Abstract
Pinus eldarica Medw. (Iranian pine) is native to Transcaucasian region and has been vastly planted in Iran, Afghanistan, and Pakistan. Various parts of this plant have been widely used in traditional medicine for the treatment of various diseases including infectious conditions (e.g. infectious wounds). In this study we aimed to investigate the antibacterial activity of P. eldarica bark extract, essential oil and proanthocyanidins on three important bacteria, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Antibacterial analysis was performed using standard disk diffusion method with different concentrations of essential oil, bark total hydroalcoholic extract, and bark proanthocyanidins (0.5, 1, 2 and 3 mg/ml). After incubation at 37°C for 24 h, the antibacterial activity was assessed by measuring the zone of growth inhibition surrounding the disks. The results indicated that the essential oil, total hydroalcoholic extract, and proanthocyanidins of the bark of the P. eldarica were effective against the gram negative bacteria, P. aeruginosa, and significantly inhibited its growth in disk diffusion method (P<0.001) of which the essential oil had the most potent inhibitory effect. However, none of the bark preparations could significantly inhibit the growth of S. aureus or E. coli. Our findings showed that P. eldarica bark components have significant anti-pseudomonas activity having potentials for new sources of antibacterial agents or antibacterial herbal preparations.
Keywords: Antibacterial activity, Anti-pseudomonas, Disk diffusion, Proanthocyanidins, Pinus eldarica
INTRODUCTION
Antimicrobial resistance causes increased morbidity, mortality and costs of health care and has been detected in both gram-positive and gram-negative bacteria. The 1990s was the era of multiple drug resistance. Resistance to antimicrobials was started by identification of penicillin resistant Escherichia coli in 1940 and continued by isolation of penicillin resistant Staphylococus aureus in 1944. Over the last decade, it has become clear that antibiotics are losing their effectiveness as pathogens evolve resistance against them, a problem compounded by the fact that new drugs only rarely reach the market (1,2,3). It is also well known that many currently available antifungal and antibacterial agents have undesirable toxicities. Therefore, pharmaceutical companies have recently made lots of efforts to develop more efficient new antibiotics (1,2).
Plants are considered as an important source of interesting and diverse bioactive compounds with different pharmacological activities. More than 300 natural metabolites with antimicrobial activities have been reported in 2000–2008 and many compounds have so far been tested in humans (1,2,4,5).
The plant family Pinaceae includes many conifers such as Cedrus, Abies, Larix, Picea and Pinus of which different species of Pines and their products have been used traditionally for treatment of many kind of diseases (6). Among the Pines, Pinus eldarica Medw. (Iranian pine) is native to Transcaucasian region between Europe and Asia, and has been widely planted in Iran, Afghanistan and Pakistan. Various parts of this plant (needles, buds, resin and nuts) have been widely used in traditional medicine for the treatment of many diseases including bronchial asthma, skin wounds, skin irritations, allergic rashes and dermatitis. Potent prophylactic effect of P. eldarica fruits extract on calcium oxalate stone formation confirming it as an antiurolithiasis, antidepressant activity of the pine needles and glucose lowering effects of the pine nut extract are new pharmacological activities of this plant which has been demonstrated in recent studies (6,7,8).
Nowadays, among numerous products obtained from pines, pine bark extracts are of special importance. These extracts containing phenolic compounds such as catechin, epicatechin, taxifolin, phenolic acids, have received considerable attention because of their antimutagenic, anticarcinogenic, antitumor, anti-aging, anti-inflammatory and high antioxidant activity which is mainly due to their high potency for removing superfluous free radicals and enhancing immunity (5,9,10,11).
The most common commercially available pine bark extract, Pycnogenol®, is a standardized extract of Pinus pinaster bark that contains naturally occurring proanthocyanidins as the main compounds. It is used worldwide as a herbal remedy and nutrition supplement in many kinds of degenerative diseases and has been reported to have some important pharmacological effects such as cardiovascular and cholesterol lowering benefits, antioxidant, anti-inflammatory, antimicrobial effects, the ability to enhance microcirculation by increasing capillary permeability and significant free radical scavenging activity (5,9,12).
The essential oils of different pines are widely used in a variety of products having many useful biological effects. These compounds are generally strong antimicrobial agents with broad spectrum activity and possible potential for controlling pathogens in plants and also treating human pathogenic diseases and often are more economical and environmentally safe, as antimicrobial agents (13).
Traditional use of P. eldarica preparations for the treatment of infectious conditions (e.g. infectious wounds) encouraged us to investigate the antibacterial activity of its bark extract, essential oil, and proanthocyanidins on three common and important bacteria. S. aureus is a gram positive bacterium that causes a variety of suppurative infections in human such as superficial skin lesions, pneumonia, meningitis, urinary tract infections and food poisoning; E. coli, a gram negative bacterium generally the main cause of food poisoning and intestinal infectious diseases, and P. aeruginosa, a gram negative bacterium, an important pathogen that is frequently involved in infections of hospitalized patients, infections of patients with weakened immune system, and a common cause of nosocomial infections such as pneumonia, urinary tract infections (UTIs) and bacteriemia (5,13,14).
MATERIALS AND METHODS
Pine bark samples
P. eldarica bark specimens were collected in April 2006 in Isfahan province. The specimens were left overnight to dry at room temperature, ground using a conventional grinder, and stored in airtight bottle, in refrigerator, until the use.
Prepration of bark total hydroalcoholic extract
100 g of pine bark powder was macerated with 500 ml of ethanol:water (70:30) and maintained at room temperature for 48 h, with occasional shaking. The mixture was filtered and the procedure repeated again. Resulting extracts were combined, concentrated with rotary evaporator at 40°C and the final extract was completely dried by freeze drying.
Isolation of bark proanthocyanidins
P. eldarica proanthocyanidins was obtained according to the method used by Iravani (15). Briefly, 100 g of pine bark powder extracted with 600 ml of boiling water, and then cooled down to 20°C. After filtration, 250 ml of liquid was collected; certain amount of sodium chloride was added up to saturation, and the precipitate formed was removed by filtration on Whatman® cellulose filter paper. Subsequently, the filtrate was extracted three times with ethyl acetate. The ethyl acetate phase was collected and dried using anhydrous sodium sulfate and reduced to 1/5 of its original volume using a rotary evaporator. The extract was then poured into three volumes of chloroform, while stirring mechanically. The proanthocyanidins were precipitated as a light beige color powder, collected by filtration and stored at −20°C. All chemicals used were of analytical grade purity (Merck, Germany).
Isolation of essential oil
An aliquot of 200 g of the pine bark powder was subjected to hydrodistillation on a Clevenger type apparatus for 4 h. The resulting essential oil was dissolved in diethyl ether, collected and treated by anhydrous sodium sulfate to remove excess water. Diethyl ether was removed carefully in room temperature and the remaining essential oil stored at refrigerator until analysis.
Antibacterial analysis
All bacterial samples were obtained from the bacteriology laboratory; School of Pharmacy, Isfahan University of Medical Sciences, Iran. The bacteria were first grown on Muller Hinton Agar medium at 37°C for 24 h prior to seeding on to the nutrient agar.
Antibacterial assessments were performed using standard disk diffusion method. Briefly, bacterial suspensions (100 μl), containing 6 × 10 CFU/ml of S. aureus (Persian Type Culture Collection (PTCC) = 1337), E. coli (PTCC = 1338) and P. aeruginosa (PTCC = 1047) were separately spread on petri dishes (9 cm in diameter) homogenously with the help of a glass spreader in aseptic condition. A sterile 6-mm diameter filter disk (Whatman® paper no 3) was placed on the infusion agar seeded with bacteria, and different concentrations (0.5, 1, 2 and 3 mg/ml) of essential oil, bark total hydroalcoholic extract, or bark proanthocyanidins, prepared in water with the aid of dimethyl sulfoxide (DMSO) (2%) as co-solvent, were dropped on to each paper disk (40 μl per disk). The prepared petri dishes were kept at 4°C for 1 h, and incubated at 37°C for 24 h. The antibacterial activity was assessed by measuring the zone of growth inhibition surrounding the disks. Each experiment was carried out in triplicate. A negative control was also included in the test using a filter paper disk saturated with DMSO (2%) in water, to check possible activity of this solvent against the bacteria assayed. Vancomycin disk (30 μg/disk) was also used as positive control (16).
Statistical analysis
Statistical analysis was performed by Statistical Package for the Social Science software (SPSS, version 13.0, SPSS Inc.), using one-way analysis of variance (ANOVA) followed by Tukey-Kramer post-hoc test for multiple comparisons. P values less than 0.05 were considered statistically significant.
RESULTS
The quantification of antibacterial activity of P. eldarica essential oil, bark total hydroalcoholic extract and bark proanthocyanidins was measured by the agar disk diffusion method. The effectiveness of P. eldarica bark preparations was demonstrated by the size of the microorganism growth inhibition zone around the filter paper disk, which is typically expressed as the diameter of the inhibition zone in millimeter.
The results indicated that all bark preparations including essential oil (Fig. 1), bark proanthocyanidins (Fig. 2), bark total hydroalcoholic extract (Fig. 3) were effective against the gram negative bacterium P. aeruginosa, and inhibited its growth significantly in disk diffusion method (P<0.001) compared to the control and amongst all three bark preparations of P. eldarica examined, the essential oil had the highest inhibitory effect. Although, the inhibitory effect of three bark preparations of P. eldarica tested in this study on P. aeruginosa were significant, but did not reach to the effect was observed with the positive control vancomycin. However, none of the P. eldarica bark preparations could significantly inhibit the growth of S. aureus or E. coli, while vancomycin, as the positive control, significantly inhibited the growth of all three bacteria species (P<0.001).
Fig. 1.
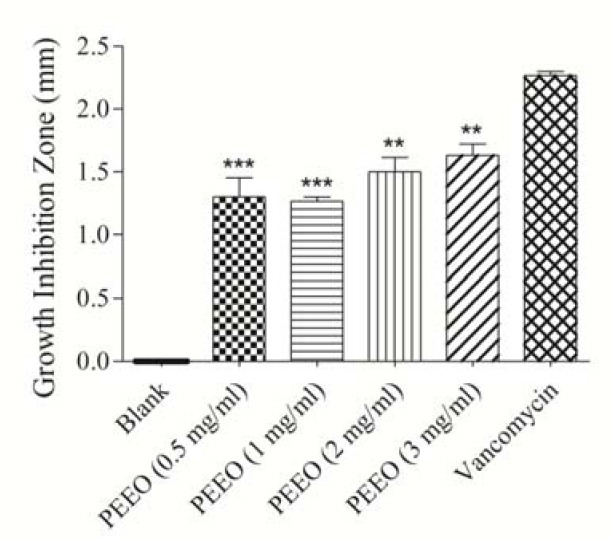
Antibacterial effects of Pinus eldarica bark essential oil (PEEO) on Pesudomonas aeroginosa determined by disc diffusion method. Values are expressed as mean ± S.E.M. PEEO and vancomycin samples have significantly increased the growth inhibition zone compared to the blank sample (P<0.001). **P<0.01 compared with vancomycin disc; ***P<0.001 compared with vancomycin disc, Statistical analyses performed with one-way ANOVA and Tukey's post hoc test.
Fig. 2.
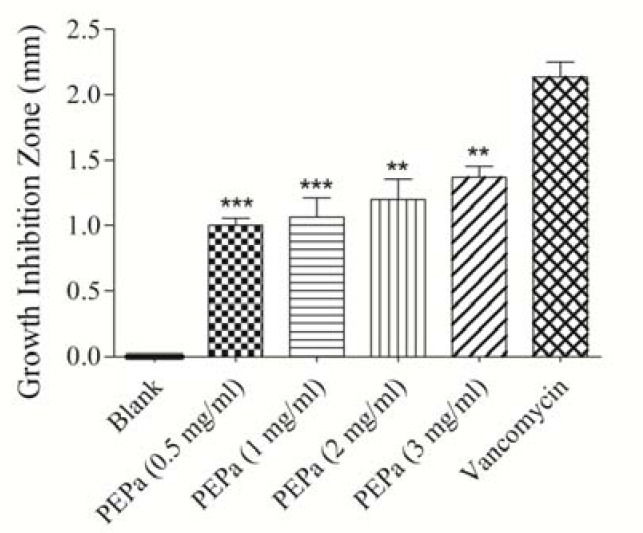
Antibacterial effects of Pinus eldarica bark proanthocyanidins (PEPa) on Pesudomonas aeroginosa determined by disc diffusion method. Values are expressed as mean ± S.E.M. PEPa and vancomycin samples have significantly increased the growth inhibition zone compared to the blank sample (P<0.001). **P<0.01 compared with vancomycin disc; ***P<0.001 compared with vancomycin disc, Statistical analyses performed with one-way ANOVA and Tukey's post hoc test.
Fig. 3.
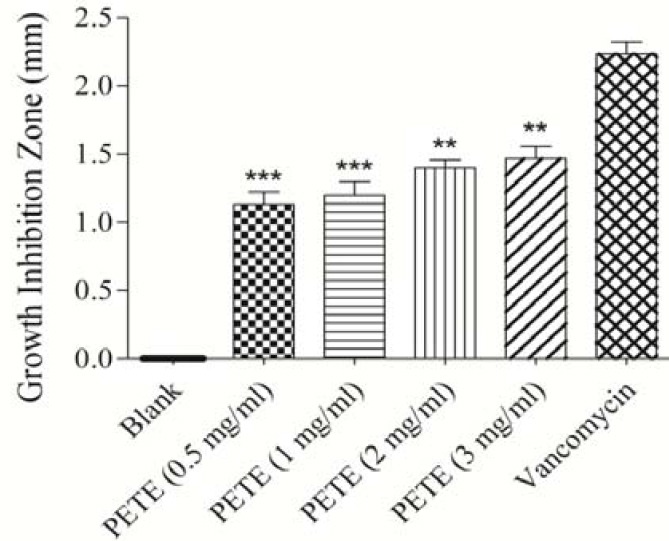
Antibacterial effects of Pinus eldarica bark total hydroalcoholic extract (PETE) on Pesudomonas aeroginosa determined by disc diffusion method. Values are expressed as mean ± S.E.M. PETE and vancomycin samples have significantly increased the growth inhibition zone compared to the blank sample (P<0.001). **P<0.01 compared with vancomycin disc; ***P<0.001 compared with vancomycin disc, Statistical analyses performed with one-way ANOVA and Tukey's post hoc test.
DISCUSSION
P. aeruginosa is the most common cause of infectious diseases in hospitalized patients and a frequent cause of nosocomial infections such as pneumonia, urinary tract infections, and bacteremia. This bacterium is also an important pathogen that frequently involved in the infections of weakened immune system patients and one the most important bacteria causing infection in post-trauma wounds (14,17,18).
P. aeruginosa has developed significant resistance to many types of antimicrobial agents and antibiotics. This intrinsic resistance to a wide range of biocides is associated with the nature of outer membrane in this strain referred to as “hydrophilic permeability barrier”. This barrier protects the bacterium against toxic agents and enables the microorganism to form biofilm. The occurrence of extended-spectrum β-lactamases in P. aeruginosa has also resulted in the lack of specific antibiotics for this organism (19,20).
P. eldarica Medw. is native to Iran and called Iranian pine or Tehran pine. Various parts of this plant (needles, buds, resin and nuts) have been widely used, in the traditional medicine, for the treatment of many diseases including infectious conditions such as wound infections (5).
Although, the antimicrobial activity of pine bark extracts and essential oils of pine needles have been studied in different researches (5,12,21,22), literature survey indicated that antibacterial effects of P. eldarica bark extracts have not been yet studied.
Bark extract of Pinus species contains numerous phenolic compounds such as catechins, taxifolin and phenolic acids that have been demonstrated to exhibit many interesting biological activities. Among polyphenols, flavonoids and proanthocyanidins are of considerable importance due to their wide range of biological activities including antioxidant, anti-inflammatory, vasorelaxant, antimicrobial, antiviral, anticarcinogenic and antimutagenic effects (15,23,24). These naturally occurring substances are generally among antimicrobial natural compounds which have been suggested to inhibit the growth of fungi, yeast, and bacteria (12,15).
P. pinaster bark extract as the most famous pine bark extract used worldwide has been shown to possess marked bacteriostatic activity against both gram-positive and gram-negative strains (12). The composition of P. eldarica bark extract is mainly composed of catechin, taxifolin, caffeic acid and ferulic acid and has a great similarity to the phenolic compounds of famous P. pinaster bark extract. Due to this significant similarity of main phenolic compounds (15,24), it seems that the anti-pseudomonas activity of P. eldarica bark extract observed in the current study could be attributed to its natural phenolic contents.
Proanthocyanidins are the main active components in pine bark extracts and are among the most abundant compounds in various pine species. Many biological and therapeutic activities including antibacterial properties, antivirus properties against Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV-1), immunomodulatory effects, anti-inflammatory activity, inhibitory effects on carcinogenesis and the development and migration of tumors, antimutation properties, cardiovascular protective effects, neuroprotective effects and improvement in perimenopausal symptoms (25) have so far been reported for pine bark proanthocyanidins.
Proanthocyanidins, and generally polyphenols, act as antimicrobial agents mainly due to their ability to complex with proteins, resulting loss of protein function. They inactivate surface-exposed adhesions, cell envelope transport proteins, membrane-bound enzymes and intracelular enzymes, causing alterations both in membrane permeability and in metabolic pathways. They can also disrupt microbial membranes by interacting with membrane lipids (23).
Essential oils are also among the biologically important plant secondary metabolites and are mainly composed of terpenoids, phenylpropanoids, fatty acid and amino acid derivatives. These compounds are generally strong antimicrobial agents and often are more economical and environmentally safe, as antimicrobial agents (13).
The bark essential oil of P. eldarica is mainly composed of high content of monoterpenes (62.2%), specially α-pinene (24.6%), δ-3-carene (10.7%) and myrtenal (3.1%), and significant amounts of sesquiterpenoids including (E)-β-caryophyllene (7.9%) and caryophyllene oxide (14.0%) (15). It seems that the terpenoid components of the essential oil contribute to the anti-pseudomonas activity of the bark essential oil which is in accordance with previous studies about the antibacterial activities of natural terpenoids (26,27,28,29). Antimicrobial activity of terpenoids is often due to their ability to penetrate into the lipid assemblies and consequent perturbation of the lipid fraction of the plasma membranes that is significantly influenced by their physicochemical characteristics and the composition of bacterial membranes (28,30).
Antibacterial activities of pine bark preparations have been demonstrated for many pine species. Generally pine bark extracts has antibacterial effects on gram positive bacteria and the number of species which are active against the gram negative bacteria, specially P. aeroginosa, are scarce. Antibacterial activity of P. halepensis (20), P. massoniana (31), P. nigra ssp. Dalmatica (32), P. nigra (33), P. cembra (23) and P. koraicenis (34) preparations are examples of antibacterial activities of various Pinus species.
Anti-pseudomonas activity of P. roxburghii (35), P. parviflora (36) and P. caribaea (37) has also been demonstrate which is in accordance to what we have observed in the present study.
CONCLUSION
Due to the spread of antibiotic resistance among many species of bacteria, the discovery of new antibiotic agents are the subject of intensive research. Multidrug resistance is quite common among non-fermenting gram-negative bacteria, especially clinically relevant species such as P. aeruginosa. The results of this study shows that P. eldarica bark extracts including total hydroalcoholic bark extract, proanthocyanidins, and volatile components of the bark have significant anti-pseudomonas activity which is important findings as gram-negative bacteria are more resistant to the common antibiotics than gram-positive bacteria. Our findings showed that P. eldarica bark components have significant anti-pseudomonas activity having potentials for new sources of antibacterial agents or antibacterial herbal preparations.
ACKNOWLEDGEMENTS
This study was conducted by the co-operation of Isfahan University of Medical Sciences and AJA University of Medical Sciences. The supports and help of stuffs are gratefully acknowledged. Special thanks are given to Dr Ali Hosseini Sharifabad for his guide and supports.
REFERENCES
- 1.Saleem M, Nazir M, Ali MS, Hussain H, Lee YS, Riaz N, et al. Antimicrobial natural products: an update on future antibiotic drug candidates. Nat Prod Rep. 2010;27:238–254. doi: 10.1039/b916096e. [DOI] [PubMed] [Google Scholar]
- 2.Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Domin MA. Highly virulent pathogens-a post antibiotic era. Br J Theatre Nurs? 1998;8:14–18. doi: 10.1177/175045899800800201. [DOI] [PubMed] [Google Scholar]
- 4.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hames-Kocabas EE, Yesil-Seliktas O, Isleten M, Vardar-Sukan F. Antimicrobial activity of pine bark extract and assesment of potential application in cooked red meat. Gida. 2008;33:123–127. [Google Scholar]
- 6.Hosseinzadeh H, Khooei AR, Khashayarmanesh Z, Motamed-Shariaty V. Antiurolithiatic activity of Pinus eldarica medw: fruits aqueous extract in rats. Urol J. 2010;7:232–237. [PubMed] [Google Scholar]
- 7.Fallah Huseini H, Mehrzadi S, Ghaznavi H, Tajallizadehkhoob Y, Fakhrzadeh H. Effects of Pinus eldarica Medw. Nut extract on blood glucose and cholesterol levels in hypercholesterolemic alloxan-induced diabetic rats. J Med Plant. 2013;1:68–74. [Google Scholar]
- 8.Bolandghamat S, Moghimi A, Iranshahi M. Effects of ethanolic extract of pine needles (Pinus eldarica Medw.) on reserpine-induced depression-like behavior in male Wistar rats. Pharmacogn Mag. 2011;7:248–253. doi: 10.4103/0973-1296.84240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zolfaghari B, Iravani S. Essential oil constituents of the bark of Pinus pinaster from Iran. J Essent Oil Bear Pl. 2012;15:348–351. [Google Scholar]
- 10.Lee JH, Yang HY, Lee HS, Hong SK. Chemical composition and antimicrobial activity of essential oil from cones of Pinus koraiensis. J Microbiol Biotechnol. 2008;18:497–502. [PubMed] [Google Scholar]
- 11.Vigo E, Cepeda A, Gualillo O, Perez-Fernandez R. In vitro anti-inflammatory activity of Pinus sylvestris and Plantago lanceolata extracts: effect on inducible NOS, COX-1, COX-2 and their products in J774A.1 murine macrophages. J Pharm Pharmacol. 2005;57:383–391. doi: 10.1211/0022357055605. [DOI] [PubMed] [Google Scholar]
- 12.Torras MAC, Faura CA, Schonlau F, Rohdewald P. Antimicrobial activity of Pycnogenol®. Phytother Res. 2005;19:647–648. doi: 10.1002/ptr.1662. [DOI] [PubMed] [Google Scholar]
- 13.Lodhia MH, Bhatt KR, Thaker VS. Antibacterial activity of essential oils from palmarosa, evening primrose, lavender and tuberose. Indian J Pharm Sci. 2009;71:134–136. doi: 10.4103/0250-474X.54278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golshani Z, Dawoodi V. Anti-Pseudomonal activity of leaf extracts of Myrtaceae plants against B-lactamase-producing strains. Zahedan J Res Med Sci. 2014;16:33–37. [Google Scholar]
- 15.Iravani S, Zolfaghari B. Phytochemical analysis of Pinus eldarica bark. Res Pharm Sci. 2014;9:243–250. [PMC free article] [PubMed] [Google Scholar]
- 16.Zellagui A, Gherraf N, Ladjel S, Hameurlaine S. Chemical composition and antibacterial activity of the essential oils from Launaea resedifolia L. Org Med Chem Lett. 2012;2:2–4. doi: 10.1186/2191-2858-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aronson NE, Sanders JW, Moran KA. In harm's way: infections in deployed American military forces. Clin Infect Dis. 2006;43:1045–1051. doi: 10.1086/507539. [DOI] [PubMed] [Google Scholar]
- 18.Koehnke A, Friedrich RE. Antibiotic Discovery in the age of structural biology - a comprehensive overview with special reference to development of drugs for the treatment of Pseudomonas aeruginosa infection. In Vivo. 2015;29:161–167. [PubMed] [Google Scholar]
- 19.Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int J Antimicrob Agents. 2015;24:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Abi-Ayad M, Abi-Ayad FZ, Lazzouni HA, Rebiahi SA. Antibacterial activity of Pinus halepensis essential oil from Algeria(Tlemcen) J Nat Prod Plant Resour. 2011;1:33–36. [Google Scholar]
- 21.Ahn J, Gru IU, Mustapha A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 2007;24:7–14. doi: 10.1016/j.fm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 23.Apetrei CL, Tuchilus C, Aprotosoaie AC, Oprea A, Malterud KE, Miron A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules. 2011;16:7773–7788. doi: 10.3390/molecules16097773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iravani S, Zolfaghari B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. Biomed Res Int 2013. 2013 doi: 10.1155/2013/639725. ID 639725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YY, Feng J, Zhang XL, Cui YY. Pine bark extracts: nutraceutical, pharmacological, and toxicological evaluation. J Pharmacol Exp Ther. 2015;353:9–16. doi: 10.1124/jpet.114.220277. [DOI] [PubMed] [Google Scholar]
- 26.Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z Naturforsch C. 2007;62:507–513. doi: 10.1515/znc-2007-7-808. [DOI] [PubMed] [Google Scholar]
- 27.Rivas da Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, Alviano DS. Biological activities of alpha-pinene and beta-pinene enantiomers. Molecules. 2012;17:6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolara P, Corte B, Ghelardini C, Pugliese AM, Cerbai E, Menichetti S, et al. Local anaesthetic, antibacterial and antifungal properties of sesquiterpenes from myrrh. Planta Med. 2000;66:356–358. doi: 10.1055/s-2000-8532. [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima H, Shimada J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol Lett. 2004;237:325–331. doi: 10.1016/j.femsle.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 31.Feng S, Zeng W, Luo F, Zhao J, Yang Z, Sun Q. Antibacterial activity of organic acids in aqueous extracts from pine needles (Pinus massoniana Lamb) Food Sci Biotechnol. 2010;19:35–41. [Google Scholar]
- 32.Politeo O, Skocibusic M, Maravic A, Ruscic M, Milos M. Chemical composition and antimicrobial activity of the essential oil of endemic Dalmatian black pine (Pinus nigra ssp. dalmatica) Chem Biodivers. 2011;8:540–547. doi: 10.1002/cbdv.201000185. [DOI] [PubMed] [Google Scholar]
- 33.Nasrollahzadeh-Sabet M, Tabaei-Aghdaei S, Imani A. Investigate the antibacterial effect of black pine essence (Pinus nigra) against the bacteria E. coli, Staphylococcus aureus and Enterococcus faecalis in three province of Ardabil, Gilan and Tehran. Europ J Zoologic Res. 2013;2:76–81. [Google Scholar]
- 34.Sakagami H, Yoshihara M, Fujimaki M, Wada C, Komatsu N, Nakashima H, et al. Effect of pine seed shell extract on microbial and viral infection. In Vivo. 1992;6:13–16. [PubMed] [Google Scholar]
- 35.Zulqarnain, Rahim A, Ahmad K, Ullah F, Ullah H, Nishan U. In vitro antibacterial activity of selected medicinal plants from lower Himalayas. Pak J Pharm Sci. 2015;28:581–587. [PubMed] [Google Scholar]
- 36.Oh-Hara T, Sakagami HF, Kawazoe YF, Kaiya TF, Komatsu NF, Ohsawa NF, et al. Antimicrobial spectrum of lignin-related pine cone extracts of Pinus parviflora Sieb. et Zucc. In Vivo. 1990;4:7–12. [PubMed] [Google Scholar]
- 37.Sonibare OO, Olakunle K. Chemical composition and antibacterial activity of the essential oil of Pinus caribaea from Nigeria. Afr J Biotechnol. 2008;7:2462–2464. [Google Scholar]


