Abstract
Iron is a transition metal that due to its inherent ability to exchange electrons with a variety of molecules is essential to support life. In mammals, iron exists mostly in the form of heme, enclosed within an organic protoporphyrin ring and functioning primarily as a prosthetic group in proteins. Paradoxically, free iron also has the potential to become cytotoxic when electron exchange with oxygen is unrestricted and catalyzes the production of reactive oxygen species. These biological properties demand that iron metabolism is tightly regulated such that iron is available for core biological functions whilst preventing its cytotoxic effects. Macrophages play a central role in establishing this delicate balance. Here, we review the impact of macrophages on heme-iron metabolism and, reciprocally, how heme-iron modulates macrophage function.
Introduction
As originally proposed by Metchnikoff in the 19th century, macrophages play an essential role in the regulation of homeostasis (Lavin et al., 2015; Okabe and Medzhitov, 2015; Wynn et al., 2013). Tissue-resident macrophages can sense and react to a broad range of environmental cues, as to assist bystander parenchyma cells in their functional outputs, and in the event of tissue injury, contribute to tissue repair and regeneration (Chovatiya and Medzhitov, 2014; Davies et al., 2013; Kotas and Medzhitov, 2015; Okabe and Medzhitov, 2015; Wynn et al., 2013). These adaptive responses were characterized mostly in the context of infection, where macrophages use pattern recognition receptors (PRR) to sense pathogen-associated molecular patterns (PAMP) (Janeway and Medzhitov, 2002). In such cases, the ensuing adaptive response of macrophages is aimed at containing or eliminating pathogens, while limiting tissue dysfunction and preserving homeostasis (Davies et al., 2013; Medzhitov, 2008). While infections are an extreme and yet recurrent situation where macrophages are called upon to restore homeostasis, there is now increasing evidence to suggest that this a far more general principle, namely, that tissue-resident macrophages sense and act upon any perturbation from the norm to restore homeostasis (Chovatiya and Medzhitov, 2014; Okabe and Medzhitov, 2015; Wynn et al., 2013). As reviewed herein, this broad homeostatic control function is particularly well-illustrated in the context of iron metabolism, regulated by macrophages both at steady-state (Korolnek and Hamza, 2015) and in response to infections (Cassat and Skaar, 2013; Ganz and Nemeth, 2015; Soares and Weiss, 2015).
Likely due to its relative abundance on earth and inherent chemical properties, iron became, from an evolutionary perspective, increasingly used to support electron exchanges in core biological processes such as the production of ATP by the mitochondria electron transport chain or the transport of oxygen (O2) and other gaseous molecules by hemoglobin (Kosman, 2010). In mammals, the majority of the body iron is in the form of heme, a protoporphyrin IX ring surrounding an iron atom (Korolnek and Hamza, 2015). Protoporphyrin IX can be generated physiologically through a series of evolutionarily conserved enzymatic reactions, with the last step culminating in the enzymatic insertion of iron into the protoporphyrin IX (Hamza and Dailey, 2012). This biosynthetic process is highly coordinated and regulated by the heme and iron status of the cell, at both transcriptional and post-transcriptional levels (Hamza and Dailey, 2012). Thus, understanding how iron metabolism and macrophage function cross-regulate each other requires a holistic viewpoint, taking into account both iron and heme molecules, which in truth are “two sides of the same coin”.
Depending upon the chemical reaction, iron and the surrounding porphyrin macrocycle of heme endow hemoproteins with the capacity to exchange electrons with other molecules (Kleingardner and Bren, 2015; Senge et al., 2015). While electron transfer reactions are also the hallmark of another class of iron-containing prosthetic groups, termed iron-sulfur clusters, we will specifically focus this review on heme.
Iron exchanges electrons primarily with divalent gaseous molecules such as O2, nitric oxide (NO•) or carbon monoxide (CO) (Kleingardner and Bren, 2015; Senge et al., 2015). As gaseous molecules became, through evolution, embedded in vital biological functions, most life forms evolved to generate them endogenously, as exemplified for O2 in plants and for NO• and CO in most other life forms. These are referred to as gasotransmitters, to indicate that they can exert biological functions when generated under physiological conditions.
Electron exchange between iron and O2 must be tightly regulated as to avoid the unfettered production of reactive oxygen species (ROS), eventually leading to oxidative stress (Kosman, 2010). The evolutionary solution to this ancient problem is to control and to some extent restrict the access of O2 and other gaseous molecules to iron. This is provided by the molecular environment of the protoporphyrin ring of heme as well as by the amino acids in the heme pockets of hemoproteins (Kleingardner and Bren, 2015; Senge et al., 2015). Electron exchange between gasotransmitters and heme-iron is perhaps best characterized when occurring between O2, and hemoglobin or myoglobin, but this process also occurs between CO or NO• and these as well as other hemoproteins. As discussed in further detail below, some of these hemoproteins play a central role in the regulation of macrophage function.
In mammals, hemoglobin accounts for the largest pool of heme and, consequently, iron (Hamza and Dailey, 2012). The hemoglobin content of erythrocytes is exceedingly high, in the range of 2.5×108 molecules per red blood cell (RBC). Considering four prosthetic heme groups per α2β2 globin tetramer, each mature RBC is thought to contain about 1.2×109 heme moieties (Korolnek and Hamza, 2015). Release of such amounts of heme upon RBC senescence or damage constitutes a permanent and considerable threat as it creates the potential for iron cytotoxicity, eventually driven by a specific form of programmed cell death termed ferroptosis (Dixon et al., 2012).
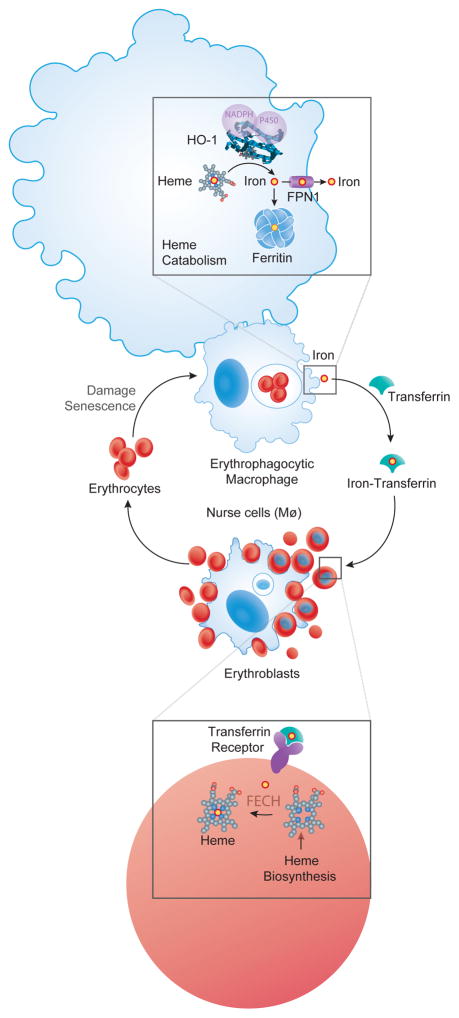
Erythrophagocytic macrophages play an essential role in preventing the release of hemoglobin from RBCs. This occurs via a mechanism in which slight modification of RBC cellular membrane, such those associated with RBC senescence or damage, are sensed by erythrophagocytic macrophages that phagocytose those RBC (Korolnek and Hamza, 2015). This macrophage lineage, previously referred as reticuloendothelial macrophages, is highly specialized in the phagocytosis of RBC, digesting their hemoglobin content and recycling the iron back to erythroid progenitors, for heme synthesis and hemoglobin production (Korolnek and Hamza, 2015)(Figure 1).
Figure 1. Macrophage regulation of heme-iron homeostasis.
Processing of damaged or senescent RBC in the phagolysosomes, represented as a white circle inside the erythrophagocytic macrophage, is associated with the release of heme from hemoglobin and its translocation into the cytoplasm, via a mechanism involving the heme transporter HRG1 (Korolnek and Hamza, 2015). Heme is catabolized by HO-1 and the iron produced via this process is either excreted from macrophages, via FPN1, or stored intracellularly, by ferritin. When released from erythrophagocytic macrophages iron is transported in plasma by transferrin and internalized by erythroblasts, via the transferrin receptor (Korolnek and Hamza, 2015). The ferrochelatase (FECH) enzyme uses iron to catalyze the last step of heme biosynthesis in the mitochondria of erythroblasts, converting protoporphyrin IX into heme (Hamza and Dailey, 2012). Erythroblasts are in close contact with a specialized population of tissue resident macrophages in the bone marrow, known as nurse cells. These phagocytose and digest the nuclei of erythroblasts in phagolysosomes (white circle inside macrophages) while plausibly delivering iron and heme required to support hemoglobin synthesis by erythroblasts (Korolnek and Hamza, 2015).
Macrophage regulation of heme-iron homeostasis
The central role played by macrophages in supporting homeostasis stems, in part, from their involvement in the regulation of iron metabolism that occurs at least at two levels. First, a specialized population of tissue-resident macrophages in the bone marrow, known as nurse cells, forms erythroblastic islands that are required to support erythropoiesis, phagocytosing and digesting the nuclei of erythroblasts while plausibly delivering the iron and heme required to support hemoglobin synthesis by erythroblasts (Korolnek and Hamza, 2015) (Figure 1). Secondly, the majority of the iron required to support heme biosynthesis in erythroblasts originates from senescent RBC engulfed by erythrophagocytic macrophages in the red pulp of the spleen, the bone marrow and to some extent in the liver (Korolnek and Hamza, 2015) (Figure 1).
The erythrophagocytic macrophage lineage develops essentially from bone marrow-derived monocyte progenitor cells via a developmental process regulated by the heme-responsive transcription factor SPI-C (Haldar et al., 2014; Kohyama et al., 2009). While erythrophagocytic macrophages share the capacity to phagocytose and process dying cells with other macrophage lineages (Haldar et al., 2014; Kohyama et al., 2009), their hallmark is to be able to do this with RBCs (Korolnek and Hamza, 2015). This is made possible through the expression of macrophage lineage-specific genetic program controlled by SPI-C and allowing heme-iron recycling from hemoglobin while preventing its cytotoxic effects (Haldar et al., 2014; Kohyama et al., 2009).
RBC processing in erythrophagocytic macrophages is associated with the release of heme from hemoglobin and with its subsequent translocation from phagolysosomes into the cytoplasm, via a mechanism assisted by the heme responsive gene-1 (HRG1) transporter (Rajagopal et al., 2008; White et al., 2013). Heme accumulation in the cytoplasm induces the expression of downstream heme-responsive genes (Korolnek and Hamza, 2015), which include heme oxygenase-1 (HO-1 encoded by HMOX1), a heme-catabolizing enzyme that extracts iron from protoporphyrin and generates equimolar amounts of biliverdin and CO (Gozzelino et al., 2010). The iron produced via heme catabolism is either excreted from macrophages via the transmembrane protein ferroportin-1 (FPN1) encoded by solute carrier family 40 member 1 (SLC40A1) gene (Knutson et al., 2005), or is stored intracellularly by ferritin, a multimeric protein composed of 24 heavy or heart (FTH) and light or liver (FTL) chains (Gozzelino and Soares, 2014; Harrison and Arosio, 1996). The iron-storing capacity of ferritin is assisted by the ferroxidase activity of FTH, which converts reactive iron (Fe2+) into inert, nucleated iron (Fe3+), no longer available to catalyze the production of free radicals via Fenton chemistry (Gozzelino and Soares, 2014; Harrison and Arosio, 1996).
Ferritin expression is regulated by several molecular players, which act both at transcriptional and post-transcriptional levels (Gozzelino and Soares, 2014; Harrison and Arosio, 1996). Transcriptional regulation involves the activation of the nuclear factor kappa B (NF-κB) family of transcription factors (Pham et al., 2004) and the transcription factor nuclear factor E2-related factor-2 (Nrf2) (Sakamoto et al., 2009), as well as the BTB and CNC homology 1 basic leucine zipper (bZIP) transcription factor 1 (Bach1) (Sakamoto et al., 2009), a transcriptional master regulator of the macrophage lineage (Gautier et al., 2012). Post-transcriptional regulation occurs via a mechanism involving inhibition of the binding of iron regulatory protein (IRP) to the 5′UTR of Ftl and Fth mRNA. This de-represses Ftl and Fth mRNA translation, the predominant mechanism for the induction of ferritin expression in response to intracellular iron accumulation (Harrison and Arosio, 1996). Ferritin expression is negatively regulated via a mechanism acting post-translationally and involving the nuclear receptor co-activator 4 (NCOA4), a selective FTH cargo receptor that shuttles ferritin for autophagy (Mancias et al., 2014). This process, termed ferritiophagy, is critical in delivering iron from ferritin once intracellular iron content drops (Mancias et al., 2014).
Hmox1 deletion is associated with progressive depletion of erythrophagocytic macrophages in mice (Kovtunovych et al., 2010), arguing that heme detoxification by HO-1 is required to maintain the integrity of this macrophage lineage. Moreover, both Hmox1 deletion in mice (Poss and Tonegawa, 1997) and loss-of-function mutations in human HMOX1 (Yachie et al., 1999) are associated with a profound deregulation of heme-iron metabolism and homeostasis. While this has been interpreted as revealing the role of erythrophagocytic macrophages in the regulation of heme-iron metabolism and homeostasis, it is unclear from these studies whether the pathological outcomes associated with HMOX1 deletion are due to a defect of heme catabolism specifically in this macrophage lineage.
Erythrophagocytic macrophages express have a relatively high ferritin content, but whether this is required to support heme-iron metabolism and homeostasis remains unclear. Ferritin can be secreted from erythrophagocytic macrophages, which probably explains its accumulation in plasma (Cohen et al., 2010). Again, the physiological role of plasma ferritin is elusive but has been proposed to serve as a vehicle for intercellular iron transport (Leimberg et al., 2008).
Heme-iron regulation of macrophage function
Regulation of heme-iron metabolism plays an essential role in controlling the expression and activity of hemoproteins. As a general principle, this process is regulated at different levels via the expression of genes controlling the relative rate of cellular heme-iron i) import, ii) biosynthesis, iii) catabolism, and/or iv) export. In this section we highlight how these impact the expression and activity of hemoproteins that modulate macrophage function.
Signaling via PRR activates specific genetic programs associated with the production reactive oxygen species (ROS) by macrophages. This is largely dependent upon the expression of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) 2 family member (gp91phox), encoded by CYBB (Yu et al., 1998). As for other NOX family members, NOX2 is a transmembrane hemoprotein that uses heme-iron to transport electrons across biological membranes and catalyze the generation of superoxide, •O2−, via the following reaction: O2 + e− → •O2− (Bedard and Krause, 2007). As it accumulates in macrophages, •O2− can give rise to other ROS, including hydrogen peroxide (H2O2), which reacts avidly with iron to generate hydroxyl radicals (•OH) and hydroxide ions (OH−), eventually leading to the production of hydrogen peroxide radicals (HOO•). This latter step occurs via two iron-catalyzed reactions; i) Fe2+ + H2O2 → Fe3+ + HO• + OH− and ii) (Fe3+ + H2O2 → Fe2+ + HOO• + H+) (Bedard and Krause, 2007).
ROS production by macrophages targets pathogens for destruction in phagolysosomes while supporting other macrophage functions as well, such as phagocytosis and disassembling of dying cells (Bedard and Krause, 2007). However, it also provides a potential source of oxidative stress and in order to minimize this, NOX2 expression and activity must be tightly regulated. This occurs mainly at the level of CYBB transcription, via a mechanism involving the lineage commitment ETS family transcription factor PU.1, a transcriptional master regulator of the macrophage lineage (Lawrence and Natoli, 2011). PU.1 marks gene enhancers, including in CYBB, with epigenetic modifications (Lawrence and Natoli, 2011), enabling other transcription factors, such as NF-κB (Anrather et al., 2006) to access these enhancers and regulate CYBB transcription. As with other hemoproteins, NOX2 expression and enzymatic activity are post-transcriptionally regulated in macrophages by mechanisms involving cellular heme-iron metabolic pathways. In support of this notion, heme catabolism by HO-1 limits the availability of heme required to support NOX2 expression and activity, acting therefore as a negative regulator of ROS production in macrophages (Taille et al., 2004). In addition, heme catabolism by HO-1 generates the gasotransmitter CO that can bind iron within the heme groups of various hemoproteins and modulate in this manner their activity, as exemplified for the inhibition of NOX2 activity (Taille et al., 2005). Moreover, CO is cytoprotective (Brouard et al., 2000), suggesting that heme catabolism by HO-1 protects macrophages from oxidative stress associated with NOX2 activity.
Cyclooxygenases (COXs) 1 and 2 isoforms, are membrane hemoproteins encoded by the prostaglandin synthase (PTGS) 1 and 2, respectively (van der Ouderaa et al., 1979). These catalyze the oxygenation and reduction of arachidonic acid to prostaglandins (PG)G2 and PGH2 (Rouzer and Marnett, 2009). COX2 is upregulated in response to PRR signaling and is an immediate-early responsive gene associated with macrophage polarization towards microbicidal functions (Cairo et al., 2011; Martinez et al., 2006; Rouzer and Marnett, 2009). Heme-iron metabolism regulates COX2 expression and activity, as reflected by modulation of prostaglandin synthesis. This notion is supported by the observation that heme catabolism by HO-1 inhibits COX2 expression and prostaglandin synthesis (Haider et al., 2002). Given the major biological roles played by prostaglandins, the regulatory effect exerted by heme catabolism HO-1 on COX2 likely has major repercussions for the outcome of inflammatory responses.
Of note, PGD synthases convert PGH2 into PGD2, which can be dehydrated to yield 15-Deoxy-Δ12,14-prostaglandin J2 (15dPGJ2) and induce the expression of HO-1 in macrophages (Koizumi et al., 1995; Lee et al., 2003). This suggests that 15dPGJ2 is part of a negative feedback loop, involving HO-1, and controlling the expression of COX2 (Inoue et al., 2000) as well as that of other genes associated with macrophage activation (Lee et al., 2003).
Heme dioxygenases are a family enzymes that include indoleamine 2,3-dioxygenase 1 and 2 (IDO1 and IDO2) as well as tryptophan 2,3-dioxygenase (TDO). These catalyze the initial and rate-limiting step of tryptophan degradation in the kynurenine pathway: L-tryptophan + O2 -> N-formyl-L-kynuren (Munn and Mellor, 2013). IDO1 expression is induced by various agonists in macrophages while TDO is expressed mainly in parenchyma cells (Munn and Mellor, 2013). The product of heme dioxygenases, i.e. N-formyl-L-kynuren, is sensed in macrophages via the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that modulates macrophage activation (Bessede et al., 2014; Stockinger et al., 2014). Heme-iron metabolism can regulate this signal transduction pathway at different levels: i) restricting heme availability for expression and activity of heme dioxygenases; ii) Modulating enzymatic activity via CO binding to ferrous heme (Brady, 1975) or iii) via the production of biliverdin, an end product of heme catabolism by HO-1 sensed by AhR (Phelan et al., 1998). Given the central role played by AhR in the regulation of both macrophage and T cell activation, modulation of this pathway by heme-iron catabolism is likely to impact on macrophage and/or T cell activation. This remains however to be tested experimentally.
The enzyme cystathionine β-synthase (CBS) is one of the major sources of hydrogen sulfide (H2S) generated by the transsulfuration pathway of the methionine cycle (Mancardi et al., 2009). Expression of CBS is induced when circulating monocytes differentiate into tissue-resident like macrophages in vitro and is reduced upon TLR4 signaling (Garg et al., 2006). H2S, along with NO• and CO, is implicated in various pathophysiological processes (Mancardi et al., 2009), including bacterial clearance by macrophages (Garg et al., 2006). Human CBS contains three domains: a catalytic pyridoxal 5′-phosphate, S-adenosyl-L-methionine, and heme-binding domains (Singh et al., 2007). The enzyme is fully active when the bound heme is oxidized, but reduction of this ferric heme to its ferrous form results in slow inactivation of the enzyme. Moreover, CBS activity is sensitive to inhibition by CO and NO• binding to ferrous heme. Thus, CBS-heme is sensitive to cellular redox positioning it at the crossroads of regulation for all three physiologically relevant gasotransmitters produced by heme-containing enzymes in macrophages. Despite this fact, the precise role of CBS and its end product H2S in macrophage function remains largely unexplored.
Nitric oxide synthases (NOS) are a family of hemoprotein enzymes that generate NO• from the oxidation of L-arginine into citrulline (Nathan and Xie, 1994). Recognition of bacterial lipopolysaccharide (LPS) by the PRR Toll like receptor 4 (TLR4), combined with the engagement of the interferon-γ (IFN-γ) receptor, induces the expression of the inducible NOS2 (iNOS or NOS2) isoform in macrophages (Nathan and Xie, 1994). This occurs, essentially, at the transcriptional level, via a mechanism involving both TLR4-mediated activation of NF-κB, and activation of the signal transducers and activators of transcription (STAT) family of transcription factors, downstream of the IFN-γ receptor. Iron accumulation in macrophages inhibits NOS2 transcription via a mechanism that is not clearly established but that involves the down-regulation of STAT activation in response to IFN-γ receptor signaling (Weiss et al., 1992; Xie et al., 1994). Macrophage heme-iron content also regulates NOS2 post-transcriptionally via mechanisms that support the expression and activity of this hemoprotein (White and Marletta, 1992).
NO• acts both as an intracellular and extracellular signaling molecule, through different mechanisms. NO• can signal intracellularly via its direct interaction with heme-iron in hemoproteins (Beckman and Koppenol, 1996), as illustrated for guanylate cyclase, an enzyme that synthesizes cyclic guanylate cyclase (cGMP) (Stone and Marletta, 1994) or for cytochrome c oxidase, the terminal enzyme in the mitochondrial respiratory chain (Palacios-Callender et al., 2004). When NO• interacts with heme-iron in cytochrome c oxidase, as well as with other iron containing components of the mitochondria electron transport chain, this gasotransmitter acts as a physiological inhibitor of mitochondrial respiration, while inducing ROS production (Moncada and Erusalimsky, 2002). Presumably, this contributes to the metabolic switch towards glycolysis observed upon macrophage activation by PRR and other sensors (Kelly and O’Neill, 2015).
Another mechanism via which NO signaling can induce changes in macrophage function relates to its reaction with the •O2− produced by NOX2. This reaction generates peroxynitrite (ONNO-), a stable reactive nitrogen species (RNS) that triggers S-nitrosylation and 3-nitration of cysteine and tyrosine residues (Mustafa et al., 2009) of proteins involved in metabolic pathways such as glycolysis and the tricarboxylic acid cycle (Kelly and O’Neill, 2015). Presumably this contributes to the metabolic switch associated with macrophage activation by PRR and other stimuli (Kelly and O’Neill, 2015).
Yet another mechanism underlying NO• signaling involves the thiol (-SH) groups of reactive cysteines in several proteins. These can be targeted by RNS, generating thiol oxidation products and eventually leading to the formation of disulfide bonds that alter protein tertiary structures and hence their activity. This thiol-based signal transduction mechanism is perhaps best illustrated in the context of the regulation of Kelch-like ECH-associated protein 1 (Keap1) activity, as described in detail below.
Intracellular accumulation of ROS and RNS is sensed in macrophages by Keap1, an adaptor for the Cullin (Cul)3-RING (really interesting new gene)-box protein (Rbx)1 ubiquitin ligase complex that ubiquitinates constitutively the transcription factor nuclear factor E2-related factor-2 (Nrf2), inducing its proteolytic degradation by the 26S proteasome (Hayes and Dinkova-Kostova, 2014) (Figure 2). Nrf2 activation triggers the transcription of genes involved in the regulation of cellular heme-iron metabolism, such as HMOX1 (Alam et al., 1999), FTH (Sakamoto et al., 2009), SLC40A1 (Marro et al., 2010) and HRG1 (Warnatz et al., 2011), amongst others. This argues that activation of the signal transduction pathway involving Keap1 and Nrf2 plays an important role in the modulation of cellular heme-iron metabolism in macrophages. Moreover, accumulation of intracellular heme-iron represses Keap1 and activates Nrf2, arguing for the existence of feedback loops between this signal transduction pathway and heme-iron metabolism.
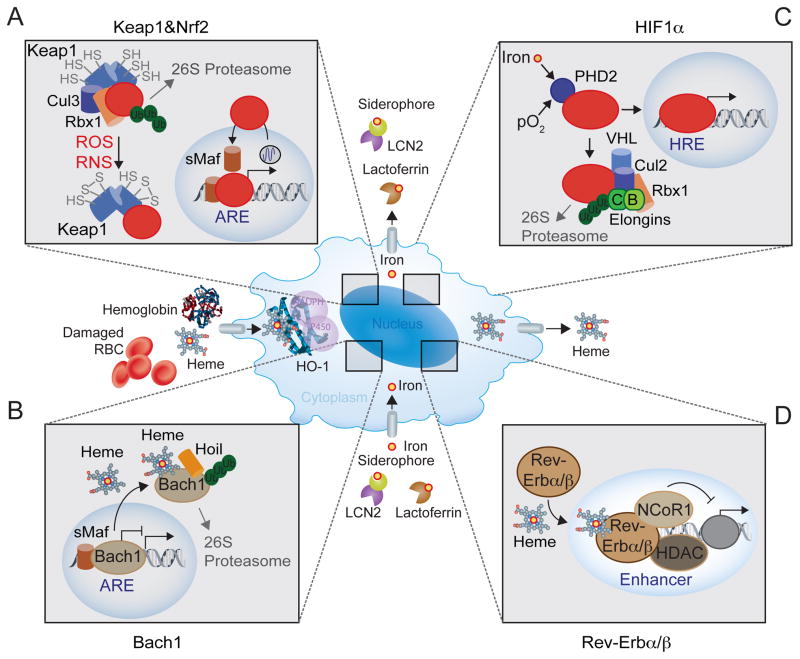
Figure 2. Heme-iron regulation of macrophage function.
(A) ROS and RNS target reactive cysteines in Keap1, inhibiting its activity (Kensler et al., 2007) and arresting Nrf2 ubiquitination as well as proteolytic degradation (Hayes and Dinkova-Kostova, 2014; Itoh et al., 1999). Depending on its rate of transcription, which falls under circadian control (Pekovic-Vaughan et al., 2014), newly generated Nrf2 is no longer repressed by Keap1, undergoing nuclear translocation, where it binds to small musculoaponeurotic fibrosarcoma (sMaf) transcription factors, including MafF, MafG and MafK (Sykiotis and Bohmann, 2010). These Nrf2 containing heterodimers activate the transcription of genes containing antioxidant responsive element (ARE) DNA binding motifs in their promoter region. (B) Bach1 competes with other CNC-bZIP proteins, including Nrf2, for binding to sMaf transcription factors and ARE DNA binding motifs (Sun et al., 2004). As Bach1 lacks a transcription activation domain, it acts essentially as a transcriptional repressor when bound to ARE DNA binding motifs in the promoter of genes such as FTH, SLC40A1, HRG1 or HMOX1 (Sun et al., 2002). Transcriptional repression is reversed by heme (Ogawa et al., 2001), which binds Bach1 with high affinity, inhibiting DNA binding and promoting its recognition by the heme-oxidized IRP2 ubiquitin ligase-1 (Hoil-1), an E3 ubiquitin-protein ligase encoded by RBCK1 (Elton et al., 2015). Hoil-1 catalyzes head-to-tail linear polyubiquitination, driving Bach1 for proteolytic degradation by the 26S proteasome (Zenke-Kawasaki et al., 2007). (C) Hypoxia is sensed via the iron-responsive prolyl hydroxylase (PHD)2 (Semenza, 2012). Under physiological pO2, PHD2 hydroxylates the hypoxia-inducible factor (HIF) family of transcription factors, which are recognized by the von Hippel-Lindau (VHL), as part of a E3 ubiquitin complex composed of Cullin-2 (CUL2), the ring-box1 E3 ubiquitin protein ligase (RBX1) and Elongin B and C, resulting in HIF ubiquitination and proteolytic degradation by the 26S proteasome (Greer et al., 2012; Semenza, 2012). Under hypoxia the activity of PHD2 is inhibited releasing HIFs from VHL and allowing for HIFs nuclear translocation and binding to DNA hypoxia responsive elements (HRE) in the promoter of effector genes regulating metabolic adaptation to hypoxia (Greer et al., 2012; Semenza, 2012). Iron activates PHD2 and as such reducing macrophage heme-iron content, represses PHD2 activity and induces HIF1α activation under normoxic conditions. (D) Upon heme binding, RevErbα (Raghuram et al., 2007; Yin et al., 2007) and Rev-Erbβ (Carter et al., 2015) recruit the nuclear receptor co-repressor 1 (NCoR1) and the histone deacetylase 3 (HDAC3), forming protein complexes that inhibit the transcription of a broad range of genes in macrophages (Lam et al., 2013). Transcription repression occurs via a mechanism that involves the macrophage lineage-specific transcription PU.1, which renders gene enhancers available for Rev-Erbα and Rev-Erbβ targeting (Lam et al., 2013).
Nrf2 activation in response to PRR signaling partakes in a negative feedback loop restraining sustained expression of PRR-responsive genes in macrophages (Ashino et al., 2008; Thimmulappa et al., 2006). For example, TLR4 activates Nrf2 in macrophages, indirectly via the induction of NOS2 and the production of NO•, inhibiting further NOS2 expression and NO• production (Ashino et al., 2008). This occurs most likely via the expression of Nrf2-regulated genes, which are likely to include the activating transcription factor 3 (ATF3) (Kim et al., 2010), a negative regulator of TLR4 signaling in macrophages (Gilchrist et al., 2006; Hoetzenecker et al., 2012). There are other Nrf2-regulated genes that modulate macrophage responses, such as HO-1, which limits heme availability and presumably therefore the expression and activity of hemoproteins, such as NOS2 (Ashino et al., 2008), NOX2 (Taille et al., 2004) or COX2 (Haider et al., 2002). On the other hand, Nrf2 activation in macrophages also promotes the activation of the NACHT, LRR and PYD domains-containing protein 3 (NALP3), leading to caspase 1 activation, pro-IL-1β cleavage and IL-1β secretion (Zhao et al., 2014). This argues that Nrf2 activation interferes with several signal transduction pathways modulating different adaptive responses of macrophages.
Several Nrf2-regulated genes controlling heme-iron metabolism in macrophages are constitutively repressed by the transcriptional macrophage lineage regulator Bach1 (Gautier et al., 2012), as illustrated for HmoxMOX1 (Sun et al., 2002), FTH (Sakamoto et al., 2009), Slc40a1 (Marro et al., 2010) and HRG1 (Warnatz et al., 2011) (Figure 2). Heme-driven Bach1 inhibition enables the activation of Nrf2 (Gozzelino et al., 2010), as well as that of other transcription factors such as SPI-C (Haldar et al., 2014), driving the transcription of a variety of genes regulating heme-iron metabolism in macrophages.
Hypoxia refers to a decrease of O2 pressure (pO2) below a physiological threshold and is sensed via the iron-responsive prolyl hydroxylase (PHD)2 (Semenza, 2012), which controls the activation of the hypoxia-inducible factor (HIF) family of transcription factors (Figure 2). Activation of the HIF1α isoform triggers a profound metabolic reprogramming in macrophages favoring ATP production via aerobic glycolytic metabolism (Cheng et al., 2014; Cramer et al., 2003; Nizet and Johnson, 2009) thereby modulating macrophage function (Cheng et al., 2014; Kelly and O’Neill, 2015).
While activation HIF1α was originally thought to emanate exclusively from hypoxia, it has become apparent that under physiological pO2, several other agonists sensed by macrophages activate this signal transduction pathway. For example, signaling via different PRR activates HIF1α (Cheng et al., 2014; Rius et al., 2008), which is also the case for iron chelation (Peyssonnaux et al., 2005; Semenza, 2012). These two apparently disparate phenomena are probably linked functionally by ferritin. Namely, PRR signaling induces the production of tumor necrosis factor (TNF) in macrophages, which, induces NF-κB activation and ferritin transcription, downstream from TNF receptor signaling (Kwak et al., 1995; Pham et al., 2004). Subsequently, iron sequestration by ferritin inhibits the activity of the iron-responsive PHD2 and stabilizes HIF1α (Siegert et al., 2015). There are other aspects of heme-iron metabolism that activate HIF1α in macrophages, such as CO, an end product of heme catabolism that in a similar manner to NO (Palacios-Callender et al., 2004), CO inhibits the activity of the mitochondrial cytochrome c oxidase, creating a state of metabolic hypoxia activating HIF1α (Chin et al., 2007). Presumably this contributes critically to the immunomodulatory effects exerted by CO in macrophages (Otterbein et al., 2000).
There is a reciprocal relationship between heme-responsive transcription factors controlling circadian rhythms and macrophage heme-iron metabolism (Kaasik and Lee, 2004). Heme-responsive transcription factors include the nuclear receptors RevErbα (Raghuram et al., 2007; Yin et al., 2007) and Rev-Erbβ (Carter et al., 2015), encoded by the nuclear receptor subfamily 1 group D member 1 (NR1D1) and 2 (NR1D2), respectively (Yin et al., 2007), as well as the circadian factor period 2 (Per2) (Yang et al., 2008). Upon heme binding, Rev-Erbα (Raghuram et al., 2007; Yin et al., 2007) and Rev-Erbβ (Carter et al., 2015) inhibit the transcription of a broad range of genes in macrophages (Lam et al., 2013) (Figure 2). These include the circadian rhythm regulators Aryl hydrocarbon receptor nuclear translocator-like (ARNTL), also known as BMAL1, the metabolic regulators peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1α) and the first enzyme in heme synthesis (5′-aminolevulinate synthase 1; ALAS1) (Raghuram et al., 2007), the chemokine (C-C motif) ligand 2 (CCL2 or MCP1), IL-6 and IL-10 (Curtis et al., 2014). These examples further depicts the intricate functional relationship between heme-iron metabolism and central aspects of macrophage function (Curtis et al., 2014).
Sensing labile heme as an alarmin
macrophages can sense a range of structurally unrelated intracellular molecules, referred to as alarmins, which alert for tissue stress or damage when detected extracellularly. Due to the high intracellular heme content of RBCs and muscle cells, detection of extracellular heme by macrophages probably reports on RBC and/or muscle cell damage, associated with hemolysis or rhabdomyolysis, respectively (Soares and Bozza, 2016). When released from damaged RBC or muscle cells, extracellular hemoglobin and myoglobin are prone to oxidation, releasing their heme prosthetic groups. The resulting labile heme can be sensed in macrophages via Bach1 (Ogawa et al., 2001), Rev-Erbα (Yin et al., 2007) and Rev-Erbβ (Carter et al., 2015). In addition, heme is also sensed in macrophages via PRR, including TLR4 (Figueiredo et al., 2007; Fortes et al., 2012) and NLRP3 (Dutra et al., 2014). Heme-sensing by macrophages is associated to the induction of HO-1 expression, which limits the pathogenic effects of hemolysis (Belcher et al., 2006; Pamplona et al., 2007) and rhabdomyolysis (Nath et al., 1992; Soares and Bozza, 2016). A similar adaptive response, involving heme-sensing and HO-1 induction in macrophages, contributes critically to the protective effect exerted by sickle cell hemoglobin against malaria (Ferreira et al., 2008; Ferreira et al., 2011). It is likely that this protective response involves heme-sensing by macrophages, though this is yet to be experimentally established.
Heme-iron can further interfere with PRR signaling to modulate macrophage responses: extracellular hemoglobin binds specifically to bacterial LPS altering its tertiary structure (Du et al., 2010; Kaca et al., 1994) and enhancing the pro-inflammatory activity of this PAMP (Kaca et al., 1994). Labile heme can have a similar effect, synergizing with LPS to induce cytokine production by tissue-resident macrophages (Fernandez et al., 2010). Apparently, this provides the means for amplifying responses upon microbe-sensing when pathogens are present at seemingly low numbers (Fernandez et al., 2010).
Induction of HO-1 expression in macrophages interferes with TLR4 signaling in different ways, including via the production of CO that down regulates TLR4 signaling via mechanisms that involve the production of low amounts of mitochondrial-driven ROS (Zuckerbraun et al., 2007), activation of mitogen activated protein kinases (Otterbein et al., 2000), HIF-1α (Chin et al., 2007) and peroxisome proliferator-activated receptor gamma (PPAR-γ)(Bilban et al., 2006). This provides yet again another example as to the intimate connection between heme-iron metabolism and macrophage responses regulating homeostasis.
Heme-iron in macrophage differentiation and polarization
Tissue-resident macrophage differentiation is driven by specific genetic programs that are tailored, to at least some extent, by microenvironmental cues emanating from parenchyma cells in different tissues (Davies et al., 2013; Okabe and Medzhitov, 2015). This is required so that tissue-resident macrophages optimize their house-keeping functions to suit the particular demands of parenchyma cells as they vary from tissue to tissue (Okabe and Medzhitov, 2015). Another level of diversity in macrophages responses exists, referred to as macrophage polarization. This second layer of regulation adapts macrophage responses when sensing different environmental cues, resulting in a wide-spectrum of responses, ranging from anti-microbial and sometimes deleterious to tissues to adaptive responses supporting tissue repair and regeneration (Okabe and Medzhitov, 2015). Heme-iron metabolism plays a central role in both differentiation and polarization of tissue-resident macrophages.
Heme induces monocyte differentiation into the erythrophagocytic macrophage lineage via activation of a signal transduction pathway releasing Bach1 and allowing the activation of SPI-C (Haldar et al., 2014; Kohyama et al., 2009). Subsequent polarization of macrophage responses towards microbicidal function is associated with intracellular iron retention by ferritin and reduced iron excretion via FPN1, while macrophage polarization towards tissue repair and regeneration is associated with increased heme catabolism by HO-1, iron retention by ferritin and enhanced iron secretion via FPN1 (Cairo et al., 2011; Recalcati et al., 2010). By some estimates over 60% of iron homeostasis genes, including HMOX1, FTH1 and SLC40A1 are differentially expressed between these two end-stages of macrophage polarization, arguing that regulation of cellular heme-iron metabolism is intimately linked to macrophage polarization (Cairo et al., 2011; Recalcati et al., 2010).
Heme catabolism probably contributes to macrophage polarization via the establishment of positive forward signaling feedback loops such as the one involving IL-10 and HO-1. Briefly, CO generated in macrophages via heme catabolism by HO-1 promotes the secretion of IL-10 in response to LPS (Otterbein et al., 2000), which feeds back to induce the expression of HO-1, via mechanism involving the p38 mitogen activated protein kinase (MAPK) (Lee and Chau, 2002) and the signal transducer and activator of transcription (STAT)-3 (Ricchetti et al., 2004).
It is worth noting that while HO-1 expression has been associated with polarization of macrophages responses towards a “tissue healing” function this is not always the case. For example, expression of HO-1 is pathogenic in the context of chronic metabolic inflammation associated with obesity (Jais et al., 2014). One reasonable explanation is that while salutary in the context of macrophage responses against infections, sustained expression of HO-1 in macrophages exposed chronically to stimuli associated with obesity, is part of maladaptive response promoting metabolic inflammation (Medzhitov, 2008).
Macrophage heme-iron metabolism and resistance to infection
Regulatory mechanisms controlling iron metabolism are central to the outcome of host-microbe interactions (Cassat and Skaar, 2013; Ganz and Nemeth, 2015; Soares and Weiss, 2015). Microbicidal responses of tissue-resident macrophages are in most cases associated with modulation of heme-iron metabolism, which feedback and regulate other adaptive responses of macrophages (Soares and Weiss, 2015).
Pathogen-sensing is thought to include some level of “qualitative information” reporting on the metabolic status of pathogens so as to modulate the adaptive responses of macrophages accordingly. For example, the macrophage response towards live pathogens should exert microbicidal effects and eventually activate adaptive immunity, while this should not be the case when responding to dead pathogens. While this argues for fine-tuning of macrophage responses towards live versus dead pathogens the mechanisms via which this occurs are unclear. Macrophages induce the expression of HO-1 (Gozzelino et al., 2010) upon the engagement of PRR (Rushworth et al., 2005) or other stress sensors (Chovatiya and Medzhitov, 2014; Soares et al., 2014). The CO produced via heme catabolism by HO-1 diffuses across cellular membranes and accesses the heme-containing respiratory complexes of bacteria (Wegiel et al., 2014). This induces bacteria to produce ATP, which is sensed back by the macrophage P2X7 purinergic receptor, modulating a K+ efflux pump that activates NLRP3 and promotes pro-IL-1β cleavage by caspase 1, culminating in IL-1β secretion (Wegiel et al., 2014). Signaling via the IL1 receptor triggers a microbicidal response that is not observed when macrophages respond to dead bacteria or debris thereof (Wegiel et al., 2014). This metabolic-sensing mechanism can be subverted by pathogens such as Mycobacteria, in which CO triggers a “dormant” state (Shiloh et al., 2008). Whether CO acts as a general metabolic-sensing mechanism towards pathogens, other than bacteria, remains to be established.
Macrophages also trigger cell autonomous and systemic responses that restrict pathogens from accessing heme-iron (Soares and Weiss, 2015) (Figure 3). This evolutionarily conserved defense strategy, referred to as nutritional immunity (Weinberg, 1975), is tailored specifically towards intracellular versus extracellular pathogens via mutually exclusive mechanisms (Figure 3). Namely, resetting iron metabolism as a protective response against intracellular pathogens is likely deleterious against extracellular pathogens and vice versa.
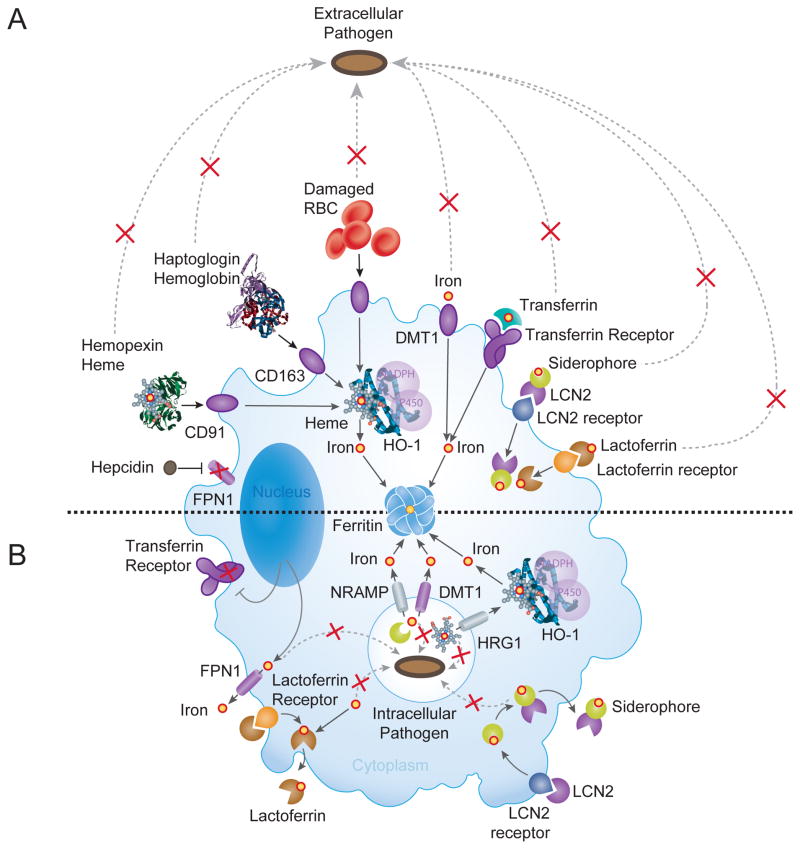
Figure 3. Iron-based resistance mechanisms against Infection.
(A) Infections by extracellular pathogens are associated with the induction of cellular iron import and retention in macrophages through recognition and internalization of plasma iron-binding proteins by specific macrophage receptors. These include i) transferrin, recognized by the transferrin receptor; ii) lactoferrin, a member of the transferrin family of iron-binding proteins secreted mainly into exocrine fluids and recognized by the lactoferrin receptor (Brock, 2002); and iii) Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL), a peptide that captures iron-laden siderophores secreted by gram-negative bacteria (Berger et al., 2006; Flo et al., 2004) and recognized by the LCN2 receptor (Cassat and Skaar, 2013; Soares and Weiss, 2015). In addition, extracellular iron can be imported via the DMT1 iron transporter. In the event of hemolysis haptoglobin and hemopexin scavenge extracellular hemoglobin and labile heme and the ensuing hemoglobin-haptoglobin and heme-hemopexin complexes are captured by tissue-resident macrophages, via CD163 (Kristiansen et al., 2001) and CD91 (Hvidberg et al., 2005), respectively. Tissue resident macrophages phagocytose damaged RBC. Inhibition of macrophage iron export is accomplished via a mechanism involving hepcidin, which binds and triggers the degradation of FPN1 (Drakesmith and Prentice, 2012). Intracellular heme is catabolized by HO-1 and iron is stored by ferritin. (B) Infections by intracellular pathogens are associated with reduced macrophage intracellular heme-iron content. This defense strategy relies on mechanisms that suppress macrophage heme-iron import by the transferrin receptor while promoting heme-iron export, first from phagolysosomes (white circle containing a pathogen) and then from the cytoplasm of macrophages. Export form phagolysosomes involves NRAMP1 (Jabado et al., 2000) and presumably its paralog NRAMP2, also known as DMT1, two divalent metal transporters that translocate iron across the endolysosomal membranes into the cytoplasm. Other iron transporters such as FPN1 and LCN2 can contribute to this process (Soares and Weiss, 2015). Cytoplasmic iron is stored by ferritin or secreted extracellularly by FPN1. The phagolysosome heme transporter HRG1 translocates heme from effete RBCs into the cytoplasm but contribution to resistance against intracellular pathogens is unclear at the moment. Moreover, the precise roles for recently discovered heme exporters, feline leukemia virus subgroup C cellular receptor 1 (FLVCR1), ABCG2 and ABCC5 in macrophage function are also unknown. Heme catabolism by HO-1 is used to release the iron from heme before it can be stored into ferritin or secreted via FPN1. Lactoferrin and lipocalin (LCN2) receptors are used to internalize apo-lactoferrin and apo-lipocalin, respectively. These bind intracellular labile iron and the iron contained in siderophores, and are secreted from macrophages, respectively. Red Xs indicates blockades for iron acquisition by pathogens.
Iron-based resistance mechanisms against extracellular pathogens involve a systemic reduction of circulating iron (Soares and Weiss, 2015). The mechanisms underlying this adaptive response rely on rewiring of iron metabolism towards intracellular iron retention in macrophages (Figure 3). The process falls under the control of two general principles: i) inhibition of cellular iron export and ii) induction of cellular iron import and retention by macrophages (Figure 3) as well as parenchyma cells. Inhibition of cellular iron export is orchestrated by hepcidin, a peptide secreted by hepatocytes in response to a variety of agonists associated with infections (Drakesmith and Prentice, 2012), including cytokines, e.g. IL-1, IL-6, IL-22; LPS or iron accumulation in the circulation (Armitage et al., 2011; Drakesmith and Prentice, 2012; Nemeth et al., 2003). Hepcidin binds and induces the degradation of FPN1, thereby suppressing cellular iron export from macrophages and parenchyma cells (Drakesmith and Prentice, 2012) (Figure 3).
Induction of cellular iron import and retention in macrophages is accomplished through several distinct mechanisms that rely on the cognate recognition and internalization of plasma iron-binding proteins by specific macrophage receptors (Cassat and Skaar, 2013; Soares and Weiss, 2015) (Figure 3). In order to access heme-iron several extracellular pathogens induce hemolysis (Cassat and Skaar, 2013; Soares and Weiss, 2015). This highly pathogenic process is likely countered by haptoglobin and hemopexin, two acute phase proteins that scavenge extracellular hemoglobin and labile heme in plasma, respectively (Gozzelino et al., 2010). Tissue-resident macrophages capture hemoglobin-haptoglobin and heme-hemopexin complexes, via the hemoglobin scavenger receptor CD163 (Kristiansen et al., 2001) and the hemopexin scavenger receptor CD91 (Hvidberg et al., 2005), respectively (Figure 3).
A common denominator shared by heme-iron import and retention mechanisms in macrophages is the induction of ferritin, which sequesters intracellular iron in a non-reactive and, importantly, non-cytotoxic form (Gozzelino and Soares, 2014) (Figure 3). When macrophages acquire extracellular heme, rather than iron, HO-1 is induced, to release iron from protoporphyrin IX before it can be stored into ferritin (Gozzelino and Soares, 2014). Cytokines such as IL-6 and IL-10 induce extracellular heme uptake and HO-1 expression in macrophages (Lee and Chau, 2002), while IL-4, IL-13 and IL-10 promote transferrin receptor-mediated iron uptake and intracellular retention by ferritin (Weiss and Schett, 2013).
Accumulation of intracellular iron has further implications for multiple aspects of macrophage microbicidal activity (Recalcati et al., 2010). In one instance, intracellular iron maybe used to support the activity of hemoproteins that exert cytotoxic effects on pathogens, as illustrated for NOX2 or NOS2. However, intracellular iron also downregulates NOS2 transcription in response to interferon γ (IFN-γ) (Weiss et al., 1992), via a mechanism involving STAT inhibition (Xie et al., 1994).
Heme-iron-based resistance mechanisms directed against strictly or facultative intracellular pathogens include the reduction of macrophage intracellular heme-iron content, while increasing extracellular heme-iron (Figure 3). This defense strategy relies on mechanisms that suppress macrophage heme-iron import while promoting heme-iron export from phagolysosomes and eventually from macrophages (Figure 3).
Iron export from phagolysosomes is promoted, among other iron and heme transporters, by the Slc11a1 gene encoding for natural resistance associated macrophages protein 1 (NRAMP1)(Forbes and Gros, 2001) (Figure 3). Of note, C57BL/6 mice have a loss-of-function mutation in the Slc11a1 gene, associated with inability to clear some types of intracellular infections (Vidal et al., 1996), a phenotype also observed in humans with SLC11A1 mutations (Soares and Weiss, 2015). The putative role for recently discovered heme exporters, including HRG1 (Figure 3), feline leukemia virus subgroup C cellular receptor 1 (FLVCR1), ATP binding cassette subfamily G member 2 (ABCG2), also known as breast cancer resistance protein or the ATP binding cassette subfamily C member 5 (ABCC5), also known as multidrug resistance associated protein 5 (MRP5), in modulating resistance to intracellular pathogens is unclear at the moment.
Heme catabolism by HO-1 can modulate resistance against intracellular pathogens in macrophages such as illustrated for Mycobacteria (Regev et al., 2012; Silva-Gomes et al., 2013). While it is unclear how this process occurs, it is plausible that HO-1 restrains Mycobacteria’s access to intracellular heme. Another non-mutually exclusive explanation is that the salutary effect of HO-1 acts via the cytoprotective effect of CO (Brouard et al., 2000). This should protect macrophages from the cytotoxic effects of heme (Fortes et al., 2012), likely supporting granuloma formation and resistance to Mycobacteria (Regev et al., 2012; Roca and Ramakrishnan, 2013; Silva-Gomes et al., 2013).
In addition to restraining intracellular pathogens from accessing heme-iron, mechanisms reducing macrophage heme-iron content also regulate resistance mechanisms against intracellular pathogens (Soares and Weiss, 2015). Namely, lowering intracellular heme-iron in macrophages promotes the induction of NOS2, TNF, IL-6, IL-12 and histocompatibility (MHC) class II expression in response to IFNγ, an effect mediated in part by NRAMP1 (Fritsche et al., 2008) and involving the transcription factors HIF1α, nuclear factor for IL-6 (NF-IL6) and STAT family of transcription factors (Soares and Weiss, 2015).
Arguing further for a tight functional relationship between cellular heme-iron metabolism and the anti-microbial activity of macrophages is the recent finding on the mechanism via which NO• confers resistance to the facultative intracellular bacteria Salmonella. It is well established that NO• and ONNO− contribute critically to the microbicidal activity of macrophages against intracellular Salmonella, Leishmania or Mycobacteria (Nathan and Shiloh, 2000). In keeping with this notion, deletion of the Nos2 allele in mice is associated with exacerbated susceptibility to these pathogens (Nathan and Shiloh, 2000). There is, however, another mechanism via which NO• confers resistance to such pathogens that does not rely on the cytotoxic activity of ONNO-, but instead acts via an indirect mechanism that relies on the modulation of macrophage iron metabolism (Nairz et al., 2013). As discussed above, NO• and ONNO− can activate the transcription factor Nrf2, which induces the expression of FPN1 and reduces the intracellular iron content of macrophages (Nairz et al., 2013). Importantly, impaired resistance of Nos2-deficient macrophages against intracellular Salmonella (Nathan and Shiloh, 2000) relates functionally to increased macrophage intracellular iron content (Nairz et al., 2013). Whether this mechanism, through which NO• prevents pathogens from accessing iron, is operational against other intracellular or facultative intracellular pathogens remains to be established. In support of this notion, induction of NOS2 by IFNγ is associated with increased iron export from macrophages via LCN2 and with inhibition of cellular iron import by the transferrin receptor (Soares and Weiss, 2015).
Heme-iron metabolism in tissue damage control and disease tolerance
Immune-driven resistance mechanisms and nutritional immunity can be associated with more or less pronounced trade-offs eventually compromising host homeostasis (Kotas and Medzhitov, 2015; Soares et al., 2014). Therefore, these must be coupled to an additional defense strategy referred to as disease tolerance, which supports host homeostasis irrespectively of the host pathogen load (Medzhitov et al., 2012; Schneider and Ayres, 2008). As an example, when macrophages and other cells retain heme-iron intracellularly as a defense strategy against extracellular pathogens, tissue heme-iron overload can ensue (Soares and Weiss, 2015). Accumulation of heme in parenchyma cells is cytotoxic (Larsen et al., 2010) and can promote tissue damage, leading to organ dysfunction and eventually compromising host survival (Soares and Weiss, 2015). This pathologic effect is countered by tissue damage control mechanisms that converge at the level of heme catabolism by HO-1 (Ferreira et al., 2011; Gozzelino et al., 2010; Pamplona et al., 2007; Seixas et al., 2009) and are coupled to iron sequestration by ferritin (Gozzelino et al., 2010). These heme-iron regulatory genes confer disease tolerance to systemic infections, that is, maintain the functional outputs of parenchyma tissues and preserve homeostasis, without interfering with the host pathogen load (Soares et al., 2014). To what extent tissue resident macrophages are involved in this process remains to be established.
Concluding remarks
Although much has been written about macrophage biology and the role of these multi-functional cells in iron and heme metabolism, we attempted herein to flesh-out how iron and heme modulate macrophage function. Although some fundamental concepts, such as heme-iron recycling by macrophage cannot be ignored, we have instead chosen to deliberately elaborate such macrophage functions as a means to regulate cellular iron and heme, impacting on macrophage function(s). Ultimately, our goals are to blend the rich history of macrophage biology with the established concepts in heme-iron metabolism and emerging paradigms of heme homeostasis. Through our lens, the fields are ripe for picking.
Acknowledgments
The authors thank Jessica Thompson and Faouzi Brazza for insightful comments incorporated into the final version of the manuscript; and Ana Rita Carlos and Susana Ramos for careful review and editing of the manuscript (Instituto Gulbenkian de Ciência). MPS is supported by Fundação Calouste Gulbenkian, by Fundação para a Ciência e Tecnologia (PTDC/SAU TOX/116627/2010, HMSP-ICT/0022/2010) and by the European Community 7th Framework (ERC-2011-AdG 294709-DAMAGECONTROL). IH is supported by grants from the US National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. Journal of Biological Chemistry. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–4139. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- Ashino T, Yamanaka R, Yamamoto M, Shimokawa H, Sekikawa K, Iwakura Y, Shioda S, Numazawa S, Yoshida T. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol Immunol. 2008;45:2106–2115. doi: 10.1016/j.molimm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:1834–1839. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilban M, Bach FH, Otterbein SL, Ifedigbo E, de Costa d’Avila J, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, Otterbein LE. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Brady FO. Tryptophan 2,3-dioxygenase: a review of the roles of the heme and copper cofactors in catalysis. Bioinorg Chem. 1975;5:167–182. doi: 10.1016/s0006-3061(00)80058-7. [DOI] [PubMed] [Google Scholar]
- Brock JH. The physiology of lactoferrin. Biochem Cell Biol. 2002;80:1–6. doi: 10.1139/o01-212. [DOI] [PubMed] [Google Scholar]
- Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32:241–247. doi: 10.1016/j.it.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Carter EL, Gupta N, Ragsdale SW. High Affinity Heme Binding to a Heme Regulatory Motif on the Nuclear Receptor Rev-erbbeta Leads to its Degradation and Indirectly Regulates its Interaction with Nuclear Receptor Corepressor. J Biol Chem. 2015 doi: 10.1074/jbc.M115.670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- Du R, Ho B, Ding JL. Rapid reprogramming of haemoglobin structure-function exposes multiple dual-antimicrobial potencies. EMBO J. 2010;29:632–642. doi: 10.1038/emboj.2009.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton L, Carpentier I, Verhelst K, Staal J, Beyaert R. The multifaceted role of the E3 ubiquitin ligase HOIL-1: beyond linear ubiquitination. Immunol Rev. 2015;266:208–221. doi: 10.1111/imr.12307. [DOI] [PubMed] [Google Scholar]
- Fernandez PL, Dutra FF, Alves L, Figueiredo RT, Mourao-Sa D, Fortes GB, Bergstrand S, Lonn D, Cevallos RR, Pereira RM, et al. Heme amplifies the innate immune response to microbial molecules through spleen tyrosine kinase (Syk)-dependent reactive oxygen species generation. J Biol Chem. 2010;285:32844–32851. doi: 10.1074/jbc.M110.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Balla J, Jeney V, Balla G, Soares MP. A central role for free heme in the pathogenesis of severe malaria: the missing link? J Mol Med. 2008;86:1097–1111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Marguti I, Bechmann I, Jeney V, Chora A, Palha NR, Rebelo S, Henri A, Beuzard Y, Soares MP. Sickle Hemoglobin Confers Tolerance to Plasmodium Infection. Cell. 2011;145:398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- Fortes GB, Alves LS, de Oliveira R, Dutra FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher M, Golenbock D, et al. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood. 2012;119:2368–2375. doi: 10.1182/blood-2011-08-375303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche G, Nairz M, Werner ER, Barton HC, Weiss G. Nramp1-functionality increases iNOS expression via repression of IL-10 formation. Eur J Immunol. 2008;38:3060–3067. doi: 10.1002/eji.200838449. [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15:500–510. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Vitvitsky V, Gendelman HE, Banerjee R. Monocyte differentiation, activation, and mycobacterial killing are linked to transsulfuration-dependent redox metabolism. J Biol Chem. 2006;281:38712–38720. doi: 10.1074/jbc.M606235200. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754–1769. doi: 10.1089/ars.2013.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A, Olszanecki R, Gryglewski R, Schwartzman ML, Lianos E, Kappas A, Nasjletti A, Abraham NG. Regulation of cyclooxygenase by the heme-heme oxygenase system in microvessel endothelial cells. J Pharmacol Exp Ther. 2002;300:188–194. doi: 10.1124/jpet.300.1.188. [DOI] [PubMed] [Google Scholar]
- Haldar M, Kohyama M, So AY, Kc W, Wu X, Briseno CG, Satpathy AT, Kretzer NM, Arase H, Rajasekaran NS, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I, Dailey HA. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim Biophys Acta. 2012;1823:1617–1632. doi: 10.1016/j.bbamcr.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PM, Arosio P. Ferritins - Molecular Properties, Iron Storage Function and Cellular Regulation. Biochimica et Biophysica Acta - Bioenergetics. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Hoetzenecker W, Echtenacher B, Guenova E, Hoetzenecker K, Woelbing F, Bruck J, Teske A, Valtcheva N, Fuchs K, Kneilling M, et al. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat Med. 2012;18:128–134. doi: 10.1038/nm.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem. 2000;275:28028–28032. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kaasik K, Lee CC. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature. 2004;430:467–471. doi: 10.1038/nature02724. [DOI] [PubMed] [Google Scholar]
- Kaca W, Roth RI, Levin J. Hemoglobin, a newly recognized lipopolysaccharide (LPS)-binding protein that enhances LPS biological activity. J Biol Chem. 1994;269:25078–25084. [PubMed] [Google Scholar]
- Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kim KH, Jeong JY, Surh YJ, Kim KW. Expression of stress-response ATF3 is mediated by Nrf2 in astrocytes. Nucleic Acids Res. 2010;38:48–59. doi: 10.1093/nar/gkp865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleingardner JG, Bren KL. Biological significance and applications of heme c proteins and peptides. Acc Chem Res. 2015;48:1845–1852. doi: 10.1021/acs.accounts.5b00106. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, Frazier WA, Murphy TL, Murphy KM. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi T, Odani N, Okuyama T, Ichikawa A, Negishi M. Identification of a cis-regulatory element for delta 12-prostaglandin J2-induced expression of the rat heme oxygenase gene. J Biol Chem. 1995;270:21779–21784. doi: 10.1074/jbc.270.37.21779. [DOI] [PubMed] [Google Scholar]
- Korolnek T, Hamza I. Macrophages and iron trafficking at the birth and death of red cells. Blood. 2015;125:2893–2897. doi: 10.1182/blood-2014-12-567776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosman DJ. Redox cycling in iron uptake, efflux, and trafficking. J Biol Chem. 2010;285:26729–26735. doi: 10.1074/jbc.R110.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas ME, Medzhitov R. Homeostasis, Inflammation, and Disease Susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood. 2010;116:6054–6062. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Larochelle DA, Beaumont C, Torti SV, Torti FM. Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. Journal of Biological Chemistry. 1995;270:15285–15293. doi: 10.1074/jbc.270.25.15285. [DOI] [PubMed] [Google Scholar]
- Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Lee TS, Tsai HL, Chau LY. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Delta 12,14-prostaglandin J2. J Biol Chem. 2003;278:19325–19330. doi: 10.1074/jbc.M300498200. [DOI] [PubMed] [Google Scholar]
- Leimberg MJ, Prus E, Konijn AM, Fibach E. Macrophages function as a ferritin iron source for cultured human erythroid precursors. J Cell Biochem. 2008;103:1211–1218. doi: 10.1002/jcb.21499. [DOI] [PubMed] [Google Scholar]
- Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P. Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta. 2009;1787:864–872. doi: 10.1016/j.bbabio.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marro S, Chiabrando D, Messana E, Stolte J, Turco E, Tolosano E, Muckenthaler MU. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95:1261–1268. doi: 10.3324/haematol.2009.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU, et al. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med. 2013;210:855–873. doi: 10.1084/jem.20121946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. Journal of Clinical Investigation. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Sun J, Taketani S, Nakajima O, Nishitani C, Sassa S, Hayashi N, Yamamoto M, Shibahara S, Fujita H, Igarashi K. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. Embo J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2015;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares MP, Tao HL, Wysk M, Davis R, Flavell R, Choi AMK. Carbon monoxide mediates anti-inflammatory effects via the mitogen activated protein kinase pathway. Nature Medicine. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Palacios-Callender M, Quintero M, Hollis VS, Springett RJ, Moncada S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A. 2004;101:7630–7635. doi: 10.1073/pnas.0401723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28:548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S, Locati M, Marini A, Santambrogio P, Zaninotto F, De Pizzol M, Zammataro L, Girelli D, Cairo G. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40:824–835. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]
- Regev D, Surolia R, Karki S, Zolak J, Montes-Worboys A, Oliva O, Guroji P, Saini V, Steyn AJ, Agarwal A, Antony VB. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria. Lab Invest. 2012;92:1541–1552. doi: 10.1038/labinvest.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchetti GA, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004 doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50(Suppl):S29–34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth SA, Chen XL, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol. 2005;175:4408–4415. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Iwasaki K, Sugiyama H, Tsuji Y. Role of the tumor suppressor PTEN in antioxidant responsive element-mediated transcription and associated histone modifications. Mol Biol Cell. 2009;20:1606–1617. doi: 10.1091/mbc.E08-07-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, Rebelo S, Penido C, Smith NR, Coutinho A, Soares MP. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci U S A. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senge MO, MacGowan SA, O’Brien JM. Conformational control of cofactors in nature - the influence of protein-induced macrocycle distortion on the biological function of tetrapyrroles. Chem Commun (Camb) 2015;51:17031–17063. doi: 10.1039/c5cc06254c. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert I, Schodel J, Nairz M, Schatz V, Dettmer K, Dick C, Kalucka J, Franke K, Ehrenschwender M, Schley G, et al. Ferritin-Mediated Iron Sequestration Stabilizes Hypoxia-Inducible Factor-1alpha upon LPS Activation in the Presence of Ample Oxygen. Cell Rep. 2015;13:2048–2055. doi: 10.1016/j.celrep.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection. Infect Immun. 2013;81:2536–2545. doi: 10.1128/IAI.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Madzelan P, Banerjee R. Properties of an unusual heme cofactor in PLP-dependent cystathionine beta-synthase. Nat Prod Rep. 2007;24:631–639. doi: 10.1039/b604182p. [DOI] [PubMed] [Google Scholar]
- Soares MP, Bozza MT. Red alert: labile heme is an alarmin. Curr Opin Immunol. 2016;38:94–100. doi: 10.1016/j.coi.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Soares MP, Gozzelino R, Weis S. Tissue damage control in disease tolerance. Trends Immunol. 2014;35:483–494. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Soares MP, Weiss G. The Iron age of host-microbe interactions. EMBO Rep. 2015;16:1482–1500. doi: 10.15252/embr.201540558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc Natl Acad Sci U S A. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. Embo J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- Taille C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem. 2004;279:28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ouderaa FJ, Buytenhek M, Slikkerveer FJ, van Dorp DA. On the haemoprotein character of prostaglandin endoperoxide synthetase. Biochim Biophys Acta. 1979;572:29–42. doi: 10.1016/0005-2760(79)90197-8. [DOI] [PubMed] [Google Scholar]
- Vidal SM, Pinner E, Lepage P, Gauthier S, Gros P. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J Immunol. 1996;157:3559–3568. [PubMed] [Google Scholar]
- Warnatz HJ, Schmidt D, Manke T, Piccini I, Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M, et al. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J Biol Chem. 2011;286:23521–23532. doi: 10.1074/jbc.M111.220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124:4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- Weiss G, Fuchs D, Hausen A, Reibnegger G, Werner ER, Werner-Felmayer G, Wachter H. Iron modulates interferon-gamma effects in the human myelomonocytic cell line THP-1. Exp Hematol. 1992;20:605–610. [PubMed] [Google Scholar]
- Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205–215. doi: 10.1038/nrrheum.2012.183. [DOI] [PubMed] [Google Scholar]
- White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013;17:261–270. doi: 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KA, Marletta MA. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992;31:6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. Journal of Clinical Investigation. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Kim KD, Lucas A, Drahos KE, Santos CS, Mury SP, Capelluto DG, Finkielstein CV. A novel heme-regulatory motif mediates heme-dependent degradation of the circadian factor period 2. Mol Cell Biol. 2008;28:4697–4711. doi: 10.1128/MCB.00236-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acad Sci U S A. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, Igarashi K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol Cell Biol. 2007;27:6962–6971. doi: 10.1128/MCB.02415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Gillette DD, Li X, Zhang Z, Wen H. Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J Biol Chem. 2014;289:17020–17029. doi: 10.1074/jbc.M114.563114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerbraun BS, Chin BY, Bilban M, de Costa d’Avila J, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. Faseb J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]