and in differentiating MMD from other intracranial artery steno-occlusive diseases, especially ICAD.
Financial support and sponsorship
This study was supported by the Program of Beijing Municipal Science and Technology Commission (No. Z13110200680000).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–99. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 2.Ikezaki K, Han DH, Kawano T, Inamura T, Fukui M. Epidemiological survey of moyamoya disease in Korea. Clin Neurol Neurosurg. 1997;99(Suppl 2):S6–10. doi: 10.1016/s0303-8467(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 3.Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (’moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg. 1997;99(Suppl 2):S238–40. [PubMed] [Google Scholar]
- 4.Kim YJ, Lee DH, Kwon JY, Kang DW, Suh DC, Kim JS, et al. High resolution MRI difference between moyamoya disease and intracranial atherosclerosis. Eur J Neurol. 2013;20:1311–8. doi: 10.1111/ene.12202. [DOI] [PubMed] [Google Scholar]
- 5.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–34. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 6.Klein IF, Lavallée PC, Touboul PJ, Schouman-Claeys E, Amarenco P. In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology. 2006;67:327–9. doi: 10.1212/01.wnl.0000225074.47396.71. [DOI] [PubMed] [Google Scholar]
- 7.Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF, et al. Arterial remodeling of advanced basilar atherosclerosis: A 3-tesla MRI study. Neurology. 2010;75:253–8. doi: 10.1212/WNL.0b013e3181e8e714. [DOI] [PubMed] [Google Scholar]
- 8.Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: A high-resolution magnetic resonance imaging study. Stroke. 2015;46:697–703. doi: 10.1161/STROKEAHA.114.008181. [DOI] [PubMed] [Google Scholar]
- 9.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: An intravascular ultrasound study. Circulation. 2000;101:598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 10.Kaku Y, Morioka M, Ohmori Y, Kawano T, Kai Y, Fukuoka H, et al. Outer-diameter narrowing of the internal carotid and middle cerebral arteries in moyamoya disease detected on 3D constructive interference in steady-state MR image: Is arterial constrictive remodeling a major pathogenesis? Acta Neurochir (Wien) 2012;154:2151–7. doi: 10.1007/s00701-012-1472-4. [DOI] [PubMed] [Google Scholar]
- 11.Hardie AD, Kramer CM, Raghavan P, Baskurt E, Nandalur KR. The impact of expansive arterial remodeling on clinical presentation in carotid artery disease: A multidetector CT angiography study. AJNR Am J Neuroradiol. 2007;28:1067–70. doi: 10.3174/ajnr.A0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010;212:507–11. doi: 10.1016/j.atherosclerosis.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Chung GH, Kwak HS, Hwang SB, Jin GY. High resolution MR imaging in patients with symptomatic middle cerebral artery stenosis. Eur J Radiol. 2012;81:4069–74. doi: 10.1016/j.ejrad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Ryu CW, Jahng GH, Kim EJ, Choi WS, Yang DM. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovasc Dis. 2009;27:433–42. doi: 10.1159/000209238. [DOI] [PubMed] [Google Scholar]
- 15.Yuan M, Liu ZQ, Wang ZQ, Li B, Xu LJ, Xiao XL. High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci Lett. 2015;584:77–82. doi: 10.1016/j.neulet.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH, et al. High-resolution magnetic resonance wall imaging findings of moyamoya disease. Stroke. 2014;45:2457–60. doi: 10.1161/STROKEAHA.114.004761. [DOI] [PubMed] [Google Scholar]
- 17.Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Histological features of middle cerebral arteries from patients treated for moyamoya disease. Neurol Med Chir (Tokyo) 2007;47:1–4. doi: 10.2176/nmc.47.1. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda S, Houkin K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008;7:1056–66. doi: 10.1016/S1474-4422(08)70240-0. [DOI] [PubMed] [Google Scholar]
- 19.Li ML, Xu WH, Song L, Feng F, You H, Ni J, et al. Atherosclerosis of middle cerebral artery: Evaluation with high-resolution MR imaging at 3T. Atherosclerosis. 2009;204:447–52. doi: 10.1016/j.atherosclerosis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Fukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology. 2000;20(Suppl):S61–4. doi: 10.1046/j.1440-1789.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin R, Xie Z, Zhang J, Xu H, Su H, Tan X, et al. Clinical and immunopathological features of moyamoya disease. PLoS One. 2012;7:e36386. doi: 10.1371/journal.pone.0036386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita M, Oka K, Tanaka K. Histopathology of the brain vascular network in moyamoya disease. Stroke. 1983;14:50–8. doi: 10.1161/01.str.14.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. Atherosclerosis – An inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 25.Millon A, Mathevet JL, Boussel L, Faries PL, Fayad ZA, Douek PC, et al. High-resolution magnetic resonance imaging of carotid atherosclerosis identifies vulnerable carotid plaques. J Vasc Surg. 2013;57:1046–1051.e2. doi: 10.1016/j.jvs.2012.10.088. [DOI] [PubMed] [Google Scholar]
- 26.Millon A, Boussel L, Brevet M, Mathevet JL, Canet-Soulas E, Mory C, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke. 2012;43:3023–8. doi: 10.1161/STROKEAHA.112.662692. [DOI] [PubMed] [Google Scholar]
- 27.Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR. Moyamoya disease: A summary. Neurosurg Focus. 2009;26:E11. doi: 10.3171/2009.1.FOCUS08310. [DOI] [PubMed] [Google Scholar]
- 28.Yamada I, Nakagawa T, Matsushima Y, Shibuya H. High-resolution turbo magnetic resonance angiography for diagnosis of Moyamoya disease. Stroke. 2001;32:1825–31. doi: 10.1161/01.str.32.8.1825. [DOI] [PubMed] [Google Scholar]