Abstract
Human adolescence is arguably one of the most challenging periods of development. The young adult is exposed to a variety of stressors and environmental stimuli on a backdrop of significant physiological change and development, which is especially apparent in the brain. It is therefore unsurprising that many psychiatric disorders are first observable during this time. The human intestine is inhabited by trillions of microorganisms, and evidence from both preclinical and clinical research focusing on the established microbiota-gut-brain axis suggests that the etiology and pathophysiology of psychiatric disorders may be influenced by intestinal dysbiosis. Provocatively, many if not all of the challenges faced by the developing teen have a documented impact on these intestinal commensal microbiota. In this review, we briefly summarize what is known about the developing adolescent brain and intestinal microbiota, discuss recent research investigating the microbiota-gut-brain axis during puberty, and propose that pre- and probiotics may prove useful in both the prevention and treatment of psychiatric disorders specifically benefitting the young adult.
Keywords: adolescence, microbiota-gut-brain axis, development, psychiatric illnesses, early life challenges, probiotics, hypothalamic-pituitary-adrenal axis, brain plasticity, critical windows
Abstract
L’adolescence humaine est sans aucun doute l’une des périodes les plus difficiles du développement. Le jeune adulte est exposé à une variété de stresseurs et de stimuli environnementaux dans un contexte de changement et de développement physiologique significatif, qui est spécialement apparent dans le cerveau. Il n’est donc pas étonnant que nombre de troubles psychiatriques sont d’abord observables à cette période. L’intestin humain est habité de mille milliards de microorganismes, et des données probantes d’études cliniques et précliniques portant sur l’axe établi microbiote-intestin-cerveau suggèrent que l’étiologie et la pathophysiologie des troubles psychiatriques peuvent être influencées par la dysbiose intestinale. Disons audacieusement que les nombreuses sinon toutes les difficultés avec lesquelles l’adolescent en développement est aux prises ont un impact documenté sur ces microbiotes intestinaux commensaux. Dans cette revue, nous résumons brièvement ce qui est connu du cerveau en développement de l’adolescent et des microbiotes intestinaux, nous discutons de la recherche récente sur l’axe microbiote-intestin-cerveau durant la puberté, et nous proposons que les pré-biotiques et les pro-biotiques peuvent se révéler utiles dans la prévention et le traitement des troubles psychiatriques qui touchent spécifiquement le jeune adulte.
Highlights
The young adult is exposed to numerous challenges and stressors during adolescence, a period in which the brain is still developing and many psychiatric disorders first manifest.
The composition and diversity of the gut microbiota are also changing during adolescence, and the trajectory of these changes is affected by environmental challenges.
Alterations to the gut microbiota during adolescence can influence the development of the brain and may contribute to the manifestation of various psychiatric illnesses associated with puberty.
Microbial-based treatments may provide safe and effective methods for improving teenage psychiatric health and resiliency to the ill effects of experienced adverse events.
Introduction
Human adolescence is known to be a challenging period of development; it is a time when the young adult is frequently exposed to a variety of psychological stressors, often less than optimal nutrition and sleep, and increased opportunity for exposure to known neurodevelopmental teratogens such as alcohol and drugs. These challenges and exposures are occurring on a background of critical wiring for brain development—in fact, together with the neonatal period, adolescence is one of the most dynamic and important periods for growth and development in the brain.1–7
Perhaps unsurprisingly, adolescence is also a period of development when psychiatric illnesses frequently first manifest. Symptoms of anxiety, depression, eating disorders, psychosis and substance abuse are often first observed during this critical window.2,8 We are beginning to increasingly understand that the pathophysiology of these disorders can be traced to changes that are unfolding in the adolescent brain,8,9 alterations that can easily be influenced by the challenges and exposures frequently faced during the adolescent period (see Figure 1).1,8,10
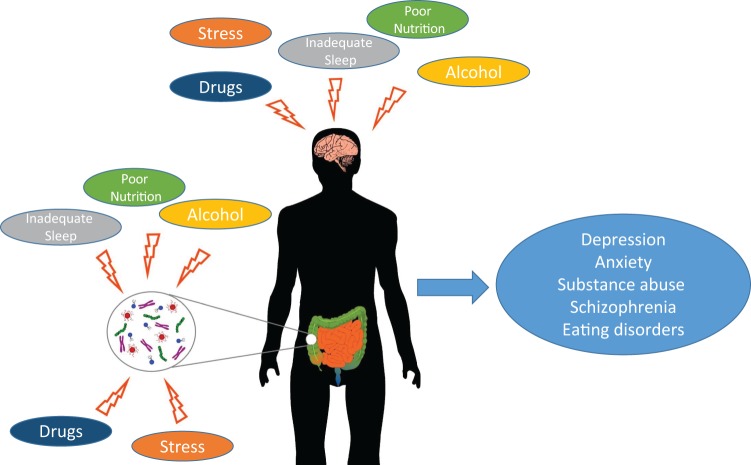
Figure 1.
Adolescent exposures to stress, poor nutrition, inadequate sleep, and potentially alcohol and drugs affect both gut microbiota and brain developmental trajectories, which may have deleterious effects on mental health outcomes.
There has been a recent surge in evidence that the trajectory of postnatal nervous system development is influenced by the intestinal microbial status.11–14 Work in preclinical models of intestinal microbial dysbiosis, such as those using germ-free (GF) rodents or antibiotic-treated animals, has shown alterations to behaviour in addition to changes in associated brain neurochemistry.13,15–19 Clinical studies examining intestinal microbial status in relation to a number of gastrointestinal and psychiatric disorders indicate that the bacteria housed in the gut likely play a significant role in disease presentation and/or pathogenesis.20–27 In fact, the well-established gut-brain axis, or the bidirectional highway of communication connecting the gut and brain, has been recently extended to include the bacterial components of the intestinal lumen, leading to the now extended concept of a microbiota-gut-brain axis.28–31 Mechanisms by which communication occurs in this axis is the focus of robust scientific efforts and likely involves a combination of activity from immune, endocrine, metabolic, and neural pathways.32
In this review, we consider the rapidly expanding evidence of the microbiota-gut-brain axis, particularly with respect to the developing adolescent brain. We briefly summarize what is known about neural development during this critical window and the increasing information we have about the intestinal microbial status at this time point. We hypothesize that alterations to the bacterial load of the gut during this time may be influencing the development of the brain and may play a mechanistic role in the manifestation of various psychiatric illnesses associated with puberty. Last, we propose that therapeutics aimed at the intestinal microbiota will provide alternatives for both the prevention and treatment of psychiatric illnesses associated with adolescence.
Brain Development during Adolescence
The physiological changes that occur in the adolescent are thought to reflect the evolution of the young adult readying to leave the safety of home and to lead to the ultimate achievement of independence from caregivers. Similarly, significant social and cognitive changes are associated with puberty in the transition to adulthood that allow for that same independence.3,33 Historically, the brain was considered to have been largely finished in development by puberty, but we now know that this period of profound transition is also reflected in neuroanatomical change and maturation.
Postmortem Evidence for Structural Brain Development in Adolescence
Research on postmortem human brains in the late 1960s and early 1970s first revealed that the frontal lobes of the brain continue to develop well past adolescence and into early adulthood.34,35 The frontal lobes are key structures involved in executive functioning, cognition, and working memory,36,37 and 2 main structural alterations were found to occur there before and after puberty: changes in synaptic density and axonal myelination.
The developmental focus in early life is on synaptogenesis, but this is soon followed by a period of synaptic elimination and pruning.33,38 Synaptic pruning is thought to be critical for the fine-tuning of neural networks in the brain, rendering the communication between the remaining synaptic circuits more efficient,39 as it involves the elimination of many of the synaptic connections formed early in development. While the exact mechanisms by which pruning occurs are unknown, we do know that resident microglia play a role in the eradication of synapses and the strengthening and maintenance of others.40,41 In sensory regions, synaptogenesis occurs during the first few months following birth, after which time pruning begins, with synaptic density reaching adult levels before or around adolescence.35,42,43 The time course of synaptic development in the frontal lobes, however, is strikingly different. In the prefrontal cortex, there is a proliferation of synapses during childhood and again in puberty, followed by a plateau in synaptic density and a subsequent postpubertal elimination of synaptic connections.34,44,45 These data suggest that synaptic pruning occurs primarily during adolescence in the frontal lobes and results in a reduction in the total amount of synaptic connections in adulthood.
The second main difference between brain structure in the pre- and postpubertal periods pertains to changes in myelin. As neurons develop, glial cells produce myelin, which wraps around axons to increase the speed of neural communication. In the first few years of life, sensory and motor areas are already fully myelinated, while the axons in the frontal cortex continue to undergo myelination throughout adolescence.46 The implication of these findings is that the speed of neural communication in the frontal lobes is increasing from childhood to adolescence.
Imaging the Adolescent Brain
The advent of noninvasive brain imaging such as magnetic resonance imaging (MRI) has provided further evidence of the ongoing maturation of the frontal cortex in adolescence. During childhood, the frontal and parietal lobes both show general increases in grey matter cortical volume, which peak in puberty and then begin to decrease.47–53 Concurrent with this, volumes of white matter show a relatively constant increase.47,54 These changes have widely been attributed to alterations in synaptic pruning and myelination.8,33,38 It is likely that axonal myelination during adolescence results in an increase in white matter and a simultaneous decrease in grey matter as observed with MRI. Another possible interpretation is that changes to grey matter volume reflect synaptic remodeling; the increase and decrease of grey matter volume at the onset and after puberty have been attributed to synaptogenesis and synaptic pruning, respectively.33,38
Pubertal Changes in Stress Reactivity
Adolescence and puberty are marked by significant changes in the endocrine system, resulting in altered hormonal signaling. Although most studies have focused on gonadal hormones, recent attention has been drawn to understanding the development of the hypothalamic-pituitary-adrenal (HPA) axis and stress responsivity during adolescence (for reviews, Eiland and Romeo,55 McCormick and Mathews,56 Romeo and McEwen,57 and Romeo58). The majority of evidence for development of stress responsivity in adolescence comes from research in rodent models. During puberty, gonadal hormones increase dramatically, whereas basal levels of stress hormones remain relatively constant.59 In contrast, the increase in corticosterone to both physical and psychological stressors is prolonged in prepubertal compared to adult rats.60–62 Interestingly, this elongation of stress hormone signaling is not mediated by the different gonadal hormonal milieus of these 2 developmental stages.61 Repeated exposure to the same stressor induces habituation of the hormonal stress response in adult rats; however, prepubertal males, but not females, display a higher peak level of corticosterone but a faster return to baseline.63 In line with these findings, studies have found increased anxiety- and depressive-like behaviours in prepubertal rats previously exposed to a stressor.64–66 Taken together, the literature clearly shows that HPA axis signaling and stress responsivity undergo development during adolescence, but the mechanisms underlying these changes remain to be elucidated.
Dynamics of Gut Microbiota during the Adolescent Period
Recent improvements in next-generation microbial sequencing technology have allowed us to more reliably examine the constituent bacteria in the human gut and to look for patterns in diversity over the course of the life span.67 We now know that while the intestinal microbiota of an adult is abundant, highly varied, and relatively stable, the infant microbiota is much simpler and less stable.9,68 Colonization of the infant is considered to occur primarily during birth and the immediate postnatal period, and historically, the infant’s bacterial load was thought to be determined primarily by that which is resident in the mother. It is now becoming clear that this may only be true if the infant is vaginally born, as mode of delivery (i.e., vaginal or caesarean) has a distinct impact on bacterial colonization patterns.69–71 Various additional factors have been identified as influencing the continued development of microbiota during early life, including if the infant was hospital or home born,72,73 formula or breastfed,74–78 and if there was any antibiotic use during this period.79–81
During childhood, we generally observe less diversity in microbial species compared to that which is seen in adults,82 but during development and growth into the adolescent period, the intestinal microbiota similarly develops and slowly changes, with decreases occurring in numbers of aerobes and facultative anaerobes, as well as concurrent increases in anaerobic species.83 A recent high-throughput analysis of distal gut microbiota that compared adults to adolescents found a significantly higher abundance of both Bifidobacterium and Clostridium genera in the adolescents, while the number of species was similar between the 2 groups.84
While we are still far from an exhaustive list of changes that occur over the life span in terms of colonizing intestinal microbiota, what is abundantly clear is that the gut houses a community of diverse bacteria that are changing throughout life in response to physiological and environmental cues. Intriguingly, many if not all of the stressors that teenagers are exposed to are documented to affect the bacterial constituents of the intestinal lumen. Changes to diet, sleep patterns, stress, alcohol and drug use, and prescription antibiotic use all result in alterations to the intestinal microbiota.15,85–88 Given that adolescents are exposed to such a vast array of external challenges and stressors, all with known effects on the intestinal microbiota, we are then left with pertinent questions regarding the potential role of these bacteria in the psychiatric disorders that often emerge during the teenage years.
Adolescent Behaviour and the Microbiota-Gut-Brain Axis
A large-scale National Comorbidity Study conducted in the United States from 2001 to 2003 indicated that the peak age of onset for psychiatric disease as a whole is 14 years.89 The mood disorders, including anxiety and depression, schizophrenia, and eating disorders,8 often first manifest at this time. In healthy adolescents without clinical psychiatric illness, alterations in behaviour corresponding to increased social behaviours, risk taking,7,90 sensation seeking,91 impulsivity,92 and emotional instability7 are frequently observed.
In rodent models, the emergence of maladaptive behaviours during adolescence can be directly linked to the occurrence of early life challenges, with many of the preclinical studies examining the emergence of behavioural alterations during adolescence found in the social behaviour and autism literature. Injecting pregnant dams with valproic acid (VPA) is one of the most robust and well-characterized models for studying autistic-like symptoms in rodent offspring. It has been established that not only does this prenatal challenge result in altered social behaviours in adolescent offspring, but it also induces changes to the commensal intestinal microbiota.93 Similarly, a recent study by Foley et al.94 demonstrated that injecting rat dams with either lipopolysaccharide or propionic acid (a fatty acid product of many enteric bacteria) also resulted in long-term alterations to social behaviours in offspring, with the aberrations particularly apparent during the adolescent period. Both of these treatments also showed marked effects on the composition of the intestinal microbiota. Intriguingly, a recent study using maternal immune activation to induce autism-like symptoms in young adult offspring demonstrated that feeding the pups commensal bacteria Bacteroides fragilis ameliorated the otherwise observed behavioural deficits.95
Given our growing understanding of the relationship between the microbiota and behaviour, there are still remarkably few studies examining the role of the microbiota-gut-brain axis in the development and function of the adolescent brain. To investigate the effects of manipulating the bacterial contents of the gut during this critical window of development, researchers have examined the impact of adolescent bacterial colonization of GF mice (mice bred and raised in the total absence of bacteria), while others have examined the impact of antibiotic treatment in otherwise normal juvenile animals.
GF mice show distinct abnormalities in HPA axis activity. Specifically, these animals show lifelong stress hyperresponsivity, with increased plasma adrenocorticotropin hormone (ACTH) and corticosterone following exposure to a stressor.14 These alterations are accompanied by an abnormal behavioural phenotype, such as anxiolytic-like behaviour,13,16 social behaviour deficits,17 and cognitive dysfunction.19 Intriguingly, if these mice are introduced to colonizing bacteria by the adolescent time period (in mice roughly 4 weeks of age), the dysfunctions in stress responsivity can be reversed, but not if the bacteria are introduced to GF mice in adulthood (i.e., past 6 weeks of age). This finding is important because it indicates that there may be a brief window in life, up to and encompassing the adolescent period, whereby commensal intestinal microbiota may have a lasting effect on central stress circuitry. Thus, given that the dysregulation of brain circuitry responsible for stress responsivity is implicated in all of the psychiatric illnesses that often first appear in adolescence, the fact that these circuits are responsive to changes in gut microbiota during this same time point is highly provocative.
A recent study examining the effects of antibiotics administered at weaning in mice (generally between 3 and 4 weeks of age and again considered roughly equivalent to an adolescent period) found significant differences in adult intestinal microbial content, behaviour, and brain neurochemistry posttreatment compared to controls.15 Specifically, mice were given a combination of antibiotics administered in drinking water for 4 weeks, after which adult behaviours were assessed. Mice receiving antibiotic showed reduced anxiety-like behaviour in the light-dark test and altered cognitive functioning and social behaviours, as assessed in a novel object recognition test and social transmission of food preference test, respectively. In addition, mice treated with antibiotics in young adulthood showed increased serum tryptophan and decreased kynurenine levels relative to controls and significantly reduced hippocampal brain-derived neurotrophic factor (BDNF) messenger RNA (mRNA). Alterations in brain monoamines and monoamine metabolites as well as hypothalamic oxytocin and vasopressin mRNA expression were also observed following antibiotic treatment.15 This study demonstrates that altering the intestinal microbial population through antibiotic treatment in the adolescent time period can have long-term effects on brain function and gene expression. Intriguingly, the anxiolytic-like behaviour observed in these mice mirrors those observed in GF mice, which not only demonstrate reduced anxiety-like behaviour but also associated changes in hippocampal BDNF expression.13,16
Future Directions
Given our expanding understanding of microbiota-gut-brain communication, we are now at an exciting time for the design and development of potential therapeutics targeting psychiatric disease. There is currently a high level of interest in the use of pre- and/or probiotics as a novel avenue of therapeutic possibilities in the often refractory psychiatric illnesses. Indeed, the therapeutic potential of bacteria in the treatment of psychiatric illnesses has led to the development of the term psychobiotic and has been initially examined in a few recent studies.96 Most clinical studies examining the potential impact of commensal bacteria on behavioural symptoms have been done on patients with irritable bowel syndrome (IBS). Patients with IBS frequently present with comorbid anxiety and depressive illnesses, and several different strains of bacteria have proven to be useful in combatting the psychological symptoms associated with the disorder.97–100 Similarly, a randomized, double-blind, placebo-controlled study of patients with chronic fatigue syndrome also found commensal bacteria to be beneficial in the treatment of anxiety symptoms.101 Studies in healthy volunteers have also shown psychological benefits of certain bacterial strains, with probiotic treatment associated with reduced levels of psychological distress and improved overall mood.101,102
Concluding Remarks
While there are many examples of preventative measures directed at adolescents for navigating through the various challenges of this tumultuous time in life, we would be wise to direct our consideration to potential therapeutics aimed at the intestinal microbiota. Possible preventative and/or ameliorative treatment effects of pre- and probiotics have been heretofore unexamined for adolescents and would provide an exciting, safe, and potentially highly effective method for improving teenage resiliency to the multiple and varied challenges they face. The vulnerability of the adolescent brain due to its higher degree of plasticity can be exploited to our advantage. The malleability of the nervous system at this critical period can be harnessed, allowing us to intervene in high-risk individuals. Specifically, we could target the intestinal bacteria and thus the microbiota-gut-brain axis to both incur protection to the brain from challenges and to ameliorate the ill effects of experienced adverse events. Improving teenage mental resiliency through microbial-based interventions is an exciting and heretofore unexamined possibility, one with highly anticipated opportunities for better psychiatric health.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. O’Connor RM, Cryan JF. Adolescent brain vulnerability and psychopathology through the generations: role of diet and dopamine. Biol Psychiatry. 2014;75(1):4–6. [DOI] [PubMed] [Google Scholar]
- 2. Sturman DA, Moghaddam B. The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev. 2011;35(8):1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1(4):392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blakemore SJ, Choudhury S. Brain development during puberty: state of the science. Dev Sci. 2006;9(1):11–14. [DOI] [PubMed] [Google Scholar]
- 6. Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. NeuroImage. 2014;88:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. [DOI] [PubMed] [Google Scholar]
- 8. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–518. [DOI] [PubMed] [Google Scholar]
- 10. Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol. 2014;41:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26(1):98–107. [DOI] [PubMed] [Google Scholar]
- 12. McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25(2):e183–e188. [DOI] [PubMed] [Google Scholar]
- 13. Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–264, e119. [DOI] [PubMed] [Google Scholar]
- 14. Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desbonnet L, Clarke G, Traplin A, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. [DOI] [PubMed] [Google Scholar]
- 16. Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19(2):146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. [DOI] [PubMed] [Google Scholar]
- 19. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. [DOI] [PubMed] [Google Scholar]
- 20. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26(8):1155–1162. [DOI] [PubMed] [Google Scholar]
- 21. Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25(9):713–719. [DOI] [PubMed] [Google Scholar]
- 22. Kennedy PJ, Clarke G, O’Neill A, et al. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med. 2014;44(7):1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonfrate L, Tack J, Grattagliano I, Cuomo R, Portincasa P. Microbiota in health and irritable bowel syndrome: current knowledge, perspectives and therapeutic options. Scand J Gastroenterol. 2013;48(9):995–1009. [DOI] [PubMed] [Google Scholar]
- 24. Jeffery IB, Quigley EM, Ohman L, Simren M, O’Toole PW. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes. 2012;3(6):572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11(8):497–505. [DOI] [PubMed] [Google Scholar]
- 26. Finegold SM. State of the art; microbiology in health and disease: intestinal bacterial flora in autism. Anaerobe. 2011;17(6):367–368. [DOI] [PubMed] [Google Scholar]
- 27. Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? BioEssays. 2014;36(10):933–939. [DOI] [PubMed] [Google Scholar]
- 28. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. [DOI] [PubMed] [Google Scholar]
- 30. Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut. 2011;60(3):288–289. [DOI] [PubMed] [Google Scholar]
- 31. De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol. 2014;592(Pt 14):2989–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. [DOI] [PubMed] [Google Scholar]
- 33. Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neurosci Biobehav Rev. 2011;35(8):1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. [DOI] [PubMed] [Google Scholar]
- 35. Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett. 1982;33(3):247–252. [DOI] [PubMed] [Google Scholar]
- 36. Tamminga CA, Buchsbaum MS. Frontal cortex function. Am J Psychiatry. 2004;161(12):2178. [DOI] [PubMed] [Google Scholar]
- 37. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 38. Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychology Psychiatry. 2006;47(3-4):296–312. [DOI] [PubMed] [Google Scholar]
- 39. Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. [DOI] [PubMed] [Google Scholar]
- 40. Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7(4):327–332. [DOI] [PubMed] [Google Scholar]
- 42. Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13(7):2801–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cragg BG. The development of synapses in the visual system of the cat. J Comp Neurol. 1975;160(2):147–166. [DOI] [PubMed] [Google Scholar]
- 44. Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4(1):78–96. [DOI] [PubMed] [Google Scholar]
- 45. Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80(4):1149–1158. [DOI] [PubMed] [Google Scholar]
- 46. Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophrenia Bull. 1989;15(4):585–593. [DOI] [PubMed] [Google Scholar]
- 47. Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. [DOI] [PubMed] [Google Scholar]
- 48. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6(4):551–560. [DOI] [PubMed] [Google Scholar]
- 49. Giorgio A, Watkins KE, Chadwick M, et al. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2010;49(1):94–103. [DOI] [PubMed] [Google Scholar]
- 50. Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. [DOI] [PubMed] [Google Scholar]
- 51. Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. [DOI] [PubMed] [Google Scholar]
- 52. Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. [DOI] [PubMed] [Google Scholar]
- 53. Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15(12):1848–1854. [DOI] [PubMed] [Google Scholar]
- 55. Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–233. [DOI] [PubMed] [Google Scholar]
- 57. Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. [DOI] [PubMed] [Google Scholar]
- 58. Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52(3):244–253. [DOI] [PubMed] [Google Scholar]
- 59. Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. Adrenarche in the rat. J Endocrinol. 2006;191(1):301–308. [DOI] [PubMed] [Google Scholar]
- 60. Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12(3):199–211. [DOI] [PubMed] [Google Scholar]
- 61. Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79(3):125–132. [DOI] [PubMed] [Google Scholar]
- 62. Vazquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res. 1993;34(5):646–653. [DOI] [PubMed] [Google Scholar]
- 63. Romeo RD, Bellani R, Karatsoreos IN, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147(4):1664–1674. [DOI] [PubMed] [Google Scholar]
- 64. Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62(1):22–30. [DOI] [PubMed] [Google Scholar]
- 65. McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187(2):228–238. [DOI] [PubMed] [Google Scholar]
- 66. Vidal J, Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92(5):824–830. [DOI] [PubMed] [Google Scholar]
- 67. Forde BM, O’Toole PW. Next-generation sequencing technologies and their impact on microbial genomics. Briefings Functional Genomics. 2013;12(5):440–453. [DOI] [PubMed] [Google Scholar]
- 68. Fouhy F, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes. 2012;3(3):203–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Prince AL, Antony KM, Chu DM, Aagaard KM. The microbiome, parturition, and timing of birth: more questions than answers. J Reprod Immunol. 2014;104-105:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatrica. 2009;98(2):229–238. [DOI] [PubMed] [Google Scholar]
- 72. Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Can Med Assoc J. 2013;185(5):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. [DOI] [PubMed] [Google Scholar]
- 74. Stark PL, Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982;15(2):189–203. [DOI] [PubMed] [Google Scholar]
- 75. Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology. 2010;156(Pt 11):3329–3341. [DOI] [PubMed] [Google Scholar]
- 76. Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe. 2011;17(6):478–482. [DOI] [PubMed] [Google Scholar]
- 77. Fallani M, Young D, Scott J, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010;51(1):77–84. [DOI] [PubMed] [Google Scholar]
- 78. Le Huerou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23(1):23–36. [DOI] [PubMed] [Google Scholar]
- 79. Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes. 2015;39(4):665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arboleya S, Sanchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538–544. [DOI] [PubMed] [Google Scholar]
- 81. Hussey S, Wall R, Gruffman E, et al. Parenteral antibiotics reduce bifidobacteria colonization and diversity in neonates. Int J Microbiol. 2011;2011:130574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ringel-Kulka T, Cheng J, Ringel Y, et al. Intestinal microbiota in healthy U.S. young children and adults—a high throughput microarray analysis. PLoS One. 2013;8(5):e64315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hopkins MJ, Sharp R, Macfarlane GT. Variation in human intestinal microbiota with age. Dig Liver Dis. 2002;34(Suppl 2):S12–S18. [DOI] [PubMed] [Google Scholar]
- 84. Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol. 2011;77(2):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42): E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45(4):1055–1065. [DOI] [PubMed] [Google Scholar]
- 87. Golubeva AV, Crampton S, Desbonnet L, et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology. 2015;60:58–74. [DOI] [PubMed] [Google Scholar]
- 88. O’Mahony SM, Marchesi JR, Scully P, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65(3):263–267. [DOI] [PubMed] [Google Scholar]
- 89. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 90. Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28(1):78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Giedd JN. Linking adolescent sleep, brain maturation, and behavior. J Adolesc Health. 2009;45(4):319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52(3):216–224. [DOI] [PubMed] [Google Scholar]
- 93. de Theije CG, Wopereis H, Ramadan M, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206. [DOI] [PubMed] [Google Scholar]
- 94. Foley KA, MacFabe DF, Vaz A, Ossenkopp KP, Kavaliers M. Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: implications for autism spectrum disorders. Int J Dev Neurosci. 2014;39:68–78. [DOI] [PubMed] [Google Scholar]
- 95. Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. [DOI] [PubMed] [Google Scholar]
- 97. Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18(17):2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome—focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35(4):403–413. [DOI] [PubMed] [Google Scholar]
- 99. Mohammadi AA, Jazayeri S, Khosravi-Darani K, et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. 2015. April 16 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 100. Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–264. [DOI] [PubMed] [Google Scholar]
- 101. Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathogens. 2009;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2(4):256–261. [DOI] [PubMed] [Google Scholar]