Abstract
A fundamental goal of research into the basic mechanisms of aging is to develop translational strategies that improve human health by delaying the onset and progression of age-related pathology. Several interventions have been discovered that increase lifespan in invertebrate organisms, some of which have similar effects in mice. These include dietary restriction and inhibition of the mechanistic target of rapamycin by treatment with rapamycin. Key challenges moving forward will be to assess the extent to which these and other interventions improve healthy longevity in addition to increasing lifespan in mice, and to develop practical strategies for extending this work to the clinic. Companion animals may provide an optimal intermediate between laboratory models and humans. By improving healthy longevity in companion animals, important insights will be gained regarding human aging while simultaneously improving quality of life for people and their pets.
Keywords: lifespan, healthspan, dietary restriction, caloric restriction, mTOR, target of rapamycin, sirtuins, mice, dogs
Introduction
Research on the basic biology of aging aims to understand mechanisms that cause organisms to decline in function over time and lead to increasing risk of morbidity and mortality 61. This is, of course, intimately connected to pathology because aging promotes disease. Most leading causes of mortality in developed nations share a single greatest risk factor, and it isn’t how much you eat, drink, smoke or exercise; it’s how old you are 39. Diabetes, heart disease, kidney disease, stroke, Alzheimer’s disease, Parkinson’s disease, and most forms of cancer, along with several other diseases, all show an exponential increase in risk with age over much of the human lifespan (Figure 1, 2).
Figure 1. Aging drives disease.
(A) Age is the single greatest risk factor for most causes of death and disability in developed countries.
Figure 2.
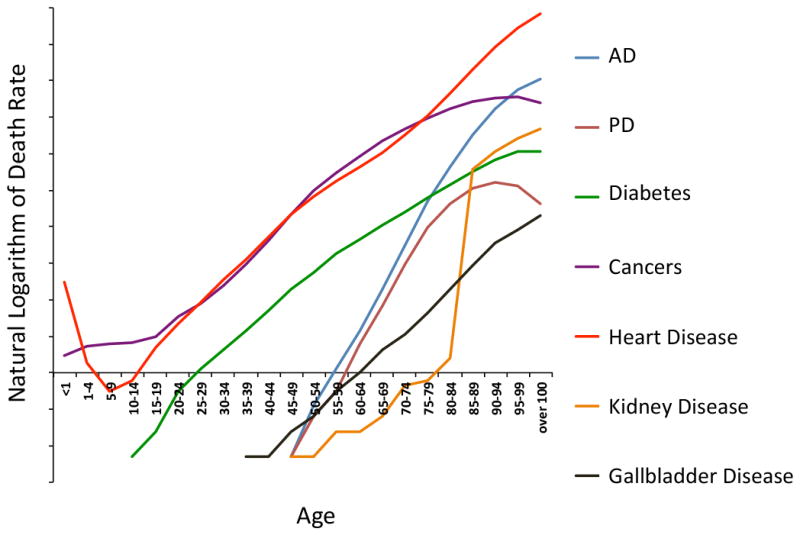
Age-specific death rates for some leading causes of death increase exponentially with age. Age-specific death rates for leading causes of death in the United States. Natural logarithm of the death rate refers to the natural logarithm of an individual’s risk of dying from a given disease at the specified age. Death rate data from the United States Center for Disease Control. AD = Alzheimer’s Disease. PD = Parkinson’s Disease.
Understanding why this relationship between age and disease exists, and, ultimately, intervening in the aging process at a molecular level to promote healthy longevity, is a primary goal of aging research. In this way, aging research is the ultimate form of preventative medicine: if the rate of aging can be slowed, then many of the diseases and declines in function associated with aging should be simultaneously delayed. Thus, interventions that target the molecular mechanisms of aging have the potential to increase both lifespan and healthspan, which can be defined as the period of life free from chronic disease and disability.
The field of Pathology plays a critical role in aging research, both from the perspective of understanding the spectrum of diseases that occur during aging in different model organisms and different individuals within a population, and in evaluating the impact of genetic and environmental factors on healthspan. This is particularly true in laboratory animal models where it is often challenging to quantify healthspan. Diagnosing and quantifying disease rates in aged animals, in particular, is essential for understanding the impact of factors that may increase the risk of age-associated disease, as well as for assessing whether interventions designed to delay aging are actually having the desired effect.
Conserved longevity pathways
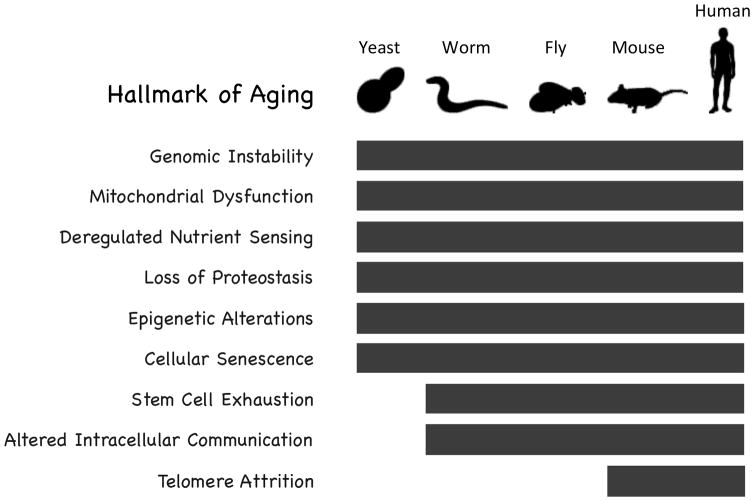
Studies of aging in yeast and invertebrate model organisms such as Caenorhabditis elegans and Drosophila melanogaster have had a large impact on the field by allowing detailed mechanistic studies of molecular mechanisms of aging and high-throughput genetic analysis of modifiers of lifespan 52,86. One of the most important findings to emerge from these model systems is that many aspects of aging are highly conserved across broad evolutionary distances. This includes both genetic modifiers of longevity, as well as specific cellular processes that degrade with age and likely contribute to age-associated pathology. Indeed, a recent review categorized these conserved features of aging into a set of nine “Hallmarks of Aging” 57, many of which span the evolutionary distance from yeast to people (Figure 3). The nine Hallmarks of Aging identified by Lopez Otin and colleagues are genomic instability, mitochondrial dysfunction, deregulated nutrient sensing, loss of proteostasis, epigenetic alterations, cellular senescence, stem cell exhaustion, altered intracellular communication, and telomere attrition. Among these, only telomere attrition has not been definitively associated with aging in invertebrate model organisms.
Figure 3. Conserved mechanisms of aging.
Eight of the nine identified “Hallmarks of Aging” have been definitively implicated in aging of non-mammalian species.
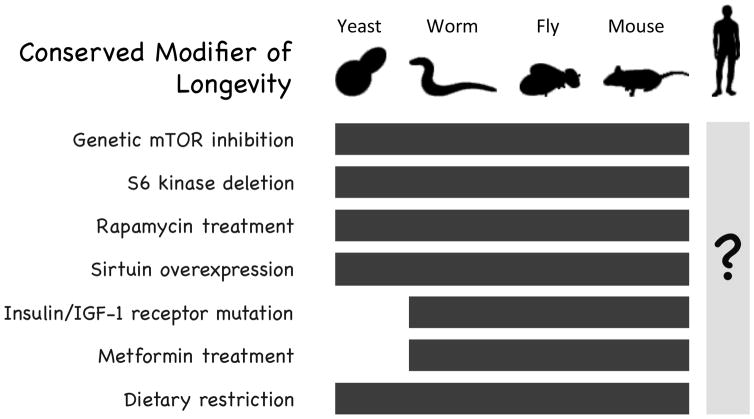
In addition to providing insight into fundamental mechanisms of aging, there are several examples where studies of aging in non-mammalian model systems have led to the identification of interventions that extend lifespan in mammals. Among these are genetic or pharmacological inhibition of the mechanistic target of rapamycin (mTOR), deletion of the mTOR substrate S6 kinase, mutations that reduce insulin/IGF-1-like signaling (IIS), including loss of function mutations to the insulin/IGF-1 receptors, overexpression of sirtuin family enzymes, and treatment with the anti-diabetic drug metformin 72 (Figure 4 and additional references2,4,6,14,20,32,43,44,46,47,51,56,58,59,62,71,73,77–79,84,85,92,93,96,99). The discovery of pathways and pharmacological interventions that delay aging in non-mammalian species and the subsequent demonstration of their efficacy in rodent models (Text Box 1) further supports the idea that fundamental aspects of aging are conserved at a genetic and molecular level, and suggests that screening for longevity-promoting compounds in yeast or invertebrate species may prove fruitful for initial identification of candidates for further analysis in mice.
Figure 4. Conserved modifiers of aging.
Seven genetic and environmental modifiers of longevity modulate lifespan similarly in yeast, worms, flies, and mice. The “?” under the human figure indicates that we do not yet know whether these factors similarly modulate human aging.
Text Box 1. Genetics of aging: from simple eukaryotes to mammals.
Many of the mouse models of extended longevity were first shown to modulate aging in non-mammalian organisms. These include components of the insulin/IGF-1 signaling (IIS) pathway, the sirtuins, and mTOR.
Among genetic models of enhanced longevity in rodents, mutations that perturb growth hormone and IIS are the best documented and most robust 5,38. This pathway was first implicated in aging of C. elegans, with studies showing that mutation of insulin-like receptor gene, daf-2, or the PI-3-kinase gene, age-1, are sufficient to increase lifespan 26,51. These long-lived mouse models include the Ames and Snell Dwarf mice, growth hormone receptor knock-out mice (GHRKO), growth hormone releasing hormone (GHRH) knockout mice, IGF-1 receptor heterozygous mice, fat-specific insulin-receptor knockout (FIRKO) mice, and IGF-1-deficient mice 9,17,23,33.
The sirtuins are a family of NAD+-dependent enzymes that remove post-translational modifications from proteins 28. The major catalytic activity of most sirtuins is protein deacetylation 34; however sirtuins also have ribosyltransferase activity and, in the case of mammalian SIRT5, the ability to remove additional post-translational modifications from lysine residues including glutaryl malonyl, and succinyl groups 28. Sirtuins first became implicated in aging based on studies in budding yeast demonstrating that overexpression of the yeast Sir2 gene was sufficient to extend lifespan 43. Mammals have seven sirtuin genes, SIRT1-7. SIRT1 is the ortholog of yeast Sir2; however, whole body overexpression of SIRT1 does not extend lifespan in mice. A recent report describes increased lifespan from overexpression of SIRT1 only in specific regions of the brain 79, and whole body overexpression of another sirtuin, SIRT6, can extend lifespan in male mice 46.
In recent years, the mTOR pathway has emerged as perhaps the most important modulator of healthy aging in mice, with many clinically relevant parallels in a variety of human diseases 36,40. The mTOR protein is a nutrient and growth factor responsive kinase that exists in two complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Genetic or pharmacological (i.e. rapamycin) inhibition of mTORC1 extends lifespan in yeast, worms, flies, and mice 90. In mice, lifespan extension from genetic inhibition of mTORC1 has been documented through heterozygous knockout of mTOR along with the mTORC1 component mLST8 53, as well as in a second study of mice carrying hypomorphic alleles of mTOR 99. Deletion of the gene encoding the mTORC1 substrate protein S6k1 also increases lifespan in female mice 83. The mechanisms by which mTORC1 modulates aging remains an area of active research, and probably involves both cell intrinsic processes including regulation of mRNA translation, autophagy, and mitochondrial function, as well as systemic effects on metabolism, inflammation, and immunity.
Dietary restriction and rapamycin: Two interventions that enhance longevity
Dietary restriction
The best-characterized intervention for increasing lifespan in mammals is dietary restriction (DR, also referred to as caloric or calorie restriction), which can be defined as a reduction in nutrient availability in the absence of malnutrition. First described to increase lifespan in rats in the 1930s 65, DR has since been shown to also increase lifespan in a plethora of other species, including yeast, worms, fruit flies, mice, fish, spiders, bats, and rhesus monkeys 1,50,63,70. Several different paradigms for restricting nutrients in rodents have been described, including chronic restriction of chow from 20–50%, intermittent or every-other-day feeding, and diets low in methionine or tryptophan 74,89. Each of these has been proposed to improve health during aging and, to a varying extent, also to increase lifespan; however, the extent to which these different forms of nutrient restriction have similar causal mechanisms is still unclear. Chronic restriction is both the most studied and most robust DR paradigm for extending lifespan, with 30–50% restriction typically increasing median lifespan by approximately 30–50%.
In addition to having their lifespans extended, rodents subjected to DR have dramatically reduced rates of most age-associated cancers, do not become obese, maintain glucose homeostasis and insulin sensitivity, and are protected against age-associated cognitive and cardiac declines 27,75,87. DR has also been reported to confer protection against age-related disease models in mice, including models of Alzheimer’s and other neurodegenerative diseases 82. Similar reports of improved healthspan have also been seen in two independent studies of DR in rhesus monkeys, with the DR groups showing lower rates of diabetes, obesity, cardiovascular disease, and cancer 16. Interestingly, DR increased lifespan in only one of these two studies, although the reasons for this difference remain unclear 15,64.
Although DR has been shown to increase lifespan and delay age-associated decline in a variety of species and numerous independent studies, there is growing recognition of the importance that genetic background may play in individual response to DR. A group of studies performed in forty-one recombinant inbred lines of mice, for example, found that roughly as many of the lines had their lifespans shortened by a 40% reduced calorie DR regimen as had their lifespans extended 54. This is a similar distribution as seen in a study of DR response in more than one hundred single-gene deletion mutants in budding yeast 80. This study is noteworthy, as it also identified functional categories that showed differential responses to DR: mitochondrial mutants tended to show the largest lifespan extensions, while superoxide dismutase deficient cells or mutants defective for acidification of the vacuole (yeast lysosome) tended to have their lifespans shortened by DR 81. Whether these functional relationships observed in yeast also hold true in mammals has yet to be established, although a follow-up study based on the yeast work found that inhibition of mTOR (a downstream effect of DR, see below) rescued a severe mitochondrial disease in mice caused by deficiency of Complex I of the electron transport chain 37. It has also been speculated that genetic background differences may account for the failure of DR to increase lifespan in some inbred mouse lines, as well as in a wild-derived mouse strain 21,31,91. It is worth noting, however, that in nearly every case where DR has failed to extend lifespan in mice, only a single DR level and regimen was assessed. Thus, it may be that many genotypes alter the response profile for DR such that a different level of restriction is necessary to obtain an optimal (or perhaps any) beneficial effect on aging. This concern illustrates the seminal importance of dose response considerations in biology, something that is often lacking from studies of aging-related biology.
Rapamycin
Among pharmacological interventions that extend lifespan in mice, rapamycin is the most documented and effective to date. Rapamycin is a specific inhibitor of the mTOR complex 1 (see Text Box 1) that is approved by the United States Food and Drug Administration for clinical use to prevent organ transplant rejection, in cardiac stents, and for certain forms of cancer 40. Rapamycin was first shown to increase mouse lifespan by the National Institute on Aging Intervention Testing Program (ITP) by delivering it to UMHET3 mice in an encapsulated form in the diet beginning at 20 months of age 32,42. This regimen increased median lifespan of male mice by about 9% and that of female mice by about 14%. Subsequent studies have shown that a similar dietary regimen increases lifespan comparably when initiated at 9 months of age, and that dietary delivery at a threefold higher concentration increases median lifespan in both male and female mice by about 25% 66,67. Several other groups have also published lifespan extension from rapamycin delivered both in the diet and by injection in C57BL/6, and 129/Sv mice 3,25,68,100.
In addition to extending lifespan, rapamycin has also been shown to positively impact multiple age-related declines in function in mice, as well as mouse models of specific age-related diseases 36. This includes improved cognitive function, both during normative aging and in mouse models of Alzheimer’s disease 11,30,55,60,88. Of particular note, treatment with rapamycin during mid-life can also at least partially reverse age-related declines in immune function and cardiac function within 6 weeks of treatment in mice 13,18,24. Despite these improvements in multiple organ systems during aging, it has remained somewhat controversial whether rapamycin generally slows aging and improves healthspan or is primarily increasing lifespan through reduction in cancer incidence (discussed further in the next section) 8,19,76.
The importance of quantifying healthspan in addition to lifespan
There is growing recognition within the field that it is important to obtain quantitative measures of healthspan in addition to assessing the effects of different interventions on lifespan 10,49. As mentioned above, the interventions that robustly increase lifespan in mice also tend to delay multiple age-related declines in function along with major age-related diseases, especially cancer; however, there have been relatively few studies that have attempted to assess overall quality of life in these animals. There are at least two major reasons for this deficiency; first, mouse longevity studies to assess lifespan are already expensive, and the addition of animals and assays to measure healthspan is often prohibitive; second, there is no consensus as to which assessments best capture overall health during aging in rodents.
Recent studies on rapamycin provide a particularly good example of challenges associated with definitively assessing the effect of an intervention on healthspan in mice. As described above, rapamycin treatment has been shown to extend lifespan in several different mouse strains and to improve outcome in age-related disease models. In addition, at least two studies have attempted to directly address the question of whether rapamycin ‘slows aging’ by assessing a breadth of age-related parameters encompassing several organ systems. Although both studies detected significant increases in lifespan and improvements in at least a subset of the parameters measured, they reached opposite conclusions: Wilkinson et al., 98 reported that “rapamycin slows aging in mice”, while Neff et al. 68 reported that rapamycin “has limited effects on aging”.
Of the two studies, Neff et al. 68 performed the more comprehensive assessment of health, looking at around 40 distinct measures of healthspan, including histopathological assessments of liver, brain, muscle, heart, kidney, and endocrine organs, as well as precancerous and cancerous lesions. They detected improvements in about 40% of these healthspan measures following rapamycin treatment. Based on their inability to detect positive changes in the remaining measures of healthspan, in combination with the fact that rapamycin induced changes in some healthspan measures even in young animals, they concluded that rapamycin does not generally slow aging 68. One criticism of such an interpretation is that it relies on what are essentially a series of negative results (failure to detect a change) in the absence of a positive control (something that elicits the change they were trying to detect). The strength of this interpretation is further limited by the suboptimal dosing employed in the study. Rapamycin is known to more robustly increase lifespan at a 3-fold higher dose than was used by either Neff et al. 68 or Wilkinson et al. 98, and it seems likely that some healthspan measures may be similarly dose-responsive 41.
These examples raise some important issues that should be considered when designing and interpreting experiments aimed at assessing healthy aging in mice. The field will need to work toward consensus regarding which measures of healthspan are most informative and how many measures of healthspan are needed to obtain a meaningful picture of overall health. It will also be important to consider what it means if an intervention improves a measure of healthspan in both young and old animals. Although Neff et al. 68 argued that such a result indicates the intervention is not affecting aging, others have argued that there is no strong justification for such an interpretation 35. Researchers should also be cautious not to over-interpret their data in either direction, particularly when most interventional studies of aging in mice have not been optimized for dose or delivery and are lacking in positive control. In addition, it may be the case that some interventions increase lifespan and delay most age-associated phenotypes, while simultaneously exacerbating other age-related traits or even causing new pathological conditions. Again, rapamycin presents an interesting example, as there is evidence that chronic rapamycin treatment in mice can impair the ability of animals to respond to a glucose challenge, causes testicular atrophy, and may increase risk of cataracts 7, and there are several side effects in patients taking high doses of rapamycin to prevent organ transplant rejection 48.
A noteworthy advance in the area of experimental healthspan assessment is the recent description of a non-invasive frailty index for quantifying healthspan in mice 97. Indices of frailty have been commonly used in geriatric medicine, but are rarely applied in studies of aged rodents. The 31 point mouse frailty index developed by Whitehead et al. 97 has the major advantages of being relatively inexpensive and non-invasive. This index has been applied to assess the effects of DR and resveratrol on healthspan of mice 45, and may prove useful for quantifying healthy aging across a variety of parameters if it becomes widely utilized.
Companion Animals and Citizen Science in Aging Research
As discussed in the prior sections, the field has done an admirable job of characterizing key pathways and molecular mechanisms of aging in laboratory models and, in some cases, identifying interventions that appear to slow the rate of aging in these same systems. In addition to the need for improved and more consistent assessments of healthspan in rodents, a major barrier exists in translation of these discoveries to improve quality of life for people. There has been much discussion of the importance of targeting human aging to increase healthspan and reduce the burden of age-related disease, including the anticipated economic and social consequences 29,49,69. Unfortunately, the path toward accomplishing this goal remains unclear, with significant hurdles associated with testing putative longevity- or healthspan-promoting interventions directly in people 72.
My colleagues and I believe that companion animals, particularly pet dogs, represent an outstanding intermediate between laboratory animals and humans for understanding the basic mechanisms of aging, and in limited cases, for validating interventions expected to promote healthy longevity. Dogs have several traits that make them particularly compelling in this regard. From an evolutionary standpoint, dogs are roughly as distant from humans as mice are; however, companion dogs experience an environment strikingly similar to that of their owners; something that can’t be mimicked in rodents 94. Companion dogs also offer a wealth of genetic and phenotypic diversity, with known breed-specific disposition to certain diseases and causes of death 22,95. Dogs suffer from many of the same age-associated declines as humans, albeit at a much accelerated rate, which makes the timeframe for studies of aging in dogs much more reasonable than similar studies in humans. Importantly, the understanding of geriatric ailments and the breadth of treatment options within the canine veterinary community are second only to that of human medicine. Perhaps most importantly, there is significant intrinsic value to improving healthy aging in companion dogs, as it would not only facilitate future efforts toward similarly impacting human aging but would also significantly enhance the quality of life for the dogs and their owners.
Based on this concept, we have recently launched The Dog Aging Project (www.dogagingproject.com), an initiative with the primary goal of enhancing healthy longevity in companion dogs 12. There are two major goals of the Dog Aging Project: a longitudinal study of aging in companion dogs and an intervention trial to assess the ability of rapamycin to promote healthspan and increase lifespan in middle-aged dogs.
The Longitudinal Study of Aging in dogs will follow companion dogs throughout life in order to define the major genetic and environmental features that determine why some animals age well and have relatively long healthspan and lifespan, while other animals age poorly. The final design of the longitudinal study of aging is currently being determined, with the support of a National Institute on Aging funded R13 Networking Grant which has allowed the formation of the Canine Longevity Consortium (CLC) directed by Dr. Daniel Promislow (University of Washington). The CLC is hosting a series of meetings around this topic, and key experimental details were discussed at the most recent conference in Bethesda in March 2015. Current plans for this study involve including up to 100,000 dogs, with assessments of genotype and environmental parameters along with lifetime health measures for all animals. Subsets of animals would participate in more extensive testing of age-associated health parameters, including activity monitoring, cognitive function, metabolic health, cardiac and kidney function, and cancer incidence, as well as additional phenotypic assessments, such as serum metabolomics. In cases where owners are willing to allow autopsy, assessment of tissue pathology will play a critical role in determining disease burden at time of death, and will be important for correlating disease with genotypic and phenotypic information.
The rapamycin intervention study is a smaller, more focused study designed specifically to assess the effects of low dose rapamycin on healthy longevity in middle-aged, mid-to-large size companion dogs. In order to be eligible for the initial study, dogs must be at least 40 pounds in weight and at least 6 years old with no major pre-existing health conditions. There are no breed exclusions as long as the animals meet these two criteria. Owners can enroll their animals for consideration in the study through the Dog Aging Project website. Between November 2014 and February 2015, approximately 1,000 animals have been enrolled.
The current study design call for two phases. The first phase is primarily a dosing and safety study to ensure that the low dose rapamycin regimen does not result in significant side effects. A relatively small cohort of at least 32 animals from the Seattle area will be enrolled into either placebo or treatment groups for ten weeks, with an initial veterinary exam prior to enrollment which will include echocardiography, blood draw, and sampling of hair, nails, and feces. A second exam after two weeks of treatment will verify no significant adverse effects and provide for a second sampling of blood, hair, nails, and feces. A third exam after ten weeks of treatment will include similar sampling along with a second echocardiogram. In addition to the veterinary care, owners will be asked to carefully monitor their dog and to immediately report any abnormal behaviors or side effects of the treatment. Echocardiograms are included in the short-term study based on data in mice indicating that ten weeks of rapamycin treatment in 20 month old mice is sufficient to reverse some measures of age-related cardiac decline 18,24. The short-term trial is anticipated to be in progress prior to publication of this article.
The second phase of the rapamycin intervention study is intended to be a national study that will include several hundred dogs. The age and weight entry criteria will be similar to the short-term trial, and the rapamycin treatment duration is anticipated to be from one to three years. As in the short-term study, adverse events will be closely monitored through a combination of owner reporting and veterinary care, and blood, hair, nails, and feces will be obtained at periodic veterinary exams. In addition to cardiac function, a more detailed assessment of healthy aging will be obtained, including activity monitoring, cognitive function, metabolic health, cardiac and kidney function, and cancer incidence and mortality rates.
A notable feature of both the Longitudinal Study of Aging and the Rapamycin Intervention Trial is that they incorporate pet owners into the study as citizen scientists. Not only are owners invested in these studies through the participation of their pet, but they also participate directly in the collection of data regarding overall health, behavior, and activity. My colleagues and I believe that this feature of the Dog Aging Project, combined with the close connection that many dog owners feel to their pet, create a unique dynamic that resonates with the general public in a way that few other scientific endeavors have.
Conclusion
Substantial progress has been made in understanding the cellular mechanisms of aging, which has translated to the identification of several genetic and environmental interventions capable of extending lifespan in mice. Two of the most robust longevity-enhancing interventions are chronic DR and treatment with the mTOR inhibitor rapamycin. One challenge that the field will need to address in the near future is how best to assess the impact of these and other such interventions on healthspan and overall quality of life. Quantification of disease burden and end-of-life pathology will undoubtedly be an important part of any such metric. A second major challenge will be to develop tractable approaches to translating these discoveries to improve human health. Companion animals may be a particularly important bridge in this regard, providing both informative avenues for future human studies while simultaneously improving the quality of life for both the animals and their owners.
Literature Cited
- 1.Anderson RM, Weindruch R. The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol. 2012;24(2):101–106. doi: 10.1002/ajhb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87(3):201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 4.Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18(24):3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartke A. Pleiotropic effects of growth hormone signaling in aging. Trends Endocrinol Metab. 2011;22(11):437–442. doi: 10.1016/j.tem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging. 2012;4(5):350–358. doi: 10.18632/aging.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blagosklonny MV. Rapamycin extends life- and health span because it slows aging. Aging (Albany NY) 2013;5(8):592–598. doi: 10.18632/aging.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown-Borg H, Borg K, Meliska C, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 10.Burch JB, Augustine AD, Frieden LA, Hadley E, Howcroft TK, Johnson R, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S1–3. doi: 10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Check Hayden E. Pet dogs set to test anti-ageing drug. Nature. 2014;514(7524):546. doi: 10.1038/514546a. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, et al. Extension of lifespan by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 15.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coschigano K, Clemmons D, Bellush L, Kopchick J. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 18.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13(3):529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71(22):4325–4346. doi: 10.1007/s00018-014-1677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976;73(4):1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming JM, Creevy KE, Promislow DE. Mortality in north american dogs from 1984 to 2004. an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. 2011;25(2):187–198. doi: 10.1111/j.1939-1676.2011.0695.x. [DOI] [PubMed] [Google Scholar]
- 23.Flurkey K, Papaconstantinou J, Miller R, Harrison D. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12(5):851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2014;9(1):e83988. doi: 10.1371/journal.pone.0083988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusco S, Pani G. Brain response to calorie restriction. Cell Mol Life Sci. 2013;70(17):3157–3170. doi: 10.1007/s00018-012-1223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giblin W, Skinner ME, Lombard DB. Sirtuins. guardians of mammalian healthspan. Trends Genet. 2014;30(7):271–286. doi: 10.1016/j.tig.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman DP, Cutler D, Rowe JW, Michaud PC, Sullivan J, Peneva D, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood) 2013;32(10):1698–1705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halloran J, Hussong S, Burbank R, Podlutskaya N, Fischer K, Sloane L, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5(6):441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even P, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–189. doi: 10.1038/nature01298. f1703653-52bb-4b18-e92a-6f87ea1aef70. [DOI] [PubMed] [Google Scholar]
- 34.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 35.Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M. Preserving youth: does rapamycin deliver? Sci Transl Med. 2013;5(211):211fs240. doi: 10.1126/scitranslmed.3007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdiscip Top Gerontol. 2015;40:107–127. doi: 10.1159/000364974. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013;342(6165):1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9(6):366–376. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. doi: 10.12703/P5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaeberlein M. mTOR Inhibition: From Aging to Autism and Beyond. Scientifica (Cairo) 2013;2013:849186. doi: 10.1155/2013/849186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaeberlein M. Rapamycin and ageing: when, for how long, and how much? J Genet Genomics. 2014;41(9):459–463. doi: 10.1016/j.jgg.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaeberlein M, Kennedy BK. Ageing: A midlife longevity drug? Nature. 2009;460(7253):331–332. doi: 10.1038/460331a. [DOI] [PubMed] [Google Scholar]
- 43.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 45.Kane AE, Hilmer SN, Boyer D, Gavin K, Nines D, Howlett SE, et al. Impact of Longevity Interventions on a Validated Mouse Clinical Frailty Index. J Gerontol A Biol Sci Med Sci. 2015 doi: 10.1093/gerona/glu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 47.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando) 2014;28(3):126–133. doi: 10.1016/j.trre.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64(11):1323–1328. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 52.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 53.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging cell. 2010;9(1):92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33(9):1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289(5487):2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu JY, Lin YY, Sheu JC, Wu JT, Lee FJ, Chen Y, et al. Acetylation of yeast AMPK controls intrinsic aging independently of caloric restriction. Cell. 2011;146(6):969–979. doi: 10.1016/j.cell.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301(5640):1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- 60.Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging cell. 2012;11(2):326–335. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin GM. Frontiers of aging. Science. 2001;294(5540):13. doi: 10.1126/science.294.5540.13. [DOI] [PubMed] [Google Scholar]
- 62.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 64.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life and upon ultimate size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 66.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123(8):3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- 70.Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS letters. 2011;585(11):1537–1542. doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PloS one. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pitt JN, Kaeberlein M. Why is aging conserved and what can we do about it? PLoS Biology. 2015 doi: 10.1371/journal.pbio.1002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20(2):174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20(2):157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 75.Quarles EK, Dai DF, Tocchi A, Basisty N, Gitari L, Rabinovitch PS. Quality control systems in cardiac aging. Ageing Res Rev. 2015 doi: 10.1016/j.arr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson A. Rapamycin, anti-aging, and avoiding the fate of Tithonus. J Clin Invest. 2013;123(8):3204–3206. doi: 10.1172/JCI70800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell metabolism. 2012;15(5):713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schleit J, Johnson SC, Bennett CF, Simko M, Trongtham N, Castanza A, et al. Molecular mechanisms underlying genotype-dependent responses to dietary restriction. Aging Cell. 2013;12(6):1050–1061. doi: 10.1111/acel.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schleit J, Wasko BM, Kaeberlein M. Yeast as a model to understand the interaction between genotype and the response to calorie restriction. FEBS Lett. 2012;586(18):2868–2873. doi: 10.1016/j.febslet.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schroeder JE, Richardson JC, Virley DJ. Dietary manipulation and caloric restriction in the development of mouse models relevant to neurological diseases. Biochim Biophys Acta. 2010;1802(10):840–846. doi: 10.1016/j.bbadis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 83.Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Slack C, Foley A, Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One. 2012;7(10):e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith ED, Kaeberlein TL, Lydum BT, Sager J, Welton KL, Kennedy BK, et al. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev Biol. 2008;8:49. doi: 10.1186/1471-213X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith ED, Kennedy BK, Kaeberlein M. Genome-wide identification of conserved longevity genes in yeast and worms. Mech Ageing Dev. 2007;128(1):106–111. doi: 10.1016/j.mad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 87.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32(3):159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9(3):324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790(10):1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swindell WR. Dietary restriction in rats and mice: A meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing research reviews. 2011 doi: 10.1016/j.arr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 93.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 94.Wang AM, Promislow DE, Kaeberlein M. Fertile waters for aging research. Cell. 2015;160(5):814–815. doi: 10.1016/j.cell.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 95.Waters DJ. Aging research 2011; exploring the pet dog paradigm. ILAR J. 2011;52(1):97–105. doi: 10.1093/ilar.52.1.97. [DOI] [PubMed] [Google Scholar]
- 96.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 97.Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69(6):621–632. doi: 10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging cell. 2012;11(4):675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep. 2013;4(5):913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69(2):119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]