Abstract
The heterocyclic compounds have a great importance in medicinal chemistry. One of the most important heterocycles in medicinal chemistry are quinazolines possessing wide spectrum of biological properties like antibacterial, antifungal, anticonvulsant, anti-inflammatory, anti-HIV, anticancer and analgesic activities. This skeleton is an important pharmacophore considered as a privileged structure. This review highlights the recent advances in the synthesis of quinazolines and quinazolinone derivatives with potent antimicrobial and cytotoxic activities.
Keywords: Quinazolinones, Quinazoline, Antimicrobial, Cytotoxic
1.INTRODUCTION
Quinazolinones and quinazolines are noteworthy in medicinal chemistry, because of wide range of their antibacterial, antifungal (1,2,3,4,5,6), antiinflammatory (7,8), antimalaria (9), anti-HIV (10), antiviral (10,11), antituberculosis (1,12) properties and also their inhibitory effects on thymidylate synthase (13,14), poly-(ADP-ribose) polymerase (PARP) (15,16,17) and thyrosine kinase (18,19). There are several approved drugs with quinazoline structure in the market such as, prazosin hydrochloride, doxazosine mesylate and terazosine hydrochloride (20,21) (Fig. 1).
Fig. 1.
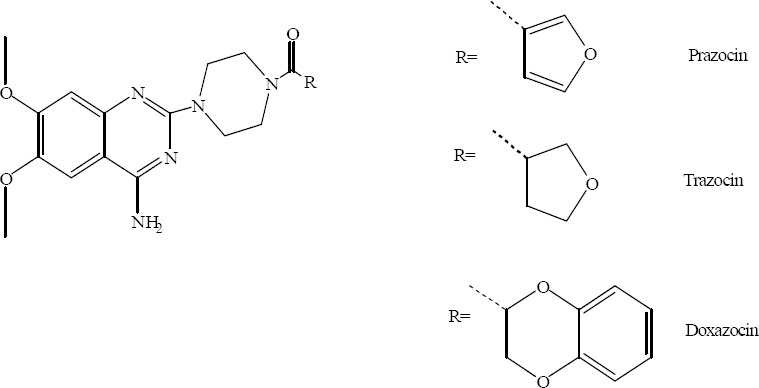
Approved marketed drugs with quinazoline structure.
This review mainly focuses on recent quinazolinones and quinazoline structures with potent antimicrobial and cytotoxic activities. Consideration has been taken to cover the most recent references.
2. Antimicrobial activity
Bacterial resistance to existing drugs is a growing problem in the world. Considerable researches have been performed on the synthesis of new quinazolinone derivatives with potent antimicrobial activity. These derivatives possess antibacterial activities, especially against the gram positive strains, and fungi through their interaction with the cell wall and DNA structures (22).
Structure activity relationship studies of quinazolinone derivatives in various literatures have revealed that substitution at positions 2 and 3, existence of halogen atom at 6 and 8 positions and substitution (mainly amine or substituted amine) at 4th position of the quinazolinone ring can improve their antimicrobial activities (Fig. 2) (3,23,24,25,26). The presence of substituted aromatic ring at position 3 (3) and methyl, amine or thiol groups at position 2 are essential for antimicrobial activities.
Fig. 2.
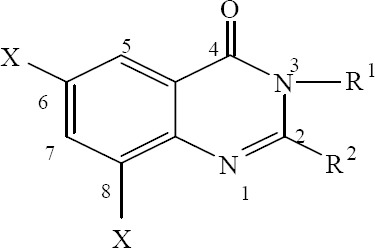
Quinazolinone basic structure.
Several N3-sulfonamide substituted quinazolinone derivatives were prepared by Zayed and colleagues. Substitution of the main aromatic ring of quinazolinone with iodine at 6 and 8 positions significantly improved antibacterial activity (Fig. 3) (23).
Fig. 3.
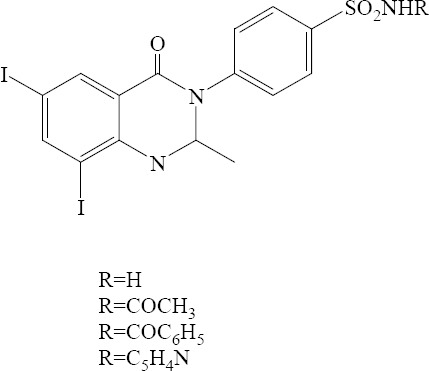
6,8-Diiodo-2-methyl-3-substituted-quinazolin-4 (3H)-ones derivatives.
Raval and coworkers developed a simple and reproducible technique for synthesis of various pyrazolyloxopropyl-quinazolin-4(3H)-one derivatives by connecting the pyrazolyl moiety at 2 position of the quinazolinone nucleus with short reaction times, excellent yields, and without formation of undesirable side products. Representative compounds showed significant activity in antimicrobial screen is depicted in Fig. 4 (27).
Fig. 4.
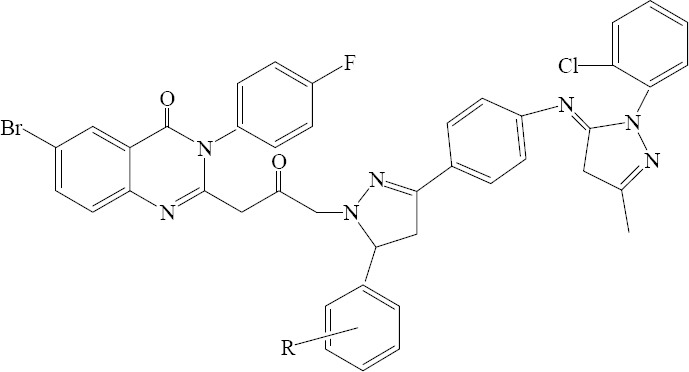
6-Bromo-2-(3-(3-(4-(1-(2-chlorophenyl) -3-methyl-1H-pyrazol-5(4H)- ylideneamino) phenyl)-5- (substituted phenyl) -4,5-dihydro-1H-pyrazol-1-yl) -2-oxopropyl) - 3-(4-fluorophenyl) quinazolin-4(3H)-one.
Sojitra and colleagues have reported a simple and efficient methodology for the synthesis of new 4-(3,5-dimethyl-1-phenyl-1H-pyrazol-4-ylazo)- N -(2-substituted-4-oxo-4H-quinazolin-3-yl) benzene sulfonamide derivatives in good yields. The in vitro antimicrobial assays of the new compounds revealed that their antibacterial effects were superior to their antifungal activities (Fig. 5) (28).
Fig. 5.
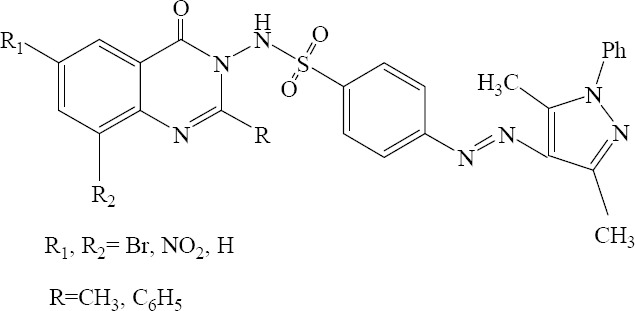
4-(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-ylazo) – N - (2 – substituted - 4 – oxo - 4H-quinazolin - 3 - yl) benzenesulfonamide derivatives.
A series of 2-benzyl-3-{4-[N′-(3-substituted-5 – oxo - 1-substituted - 1,5 – dihydropyrazol – 4 -ylidene) hydrazino] phenyl}-3H-quinazoline-4-one derivative were synthesized by Unnissa and coworkers. The synthesized compounds were screened for their antibacterial and antifungal activities against pathogenic bacteria and fungi. Compounds exhibited good antibacterial and antifungal activities against the tested microorganisms (Fig. 6) (29).
Fig. 6.
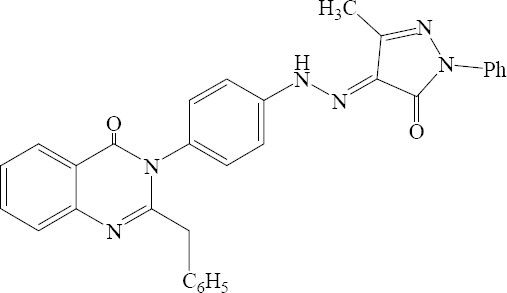
2- benzyl – 3 - {4 - [N ‘- (3-methyl - 5- oxo - 1,5-dihydropyrazole-4 ylidene) hydrazino] phenyl}-3H-quinazoline-4-one.
New derivatives with incorporation of an indole and a fluorinated aromatic ring at 2 and 3 positions of quinazolinone, respectively, were prepared by Dave and colleagues. All the newly synthesized compounds were screened for antibacterial activity. Three derivatives including a, (2-fluoro benzylideneamino) b, (benzylideneamino) and c, (2-nitro benzylideneamino) were found to be active against S. aureus (Fig. 7) (25).
Fig. 7.
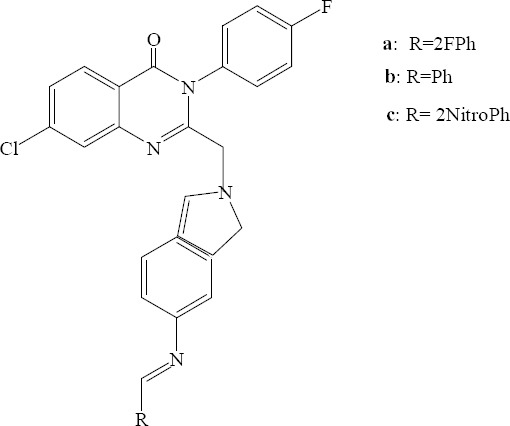
7-Chloro-3-(4-fluorophenyl)-2-((5-(substituted amino) -1H-indol-1-yl)methyl) quinazolin-4(3H)-one derivative.
Aza isatins are biological active compounds which are mainly used for their antibacterial and antifungal activities. Devi and coworkers have reported the synthesis of some new aza isatin derivatives containing 4(3H) quinazolinones. The N-hexyl substituted isatin-quinazoline derivative has been found to be relatively active against screened gram positive, gram negative bacteria and fungi species in comparison to other compounds (Fig. 8) (26).
Fig. 8.
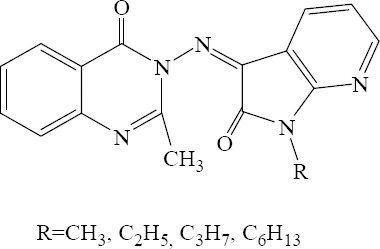
3-(1,2-Dihydro-1-substituted-2-oxopyrrolo[2,3-b]pyridin - 3 - ylideneamino) - 2 - methylquinazolin - 4(3H) -ones.
Synthesis and antimicrobial evaluation of novel 2-(chloromethyl)-3-(4-methyl-6-oxo-5-[(E) - phenyldiazenyl] – 2 – thioxo - 5,6 –dihydropyrimidine - 1(2H) - yl)quinazoline -4(3H)-ones derivatives were carried out by Kumar and coworkers (Fig. 9). The data from antibacterial examinations indicated that the substituent on the phenyl ring exerted significant influence on the antibacterial profile as compounds a, b and c with methoxy; and methyl-substituted ring were more active molecules compared to other compounds bearing other electron donating or withdrawing groups. The results also indicated that gram positive bacteria were more susceptible towards this newly synthesized quinazoline-4(3H)-ones (30).
Fig. 9.
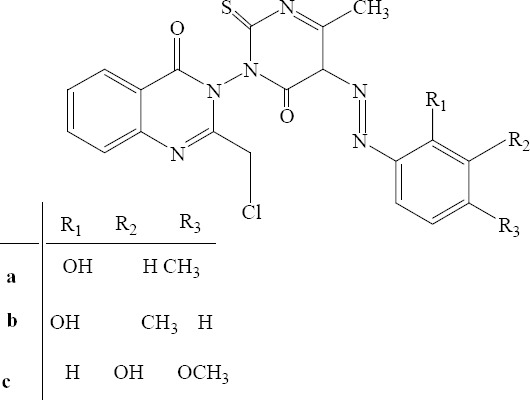
2-(Chloromethyl)-3-(4-methyl-6-oxo-5-[(E)-phenyldiazenyl]-2-thioxo-5,6-dihydropyrimidine-1(2H)-yl) quinazoline-4(3H)-ones derivatives.
Desai and colleagues have reported the synthesis and in vitro antimicrobial activity of various 2-(2-chloro-6-methyl(3-quinolyl))3-[2-(4 - chlorophenyl) - 4 -oxo(3-hydroquinazolin-3 - yl)] - 5 -[(aryl)methylene]-1,3-thiazolidin-4-ones. Some derivatives bearing chloro or hydroxy groups on R’ exhibited very good antimicrobial activities. Additionally, R′=2OH and R′=3OH substituted derivatives exhibited excellent activities against both bacterial and fungal species. It seems that the hydroxy group at ortho or meta position are important for enhancing activity against both bacterial and fungal species (Fig. 10) (31).
Fig. 10.
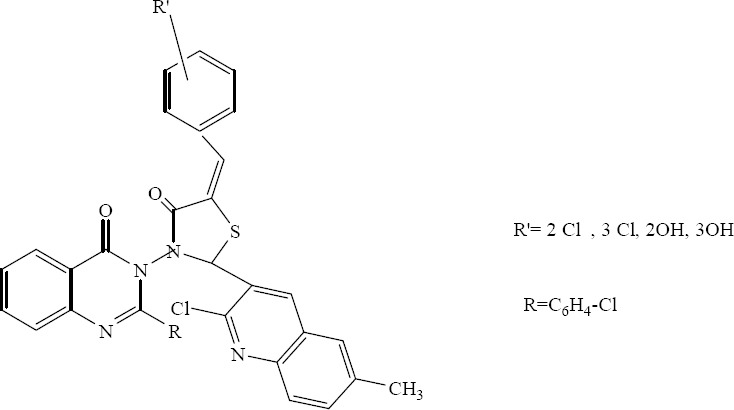
2-(2-Chloro-6-methyl (3-quinolyl))3-[2-(4-chlorophenyl)-4-oxo(3-hydroquinazolin-3-yl)]-5-[(aryl) methylene]-1,3-thiazolidin-4-ones.
A series of 2-oxo-azetidinyl-quinazolin-4(3H)-ones have been synthesized from their corresponding Schiff bases derivatives by Patel and coworkers (Fig. 11).
Fig. 11.
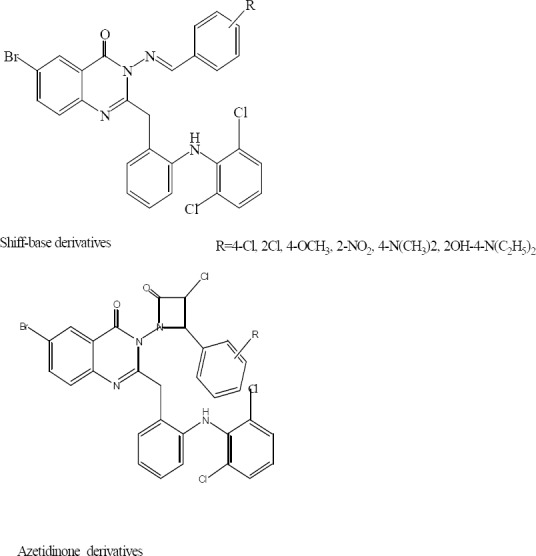
6-Bromo-2-[2-(2,6-dichlorophenyl)amino] benzyl-3-(nitro or hydroxyl or methoxy,.benzylideneamino)-quinazolin-4(3H)-one (Shiff base derivatives) and 6-bromo-3-[3-chloro-4-(-nitro, or hydroxyl or methoxy, phenyl)-2-oxoazetidin-1-yl]-2-[2-(2,6-dichloro phenyl) amino]benzyl-quinazolin-4(3H)-one one (azetidinone derivatives).
Compounds containing chloro or methoxy groups showed good antimicrobial activity in most cases. 2-Azetidinone derivatives were found to be more active than the Schiff bases. However, both Schiff bases as well as 2-azetidinones possessed moderate to poor antifungal activity. Compounds containing 4-dimethylamino or 2-hydroxy-4-diethylamino groups were found to be inactive against bacterial species (32).
Dimeric 2-(2-chlorophenyl)-quinazolin-4-ones had been prepared and introduced as potential antimicrobial agents by Desai and coworkers. The aromatic substituted derivatives as shown in Fig. 12 had very good activity against several strains of bacteria (33).
Fig. 12.
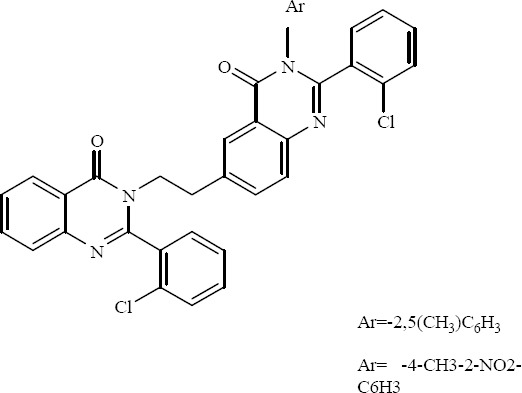
3- (Aryl) - 2-(2-chlorophenyl) – 6 - {2 - [2 - (2 -chlorophenyl)-4-oxo (3-hydroquinazolin-3yl)]ethyl}-3-hydroquinazolin-4-ones.
Some new 2,3-disubstituted (3H)-quinazolinone derivatives have been synthesized by Hassanzadeh and colleagues (34). Khodarahmi and coworkers have evaluated antibacterial and antifungal effects of these compounds. All synthesized compounds indicated mild to high antibacterial effects especially against gram-negative bacteria. All tested strains of fungi were sensitive to these compounds as well (Fig. 13) (35).
Fig. 13.
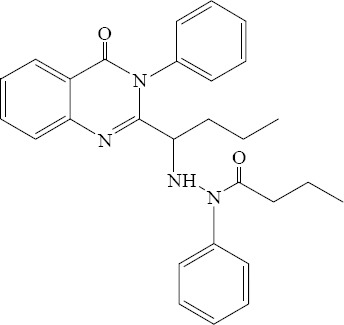
N′-(1-(4-Oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)-N-phenylbutyrohydrazide.
A series of novel derivatives of 3-substituted-2-thioxoquinazolin4(3H)-ones have been prepared by Rajasekaran and colleagues. Compound 2-[(2,3-dimethyl-phenyl)-(4-oxo-3-phenyl-2-thioxo-3,4-dihydro-2H-quinazolin - 1-ylmethyl)-amino]-benzoic acid showed broad spectrum of activity against all the tested gram positive, gram negative bacteria and the fungi (Fig. 14) (36).
Fig. 14.
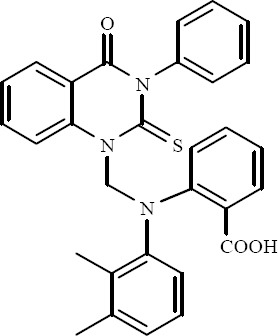
2-[(2,3-Dimethyl-phenyl)-(4-oxo-3-phenyl-2-thioxo-3,4-dihydro-2H-quinazolin-1-ylmethyl)-amino]-benzoic acid.
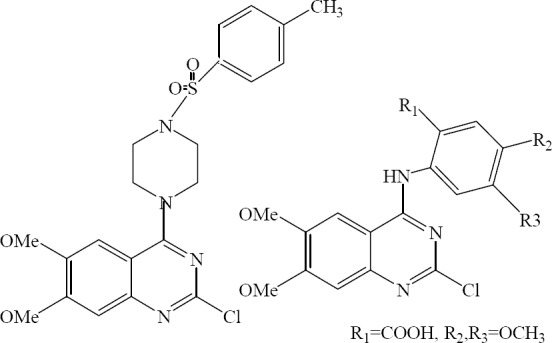
A new series of 2-heteroarylthio-6-substituted-quinazolin-4-one analogs were designed synthesized and evaluated for their in vitro antimicrobial activity by Al-Omary. 2-(6,7-Dimethoxy-3-benzyl-4-oxo-3,4-dihydro-quinazoline-2-ylthio) nicotinic acid showed broad spectrum antimicrobial activity comparable to the known standard antibiotic (Fig. 15) (37).
Fig. 15.
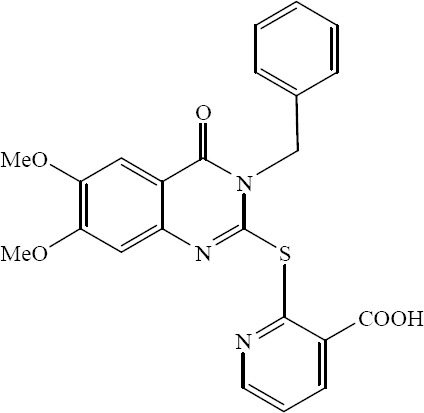
2-(6,7-Dimethoxy-3- benzyl-4-oxo-3,4-dihydro quinazoline-2-ylthio) nicotinic acid.
6,7-Bis(arylthio)-quinazoline-5,8-dione and furo-[2,3-f]quinazolin-5-ol derivatives were synthesized and tested for in vitro antifungal activity by Ryu and coworkers (Fig. 16).
Fig. 16.
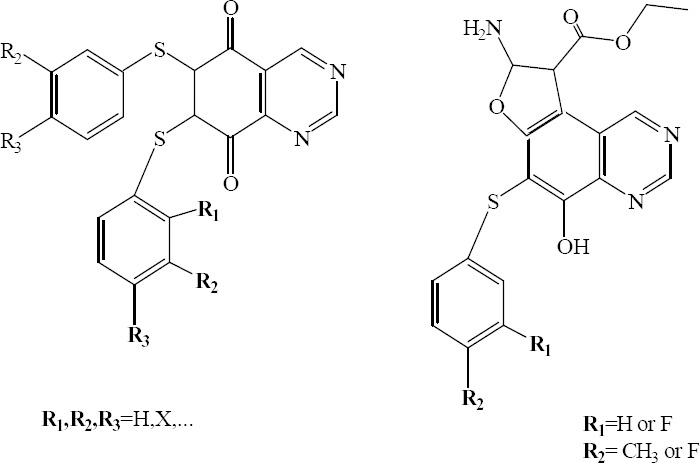
6, 7-Bis(arylthio)-quinazoline-5,8-dione (left), furo[2,3-f]quinazolin-5-ols(right).
Among tested compounds, many of furo [2, 3-f] quinazolin-5-ols and 6,7-bis(arylthio)-quinazoline-5,8-diones showed good antifungal activity against all tested fungi. The results suggested that furo [2, 3- f] quinazolin-5-ol and 6,7-bis(arylthio)-quinazoline-5,8-dione would be promising leads for the development of antifungal agents (38).
Shi and colleagues synthesized potential antimicrobial agents containing two groups: a polyhalobenzonitrile and 5-arylamine (or alkylamine)-8-aminoquinazolin-4(3H)-one. Compounds were screened for their in vitro antibacterial and antifungal activities. The results showed that most of the tested compounds had variable inhibitory effects on the growth of gram-positive, gram negative bacteria or fungal strains. Introduction of haloaniline or simple alkylamine into the 5 position and 2,4,5-trichloro-isophthalonitrile into the 8 position of quinazolin-4(3H) one, could improve antimicrobial activity in all synthetic derivatives (Fig. 17) (39).
Fig. 17.
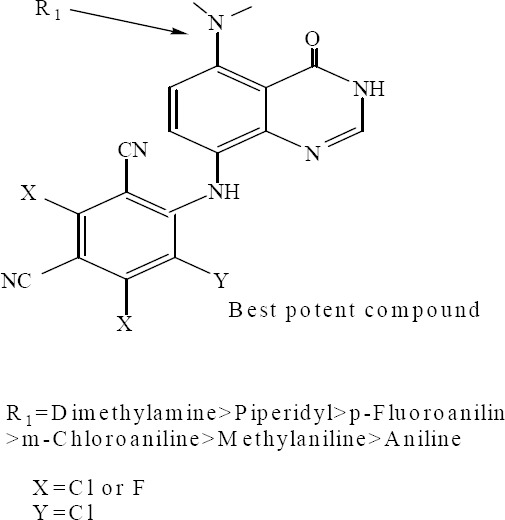
5-arylamine (or alkylamine)-8-amino polyhalo benzonitrile quinazolin-4(3H)-one derivatives.
Mohamed and coworkers have reported the synthesis of some of substituted quinazolinones. Their results indicated that although the synthesized quinazolinone derivatives (II, III and IV) (Fig. 18) were powerfully active against gram-positive bacteria as compared to streptomycin, they had no effect on Pseudomonas aeruginosa, as representative gram negative species. The order of the activity of the three derivatives was II, IV and III. Compound II was found to be the most active gyrase inhibitor as illustrated by the docking study. The reasonable antibacterial activity against gram-positive bacteria may be attributed to their better permeability to the bacterial cell wall. Structure activity relationship of these compounds demonstrated that substitution of the N-H hydrogen at position 3 of the pyrimidine ring with ethyl acetate (II) increased the activity with respect to the unsubstituted parent compound I. Therefore, the anti-bacterial activity of II may be in part due to the prevention of lactam-lactim tautomerism and the presence of active methylene at position 2, as an electron releasing group as shown in Figs. 18 and 19. The activity of compound II against fungi may be due to the presence of a highly negatively charged region of –CH2CN at position 2 beside carbonyl oxygen at position 4 in the pyrimidine ring. The -CH2CN moiety can attack the chemical components of the cell wall by either direct interaction or by disturbing the electron balance of the cell wall. However, decreased activity of derivative IV may be related to the presence of an amide group at position 2 of the pyrimidine ring which is less active than the cyano group in II (22).
Fig. 18.
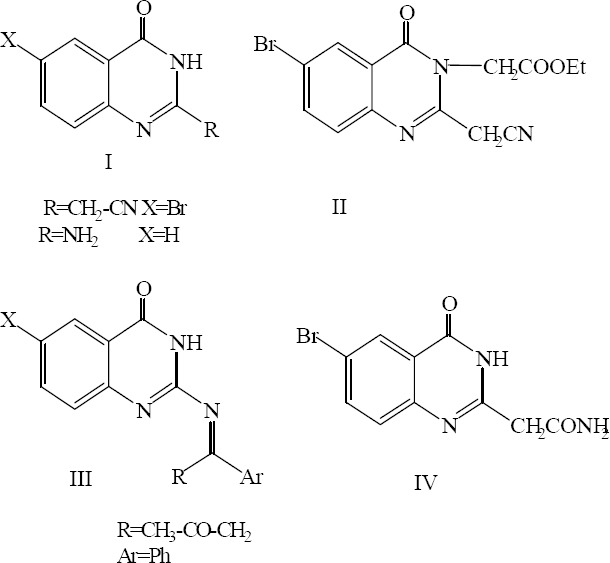
Substituted quinazolinones.
Fig. 19.
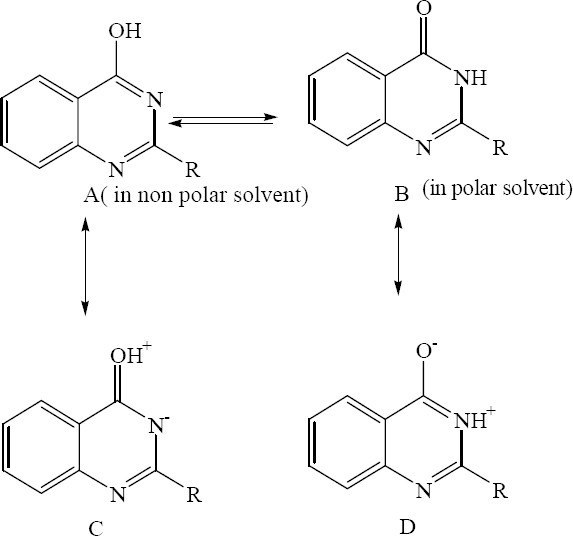
Lactam-lactim dynamic equilibrium.
Lactam-lactim dynamic equilibrium is a classical solvent dependent equilibrium in organic synthesis. Quinazolinone derivatives are normally found in more polar conformer (B) (lactam form) which is more stable in polar solvent or in aqueous media. Form (A) will be found only in non-polar solvents or in gas phase. Among the canonical forms (D) and (C), the (D) form with oxygen (negative charge) and nitrogen (positive charge) has much greater stability than the (C) form (Fig. 19), consequently, O-alkylation of quinazolinone ring should be more likely in polar solvents. Accordingly, El-Badry and coworkers have developed the synthesis of substituted quinazolines at 4-position by O-alkylation depending on the lactam-lactim dynamic equilibrium phenomena (24).
In a study of the effect of solvent polarity on lactam-lactim dynamic equilibrium of quinazolin-4(3H)-one, acylation of quinazolin-4(3H)-one with acetyl chloride (used also as solvent) generated 4-acetoxyquinazoline as a sole product while 3-acetyl quinazolinone derivative was produced from above reaction by changing solvent polarity. This illuminated that, in the presence of acetyl chloride as the solvent, quinazolinone exists in lactim form while in pyridine (polar solvent) it exists in lactam form (Fig. 20) (24).
Fig. 20.
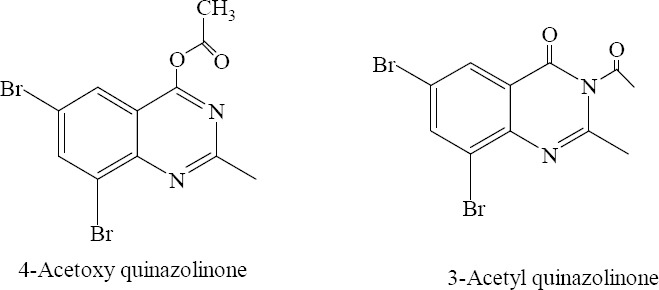
4-Acetoxy-6, 8-dibromo-2-methylquinazolin-4 (3H)-one, 3-Acetyl-6,8-dibromo-2-methylquinazolin-4 (3H)-one.
Saxena and colleagues have carried out the synthesis of some thiosemicarbazone derivatives. Thiosemicarbazones possess both – N-C=S and –CH=N- groups as pharmacophore. Synthesized compounds were evaluated against different human pathogens. Compounds having p-nitro and m-nitro substituents showed marked activity against Klebsiella pneumoniae and Curvularia lunata. All compounds showed moderate to minimum activity against Aspergillus fumigatus and Escherichia coli, respectively (Fig. 21) (40).
Fig. 21.
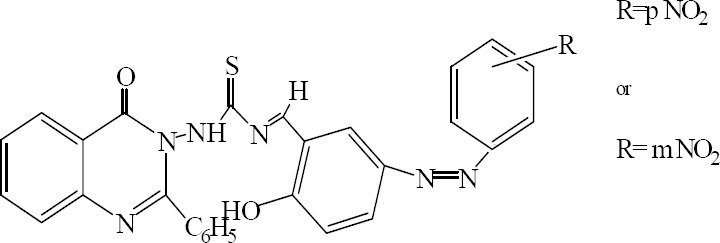
1-[2-Hydroxyl–5–(substituted phenyl)diazyl benzylidene-3-(4-oxo-2-phenylquinazolin-3(4H)-yl) thiourea.
A series of 1-(substitutedbenzylidene)-4-(4-(2-(methyl/phenyl)-4-oxoquinazolin-3(4H)-yl) phenyl) semicarbazide derivatives were synthesized by Saravanan and coworkers with the aim of developing potential antimicrobial agents. The in vitro antibacterial and antifungal properties were tested against some human pathogenic microorganisms. Based on the results obtained, compound shown in Fig. 22 was very active compared to the rest of the tested compounds (41).
Fig. 22.
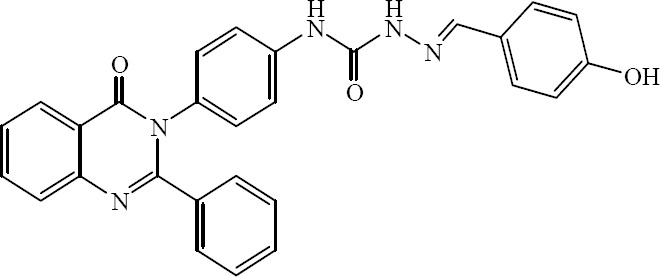
1-(4-Hydroxybenzylidene)-4-(4-(4-oxo-2-phenylquinazolin-3(4H)-yl) phenyl) semicarbazide.
A series of urea/thiourea/acetamide/sulphonamide derivatives of quinazolinones conjugated with lysine have been synthesized by Suresha and coworkers. All compounds have been evaluated against variety of pathogens for their antibacterial activity and structure-activity relationship has been developed. The activity profile revealed that the compounds containing urea and thiourea along with fluoro group exerted a highly potent activity (Fig. 23) (42).
Fig. 23.
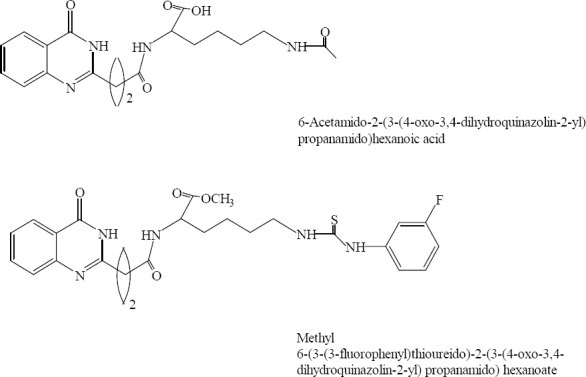
Acetamide / thiourea/sulphonamide/urea/derivatives of quinazolinones conjugated with lysine.
A series of 3-[benzimidazo(1,2-c)quinazolin-5-yl]-2H-chromene-2-one and 3-[benzothiadiazoleimidazo(1,2-c)quinazolin-5-yl] - 2H - chromene - 2 - one derivatives incorporating a variety of substituents at the coumarin moieties have been synthesized by Kuarm and colleagues. These analogs were evaluated for their antimicrobial activity against bacteria and fungi. 6,8-Dichloro analog with 3-[benzimidazo(1,2-c)quinazolin-5-yl]-2H-chromene-2-one and 6,8-dibromo analog of 3-[benzothiadiazoleimidazo(1,2-c)quinazolin-5-yl]-2H-chromene-2-one were shown to be potent antimicrobial agents (Fig. 24) (43).
Fig. 24.
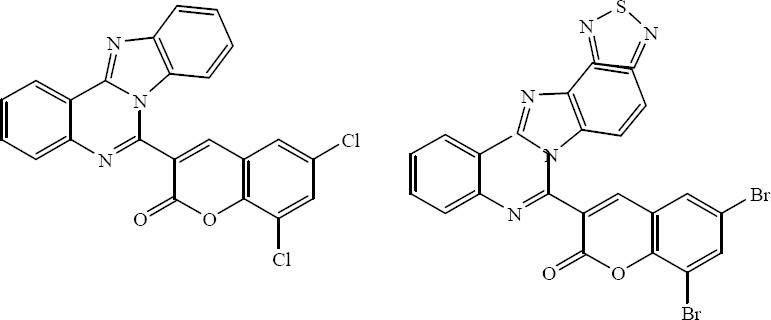
3-[Benzimidazo(1,2-c)quinazolin-5-yl]-2H-chromene-2-one (left) and 3-[benzothiadiazoleimidazo(1,2-c)quinazolin-5-yl]-2H-chromene-2-one (right).
A number of tricyclic quinazolinone derivatives including fused pyrazolo or pyridazino-quinazolinones and fused pyrrolo-quinazolinones were synthesized from substituted anthranilic acids and chloroacyl chlorides by jafari and coworkers (44) (Fig. 25). Hassanzadeh and coworkers (45) and Khodarahmi and colleagues (46) have evaluated antibacterial and antifungal effects of these compounds. The results showed that the tested compounds had better bacteriostatic activity against gram-negative bacteria. The results also revealed that compounds had more significant bacteriostatic than bactericidal activities. Overall the results showed that C. albicans and A. niger were highly sensitive fungi to the compounds.
Fig. 25.
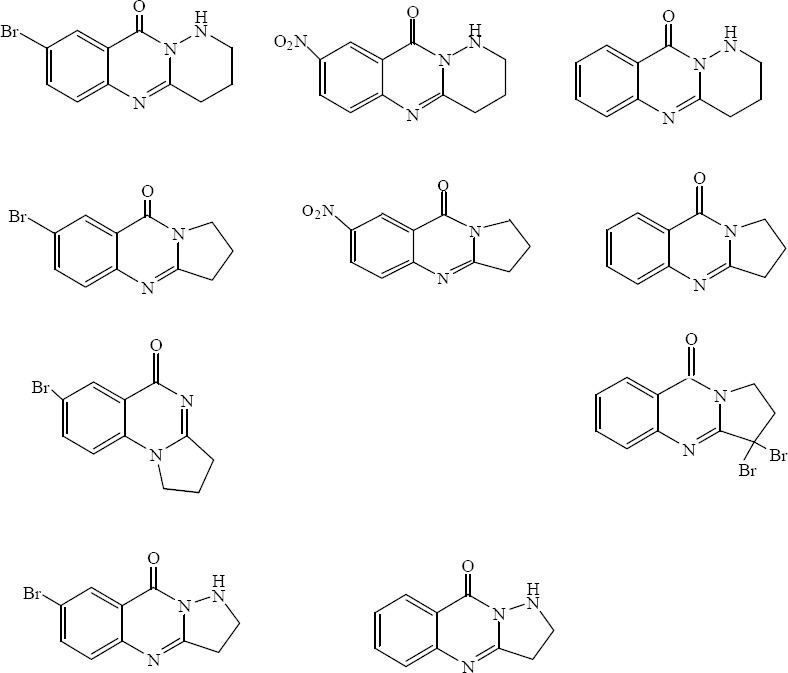
Tricyclic quinazolinone derivatives include fused pyridazino or pyrrolo -quinazolinones and fused pyrazolo–quinazolinones.
A new series of 6-iodo-2-thienylquinazolin-4(3H)-one and its fused heterocyclic analogs were prepared and tested for their antimicrobial activity by Alafeefy. Compounds shown in Fig. 26 had remarkable broad spectrum antimicrobial activities (47).
Fig. 26.
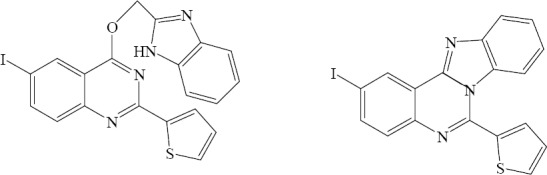
2-(2-Thienyl)-4-(2-benzimidazolylmethyloxy)-6-iodoquinazoline, 6-(2-Thienyl)-2-iodo-benzimidazo [1,2-c]-quinazoline.
3. Cytotoxic activity
During the last decade lots of anticancer drugs with quinazoline structure have been discovered. Gefitinib (Iressa) and erlotinib are important examples of this group which were introduced to the market as anticancer agents (Fig. 27) (21,48).
Fig. 27.
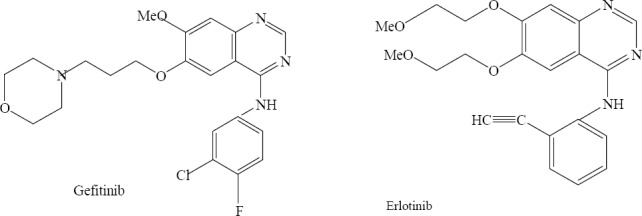
Quinazolinones with well known anticancer activity.
The proposed anti-cancer mechanisms for quinazolines include: 1) inhibition of the DNA repair enzyme system (17,49,50), 2) inhibition of epidermal growth factor receptor (EGFR) (a cellular trans-membrane tyrosine kinases that is over-expressed in a significant number of human tumors (48), 3) thymidylate synthase inhibition (37), and 4) inhibitory effects for tubulin polymerase (51), some representative examples are explained below:
Poly(ADP-ribose) polymerase-1 (PARP-1) is involved in many fundamental processes including DNA repair and transcriptional regulations. A novel series of pyrazolo [1,5-a]quinazolin-5(4H)-one derivatives introduced as potent class of PARP-1 inhibitors by Orvieto and colleagues. Phenyl substituent at the 2-position of the tricyclic system provided submicromolar inhibitors. Introduction of polar amides in position 2 of the tricyclic system led to the discovery of nanomolar inhibitors (Fig. 28) (17).
Fig. 28.
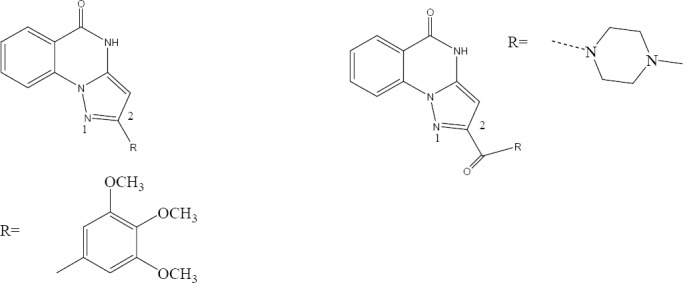
Pyrazolo[1,5-a]quinazolin-5(4H)-one derivatives.
2-{3-[4-(4-Fluorophenyl)-3,6-dihydro-1(2H)-pyridinyl]propyl}-8-methyl 4(3H)quinazolin -one is identified by Kinoshita and coworkers as potent PARP1 inhibitor with an IC50 value of 14 nM (Fig. 29) (50).
Fig. 29.
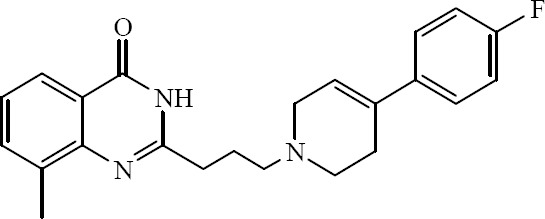
2-{3-[4-(4-Fluorophenyl)-3,6-dihydro-1(2H)-pyridinyl]propyl}-8-methyl-4(3H)-quinazolin-one.
4- Aminoquinazoline represents a new class of anticancer drugs. These compounds are good inhibitors of epidermal growth factor receptor overexpressed through the inhibition of EGFR autophosphorylation. Wissner and colleagues discovered derivatives of quinazoline which showed EGFR kinase inhibitory activity (Fig. 30) (52).
Fig. 30.
Quinazolinone with EGFR kinase inhibitory activity.
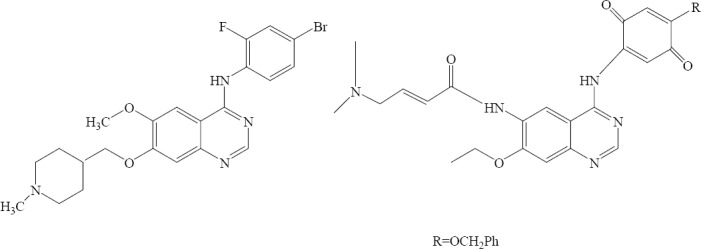
Two series of new 6-alkoxy-4-substituted-aminoquinazolines with EGFR kinase inhibitory activity were designed and synthesized by Abouzid and coworkers. Most of the tested compounds showed potent antitumor activity with IC50 values in the nanomolar range (Fig. 31) (53).
Fig. 31.
6-Alkoxy-4-substituted-aminoquinazolines.
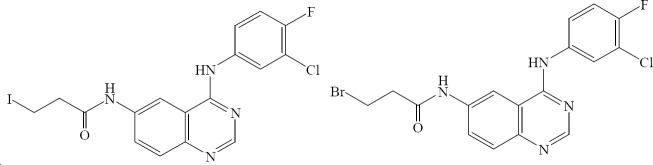
Fernandes and colleagues demonstrated that the introduction of a β-halopropionamide chain at the 6th position of the quinazoline moiety has improved the capability of inhibition of autophosphorylation of EGFR, when compared to the parent quinazoline (Fig. 32) (54).
Fig. 32.
Quinazolines with β-halopropionamide chain at the 6th position.
Al-obaid and coworkers synthesized 2-(2-thieno)-6-iodo-3-phenylamino-3,4-dihydro-quin-zolin-4-one and 2-(2-thieno)-4-[4-sulfonamido-benzylamino] - 6 - iodo - quinazoline. These compounds were most active members for inhibition of EGFR. (Fig. 33) (55).
Fig. 33.
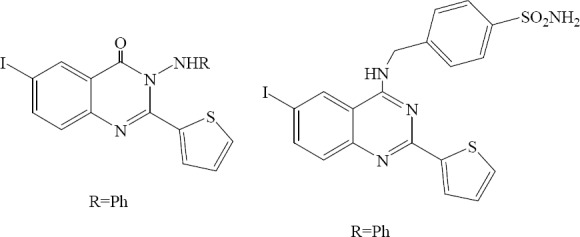
2-(2-Thieno)-6-iodo-3-phenylamino-3,4-dihydro-quina-zolin-4-one, and 2-(2-thieno)-4-[4-sulfonamidobenzylamino]-6-iodo-quinazoline.
Dihydrofolate reductase (DHFR) which catalyzes the reduction of folate or 7,8-dihydrofolate to tetrahydrofolate and intimately couples with thymidylate synthase (TS) is a target for developing new cytotoxic quinazolinones. Inhibition of DHFR or TS activity, leads to ‘thymineless cell death′. Al-Omary and colleagues designed and evaluated 3-benzyl-2-cinnamylthio-6-(methyl or nitro)-quinazolin-4(3H)-ones as active DHFR inhibitors. Raltitrexed which is an inhibitor of thymidylate synthase has been registered for the first-line treatment of advanced colorectal cancer (Fig. 34) (56).
Fig. 34.
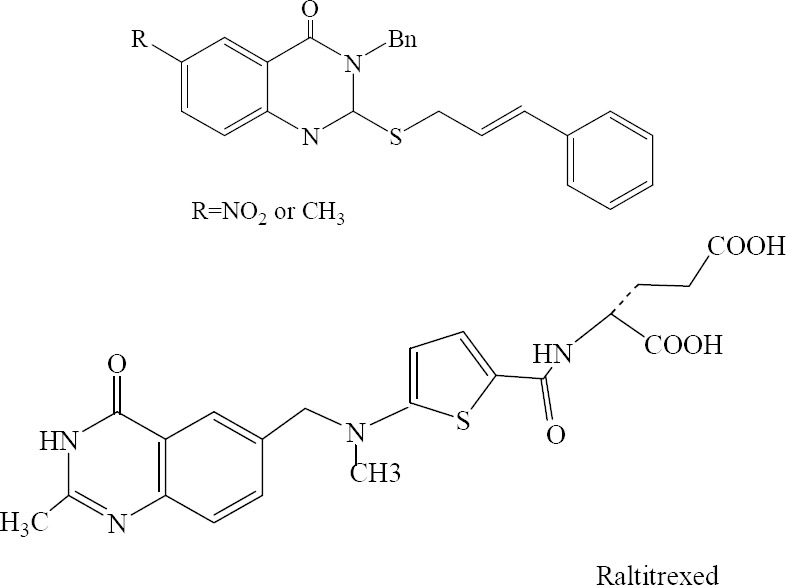
3-Benzyl-2-cinnamylthio-6-(methyl or nitro)-quinazolin-4(3H)-ones and raltitrexed as active DHFR inhibitors.
A new series of 2-heteroarylthio-6-substituted-quinazolin-4-one analogs were designed, synthesized and evaluated for their in vitro DHFR inhibition by Al-Omary and coworkers (Fig. 35) (37).
Fig. 35.
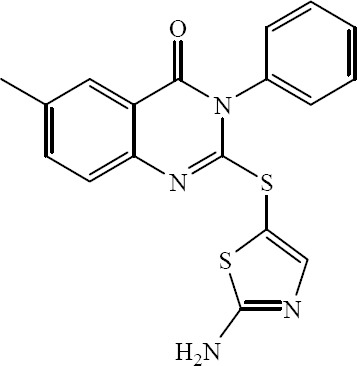
2-(2-Aminothiazol-5-ylthio)-3-phenyl -6-methyl-quinazolin-4(3H)-ones.
The essential role of microtubules in mitosis and cell division makes them important targets for anticancer drugs. Several 2-styrylquinazolin-4(3H)-ones have been prepared by Raffa and colleagues. These compounds were active against certain types of cancer by inhibiting tubulin polymerization (Fig. 36) (57).
Fig. 36.
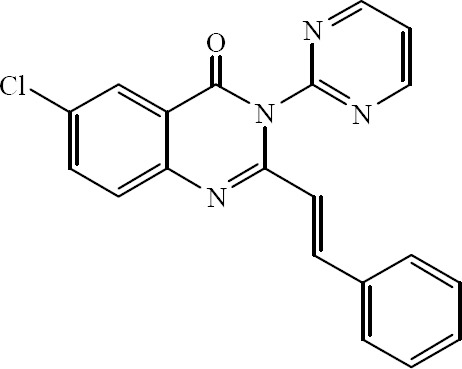
6-Chloro-3-(pyrimidin-2-yl)-2-styryl quinazolin-4(3H)-one.
4. CONCLUSION
Quinazolinone is an important pharmaco-phore which is considered to be a privileged structure in medicinal chemistry. These structures represent molecules which are capable of binding at multiple sites with high affinity and facilitate more rapid discovery of useful medicinally active compounds. Alfuzosine hydrochloride, prazosin hydrochloride, doxazosine mesylate and terazosine hydrochloride are approved drugs with quinazolinone structure in the market.
Literature review of the structure activity relationship studies of quinazolinone ring revealed that substituents at positions 2 and 3, existence of halogen atom at 6 and 8 positions and substituents, mainly amine or substituted amines, at 4th position can improve antimicrobial activities. Also the presence of substituted aromatic ring at position 3 and methyl or thiol group at position 2 are essential for antimicrobial activities.
Cytotoxic activity of the quinazolinone derivatives on various cell lines for example HeLa, L1210, and HT29 has also been reported. Gefitinib (Iressa®) and erlotinib are derivatives of quinazolinones introduced to the market as anticancer agents. Proposed anticancer mechanisms for quinazoline derivatives include: 1) inhibition of the DNA repair enzyme system, 2) inhibition of EGFR, 3) thymidylate enzyme inhibition and 4) inhibitory effects for tubulin polymerize. Future investigation of quinazolinone structure could give some more hopeful results in the field of medicinal chemistry.
REFERENCES
- 1.Samira I, Patel S, Hasmin M, Patel S. Biological profile of quinazoline. Int J Pharm Chem Sci. 2012;1:1863–1872. [Google Scholar]
- 2.Singh VK, Singh SK, Gangwar L. Synthesis and antimicrobial activity of novel fused 4-(3H) quinazolinone derivatives. Int J Sci Res. 2013;2:425–428. [Google Scholar]
- 3.Ghorab MM, Ismail Z, Radwan AA, Abdalla M. Synthesis and pharmacophore modeling of novel quinazolines bearing a biologically active sulfonamide moiety. Acta Pharm. 2013;63:1–18. doi: 10.2478/acph-2013-0006. [DOI] [PubMed] [Google Scholar]
- 4.Vijayakumar K, Ahamed AJ, Thiruneelakandan G. Synthesis, antimicrobial, and anti-HIV1 activity of quinazoline-4(3H)-one derivatives. J Applied Chem 2013. 2013 ID 387191. [Google Scholar]
- 5.Deep A, Narasimhan B, Ramasamy K, Mani V, Mishra RK, Majeed AB. Synthesis, antimicrobial, anticancer evaluation and QSAR studies of thiazolidin-4-ones clubbed with quinazolinone. Curr Top Med Chem. 2013;13:2034–2046. doi: 10.2174/15680266113139990130. [DOI] [PubMed] [Google Scholar]
- 6.Al-Amiery AA, Kadhum AAH, Shamel M, Satar M, Khalid Y, Mohamad AB. Antioxidant and antimicrobial activities of novel quinazolinones. Med Chem Res. 2014;23:236–242. [Google Scholar]
- 7.Laddha SS, Wadod Kar SG, Meghal SK. Studies on some biologically active substituted 4(3H)-quinazolinones. Part 1. Synthesis, characterization and anti-inflammatory, antimicrobial activity of 6,8-disubstituted 2-phenyl-3-[substituted- benzothiazol-2-yl]-4(3H)-quinazolinone. Arkivoc. 2006;11:1–20. [Google Scholar]
- 8.Giri RS, Thaker HM, Giordano T, Williams J, Rogers D, Sudersanam V, et al. Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazolin-4-one derivat-ives as inhibitors of NF-ĸB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur J Med Chem. 2009;44:2184–2189. doi: 10.1016/j.ejmech.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Jiang S, Zeng Q, Gettayacamin M, Tungtaeng A, Wannaying S, Lim A, et al. Antimalaria activities and therapeutic properties of febrifugine analogs. Antimicrob Agents Chemoter. 2005;49:1169–1176. doi: 10.1128/AAC.49.3.1169-1176.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deetz MJ, Malerich JP, Beatty AM, Smith BD. One-step synthesis of 4(3H) quinazolinones. Tetrahedron Lett. 2001;42:1851–1854. [Google Scholar]
- 11.Krishnan SK, Ganguly S, Veeramy R, Jan B. Synthesis, antiviral and cytotoxic investigation of 2-phenyl-3-substituted quinazolin-4(3H)-ones. Eur Rev Med Pharmacol Sci. 2011;15:673–681. [PubMed] [Google Scholar]
- 12.Khosropour AR, Mohammadpoor-Baltork I, Ghorbankhani H. Bi (TFA) 3-[nbp] Fecl4: A new, efficient and reusable promoter system for the synthesis of 4(3H)-quinazolinone derivatives. Tetrahedron Lett. 2006;47:3561–3564. [Google Scholar]
- 13.Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AAM, Abdel-hamide SG, et al. Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem. 2006;14:8608–8621. doi: 10.1016/j.bmc.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Liu F, Yu X, Ding G, Xu P, Cao J, et al. The 3D-QSAR analysis of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains on thymidylate synthase. Bioorg Med Chem. 2006;14:1425–1430. doi: 10.1016/j.bmc.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 15.Hattori K, Kido Y, Yamamoto H, Ishida J, Iwashita A, Mihara K. Rational design of conformationally restricted quinazolinone inhibitors of poly (ADP-ribose) polymerase. Bioorg Med Chem Lett. 2007;17:5577–5581. doi: 10.1016/j.bmcl.2007.07.091. [DOI] [PubMed] [Google Scholar]
- 16.Guiles J, Sun X, Critchley IA, Ochsner U, Tregay M, Stone K, et al. Quinazolin-2-ylamino-quinazolin-4-ols as novel non-nucleoside inhibitors of bacterial DNA polymerase III. Bioorg Med Chem Lett. 2009;19:800–802. doi: 10.1016/j.bmcl.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Orvieto F, Branca D, Giomini C, Jones P, Koch U, Ontoria JM, et al. Identification of substituted pyrazolo [1,5-a] quinazolin-5 (4H)-one as potent poly (ADP-ribose) polymerase-1 (PARP-1) inhibitors. Bioorg Med Chem Lett. 2009;19:4196–4200. doi: 10.1016/j.bmcl.2009.05.113. [DOI] [PubMed] [Google Scholar]
- 18.Khalil AA, Abdel hamide SG, Al-Obaid AM, El-subbagh H. Substituted quinazolinones, Part2.Synthesis and in vitro anticancer evaluation of new 2-substituted mercapto-3H-quinazolin analogs. Arch Pharm Pharm Med Chem. 2003;336:95–103. doi: 10.1002/ardp.200390011. [DOI] [PubMed] [Google Scholar]
- 19.Nagar AA, Rathi LG, Chugh N, Pise VJ, Bendale A. Microwave assisted one pot synthesis of 2, 3-di-substituted quinazolin-4- (3H)-ones and their potential biological activity. Der Pharm Chem. 2010;2:37–43. [Google Scholar]
- 20.Selvam TP, Kumar PV. Quinazoline Marketed drugs - A Review. Res Pharm. 2011;1:1–21. [Google Scholar]
- 21.Abida, Parvez N, Rana A, Imran M. An updated review: newer quinazoline derivatives under clinical trial. Int J Pharm Biol Arch. 2011;2:1651–1657. [Google Scholar]
- 22.Mohamed MA, Ghanem HM, Abd El-Ghaffar NF, Mohamed SS. Biological evaluation and molecular docking of substituted quinazolinones as antimicrobial agents. AustJ Basic Appl Sci. 2013;7:263–274. [Google Scholar]
- 23.Zayed MF, Hassan MH. Synthesis and biological evaluation studies of novel quinazolinone derivatives as antibacterial and anti-inflammatory agents. Saudi Pharm J. 2014;22:157–162. doi: 10.1016/j.jsps.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Badry YA, Anter NA, El-Sheshtawy HS. Synthesis and evaluation of new polysubstituted quinazoline derivatives as potential antimicrobial agents. Der Pharma Chemica. 2012;4:1361–1370. [Google Scholar]
- 25.Dave RS, Odedara R RJ, Kalaria RI, Upadhyay JJ. Synthesis, characterization and antimicrobial activity of new quinazolin- 4(3H)-one schiff base derivatives. J Chem Pharm Res. 2012;4:4864–4869. [Google Scholar]
- 26.Devi KA, Sriram MS. Synthesis and antimicrobial activity of some quinazolinones derivatives. Int J Drug. 2012;4:324–327. [Google Scholar]
- 27.Raval JP, Desai KG, Desai KR. Microwave synthesis, characterization and antimicrobial study of new pyrazolyl-oxopropyl-quinazolin-4(3H)-one derivatives. J Saudi Chem Soc. 2012;16:387–393. [Google Scholar]
- 28.Sojitra NA, Dixit RB, Patel RK, Patel JP, Dixit BC. Classical and microwave assisted synthesis of new 4-(3,5-dimethyl-1-phenyl-1H-pyrazol-4-ylazo)-N-(2 -substituted-4-oxo-4H-quinazolin-3-yl) benzene-sulfonamide derivatives and their antimicrobial activities. J Saudi Chem Soci. 2012 In press. [Google Scholar]
- 29.Unnissa SH, Reddy GK. Synthesis and antimicrobial screening of 3H-Quinazolin-4-ones containing 3-methyl pyrazolinone moiety. Current Pharma Res. 2012;3:690–696. [Google Scholar]
- 30.Kumar A, Sharma P, Kumari P, Lal Kalal B. Exploration of antimicrobial and antioxidant potential of newly synthesized 2,3-disubstitut- ed quinazoline-4(3H)-ones. Bioorg Med Chem. Lett. 2011;21:4353–4357. doi: 10.1016/j.bmcl.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Desai NC, Dodiya A, Shihory N. Synthesis and antimicrobial activity of novel quinazolinone-thiazolidine–quinoline compounds. J Saudi Chem Soc. 2013;17:259–267. [Google Scholar]
- 32.Patel NB, Patel JC. Synthesis and antimicrobial activity of Schiff bases and 2-azetidinones derived from quinazolin-4(3H)-one. Arabian J Chem. 2011;4:403–411. [Google Scholar]
- 33.Desai NC, Dodiya A, Bhatt N, Kumar M. Dimeric 2-(2-chlorophenyl)-quinazolin-4-ones as potential antimicrobial agents. Med Chem Res. 2012;21:1127–1135. [Google Scholar]
- 34.Hassanzadeh F, Rahmani Khajouei M, Hakimelahi GH, Jafari E, Khodarahmi GA. Synthesis of some new 2,3-disubstituted-4(3H)quinazolinone derivat-ives. Res Pharm Sci. 2012;7:23–30. [PMC free article] [PubMed] [Google Scholar]
- 35.Khodarahmi GA, Rahmani Khajouei M, Hakimelahi GH, Abedi D, Jafari E, Hassanzadeh F. Antibacterial, antifungal and cytotoxic evaluation of some new 2,3-disubstituted 4(3H)-quinazolinone derivatives. Res Pharm Sci. 2012;7:151–158. [PMC free article] [PubMed] [Google Scholar]
- 36.Rajasekaran A, Rajamanickam V, Darlinquine S. Synthesis of some new thioxoquinazolinone derivatives and a study on their anticonvulsant and antimicrobial activities. Eur Rev Med Pharmacol Sci. 2013;17:95–104. [PubMed] [Google Scholar]
- 37.Al-Omary FAM, Hassan GS, El-Messery SM, Nagi MN, Habib ESE, El-Subbagh HI. Nonclassical antifolates, part 3: Synthesis, biological evaluation and molecular modeling study of some new 2-heteroarylthio-quinazolin-4-ones. Eur J Med Chem. 2013;63:33–45. doi: 10.1016/j.ejmech.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 38.Ryu CK, Kim YH, Im HA, Kim JY, Yoon JH, Kim A. Synthesis and antifungal activity of 6,7-bis(arylthio)-quinazoline-5,8-diones and furo[2,3-f]quinazolin-5-ols. Bioorg Med Chem Lett. 2012;22:500–503. doi: 10.1016/j.bmcl.2011.10.099. [DOI] [PubMed] [Google Scholar]
- 39.Shi LP, Jiang KM, Jiang JJ, Jina Y, Tao YH, Li K, et al. Synthesis and antimicrobial activity of polyhalobenzonitrile quinazolin-4(3H)-one derivatives. Bioorg Med Chem Lett. 2013;23:5958–5963. doi: 10.1016/j.bmcl.2013.08.068. [DOI] [PubMed] [Google Scholar]
- 40.Mujalda V, Tiwari S, Sharma V, Saxena P, Shrivastava M. Synthesis and antimicrobial evaluation of some new quinazoline based thiosemicarbazones. Int J Chem Tech Res. 2012;4:1033–1037. [Google Scholar]
- 41.Saravanan G, y Alagarsamy V, Prakash CR. Synthesis, characterization and in vitro antimicrobial activity of some 1-(substitutedbenzylidene)-4-(4-(2-(methyl/phenyl)-4-oxoquinazolin-3(4H)-yl)phenyl) semicarbazide derivatives. J Saudi Chem Soci. 2015;19:3–11. [Google Scholar]
- 42.Suresha GP, Suhas R, Kapfo W, Gowda DC. Urea/thiourea derivatives of quinazolinoneelysine conjugates: Synthesis and structure activity relationships of a new series of antimicrobials. Eur Med Chem. 2011;46:2530–2540. doi: 10.1016/j.ejmech.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 43.Kuarm BS, Reddy YT, Madhav JV, Crooks PA, Rajitha B. 3-[Benzimidazo- and 3-[benzothia-diazoleimidazo-(1,2-c)quinazolin-5-yl]-2H-chromene-2-ones as potent antimicrobial agents. Bioorg Med Chem Lett. 2011;21:524–527. doi: 10.1016/j.bmcl.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 44.Jafari E, Khodarahmi GA, Hakimelahi GA, Tsai FY, Hassanzadeh F. Synthesis of some new tricyclic 4(3H)-quinazolinone derivatives. Res Pharm Sci. 2011;6:93–100. [PMC free article] [PubMed] [Google Scholar]
- 45.Hassanzadeh F, Jafari E, Hakimelahi GH, Rahmani Khajouei M, Jalali M, Khodarahmi GA. Antibacterial, antifungal and cytotoxic evaluation of some new quinazolinone derivatives. Res Pharm Sci. 2012;7:87–94. [PMC free article] [PubMed] [Google Scholar]
- 46.Khodarahmi GA, Jafari E, Hakimelahi GH, Abedi D, Rahmani Khajouei M, Hassanzadeh F. Synthesis of some new quinazolinone derivatives and evaluation of their antimicrobial activities. Iran J Pharm Res. 2012;11:789–79 7. [PMC free article] [PubMed] [Google Scholar]
- 47.Alafeefy AM, El-Azab AS, Mohamed MA, Bakhat MA, Abdel-Hamid SG. Synthesis of some new substituted iodoquinazoline derivatives and their antimicrobial screening. J Saudi Chem Soc. 2011;15:319–325. [Google Scholar]
- 48.Hu S, Xie G, Zhang DX, Davis C, Long W, Hu Y, et al. Synthesis and biological evaluation of crown ether fused quinazoline analogues as potent EGFR inhibitors. Bioorg Med Chem Lett. 2012;22:6301–6305. doi: 10.1016/j.bmcl.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 49.Kh, Atia AJ, Al-Mufrgeiy SS. Synthesis and antibacterial activities of new 3-amino-2-methyl-quinazolin-4 (3h)-one derivatives. American J Chem. 2012;2:150–156. [Google Scholar]
- 50.Kinoshita T, Nakanishi I, Warizaya M, Iwashita A, Kido Y, Hattori K, et al. Inhibitor-induced structural change of the active site of human poly(ADP-ribose) polymerase. FEBS Letters. 2004;556:43–46. doi: 10.1016/s0014-5793(03)01362-0. [DOI] [PubMed] [Google Scholar]
- 51.Jin Y, Zhou ZY, Tian W, Yu Q, Long YQ. 4′-Alkoxyl substitution enhancing the anti-mitotic effect of 5-(3′, 4′, 5′-substituted) anilino-4-hydroxy-8-nitroquinazolines as a novel class of anti-microtubule agents. Bioorg Med Chem Lett. 2006;16:5864–5869. doi: 10.1016/j.bmcl.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 52.Wissner A, Fraser HL, Ingalls CL, Dushin RG, Floyd MB, Cheung K, et al. Dual irreversible kinase inhibitors: Quinazoline-based inhibitors incorporating two independent reactive centers with each targeting different cysteine residues in the kinase domains of EGFR and VEGFR-2. Bioorg Medi Che. 2007;15:3635–3648. doi: 10.1016/j.bmc.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 53.Abouzid K, Shouman S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg Med Chem. 2008;16:7543–7551. doi: 10.1016/j.bmc.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes C, Oliveira C, Gano L, Bourkoula A, Pirmettis I, Santos I. Radioiodination of new EGFR inhibitors as potential SPECT agents for molecular imaging of breast cancer. Bioorg Med Chem. 2007;15:3974–3980. doi: 10.1016/j.bmc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Al-Obaid AM, Abdel-Hamide SG, El-Kashef HA, Abdel-Aziz AM, El-Azab AS, Al-Khamees HA, et al. Substituted quinazolines, part 3.Synthesis, in vitro antitumoractivity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur J Med Chem. 2009;44:2379–2391. doi: 10.1016/j.ejmech.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Al-Omary FAM, Abou-zeid LA, Nagi MN, Habib ESE, Abdel-Aziz AAM, El-Azab AS, et al. Non-classical antifolates.Part 2: Synthesis, biological evaluation, and molecular modeling study of some new 2,6-substituted-quinazolin-4-ones. Bioorg Med Chem. 2010;18:2849–2863. doi: 10.1016/j.bmc.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Raffa D, Edler MC, Daidone G, Maggio B, Merickech M, Plescia S, et al. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur J Med Chem. 2004;39:299–304. doi: 10.1016/j.ejmech.2003.12.009. [DOI] [PubMed] [Google Scholar]