Abstract
Epidermal growth factor (EGF), a growth factor involved in cell growth and differentiation, is a small polypeptide with molecular weight of approximately 6 kDa known to be present in a number of different mammalian species. Experimental studies in animals and humans have demonstrated that the topical application of EGF accelerates the rate of epidermal regeneration of partial-thickness wounds and second-degree burns. Due to its commercial applications, Human EGF (hEGF) has been cloned in several forms. In the present study, adenoviral based expression system was used to produce biologically active recombinant hEGF. The presence of secreted recombinant hEGF was confirmed by a dot blot and its expression level was determined by enzyme-linked immuno-sorbent assay. Moreover, biological activity of secreted hEGF was evaluated by a proliferation assay performed on A549 cells. For production of hEGF in a secretory form, a chimeric gene coding for the hEGF fused to the signal peptide was expressed using adenoviral based method. This method enables the production of hEGF at the site of interest and moreover it could be used for cell proliferation and differentiation assays in tissue engineering research experiments instead of using commercially available EGF.
Keywords: hEGF, Adenovirus, Epiderm, Proliferation
INTRODUCTION
Epidermal growth factor (EGF) is a member of a class of molecules referred to as growth factors which are involved in the cell growth and differentiation. EGF is a single polypeptide consisting of 53 amino acids with molecular weight of approximately 6 kDa which is present in a number of different mammalian species (1). The six cysteine residues present in the EGF sequence are able to form three internal disulfide bands (2). EGF was first isolated from parotid gland of male mice and showed to stimulate precocious eyelid opening and tooth eruption in newborn (3). Subsequently Human EGF (hEGF) was purified from human urine and referred to as “urogastrone” based on its ability to inhibit gastric acid secretion in human (4,5). Unlike hEGF which is derived from a 1207 amino acid precursor, mouse EGF comes from a 1217 amino acid precursor protein which contains 7 additional EGF-like domains. It has been reported that EGF can prompt abundant effects on both cells and epithelial tissue (6). Moreover, hEGF has various effects on cell regeneration including stimulation of proliferation, migration of keratinocytes, formation of granulation tissues, and stimulation of fibroblast motility, which are play major role in wound healing processes (6,7). Experimental studies in animals and humans have demonstrated that the topical application of EGF accelerates the rate of epidermal regeneration of partial-thickness wounds and second-degree burns (8). Due to its commercial applications ranging from wound healing and inhibition of gastric acid secretion to wool harvesting, hEGF has been cloned in several forms by various industrial laboratories (9,10).
Adenovirus vectors have been widely used as exogenous gene delivery system to many mammalian cells. Adenovirus vectors are known and used for their higher safety profile, high titer production and good gene transduction efficiencies in comparison to other viruses (11,12).
Adenovirus transduction occurs non-specifically to the cells expressing coxsackie-adenovirus receptor on the surface, which limits their systemic administration (13). Genetically manipulated adenoviruses could be administered in a local manner to the desired tissue avoiding any harmful side effects (14). The main aim of the current study was the development of hEGF gene template and integration of recombinant hEGF in adenoviral vector for extracellular production of hEGF protein by mammalian host cells.
MATERIAL AND METHODS
Production of recombinant adenovirus
AdenoVator™ (Q.BIO gene, CA, USA) was used according to manufacturer's instructions. Briefly, chemically synthesized EGF gene (BIOMATIK, Canada) which consisted of a signal peptide and segment coding for amino acids 971-1023 of human EGF gene (NG_011441) was inserted into the transfer vector. This expression cassette was designed to produce recombinant EGF in a secreted form by excluding other domains of an inactive large precursor called prepro-EGF. Transfer vector was linearized using restriction enzyme PmeI and it was co-transformed along with pAdenoVator ΔE1/E3 into electro competent Escherichia coli strain BJ5183. Transformed bacteria were then screened for kanamycin resistance and plasmid was extracted from resistant bacteria and further confirmed using restriction enzyme BstxI.
On the day before transfection, 1.2 × 105 HEK 293 cells/well were plated in 24 well cell culture plates and after reaching 70% confluency, 500 ng of recombinant adenoviral vector digested with PacI restriction enzyme was transfected using lipofectamine 2000 (life technologies, CA, USA). After 4–6 h of incubation at 37°C, the culture media with the incubation at 37°C, the culture media with the transfection mix was replaced with fresh cell culture medium. Transfected cells were harvested 17 days post transfection and viral particles were isolated by three cycles of rapid freeze and thaw. The presence of hEGF gene in recombinant viral DNA was verified by polymerase chain reaction (PCR). The forward primer sequence was 5′-CAAGTTTGGAAGATCTATGCT-3’ and the reverse primer sequence was 5′-GAGATCTTTAGCGCAGTTCC-3′.
Virus amplification
To produce high titer viral stocks, HEK293 cells were plated in 6 well cell culture plates (Orange Scientifique, Belgium) until confluency of 90% was achieved. Subsequently appropriate volume of recombinant adenovirus was added to each well (Table 1). Cells were incubated for 2 h at 37°C with gentle rocking every 30 min. Transduction media was replaced with fresh cell culture medium. After 36 h the media was removed and analyzed for the presence of hEGF.
Table 1.
Concentrations of virus in serial viral dilutions and related obtained virus titer.
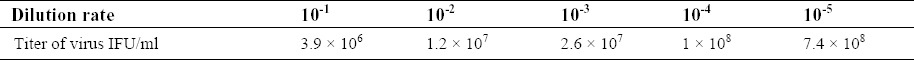
Titration of recombinant enhanced green fluorescent protein expressing adenovirus
To calculate the viral titer, enhanced green fluorescent protein (EGFP) positive adenovirus titration was performed by flow cytometry. Analysis was performed on a Cyflow apparatus and flowMax software (Quantum Analysis GmbH, version 2.70). Ten folds serial dilution of virus stock were prepared in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2% Fetal bovine serum (FBS). The media of each well of 24–well plates was aspirated and replaced with 0.5 ml of the viral dilution. In each experiment, viral dilutions were assayed in duplicate wells. We had two control wells that were not exposed to the virus. The plates were incubated for 24 h and then used in flow cytometric assay. Approximately, twenty thousand cells were counted for each sample.
Immunological assay
For analyzing the expression level of recombinant hEGF, 36 h post transduction media from HEK293 cells was subjected to a quick centrifugation to remove the cell debris. The presence of hEGF was confirmed by a dot blot and levels of secreted hEGF were determined by enzyme linked immuno-sorbent assay (ELISA).
Biological activity assay
12 × 103 A549 cells, a common cell line to assay biological activity of EGF (15,16,17), were cultured in Falcon polyurethane cell culture inserts (upper chamber, 0.4 mm pore diameter) and subsequently 1 × 105 HelaS3 cells (48 h post-transduction) were seeded in the upper chamber of the cell culture insert. This stage was repeated three times for Ad-EGF and Ad-GFP was used as a negative control. Then A549 proliferation rate was measured in each case by flow cytometry.
Statistical analysis
In all experiments, results were expressed as the Means ± S.D. Statistical differences between the data were determined by one-way ANOVA using the Tukey comparison test. P<0.05 was considered statistically significant. All statistical calculations were performed using the SPSS 15 (IBM).
RESULTS
Production of recombinant adenovirus
This study has created the expression of adenovirus construct with hEGF gene. The resulting PCR product, separated on 2 % agarose, was stained and viewed in an imaging system as depicted in Fig. 1A. Comparison of the band appeared on the gel with that of the standard marker (50 base pair marker) revealed that the gene was about 250 base pair in size. The resulting PCR product can be seen in Lanes 3, 4, 6, 10 and 11 (Fig. 1A).
Fig. 1.
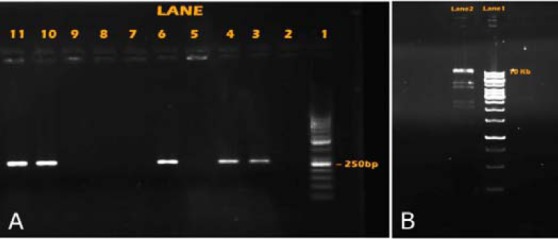
A; Ethidium bromide stained 2% agarose gel of hEGF gene template. Lane 1; 50 base pair marker. Lane; 2-11 represent replicates of hEGF gene templates with different PCR recipes. B; Ethidium bromide stained 1% agarose gel of hEGF gene template. Lane 1; 250 base pair marker. Lane 2; the results of enzymatic digestion by BstX I enzyme.
Recombinant adenoviral vector was extracted from bacteria and further confirmed using restriction enzyme BstxI. The digested product was resolved in 1 % agarose and displayed six bands between 11.9 and 1.4 kb appeared on the gel (Fig. 1B).
Titration of recombinant EGFP expressing adenovirus
Adenovirus titration was performed by flow cytometry. Analytical gates were set so that less than 1% of negative control cells exceeded the gate. The percentage of GFP-expressing cells was determined for each viral dilution. The functional viral concentration was defined according to the following formula:
GFU/ml = (percent GFP positive cells) × (number of cells per well)/(volume of viral dilution added; ml) × (viral dilution factor) (1)
In each experiment, a minimum of five serial viral dilutions were assayed in duplicate wells.
Reported GFU/ml values are the mean ± SD derived from these four viral dilutions in two experiments. We succeeded in obtaining virus titer of 107 IFU/ml.
Detection of secreted hEGF by recombinant Ad-EGF
The AdenoVator™ vector system was utilized to produce adenovirus particles capable of expressing EGF in transduced HEK 293 cells. Presence of secreted hEGF was confirmed by a dot blot. It reacted strongly to the anti-hEGF monoclonal antibody, which visualized as a distinct dot as compared with positive and negative controls (Fig. 2).
Fig. 2.
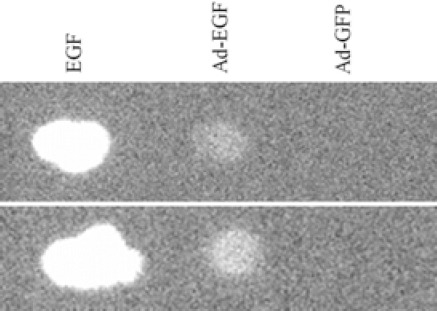
Dot blot analysis of ten microliter medium samples dotted on nitrocellulose membrane. The supernatant of HEK293 cells transduced with Ad-GFP were dotted as a negative control and commercially available EGF was used as a positive control (tests were performed in duplicate).
Sandwich ELISA was used to quantify the expressed hEGF protein. By using mouse anti-hEGF monoclonal antibody (R & D Systems Inc., USA), the expression levels of recombinant hEGF was determined by ELISA to be 1.3 ng/ml.
Evaluation of biological activity
To evaluate biological activity of recombinant hEGF a proliferation assay was performed on A549 cells. According to our results hEGF secreted by transduced HelaS3 cells caused 1.53 ± 0.18 fold increase (P<0.05) in cell proliferation of A549 cells in comparison with control group (Fig. 3).
Fig. 3.
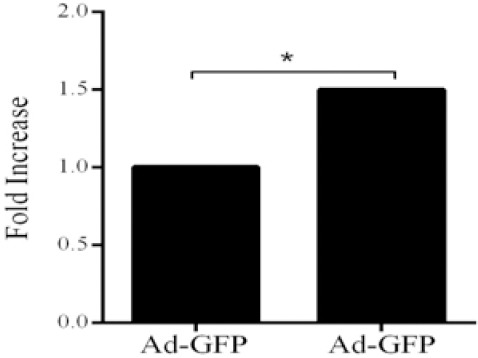
Biological activity assay of produced EGF. The fold increase in proliferation of A549 cells by exposure to recombinant EGF produced by HelaS3 cells in comparison to HelaS3 cell transduced with Ad-GFP as negative control (*P<0.05).
DISCUSSION
EGF has been isolated from numerous tissues as well as body fluids in humans and animals. EGF is known to be present in the amniotic fluid, saliva, colostrum, milk and urine. Many research groups have focused on the applications of EGF in medicine and treatment of a number of illnesses. One of the important applications of EGF is in the treatment of skin diseases and wound healing. Despite potential benefits, bioactive EGF production and clinical use of recombinant EGF has been limited primarily by the lack of practical delivery systems and proper host cells (18).
EGF is in high demand due to its several uses and has been expressed in various heterologous hosts, using recombinant DNA methods (19). To date, recombinant human EGF genes have been expressed in bacteria (20) and yeast as expression systems (21). Biologically active hEGF could be secreted by yeast host cells such as S. cerevisiae (22), Pichia pastoris, and Yarrowia lipolytica (19).
The main aim of this study was the development of hEGF gene template and integration of recombinant hEGF sequence in adenoviral vector for extracellular production of hEGF protein in mammalian host cells. In this study, expression was performed in small scale to study the production recombinant hEGF protein using expression system in adenovirus. This is because currently, there are few studies performed using adenoviral expression system, and the synthesized protein was proven to be bioactive. Over the last decade, biological assays have become more important to an effective quality control in biopharmaceutical research (23). In the present study, A549 cell proliferation assay for 24 h incubation showed significant increase of cell proliferation, which occurred at 1.3 ng/ml concentration of EGF.
The findings of this study showed that hEGF at concentration 1.3 ng/ml resulted in a 1.5-fold increase in cell proliferation. Proliferation of A549 cells was also increased in the presence of hEGF which were in agreement with findings of Ebrahimi-Rad and colleagues (24). They found that EGF was active on fibroblast proliferation. Even so, this proliferative effect of EGF was considered notable because only a small amount of hEGF was needed to increase the growth of A549. In addition, the rate of cell proliferation in untreated cultures was significantly lower than that of the treated cultures with hEGF. This result was consistent with the previous reports showing that the rate of cell proliferation was minimal in the absence of hEGF (25,26,27).
Our study promotes the use of adenoviral expression system, which is able to secrete the biologically active form of hEGF. This system was also able to successfully translocate mature, correctly processed, and folded hEGF that induced proliferation in A549 cells. In addition, adenovirus has many advantages in gene expression and introducing genes into cells: (I) simple, well tolerated with negligible apparent toxic effects, (II) infection of most cell types, broad host range and low pathogenicity in humans, (III) infection and expression of genes in replicative and non-replicative cells, (IV) replication efficiently to high titers, (V) accommodate up to 7.5 kb of foreign DNA, (VI) high expression level of functional proteins, (VII) no insertional mutagenesis and remains epichromosomal. These features are useful to produce bioactive recombinant expression with proper folding in mammalian cells for further application.
In this research a chimeric gene, coding for the human epidermal growth factor was fused to the signal peptide for production of EGF in a secretory form. This was possible by excluding other domains of inactive EGF precursor and joining a signal peptide at amino acid positions 1-22 to the sequence coding for biologically active EGF at amino acid position 971-1023. Our results suggest a novel approach of delivering EGF by using adenoviral based method which enable production of EGF at desirable site and may eliminate the need for frequent topical application of peptide form. The method allows facile delivery of EGF gene and expression of peptide in tissue by host cells and is presented to the target cells in more intimate manner than the topical application. In addition, likely it would allow a tighter control of peptide production and therefore a more regulated and balanced response to the physiological needs of tissues. More dramatic therapeutic benefits may be realized from the treatment of wounds where repair are delayed or deficient. Theoretically, a variety of cutaneous conditions including chronic non-healing ulcer keloids and hypertrophic scars might be treated with this method. Nevertheless, it could be used for cell proliferation and differentiation assays in tissue engineering research experiments instead of using commercially available EGF.
CONCLUSION
In this research, we produced the adenoviral based gene expression system in mammalian host cell integrated with recombinant hEGF gene. We showed that the secreted hEGF was biologically active. This system can be used locally to desired tissue.
ACKNOWLEDGEMENTS
We are greatly thankful of our colleagues in Virology Department, especially Dr. Bahrololoumi for revising the manuscript. This work has been granted by Tehran University of Medical Sciences and Pasteur Institute of Iran.
REFERENCES
- 1.Bell G I, Fong N M, Stempien M M, Wormsted M A, Caput D, Ku L L, et al. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986;14:8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DN, Kuo TY, Chen MC, Tang TY, Liu FH, Weng CF. Expression of porcine epidermal growth factor in Pichia pastoris and its biology activity in early-weaned piglets. Life Sci. 2006;78:649–654. doi: 10.1016/j.lfs.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF).Dev Biol. 1965;12:394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975;72:1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975;257:325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- 6.Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing-past, present and future perspectives. Surgeon. 2008;6:172–177. doi: 10.1016/s1479-666x(08)80114-x. [DOI] [PubMed] [Google Scholar]
- 7.Milani S, Calabrò A. Role of growth factors and their receptors in gastric ulcer healing. Microsc Res Tech. 2001;53:360–371. doi: 10.1002/jemt.1104. [DOI] [PubMed] [Google Scholar]
- 8.Kim DG, Kim EY, Kim YR, Kong IS. Construction of chimeric human epidermal growth factor containing short collagen-binding domain moieties for use as a wound tissue healing agent. J Microbiol Biotechnol. 2015;25:119–126. doi: 10.4014/jmb.1405.05073. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DR, Walmsley AM. Walmsley. Improved expression of recombinant plant-made hEGF. Plant Cell Rep. 2014;33:1801–1814. doi: 10.1007/s00299-014-1658-8. [DOI] [PubMed] [Google Scholar]
- 10.Razis AFA, Ismail EN, Hambali Z, Abdullah MNH, Ali AM, Lila MAM. The periplasmic expression of recombinant human epidermal growth factor (hEGF) in Escherichia coli. As Pac J Mol Biol Biotechnol. 2006;14:41–45. [Google Scholar]
- 11.Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, et al. FGF2-targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000;2:153–160. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- 12.Majhen D, Ambriovic-Ristov A. Adenoviral vectors-how to use them in cancer gene therapy? Virus Res. 2006;119:121–133. doi: 10.1016/j.virusres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 14.Hitt MM, Graham FL. Adenovirus vectors for human gene therapy. Adv Virus Res. 2000;55:479–505. doi: 10.1016/s0065-3527(00)55014-3. [DOI] [PubMed] [Google Scholar]
- 15.Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- 16.Tomshine JC, Severson SR, Wigle DA, Sun Z, Beleford DA, Shridhar V, et al. Cell proliferation and epidermal growth factor signaling in non-small cell lung adenocarcinoma cell lines are dependent on Rin1. J Biol Chem. 2009;284:26331–26339. doi: 10.1074/jbc.M109.033514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bost F, McKay R, Bost M, Potapova O, Dean NM, Mercola D, et al. The Jun kinase 2 isoform is preferentially required for epidermal growth factor-induced transformation of human A549 lung carcinoma cells. Mol Cell Biol. 1999;19:1938–1949. doi: 10.1128/mcb.19.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vachkova EG, Bivolarski BL. Origin, structure and physiological role of the epidermal growth factor. Bulg J Vet Med. 2007;10:223–233. [Google Scholar]
- 19.Hamsa PV, Kachroo P, Chattoo BB. Production and secretion of biologically active human epidermal growth factor in Yarrowia lipolytica. Curr Genet. 1998;33:231–237. doi: 10.1007/s002940050331. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu N, Fukuzono S, Harada Y, Fujimori K, Gotoh K, Yamazaki Y. Mass production of human epidermal growth factor using fed‐batch cultures of recombinant Escherichia coli. Biotechnol Bioeng. 1991;38:37–42. doi: 10.1002/bit.260380106. [DOI] [PubMed] [Google Scholar]
- 21.Heo JH, Won HS, Kang HA, Rhee SK, Chung BH. Purification of recombinant human epidermal growth factor secreted from the methylotrophic yeast Hansenula polymorpha. Protein Expr Purif. 2002;24:117–122. doi: 10.1006/prep.2001.1527. [DOI] [PubMed] [Google Scholar]
- 22.Clements JM, Catlin GH, Price MJ, Edwards RM. Secretion of human epidermal growth factor from Saccharomyces cerevisiae using synthetic leader sequences. Gene. 1991;106:267–271. doi: 10.1016/0378-1119(91)90209-t. [DOI] [PubMed] [Google Scholar]
- 23.Hann MM, Oprea TI. Pursuing the leadlikeness concept in pharmaceutical research. Curr Opin Chem Biol. 2004;8:255–263. doi: 10.1016/j.cbpa.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimi-Rad M, Azarnoosh M, Bashir A, Bakayev VV. A novel vector for expression/secretion of properly folded eukaryotic proteins: a Comparative study on cytoplasmic and periplasmic expression of human epidermal growth factor in E. coli. Iran Biomed J. 2004;8:51–61. [Google Scholar]
- 25.Sumi SI, Hasegawa A, Yagi Sh, Miyoshi K, Kanezawa A, Nakagawa Sh, et al. Overproduction of human epidermal growth factor/urogastrone in Escherichia coli and demonstration of its full biological activities. J Biotechnol. 1985;2:59–74. [Google Scholar]
- 26.Svoboda M, Bauhofer A, Schwind P, Bade E, Rasched I, Przybyiski Structural characterization and biological activity of recombinant human epidermal growth factor proteins with different N-terminal sequences. Biochimica et Biophysica Acta (BBA)-Protein. Biochimica et Biophysica Acta. 1994;1206:35–41. doi: 10.1016/0167-4838(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 27.Engler DA, Matsunami RK, Campion SR, Stringer CD, Stevens A, Niyogi SK. Cloning of authentic human epidermal growth factor as a bacterial secretory protein and its initial structure-function analysis by site-directed mutagenesis. J BiolChem. 1988;263:12384–12390. [PubMed] [Google Scholar]


