Abstract
Objective:
The objective of this study was to evaluate the safety and the effect of platelet-rich plasma (PRP) intra-articular injections obtained from blood donors (homologous PRP) on elderly patients with early or moderate knee osteoarthritis (OA) who are not candidates for autologous PRP treatment.
Methods:
A total of 60 symptomatic patients, aged 65–86 years, affected by hematologic disorders and early or moderate knee OA, were treated with 5 ml of homologous PRP intra-articular injections every 14 days for a total of three injections. Clinical evaluations before the treatment, and after 2 and 6 months were performed by International Knee Documentation Committee (IKDC), Knee injury and Osteoarthritis Outcome Score (KOOS) and Equal Visual Analogue Scale (EQ VAS) scores. Adverse events and patient satisfaction were recorded.
Results:
No severe complications were noted during the treatment and the follow-up period. A statistically significant improvement from basal evaluation to the 2-month follow-up visit was observed, whereas a statistically significant worsening from the 2-month to the 6-month follow-up visit was showed. The overall worst results were observed in patients aged 80 years or over and in those affected by minor bone attrition. It was found that 90% of patients were satisfied at the 6-month evaluation.
Conclusions:
Homologous PRP has an excellent safety profile but offers only a short-term clinical improvement in selected elderly patients with knee OA who are not candidates for autologous PRP treatment. Increasing age and developing degeneration result in a decreased potential for homologous PRP injection therapy. Further studies are needed to confirm these findings.
Keywords: aged patients, hematologic diseases, homologous platelet, knee osteoarthritis
Introduction
Osteoarthritis (OA) is the most common form of arthritis, typically seen with increasing age affecting all joints. Platelet-rich plasma (PRP) recently came under the spotlight because of its regenerative potential and promising preliminary clinical results in several conditions [Bashir et al. 2015]. Commercially available kits can provide autologous PRP (APRP) from simple blood extraction from the patient. Treatment with APRP is more effective than placebo for knee OA [Patel et al. 2013] and shows more and longer efficacy than hyaluronic acid (HA) injections [Kon et al. 2011]. However, patients with poor general health, multidrug therapy, or hematologic disorders are not candidates for APRP injections and the volume blood absence required could often cause adverse effects, in particular in repeated treatments [Martinez, 2010].
Homologous PRP (HPRP) obtained from healthy, screened and habitual blood donors presents several advantages such as ease of preparation, higher number of platelets than the therapeutic range, almost unlimited availability and limited costs. Recent clinical trials have tested HPRP as a treatment for hand and finger wounds [Balbo et al. 2010], chronic diabetic lower extremity ulcers [Shan et al. 2013], aggressive periodontitis [Markopoulou et al. 2009], and long bone defects [Gubina et al. 2014]. However, to date there are no published studies that evaluate the effect of HPRP intra-articular injections for knee OA, in particular in patients who are not candidates for APRP treatment. Moreover, no clinical trial has been performed to specifically evaluate the role of PRP in elderly patients with early or moderate OA.
The primary aim of the current research was to perform a pilot study assessing the safety of HPRP injections in patients not responsive to conventional nonsurgical treatments [McAlindon et al. 2014] and in which APRP was contraindicated. The secondary aim was to assess and evaluate the outcomes of HPRP therapy in elderly patients affected by different degrees of knee OA at short-term follow up.
Materials and methods
This prospective open-label, single-center, uncontrolled, pilot study was conducted with the highest respect for individual participants. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the eighth revision of the Declaration of Helsinki, Seoul 2008. Before the beginning of any study-related activities, each study participant signed informed consent. Inclusion criteria were: hematologic blood dyscrasias with platelet dysfunction, anemia (hemoglobin less than 10 dl), 65 years old or over, knee OA unilateral localization, history of chronic (⩾4 months) pain or knee swelling, limitation of daily activities, and radiographic imaging findings of degenerative joint changes (Ahlbäck grade I–III). Exclusion criteria were: systemic cardiovascular or neurologic disorders, diabetes, septicemia or fever, cutaneous infections in the area to be injected, use of corticosteroids for two to three weeks before the procedure, use of nonsteroidal anti-inflammatory drugs (NSAIDs) in the 3 weeks before treatment, anticoagulation use 5 days before the procedure, and previous knee surgery. Patients with Ahlbäck grade IV and V were excluded, as nonsurgical treatment was considered not ethically correct [Martinez, 2010]. Standard weight-bearing anterior-posterior radiographs with knees in full extension were performed to determine the OA grade. All patients were treated in Riuniti of Ancona University Hospital following the same inclusion criteria and were prospectively evaluated at two-month and six-month follow-up visits.
The production of HPRP followed the SIMTI recommendations on blood components for nontransfusional use [Aprili et al. 2013]. Every patient received three injections of 5 ml of HPRP poor in leukocytes, containing a concentration of 1200–1600 × 103/µl, spaced apart every 14 days and no further injection during the follow-up period. Before the injection, 10% calcium chloride (Ca++ 0.22 mEq per dose) was added to HPRP to activate coagulation. The skin was sterilely dressed and the infiltration was performed through a classic lateral approach with a 22-gage needle under ultrasound guidance (7.5 MHz transducer). All patients received a series of instructions after every injection. In case of knee pain during the treatment cycle, they were recommended to use cold therapy and to rest for at least 24 hours. Otherwise mild activities and a gradual resumption of normal sport or recreational activities were allowed as tolerated [Kon et al. 2011]. Patients were evaluated before the treatment and after 2 and 6 months from the last injection. Subjective International Knee Documentation Committee (IKDC), Knee Injury and Osteoarthritis Outcome (KOOS), and Equal Visual Analogue Scale (EQ VAS) scores were used for clinical evaluations. Adverse events and patient satisfaction were also recorded.
The Student’s t-test was performed for IKDC, KOOS, and EQ VAS scores to compare preoperative and postoperative values. Data are expressed as a mean, standard deviation (SD) and 95% confidence interval (CI) and p < 0.05 was considered significant for one-tailed tests. The statistical software SPSS (version 17.0) was used for biometric analysis.
Results
From October 2011 to May 2014, 83 patients were assessed for eligibility. A total of 17 patients declined participation and 3 patients withdrew from the study after the first injection for personal reasons. One patient was excluded for taking corticosteroids to treat lower back pain after the second injection, and two patients were lost at the 2-month follow-up visit. Therefore, the study sample consisted of 60 patients (21 men, 39 women), mean age 72 ± 5.88 (CI 70.51–73.49) affected by joint space narrowing, <3 mm (Ahlbäck I, 75%), joint space obliteration (Ahlbäck II, 18.3%) and minor bone attrition, 0–5 mm (Ahlbäck III, 6.67%). A total of 54 patients were affected by anemia (hemoglobin less than 10 dl), one by idiopathic thrombocytopenic purpura (Werlhof’s disease), three by systemic lupus erythematosus, and two by splenomegaly.
No severe complications related to the infiltrations were observed during the treatment and the follow-up period. Only minor side effects were detected in nine patients (15%), such as transitory intra-articular burning sensation immediately after the injection or mild articular pain for a few days.
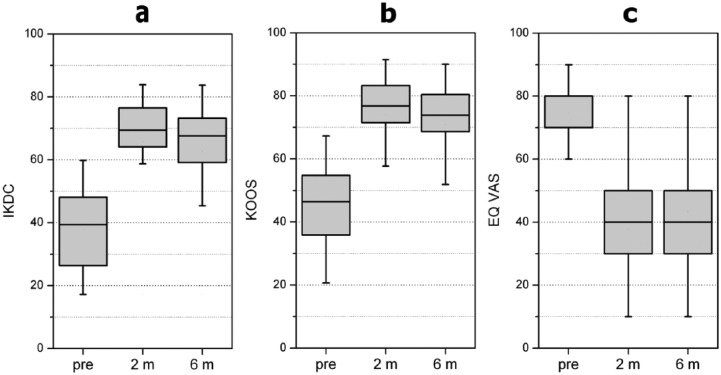
The Student’s t-test was performed for IKDC, KOOS and EQ VAS scores to find statistically significant differences (p < 0.05 for one-tailed tests) comparing pretreatment with after 2 months, pretreatment with after 6 months, and 2 months with 6 months results. The results showed that HPRP was statistically effective (Table 1 and Figure 1). In fact, for IKDC values, the results were significant at p < 0.0005 after 2 months (t = 17.52, n = 60) and after 6 months (t = 6.86, n = 60) (Table 1 and Figure 1a). Moreover, the same statistically significance was found for KOOS scores after 2 months (t = 17.49, n = 60), and after 6 months (t = 14.9, n = 60) using the same test (Table 1 and Figure 1b). Furthermore, for EQ VAS values, the related t-test on the data, after 2 months (t = 16.75, n = 60) and after 6 months (t = 13.4, n = 60), showed significant results at p < 0.0005 (Table 1, Figure 1c). A significant worsening between follow up at 2 and 6 months was also assessed using the related t-test. In fact, for IKDC (t = 7.95, n = 60), KOOS (t = 7.45, n = 60) and EQ VAS scores (t = 7.97, n = 60) obtained at 2 and 6 months, the results were significant at p < 0.0005 (Table 1).
Table 1.
Global IKDC, KOOS and EQ VAS scores at basal (PRE), 2-month (2 MO) and 6-month (6 MO) evaluations after HPRP treatment. Statistically significant differences (p < 0.05 for one-tailed tests) from basal evaluation to the 2- and 6-month follow-up visits and from the 2- to 6-month follow-up visits was assessed using the Student’s t-test.
| HPRP M ± SD [95% CI] | t value | p value | |||
|---|---|---|---|---|---|
| IKDC | PRE | 37.9 ± 12.2 [34.80–40.97] | PRE versus 2 MO | 17.52 | < 0.0005 |
| 2 MO | 65.9 ± 16.5 [61.70–70.06] | PRE versus 6 MO | 6.86 | < 0.0005 | |
| 6 MO | 62.6 ± 16.9 [58.47–67.05] | 2 MO versus 6 MO | 7.95 | < 0.0005 | |
| KOOS | PRE | 46.1 ± 11.8 [43.09–49.07] | PRE versus 2 MO | 17.49 | < 0.0005 |
| 2 MO | 74.0 ± 15.0 [70.22–77.82] | PRE versus 6 MO | 14.90 | < 0.0005 | |
| 6 MO | 70.9 ± 15.3 [67.02–74.77] | 2 MO versus 6 MO | 7.45 | < 0.0005 | |
| EQ VAS | PRE | 75.5 ± 11.4 [72.0–77.7] | PRE versus 2 MO | 16.75 | < 0.0005 |
| 2 MO | 38.6 ± 18.1 [33.3–42.3] | PRE versus 6 MO | 13.40 | < 0.0005 | |
| 6 MO | 43.2 ± 19.5 [38.6–48.1] | 2 MO versus 6 MO | 7.97 | < 0.0005 |
M, mean; SD, standard deviation; CI, confidence interval; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; EQ VAS, Equal Visual Analogue Scale.
Figure 1.
Health status evaluated with IKDC (a), KOOS (b) and EQ VAS (c) scores (0 to 100). Using the Student’s t-test and considering p < 0.05 significant for a one-tailed test, statistically significant improvements from basal evaluation to the 2- and 6-month follow-up visits were observed, whereas a significant worsening from the 2- to 6-month follow-up visits was noted. Black line, median; box limit, quartiles; extreme values, minimum–maximum; pre, pretreatment; m, months; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; EQ VAS, Equal Visual Analogue Scale.
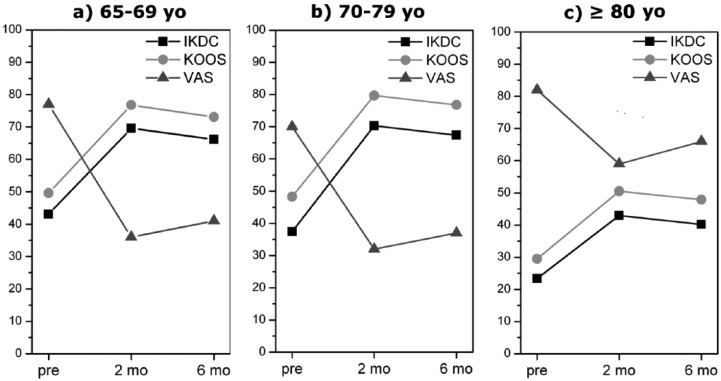
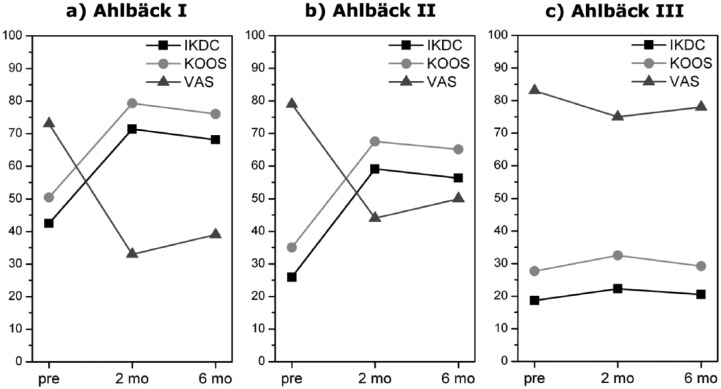
Further analyses were performed on age and knee degeneration influences on clinical outcomes. From a descriptive analysis of the mean, the overall worst results were observed in patients aged 80 years or over (n = 9). Moreover, the related t-test on 80 years or over subgroup data showed no significant differences (p > 0.05) for KOOS and EQ VAS pretreatment and post-treatment values (Figure 2c). A significant improvement was noted (p < 0.0005) for subgroups 65–69 (n = 27) and 70–79 (n = 24) years from basal to 2- and 6-month evaluations (Figure 2a and b). However, the differences between 2- and 6-month values were not significant, although the comparison between means showed that the clinical outcome after 6 months was worse than after 2 months. Furthermore, from a descriptive analysis of the means, we observed the overall best results after 2 and 6 months in Ahlbäck I subgroup and the overall worst results in Ahlbäck III subgroup (Figure 3c). The statistical analysis showed a significant improvement (p < 0.0005) in Ahlbäck I subgroup (n = 45) comparing pretreatment with 2-month and pretreatment with 6-month results, whereas a significant worsening between 2 and 6 months was noted (IKDC, p ≈ 0.0039; KOOS,p ≈ 0.007; EQ VAS, p ≈ 0.01) (Figure 3a). Ahlbäck II subgroup (n = 11) showed a significant improvement after 2 and 6 months, but the differences between 2 and 6 months were not significant (IKDC, p ≈ 0.61; KOOS, p ≈ 0.62; EQ VAS, p ≈ 0.34), although the comparison between means showed that the clinical outcome after 6 months was worse than after 2 months (Figure 3b). No statistical analysis was performed on Ahlbäck III subgroup data because the sample size was too low (four patients).
Figure 2.
Influence of age on clinical outcome. The overall worst results were observed in patients aged 80 years or over and the related t-test showed no significant differences (p > 0.05) for KOOS and EQ VAS pretreatment and post-treatment values (c). A significant improvement was noted (p < 0.0005) for subgroups 65–69 and 70–79 years from basal to 2- and 6-month results (a, b). The differences between 2- and 6-month values were not significant, although the comparison between means showed that the clinical outcome after 6 months was worse than after 2 months. yo, years old; pre, pretreatment; m, months; KOOS, Knee injury and Osteoarthritis Outcome Score; EQ VAS, Equal Visual Analogue Scale.
Figure 3.
Influence of knee degeneration on clinical outcome. The overall best results after two and six months was observed in Ahlbäck I subgroup and the overall worst results in Ahlbäck III subgroup (c). The statistical analysis showed a significant improvement (p < 0.0005) in Ahlbäck I subgroup comparing pretreatment with 2-month and pretreatment with 6-month results, whereas a significant worsening between 2 and 6 months (a). Ahlbäck II subgroup showed a significant improvement after 2 and 6 months, but the differences between 2 and 6 months were not significant, although the comparison between means showed that the clinical outcome after 6 months was worse than after 2 months (b). pre, pretreatment; m, months.
Despite the heterogeneity between subgroups, 90% of patients were found satisfied at 6-month evaluation (54 of 60) and would agree to a further HPRP treatment.
Discussion
The present article is the first description of selected patients affected by knee OA treated with HPRP injections.
Although it could be argued that what is needed now is an appropriate randomized clinical trial to assess the clinical effectiveness of HPRP in this specific subgroup of patients, the presented further pilot stage was essential.
Regarding our primary aim, we observed no severe complications related to the injections during the treatment and follow-up period, but only minor side effects also common to APRP therapy [Kon et al. 2011].
Regarding our secondary aim, a statistically significant improvement in reducing pain and recovering articular function were observed in all patients from basal evaluation to 2- and 6-month follow-up visits. However, a statistically significant worsening from 2- to 6-month evaluations was noted. Further analyses were performed to investigate the influence of age and knee degeneration on the clinical outcomes. The overall best results in terms of mean and statistically significant improvement at 2- and 6-month evaluations were observed in patients aged 65–79 years and in Ahlbäck I–II knee OA. Thus, the global statistically significant improvement after 2 and 6 months was probably due to these subgroups. The overall worst results in terms of means were observed in 80 years or over and to Ahlbäck III subgroups. Therefore, a less favorable clinical outcome might be expected in these subgroups. Moreover, apart from Ahlbäck I subgroup, no statistical differences were noted between 2- and 6-month values, although the comparison between means showed that the clinical outcome after 6 months was worse than after 2 months in all subgroups. This might be explained with the low sample size of the subgroups and therefore, any significant difference might be too small to be observed in such samples.
In the present study, we can only hypothesize the mechanism of action of PRP on the basis of previous studies in reported literature. A high percentage of vital and healthy cartilage cells could be expected in relatively younger patients with a low degree of knee OA, leading to a significant response to growth factors released by PRP. However, the PRP influence on overall joint homeostasis and thus its effect on clinical outcome could be only temporary, without affecting the cartilage tissue structure and joint degenerative progression [Ashammakhi, 2005; Spreafico et al. 2009]. It is clear that at this research stage there is no definitive answer regarding the efficacy of HPRP injections.
Our results are in agreement with previous findings in reported literature, confirming a decreased potential for PRP therapy with increasing age and developing knee degeneration [Jang et al. 2013]. Although a short-term improvement may be observed in these patients, the lack of a control group, randomization and long-term follow up prevents the assessment of the real effectiveness of this treatment. In fact, the marked improvement after 2 months could all be due to a placebo effect and therefore the efficacy of HPRP is far from being demonstrated at this pilot stage. Therefore, a careful evaluation of risks and benefits should be made before undertaking it, in view of patient comorbidity and operability. These considerations must be weighed appropriately against the potential importance of a placebo-controlled trial for any future studies.
Limitations of our study are the lack of randomization and placebo control groups other than imaging results and evaluations only at short-term follow-up. The tendency for worsening of clinical outcomes after 6 months justified the choice of this amount of time as the final evaluation and the request of a second injection cycle or a different treatment explained the impossibility of evaluating results at longer follow up. Despite the request for a second injection cycle, a further series of injections was not stated in the study protocol and therefore it was not administered. Further studies are needed to confirm these findings but also histological and biochemical investigations to determine the PRP mechanism of action, biological changes and disease-modifying properties.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Carlo Bottegoni, Department of Clinical and Molecular Sciences, Polytechnic University of Marche, Via Tronto 10/A, 60020 Ancona, Italy.
Luca Dei Giudici, Department of Clinical and Molecular Sciences, Polytechnic University of Marche, Ancona, Italy.
Sergio Salvemini, Department of Clinical and Molecular Sciences, Polytechnic University of Marche, Ancona, Italy.
Enrico Chiurazzi, Intercompany Regional Department of Transfusion Medicine, Torrette Ancona, Italy.
Rosella Bencivenga, Intercompany Regional Department of Transfusion Medicine, Torrette Ancona, Italy.
Antonio Gigante, Department of Clinical and Molecular Sciences, Polytechnic University of Marche, Ancona, Italy.
References
- Aprili G., Gandini G., Guaschino R., Mazzucco L., Salvaneschi L., Vaglio S., et al. (2013) SIMTI recommendations on blood components for non-transfusional use. Blood Transfus 3: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashammakhi N. (2005) Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials 26: 1953–1960. [DOI] [PubMed] [Google Scholar]
- Balbo R., Avonto I., Marenchino D., Maddalena L., Menardi G., Peano G. (2010) Platelet gel for the treatment of traumatic loss of finger substance. Blood Transfus 8: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir J., Panero A., Sherman A. (2015) The Emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc 115: 24–31. [DOI] [PubMed] [Google Scholar]
- Gubina B., Rožman P., Bišcević M., Domanović D., Smrke D. (2014) The influence of allogeneic platelet gel on the morphology of human long bones. Coll Antropol 38: 865–870. [PubMed] [Google Scholar]
- Jang S., Kim J., Cha S. (2013) Platelet-rich plasma (PRP) injections as an effective treatment for early osteoarthritis. Eur J Orthop Surg Traumatol 23: 573–580. [DOI] [PubMed] [Google Scholar]
- Kon E., Mandelbaum B., Buda R., Filardo G., Delcogliano M., Timoncini A., et al. (2011) Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 27: 1490–1501. [DOI] [PubMed] [Google Scholar]
- Markopoulou C., Markopoulos P., Dereka X., Pepelassi E., Vrotsos I. (2009) Effect of homologous PRP on proliferation of human periodontally affected osteoblasts. In vitro preliminary study. Report of a case. J Musculoskelet Neuronal Interact 9: 167–172. [PubMed] [Google Scholar]
- Martinez S. (2010) Practical guidelines for using PRP in the orthopaedic office. AAOS Now. Accessible at: http://www.aaos.org/news/aaosnow/sep10/clinical3.asp (accessed September 2015).
- McAlindon T., Bannuru R., Sullivan M., Arden N., Berenbaum F., Bierma-Zeinstra S., et al. (2014) OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 22: 363–388. [DOI] [PubMed] [Google Scholar]
- Patel S., Dhillon M., Aggarwal S., Marwaha N., Jain A. (2013) Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med 41: 356–364. [DOI] [PubMed] [Google Scholar]
- Shan G., Zhang Y., Ma J., Li Y., Zuo D., Qiu J., et al. (2013) Evaluation of the effects of homologous platelet gel on healing lower extremity wounds in patients with diabetes. Int J Low Extrem Wounds 12: 22–29. [DOI] [PubMed] [Google Scholar]
- Spreafico A., Chellini F., Frediani B., Bernardini G., Niccolini S., Serchi T., et al. (2009) Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J Cell Biochem 108: 1153–1165. [DOI] [PubMed] [Google Scholar]