Abstract
Tuberculosis (TB) is a major public health concern worldwide with over 2 billion people currently infected. The rise of strains of Mycobacterium tuberculosis (Mtb) that are resistant to some or all first and second line antibiotics, including multidrug-resistant (MDR), extensively drug resistant (XDR) and totally drug resistant (TDR) strains, is of particular concern and new anti-TB drugs are urgently needed. Curcumin, a natural product used in traditional medicine in India, exhibits anti-microbial activity that includes Mtb, however it is relatively unstable and suffers from poor bioavailability. To improve activity and bioavailability, mono-carbonyl analogs of curcumin were synthesized and screened for their capacity to inhibit the growth of Mtb and the related Mycobacterium marinum (Mm). Using disk diffusion and liquid culture assays, we found several analogs that inhibit in vitro growth of Mm and Mtb, including rifampicin-resistant strains. Structure activity analysis of the analogs indicated that Michael acceptor properties are critical for inhibitory activity. However, no synergistic effects were evident between the monocarbonyl analogs and rifampicin on inhibiting growth. Together, these data provide a structural basis for the development of analogs of curcumin with pronounced anti-mycobacterial activity and provide a roadmap to develop additional structural analogs that exhibit more favorable interactions with other anti-TB drugs.
Keywords: Curcumin, Curcumin analogs, Tuberculosis, Mycobacteria
1. Introduction
TB is a major public health concern, with over 2 billion people currently infected, 8.6 million new cases per year, and more than 1.3 million deaths per year. The current drug regimen combination for TB consists of isoniazid, rifampicin, ethambutol, and pyrazinamide, administered over six months [1,2]. Although this treatment has a high success rate, the utility of this regimen is limited by compliance issues, which has resulted in the rise of strains that are resistant to some or all of the first and second line antibiotics [3]. These strains, called MDR, XDR, and TDR Mtb, have worse disease outcome [4]. Recent efforts in TB drug development have resulted in the discovery of new therapeutics including bedaquiline, which retain activity against MDR and XDR strains. However, additional drugs are urgently needed.
Natural products and their plant-derived analogs are often a source of drugs or drug templates with limited toxicity, which has the potential to mitigate compliance issues during protracted administration. One natural product candidate of interest is curcumin [1,7-bis(4-hydroxy-3-methoxy phenyl)-1,6-heptadiene-3,5-dione], a phenolic compound originally extracted from the plant Curcuma longa and the primary component of the spice turmeric [5,6] (Fig. 1). For centuries, various Asian cultures have used curcumin as a traditional medicine to treat numerous disorders, particularly those associated with the skin and digestive tract. Curcumin has been found to have anti-cancer and anti-inflammatory properties, but the comparative action of mono-carbonyl derivatives demonstrates the superior efficacy of this class of molecules [7–10]. Moreover, curcumin inhibits the growth of various microorganisms including Escherichia coli, Bacillus subtilis, Helicobacter pylori, and Mtb [11–13]. In addition, curcumin acts synergistically with co-administered antibiotics to suppress growth of Staphylococcus aureus in vitro [14]. While curcumin inhibits growth of various bacterial species, this effect requires a relatively high inhibitory concentration [IC] compared to other antimicrobial agents, which is not achievable in vivo due to limited bioavailability [15] and chemical instability [16–18].
Fig. 1.
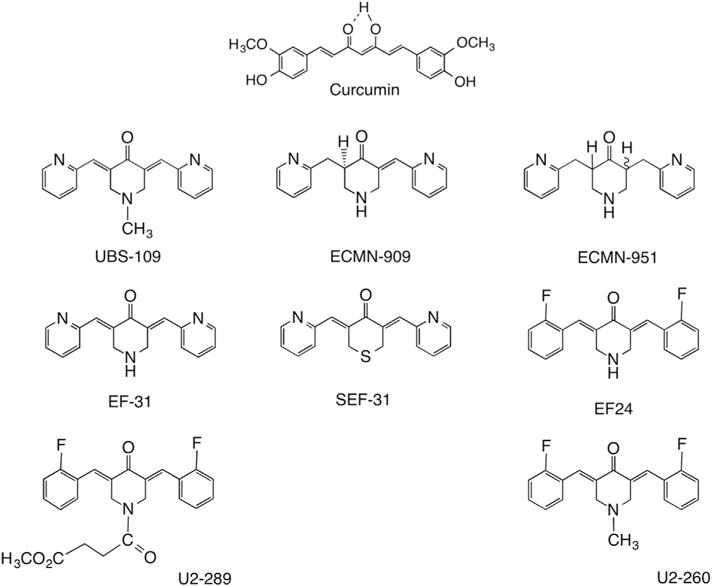
Monoketone curcumin analogs evaluated for anti-mycobacteria properties.
One approach to overcome these limitations and improve the inhibitory activity of curcumin is to develop structural analogs [10,19,20]. Curcumin has three sectors that can be targeted for modification: the β-diketone moiety, the aromatic rings, and the flanking double bonds conjugated to the β-diketone moiety. We evaluated a series of monocarbonyl analogs (Fig. 1) for their capacity to inhibit growth of the pathogenic mycobacteria Mtb and Mm.
2. Experimental procedures
2.1. Chemicals
All compounds were prepared by previously described methodology [20,21]. Briefly, the monocarbonyl analogs were generated by allowing the ketones to react with a variety of aromatic aldehydes under basic aldol condensation conditions. The structures of the final products (>95% pure, Fig. 1) have been characterized by standard analytical techniques as previously reported: UBS-109 [22–25], ECMN-909 [25], ECMN-951 [25], EF-31 [21,23,26], SEF-31 [21,27], EF-24 [20,21,28], U2–289 [29] and U2–260 [30]. Curcumin (≥94% curcuminoid content, ≥80% curcumin), Rifampicin and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MI).
2.2. Bacterial strains, and growth conditions
Mycobacterium tuberculosis strain H37Rv, H37Rv-RifR, and Beijing F2, and Mycobacterium marinum strain 1218R were grown in Difco Middlebrook 7H9 broth (Becton, Dickinson, and Company, Sparks, MD) supplemented with BBL Middlebrook ADC Enrichment (Becton, Dickinson, and Company, Sparks, MD) and 0.5% Tween 80 (Mtb) or 0.025% Tween 80 (Mm) (7H9-ADC broth). Difco Middlebrook 7H10 agar (Becton, Dickinson, and Company, Sparks, MD), supplemented with 10% oleic acid-albumin-dextrose-catalase (7H10-OADC) was used for Mm. Stocks of Mm were grown at 30 °C in 5% CO2 until OD600 of 0.8, centrifuged, the supernatant removed, and the bacteria resuspended in fresh 7H9 broth. Aliquots were stored at −80 °C. For some experiments, strains with mutations that rendered M. marinum (1218RrifR) or Mtb (H37RvrpoBH526Y) resistant to rifampicin were used [31].
2.3. Disk diffusion assay
Mm was grown in 7H9 broth until it reached an OD600 of 0.35. The culture was diluted to OD600 of 0.04, and 100 μL of the diluted stock was spread on 7H10 plates. The plates were allowed to air dry for 10 min. Next, sterilized round filter paper (6 mm diameter BBL disks; Becton, Dickinson, Sparks, MD) was placed in the center of each plate, to which 10 μl of a particular curcumin analog (100 mM in DMSO or sterilized water) was added. Three plates were assessed for each analog. The plates were then incubated at 30 °C in 5% CO2 for 7 days, after which time the extent of the zone lacking bacteria around the filter was measured (“zone of inhibition”).
2.4. Determination of 50% inhibitory concentration (IC50) for curcumin analogs against Mm and Mtb
Mm was grown in 7H9-ADC broth until OD600 of 0.35 and then diluted to an OD600 of 0.04. Cultures were grown for 72 h in 7H9-ADC broth containing no drug, 1% DMSO (vehicle control), or curcumin analogs (UBS-109, EF-24, EF-31, and ECMN-909 in DMSO) in concentrations of 0.780 μM, 1.560 μM, 3.125 μM, 6.250 μM, 12.5 μM, 25 μM, 50 μM, or 100 μM in duplicate. The percent inhibition was normalized to the level achieved with the vehicle alone. Mtb strains H37Rv, H37Rv-RifR, and Beijing F2 were grown to mid-log in 7H9-ADC broth and then diluted to an OD600 of 0.05. Cultures were grown in 7H9-ADC broth containing no drug, 1% DMSO, or curcumin analogs (UBS-109 and EF-24) in concentrations of 1 μM, 5 μM, 10 μM, 20 μM, or 50 μM in triplicate. Growth was determined by measuring the OD600 of cultures for 14 days using a spectrophotometer.
2.5. Determination of synergistic effects of UBS-109 and rifampicin on Mm
Cultures of Mm were grown in 7H9-ADC broth containing no drug, 1% DMSO (vehicle control), rifampicin alone at various concentrations (4 μg/mL, 2 μg/mL, 1 μg/mL, 0.5 μg/mL, or 0.250 μg/mL), or rifampicin at various concentrations in combination with UBS-109 at various concentrations (6.250 μM, 12.5 μM, 25 μM, 50 μM, or 100 μM) in duplicate. Growth was determined by measuring the OD600 of cultures after 72 h using a spectrophotometer. The data was normalized to the vehicle control. Synergy was determined by comparing combination treatments to rifampicin-only and UBS-109-only. Statistical analysis was done using nonparametric ANOVA. Values less than or equal to 0.05 were considered statistically significant.
3. Results
3.1. Evaluation of monocarbonyl analogs against Mm
A series of monocarbonyl analogs (Fig. 1) were synthesized and screened for anti-mycobacterial properties using a disk diffusion assay. After 7 days, the diameter of the circular zone surrounding the disk that remained free of bacterial growth (the “zone of inhibition”) was measured for each analog (Fig. 2A). Monocarbonyl analogs displayed a range of effects, with some showing little inhibition of growth compared to curcumin (e.g. U2–260) whereas others inhibited growth to substantially greater extent (e.g. UBS-109). By comparison, the vehicle control had no effect. To further characterize the growth inhibitory effect of these analogs, Mm was cultured in liquid media in the presence of various concentrations of UBS-109 or EF-24 for 72hr and the OD600 measured at various time points (Fig. 2B). The IC50 was determined from these data to be 10 μM for UBS-109 and 25 μM for EF-24.
Fig. 2.
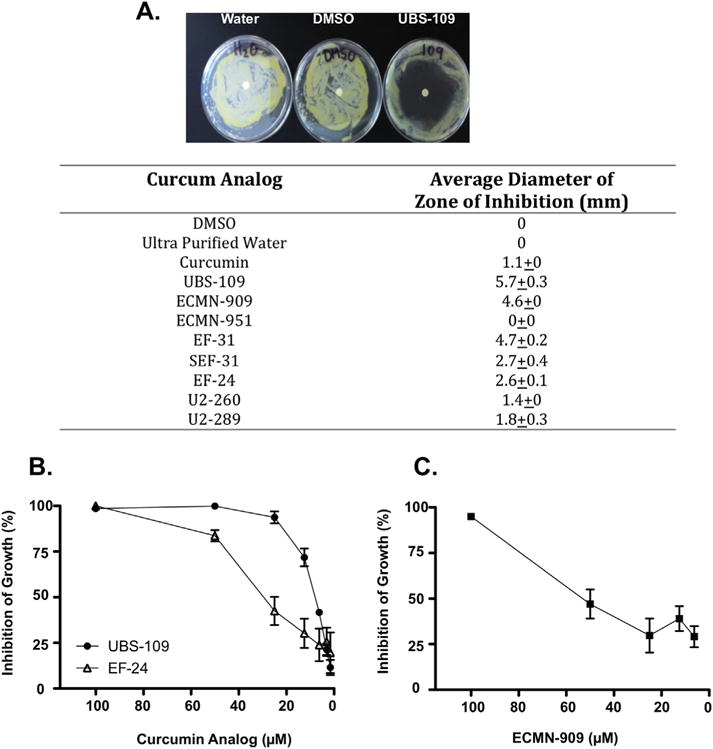
Monocarbonyl curcumin analogs inhibit the growth of Mycobacterium marinum (Mm). (A) Initial screening of inhibitory properties analyzed by disk diffusion assay. Monocarbonyl analogs (100 mM) were added to Mm containing plate, incubated for 7 days at 32 °C, and zone of inhibition measured.; (B) Assessment of the capacity of specific analogs to inhibit growth of Mm using a liquid culture assay. Indicated concentrations of analogs were added to Mm culture, incubated for 72hr, and the OD600 measured. Data normalized to untreated Mm culture. Data represents the mean ± SEM from three experiments.; (C) Assessment of the capacity of ECMN-909 to inhibit Mm growth in a liquid culture assay. Data represents the mean ± SEM from three experiments.
3.2. A Michael acceptor is required for anti-mycobacterial activity
While differences in solubility and diffusion through 7H10 agar may contribute to the variability in zones of inhibition, the ability of compounds with diverse substitution to inhibit Mm is most likely influenced by variation in protein target-ligand interactions, as has been suggested for kinase inhibition [21]. Comparison of the structural features of the analogs (Fig. 1) as it affects bacterial growth indicates that variability of all three domains within the molecules, the aromatic rings, the central ring or the Michael acceptor, can regulate antimicrobial activity. However, the Michael acceptor, comprised of two unsaturated C=C bonds flanking the carbonyl group, is known to be a highly active structural component of these analogs and is likely to play a key role in their antimicrobial activity [32–35].
To further elaborate the relationship between the Michael acceptor and antimicrobial activity, we evaluated two additional analogs, ECMN-909 and ECMN-951, in which the conjugated unsaturated ketone is altered or eliminated. Both are metabolites identified in an in vitro study of UBS-109 in liver S9 fractions from five different species [25]. ECMN-909 contains only one double bond flanking the carbonyl, which reduces the capacity of the molecule to undergo Michael additions. Likewise, ECMN-951 contains no flanking double bonds (Fig. 1), and cannot participate in a Michael reaction. In disk diffusion assays, ECMN-909 displayed the second largest zone of inhibition amongst all analogs (4.6 ± 0) mm compared to 5.7 mm for UBS-109; however, no zone of inhibition was evident with ECMN-951. Liquid culture assays confirmed these results, and indicated that ECMN-909 was somewhat less effective in inhibiting bacterial growth (IC50 = ~50 μM) compared to UBS-109 (IC50 = 10 μM) (Fig. 2C). Together, these data suggest that the terminal aromatic rings and the central 6-membered ring can influence the antimicrobial activity of UBS-109, but the Michael acceptor is required. It also suggests that the dominant and first reduced metabolite (ECMN-909) is likewise a reasonably effective anti-TB agent. We speculate that a similar train of events may apply to the other analogs in Fig. 1.
3.3. Curcumin analogs are not synergistic with rifampicin
To determine whether UBS-109 exhibits a synergistic effect when co-administered with a key anti-tuberculosis drug, Mm was cultured in the presence of rifampicin, UBS-109 or a combination of the two in various concentrations. As shown in Fig. 3, high concentrations of rifampicin or UBS-109 inhibited growth of Mm, whereas less inhibition was evident at lower concentration of either drug alone (6.25 μM for UBS-109 and 0.3 μM for rifampicin). When delivered in combination at low concentrations, no significant difference was evident compared to either drug alone. Together, these data suggest that no synergistic interaction occurs upon co-administration of UBS-109 and rifampicin.
Fig. 3.
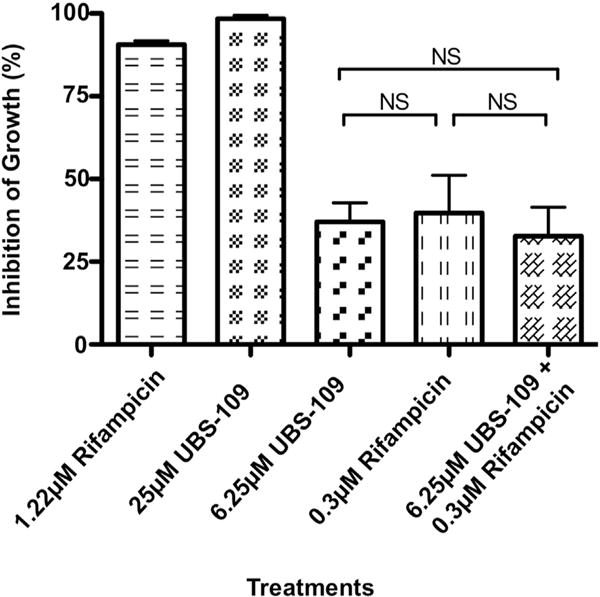
UBS-109 does not display synergistic interactions when combined rifampicin upon the inhibition growth of Mycobacterium marinum (Mm). The effect of combination of rifampicin and UBS-109 on the growth of Mm. Mm was cultured in the presence of high and low concentrations of rifampicin and UBS-109 for 72hr, then the OD600 was measured. Data normalized to untreated Mm culture. Data normalized to untreated Mm culture. Data represents the mean ± SEM from three experiments.
3.4. Evaluation of curcumin analogs on rifampicin-resistant Mm
To determine whether curcumin analogs can be used to treat drug-resistant mycobacterial species, we evaluated the ability of UBS-109 to inhibit the growth of a rifampicin-resistance strain of Mm (Mmrif). Using liquid culture assays, we grew Mmrif in the presence of DMSO, rifampicin or UBS-109. As a control, rifampicin was found to have no effect at any concentration on inhibiting the growth of Mmrif. As shown in Fig. 4, UBS-109 inhibited growth of Mmrif with an IC50 of ~4 μM.
Fig. 4.
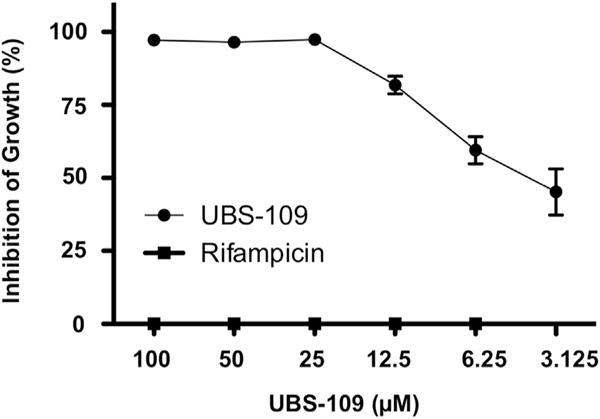
UBS-109 inhibits the growth of rifampicin-resistant Mycobacterium marinum (Mm). Liquid culture assay validating UBS-109 inhibits rifampicin-resistant Mm (different concentration of analogs were added to Mm culture, incubated for 72hr, then the OD600 was measured). Data normalized to untreated Mm culture. Data represents the mean ± SEM from three experiments.
3.5. Evaluation of curcumin analogs on M. tuberculosis
To validate the effects of UBS-109 on other clinically relevant mycobacteria, we tested the effects of UBS-109 and EF-24 on several Mtb strains using the disk diffusion and liquid assays (Fig. 5) with two different Mtb strains, H37Rv or Beijing F2. A representative disc diffusion assay with H37Rv is shown in Fig. 5A, and UBS-109 induced a marked zone of inhibition. Data from H37Rv or Beijing F2 grown in the presence of various concentrations of UBS-109 or EF-24 for two weeks and the OD600 measured daily are shown in Fig. 5B–G. The data indicate that UBS-109 inhibits the growth of H37Rv with an IC50 of ~10 μM, and the Beijing strain with an IC50 of 20 μM (Fig. 5B, C). EF-24 also blocks Mtb, though not as effectively as UBS-109 (IC50 of ~20 μM for H37Rv and 50 μM for Beijing stains; Fig. 5D, E). Finally, the effect of UBS-109 and EF-24 on the rifampcin-resistant Mtb (H37Rv RifR) was evaluated. UBS-109 depletes H37Rv RifR Mtb with an IC50 of ~7 μM (Fig. 5D). EF-24 also blocks the rifampicin-resistant Mtb strain, though with lower efficacy compared to UBS-109 (IC50 of 20 μM; Fig. 5G). These data suggest that curcumin analogs are as effective against Mm in culture as they are against Mtb H37Rv, but less effective against Mtb Beijing.
Fig. 5.
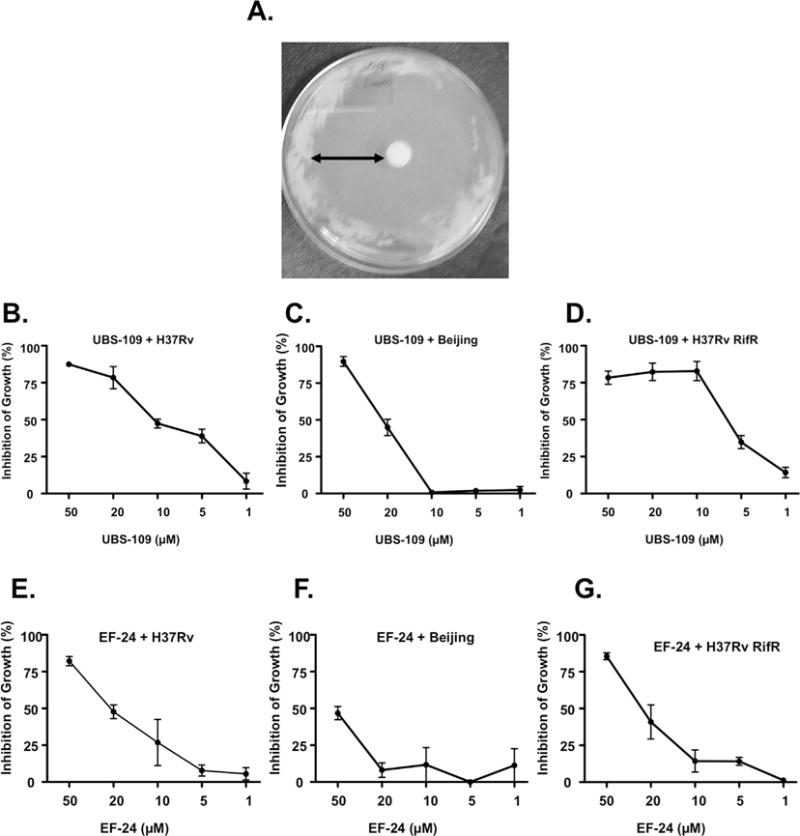
Monoketone curcumin analogs inhibit the growth of Mycobacterium tuberculosis (Mtb). (A) Initial screening of inhibitory properties against Mtb analyzed by disk diffusion assays. 100 mM of curcumin analogs was added to Mtb strain H37Rv containing plate, incubated for 28 days at 37 °C, and zone of inhibition measured; (B) Liquid culture assay validating that UBS-109 inhibits H37Rv strain of Mtb. Different concentration of analogs were added to Mtb culture, incubated for 8d, then the OD600 was measured.; (C) Liquid culture assay validating that UBS-109 inhibits Beijing strain of Mtb; (D) Liquid culture assay validating that UBS-109 inhibits rifampicin-resistence strain of H37Rv Mtb; (E) Liquid culture assay validating that EF-24 inhibits H37Rv strain of Mtb; (F) Liquid culture assay validating that EF-24 inhibits Beijing strain of Mtb; (G) Liquid culture assay validating that EF-24 inhibits rifampicin-resistence strain of H37Rv Mtb.
4. Discussion
The use of curcumin as a potential therapeutic for TB has been discounted because of poor bioavailability. One approach to overcoming this disadvantage is the synthesis and bio-evaluation of structural variations of curcumin such as members of the mono-carbonyl family depicted in Fig. 1. Analogs developed by our group and others display a range of pharmacological effects against a variety of diseases [7–10,22,24,26] and may have improved bioavailability compared to curcumin [22,28,36]. To date, only one member of this class, EF-24, has been subjected to both bioavailability evaluation and pharmacokinetics treatment. The bioavailability of the compound falls at 35% and 60% in i.p. and oral experiments, respectively [28]. The corresponding i.p. and p.o. pharmacokinetics parameters (Tmax, Cmax, t½ and AUC; Table 1S, supporting information) for EF-31 [36] and UBS-109 [28] are quite similar to those for EF-24 suggesting a comparable bioavailability.
In the present work we show that monocarbonyl curcumin analogs also inhibit growth of pathogenic mycobacteria species (Mtb and Mm). UBS-109 exhibited the best inhibitory effect, as determined by its IC50 and its activity against two species of pathogenic mycobacteria. UBS-109, and several other analogs deliver significantly lower IC50 values compared to curcumin, which has been reported to inhibit growth of Mtb [13]. We have established that all three structural domains of the analogs (Fig. 1) can be tailored to influence anti-mycobacterial activity. This observation carries over to anti-cancer and anti-inflammatory effects by this family of analogs as well [10,19,21–24,26]. First, the presence of a mono-carbonyl group linking the aromatic rings in these analogs improved growth inhibition in comparison to curcumin, which incorporates keto-enol functionality at the center of the structure (Fig. 1). Furthermore, the data suggests that fluoro-substitution of the aromatic ring reduces anti-mycobacterial activity by ~2-fold compared to UBS-109. Since the drug-target proteins of Mtb have yet to be identified, it is not yet clear what type of protein-ligand interactions within the bacteria are responsible for the halogen’s modest reduction of antibacterial activity.
Perhaps the most important structural characteristic of these analogs is the two unsaturated bonds flanking the carbonyl, which constitute a double Michael acceptor. In general, this domain is required for biological activity of curcumin analogs. While it is recognized that these bonds are required for most biological effects, they also represent a potential limitation in the usefulness of such compounds as therapeutics. Michael acceptors can render compounds unstable and facilitate their degradation. In addition, Michael acceptors can in principle undergo numerous reactions with proteins, which can contribute to nonspecific effects, including toxicity [32]. This limitation may likewise complicate the use of these particular curcumin analogs in treating TB patients. In spite of these observations, the rifamycin family of antibiotics, including Rifampicin (Fig. 3), are unsaturated amides and, thus, Michael acceptors themselves. Two clinically valuable members of the series, Rifabutin and Rifalazil, contain a second masked Michael acceptor in their naphthoquininoid rings, a feature pictured by one of the tautomers of Rifampicin as well. Recent re-evaluation of the presence of Michael acceptors in potential drugs, some of which are in medical use, suggests that such drawbacks can be overcome [32–35,37]. Our data concur with previous studies by Changtam et al., who showed that the elimination of unsaturated bonds within curcumin decreased anti-mycobacterial activity [13].
Currently, a major impediment to TB treatment is the rise of strains that are resistant to some or all first and second line antibiotics. Our data indicate that monocarbonyl curcumin analogs are effective against rifampicin-resistant strains of Mtb and Mm. Currently, drug regimens for TB consist of combinations of drugs, which reduce the likelihood of developing antibiotic resistance. The development of new therapeutics that displays synergy with co-administered antibiotics is of obvious benefit. While attractive as a therapeutic against Mtb and Mm, no additive or synergistic effect was evident when UBS-109 was administered in combination with rifampicin. Nevertheless, the prospect remains that examination of additional analogs may permit structural insights that could guide the design of analogs that do display synergistic effects when combined with other anti-TB drugs. In this context we anticipate sampling a wider range of classical antibiotics with a broader collection of monocarbonyl analogs of curcumin in search of combinations that reduce the possibility of Mtb developing resistance to both drugs.
The present study has focused on the effects of a number of monocarbonyl analogs of curcumin on bacterial growth in vitro. Future work will characterize effects of these analogs on Mtb infection in vivo. Importantly, this class of compounds has been implicated in regulating a myriad of cellular processes in mammalian cells [10]. Thus, the efficacy of these compounds in vivo may depend on whether these pathways impact Mtb infection. In this regard, curcumin has also been found to inhibit inflammatory responses; however agents that disrupt inflammation can have both positive and negative effects on Mtb infections [38]. Nevertheless, development of a new analog that facilities the clearance of Mtb by targeting both bacterial and host cellular pathways may represent a novel means to treat Mtb strains that are resistant to conventional anti-TB drugs.
Supplementary Material
Acknowledgments
We thank members of the Kalman Laboratory, R. Sonowal and G. Patel, for helpful discussions.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2015.01.020.
References
- 1.Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13:1320–1330. [PubMed] [Google Scholar]
- 2.Duncan K, Sacchettini JC. Approaches to tuberculosis drug development. In: Hatfull GF, Jacobs WR, editors. Molecular Genetics of Mycobacteria. ASM Press; Washington, DC: 2000. pp. 297–307. [Google Scholar]
- 3.Loddenkemper R, Hauer B. Drug-resistant tuberculosis: a worldwide epidemic poses a new challenge. Dtsch Arztebl Int. 2010;107:10–19. doi: 10.3238/arztebl.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sloan DJ, Davies GR, Khoo SH. Recent advances in tuberculosis: new drugs and treatment regimens. Curr Respir Med Rev. 2013;9:200–210. doi: 10.2174/1573398x113099990017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisht S, Maitra A. Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr Drug Discov Technol. 2009;6:192–199. doi: 10.2174/157016309789054933. [DOI] [PubMed] [Google Scholar]
- 6.Singh M, Sasi P, Gupta VH, Rai G, Amarapurkar DN, Wangikar PP. Protective effect of curcumin, silymarin and N-acetylcysteine on antitubercular drug-induced hepatotoxicity assessed in an in vitro model. Hum Exp Toxicol. 2012;31:788–797. doi: 10.1177/0960327111433901. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2010;30:818–860. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C, Liu Z, Liang G. Promising curcumin-based drug design: mono-carbonyl analogues of curcumin (MACs) Curr Pharm Des. 2013;19:2114–2135. [PubMed] [Google Scholar]
- 9.Zhang Y, Zhao C, He W, Wang Z, Fang Q, Xiao B, Liu Z, Liang G, Yang S. Discovery and evaluation of asymmetrical monocarbonyl analogs of curcumin as anti-inflammatory agents. Drug Des Dev Ther. 2014;8:373–382. doi: 10.2147/DDDT.S58168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shetty D, Kim Y, Shim H, Snyder J. Eliminating the heart from the curcumin molecule: monocarbonyl curcumin mimics (MACs) Molecules. 2015;20:249–292. doi: 10.3390/molecules20010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem. 2011;59:2056–2061. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- 12.De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Changtam C, Hongmanee P, Suksamrarn A. Isoxazole analogs of curcuminoids with highly potent multidrug-resistant antimycobacterial activity. Eur J Med Chem. 2010;45:4446–4457. doi: 10.1016/j.ejmech.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Moghaddam K, Iranshahi M, Yazdi M, Shaverdi A. The combination effect of curcumin with different antibiotics against Staphylococcus arueus. Int J Green Pharmacol. 2009;3:141–143. [Google Scholar]
- 15.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 16.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Analysis. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Ji HF. The pharmacology of curcumin: is it the degradation products? Trends in Molecular Medicine. 18:138–144. doi: 10.1016/j.molmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Gordon ON, Schneider C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends in Molecular Medicine. 18:361–363. doi: 10.1016/j.molmed.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosley C, Liotta D, Snyder J. Highly active anticancer curcumin analogues. In: Aggarwal B, Surh Y-J, Shishodia S, editors. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; US: 2007. pp. 77–103. [DOI] [PubMed] [Google Scholar]
- 20.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Brown A, Shi Q, Moore TW, Yoon Y, Prussia A, Maddox C, Liotta DC, Shim H, Snyder JP. Monocarbonyl curcumin analogues: heterocyclic pleiotropic kinase inhibitors that mediate anticancer properties. J Med Chem. 2013;56:3456–3466. doi: 10.1021/jm4002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu S, Moore TW, Morii N, Howard RB, Arrendale RF, Reddy P, Evers TJ, Zhang H, Sica G, Chen ZG, Sun A, Fu H, Khuri FR, Shin DM, Snyder JP, Shoji M. Synthetic curcumin analog UBS109 inhibits the growth of head and neck squamous cell carcinoma xenografts. Curr Cancer Drug Targets. 2014;14:380–393. doi: 10.2174/1568009614666140312163524. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraju GP, Zhu S, Wen J, Farris AB, Adsay VN, Diaz R, Snyder JP, Mamoru S, El-Rayes BF. Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett. 2013;341:195–203. doi: 10.1016/j.canlet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Moore TW, Sun A, Snyder JP, Shoji M. Novel curcumin analogue UBS109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: involvement in Smad activation and NF-[small kappa]B inhibition. Integr Biol. 2012;4:905–913. doi: 10.1039/c2ib20045g. [DOI] [PubMed] [Google Scholar]
- 25.Moore TW, Zhu S, Randolph R, Shoji M, Snyder JP. Liver S9 fraction-derived metabolites of curcumin analogue UBS109. ACS Med Chem Lett. 2014;5:288–292. doi: 10.1021/ml4002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivera A, Moore TW, Hu F, Brown AP, Sun A, Liotta DC, Snyder JP, Yoon Y, Shim H, Marcus AI, Miller AH, Pace TW. Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anticancer properties. Int Immunopharmacol. 2012;12:368–377. doi: 10.1016/j.intimp.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landais I, Hiddingh S, McCarroll M, Yang C, Sun A, Turker MS, Snyder JP, Hoatlin ME. Monoketone analogs of curcumin, a new class of Fanconi anemia pathway inhibitors. Mol Cancer. 2009;8:133–133. doi: 10.1186/1476-4598-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid J, Buhrow S, Gilbert J, Jia L, Shoji M, Snyder J, Ames M. Mouse pharmacokinetics and metabolism of the curcumin analog, 4-piperidinone,3,5-bis[(2-fluorophenyl)methylene]-acetate(3E,5E) (EF-24; NSC 716993) Cancer Chemother Pharmacol. 2014;73:1137–1146. doi: 10.1007/s00280-014-2447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun A, Shoji M, Lu YJ, Liotta DC, Snyder JP. Synthesis of EF24–tripeptide chloromethyl ketone: a novel curcumin-related anticancer drug delivery system. J Med Chem. 2006;49:3153–3158. doi: 10.1021/jm051141k. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Zhang Y, Cai Y, Wang J, Weng B, Tang Q, Chen X, Pan Z, Liang G, Yang S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg Med Chem. 2013;21:3058–3065. doi: 10.1016/j.bmc.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 31.Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, Bornmann W, Salgame P, Shinnick TM, Kalman D. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 2011;10:475–485. doi: 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson MH. Reversible michael additions: covalent inhibitors and prodrugs. Mini Rev Med Chem. 2012;12:1330–1344. doi: 10.2174/13895575112091330. [DOI] [PubMed] [Google Scholar]
- 33.Lee CU, Grossmann TN. Reversible covalent inhibition of a protein target. Angew Chem Int Ed. 2012;51:8699–8700. doi: 10.1002/anie.201203341. [DOI] [PubMed] [Google Scholar]
- 34.Hagel M, Niu D, St Martin T, Sheets MP, Qiao L, Bernard H, Karp RM, Zhu Z, Labenski MT, Chaturvedi P, Nacht M, Westlin WF, Petter RC, Singh J. Selective irreversible inhibition of a protease by targeting a noncatalytic cysteine. Nat Chem Biol. 2011;7:22–24. doi: 10.1038/nchembio.492. [DOI] [PubMed] [Google Scholar]
- 35.Potashman MH, Duggan ME. Covalent modifiers: an orthogonal approach to drug design. J Med Chem. 2009;52:1231–1246. doi: 10.1021/jm8008597. [DOI] [PubMed] [Google Scholar]
- 36.Zhu S, Moore TW, Lin X, Morii N, Mancini A, Howard RB, Culver D, Arrendale RF, Reddy P, Evers TJ, Zhang H, Sica G, Chen ZG, Sun A, Fu H, Khuri FR, Shin DM, Snyder JP, Shoji M. Synthetic curcumin analog EF31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integr Biol. 2012;4:633–640. doi: 10.1039/c2ib20007d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serafimova IM, Pufall MA, Krishnan S, Duda K, Cohen MS, Maglathlin RL, McFarland JM, Miller RM, Frodin M, Taunton J. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat Chem Biol. 2012;8:471–476. doi: 10.1038/nchembio.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE, III, Sher A. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


