Abstract
IMPORTANCE
X-linked retinitis pigmentosa is a severe inherited retinal degenerative disease with a frequency of 1 in 100 000 persons. Because no cure is available for this orphan disease and treatment options are limited, slowing of disease progression would be a meaningful outcome.
OBJECTIVE
To determine whether high-dose docosahexaenoic acid (DHA), an ω-3 polyunsaturated fatty acid, slows progression of X-linked retinitis pigmentosa measured by cone electroretinography (ERG).
DESIGN, SETTING, AND PARTICIPANTS
A 4-year, single-site, randomized, placebo-controlled, double-masked phase 2 clinical trial at a research center specializing in medical retina. Seventy-eight male patients diagnosed as having X-linked retinitis pigmentosa were randomized to DHA or placebo. Data were omitted for 2 patients with non–X-linked retinitis pigmentosa and 16 patients who were unable to follow protocol during the first year. The remaining participants were tested annually and composed a modified intent-to-treat cohort (DHA group, n = 33; placebo group, n = 27).
INTERVENTIONS
All participants received a multivitamin and were randomly assigned to oral DHA (30 mg/kg/d) or placebo.
MAIN OUTCOMES AND MEASURES
The primary outcome was the rate of loss of cone ERG function. Secondary outcomes were rod and maximal ERG amplitudes and cone ERG implicit times. Capsule counts and red blood cell DHA levels were assessed to monitor adherence.
RESULTS
Average (6-month to 4-year) red blood cell DHA levels were 4-fold higher in the DHA group than in the placebo group (P < .001). There was no difference between the DHA and placebo groups in the rate of cone ERG functional loss (0.028 vs 0.022 log µV/y, respectively; P = .30). No group differences were evident for change in rod ERG (P = .27), maximal ERG (P = .65), or cone implicit time (no change over 4 years). The rate of cone loss (ie, event rate) was markedly reduced compared with rates in previous studies. No severe treatment-emergent adverse events were found.
CONCLUSIONS AND RELEVANCE
Long-term DHA supplementation was not effective in slowing the loss of cone or rod ERG function associated with X-linked retinitis pigmentosa. Participant dropout and lower-than-expected disease event rate limited power to detect statistical significance. A larger sample size, longer trial, and attainment of a target blood DHA level (13%) would be desirable. While DHA supplementation at 30 mg/kg/d does not present serious adverse effects, routine monitoring of gastrointestinal tolerance is prudent.
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00100230
X-linked retinitis pigmentosa (XLRP) is an orphan retinal degenerative disease with a frequency in the population of approximately 1 in 100 000,1,2 resulting in about 3000 affected individuals in the United States. The clinical characteristics of XLRP include diminished visual acuity, progressive night blindness in early childhood due to loss of rod photoreceptor function, tunnel vision attributable to loss of cone and rod function beginning in the second or third decade of life, and concomitant appearance of retinal pigmentation in the fundus. Subsequent disease progression results in legal blindness. Although the disease has genetic origins, a persistent confounding observation is that many families with a history of retinitis pigmentosa display variations in disease severity and/or onset of symptoms despite having the same gene mutation.3,4 This inconsistency may be multifactorial and encompass additional genetic modifiers as well as environmental factors such as diet that may contribute to the dysfunction in visual processing.
Of particular significance to retinal function in XLRP is the ω-3 fatty acid docosahexaenoic acid (DHA; 22:6 ω-3), found highly enriched in cold-water fish. In humans, DHA is the most unsaturated fatty acid present in biological membranes and accounts for 1% to 5% of total fatty acids in many tissues. However, DHA accounts for 30% to 40% of fatty acids in phospholipids of the retina and is concentrated in the outer segments of rod and cone photoreceptors.5 It is found tightly associated with rhodopsin in rod outer segments,6 influences membrane fluidity and permeability,7 promotes photoreceptor differentiation,8 has antiapoptotic activity,9 and influences expression of genes associated with neurogenesis and apoptosis.10 The DHA derivative neuroprotectin 1 has neuroprotective properties including scavenging of free radicals and promoting retinal cell survival.9 Thus, optimizing the microenvironment of photoreceptor membranes may be beneficial in slowing progression in retinal degenerative diseases.
Reduced blood levels of DHA are found in many males with XLRP.11,12 Because measurement of retinal DHA in relatively healthy individuals with XLRP is unattainable, we infer that the correlation (r = 0.82) of retinal DHA with red blood cell (RBC) DHA found in nonhuman primates13 permits the use of RBCs as a surrogate index for retinal tissue levels. When adjusted for age, patients with XLRP with the lowest RBC DHA content tend to have lower rod (r = 0.57; P = .009)14 and cone (r = 0.81; P = .001)12 electroretinography (ERG) amplitudes. Substantive evidence that DHA is incorporated into the retina comes from studies in which infants given formulas enriched with DHA have improved retinal maturation compared with infants receiving little or no DHA in their diet.15
There is no cure for retinitis pigmentosa (including XLRP) and treatment options are limited. Vitamin A supplementation (15 000 IU/d) has a modest effect on the rate of cone ERG progession.16 In patients taking vitamin A, supplementation with 1200 mg of DHA per day showed no benefit over placebo on peripheral visual fields, cone ERG function, or visual acuity.17However, a post hoc analysis on a subset of patients who had not previously taken vitamin A suggested that the combination of vitamin A and DHA led to significant preservation of visual field sensitivity compared with vitamin A only.18
In a 4-year, placebo-controlled, randomized clinical trial, participants (ages 4–38 years) received 400 mg/d of DHA or placebo; however, owing to large variations in body weight, the dosage of DHA varied from 3 to 23 mg/kg/d.19 Supplementation with DHA increased the mean RBC DHA level from 2.7% to 6.9% and was associated with an average 25% reduction in the rate of cone ERG decline vs placebo (P = .12). An inverse correlation between blood DHA levels and loss of cone ERG function (r = −0.38; P = .01) suggested that an RBC DHA level of approximately 13% should minimize cone ERG loss. To attain this level, a DHA dosage of approximately 30 mg/kg/d is needed. Adverse events associated with this long-term DHA regimen were minimal20; however, this low dosage of DHA reduced plasma vitamin E levels by 15%, suggesting that the highly unsaturated nature of DHA may impede antioxidant potential. Thus, in the present study (Docosahexaenoic Acid in X-Linked Retinitis Pigmentosa [DHAX] trial), a minimal anti-oxidant status was maintained in all participants by provision of a basic multivitamin.
The objectives of the 4-year, placebo-controlled, randomized clinical DHAX trial were to use oral DHA supplementation in an attempt to increase RBC DHA levels to approximately 13% and to determine whether this level of supplementation slows the yearly loss of cone ERG function in patients with XLRP.
Methods
Study Population
The protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, Dallas. Eligible patients and/or parents of minors provided written informed consent. The use of DHA in this trial with minors was conducted under an Investigational New Drug approval from the US Food and Drug Administration. Study oversight was provided by a data and safety monitoring committee.
Male patients clinically diagnosed as having XLRP were recruited from the Southwest Eye Registry in Dallas, the Foundation Fighting Blindness database, and referrals from US and Canadian ophthalmologists. Telephone interviews or e-mails were exchanged with 216 relatives or individuals with retinal diseases. Medical and visual function records were reviewed for 128 patients, and 90 were invited to Dallas for evaluation (Figure 1). Participants (ages 7–31 years) meeting entry criteria were assigned to DHA (n = 41) or placebo (n = 37) using a computer-generated randomization schedule with varying block sizes. To maintain XLRP homogeneity, data were omitted for 2 participants determined to have mutations in genes not associated with XLRP. Sixteen participants were unable to follow protocol during the first year (eAppendix in Supplement). The remaining 60 participants were tested annually and composed a modified intent-to-treat (mITT) cohort (placebo, n = 27; DHA, n = 33). Participants who completed the trial and adhered to the protocol throughout it composed a prespecified per protocol cohort (placebo, n = 22; DHA, n = 29). Enrollment commenced August 5, 2005, and ended June 1, 2008; all annual visits ended on May 18, 2012. All testing personnel were masked to treatment assignment.
Figure 1.
Flowchart and Randomization in Docosahexaenoic Acid in X-Linked Retinitis Pigmentosa (DHAX) Trial
The modified intent-to-treat (mITT) cohort (n = 60) included data for trial participants completing at least the first-year annual visit. The per protocol cohort (n = 51) included participants following protocol and completing the 4-year trial. DHA indicates docosahexaenoic acid; XLRP, X-linked retinitis pigmentosa.
aOne due to apathy (12 months), 1 due to seeing white circles (6 months), 2 due to lost contact (12 and 10 months), 2 due to parents being too busy to travel to Dallas (12 and 8 months), 1 due to depression from continued vision loss (4 months), and 1 due to floaters and increased sensitivity to light (2 months).
bOne had a mutation in the choroideremia gene (CHD; REP-1) and the second had an autosomal recessive retinitis pigmentosa mutation (homozygous CNGB1 mutation). These mutations were identified after trial completion.
cOne moved to Iraq, 2 due to apathy (15 and 19.5 months), 1 ran away from home (38 months), and 1 due to being “terribly sick and sore when taking capsules” (35 months).
dThree lost to follow-up (0, 4, and 15 months), 1 due to apathy and difficulty in traveling to Dallas (12 months), 1 changed mind about participating and consumed no capsules (0 months), 1 due to apathy (9 months), 1 due to inconsistent bowel movements (8 months), and 1 due to dehydration and fatigue (5 months).
eOne lost to follow-up (21 months), 1 due to apathy (21 months), and 1 due to capsules possibly exacerbating inflammatory bowel disease (24 months).
fAdmitted in a posttrial interview to not taking capsules for most of the 4-year trial and was confirmed nonadherent by red blood cell DHA levels.
Intervention
DSM Nutritional Products supplied 500-mg gelatin capsules containing 200 mg of algal-derived DHA or corn/soy (placebo) triglycerides. Participants received 3 to 18 capsules per day to achieve a dosage of 30 mg/kg/d. The total amount of fat (oil) ranged from 1.5 to 9 g/d and the total DHA dosage ranged from 600 to 3600 mg/d. The DHA and placebo capsules were indistinguishable based on appearance, smell, or taste and contained vitamins E and C as antioxidants (12.5 mg each) and food-grade orange extract as flavoring (5.9 mg). A commercial multivitamin providing 100% of the recommended daily amounts of vitamins A, C, D, E, B6, and B12 was provided to all participants to maintain a minimal antioxidant status (multivitamin ingredients detailed in eAppendix in Supplement).
Clinical Evaluation
Clinical tests conducted annually included the primary outcome of cone ERG amplitudes to 31-Hz flicker stimuli and secondary ERG outcomes of rod and maximal ERG amplitudes and cone ERG implicit times. Blood DHA level and anthropometric measurements (ie, body weight, height, and body mass index) are also reported herein.
ERG Assessment
Full-field ERGs were obtained with a bipolar contact lens electrode and an Espion2 system using achromatic flashes and standards established by the International Society for Clinical Electrophysiology of Vision.21 The fundamental harmonic derived from fast Fourier transform22 spectral analysis of computer-averaged, light-adapted cone ERG amplitude to a 31-Hz flicker was the primary outcome measure. Cone b-wave implicit time was derived from the time between a test flash and corresponding b-wave peak. Rod amplitudes and maximal (mixed cone-rod) amplitudes were elicited from the dark-adapted eye.
Fatty Acid Analysis
Fatty acids in RBCs were analyzed by gas chromatography according to previously detailed procedures.23 Results are presented as relative weight percentage.
Treatment-Emergent Adverse Events
Participants self-reported health-related issues and treatment emergent adverse events (TEAEs) at annual visits and in quarterly diaries. Serious TEAEs were to be reported immediately (n = 0). Blood chemistry results were monitored annually for TEAEs according to the Division of AIDS criteria.24
Gene Mutation Analysis
Knowledge of XLRP gene mutations was not an entry criteria; thus, screening of participants was conducted during the course of the trial as an ancillary measure by colleagues at the Human Genetics Center, University of Texas Health Science Center, Houston, and at the University of Michigan Kellogg Eye Center, Ann Arbor. Methods for mutation analysis have been described.25,26
Sample Size Determination
The estimated mean (SE) rate of decline in cone ERG function in XLRP in the absence of treatment (ie, event rate) was 0.065 (0.007) log µV/y.19 Assuming a treatment effect of 40%, statistical significance for a 2-sided test using a significance level of .05 and 80% power would require 24 participants per group.27
Statistical Analysis
Data from all patients with XLRP completing at least 1 year of the trial were used in them ITT cohort analysis (n = 60). On trial termination, there was a preliminary analysis of data integrity and data lock followed by repeated-measures mixed-model regression analysis. Statistical significance was set at P < .05.
Results
Baseline Characteristics
Patients who received DHA supplementation were slightly older than patients in the placebo group (mean, 16.1 vs 14.9 years, respectively) and had marginally higher RBC DHA levels (Table 1). All other demographic and anthropometric measures were similar between groups.
Table 1.
Baseline Demographic Characteristics of Modified Intent-to-Treat Cohort
| Characteristic | Placebo (n = 27) |
DHA (n = 33) |
|---|---|---|
| Race, No. | ||
| White | 23 | 29 |
| Black | 0 | 1 |
| Other and mixed | 4 | 3 |
| Hispanic ethnicity, No. | 1 | 4 |
| Vitamin A intake >7500 IU/d from years 0 to 4, No. | ||
| Minorsa | 3 | 2 |
| Adults | 1 | 3 |
| Age, mean (SE) [range], y | 14.9 (1.1) [7–28] | 16.1 (1.4) [7–31] |
| <18 y, No. | 20 | 21 |
| ≥18 y, No. | 7 | 12 |
| Weight, mean (SE) [range], kg | 62 (6) [22–123] | 58 (5) [23–116] |
| Weight for age in minors only, mean (SE) [range], %a | 65 (6) [20–98]b | 67 (5) [30–98]c |
| Height, mean (SE) [range], cm | 161 (4) [122–194] | 159 (4) [122–191] |
| BMI, mean (SE) [range] | 23 (1) [15–39] | 22 (1) [15–34] |
| RBC DHA, mean (SE) [range], %d | 2.86 (0.10) [1.8–3.8] | 3.12 (0.15) [1.8–6.3]e |
| Mutations, No. | ||
| RPGR | 25 | 29 |
| RP2 | 1 | 3 |
| Unidentified | 1 | 1 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DHA, docosahexaenoic acid; RBC, red blood cell.
Minors are considered individuals younger than 18 years.
Sample size was 20.
Sample size was 21.
The mean (SE) RBC DHA normative value (in 29 age-matched individuals) was 2.91% (0.15%).
In a protocol deviation, 1 participant volunteered to limit his fish intake if enrolled in the trial.
RBC DHA Levels
For participants in the mITT DHA group, the mean (SE) RBC DHA level increased 3.3-fold from a baseline level of 3.12% (0.17%) to 10.10% (0.52%) by trial year 4 (P < .001) (Figure 2). At a 30-mg/kg/d dosage, RBC DHA levels reached a plateau within the first 6 months. For participants in the placebo group, RBC DHA levels at baseline and year 4 were 2.85% and 2.82%, respectively (P = .82), indicating that participants did not modify their dietary behavior. Overall, the 6-month to 4-year average increase in the RBC DHA level was 4-fold higher in the DHA group than in the placebo group (mean [SE], 10.89% [0.32%] vs 2.71% [0.13%], respectively;P < .001). The mean (SE) adherence to protocol based on capsule count monitoring was 84.6% (3.4%) for the placebo group and 89.4% (1.9%) for the DHA group (P = .19).
Figure 2.
Red Blood Cell (RBC) Docosahexaenoic Acid (DHA) Levels as a Function of Treatment Intervention
Mean RBC DHA level as a percentage of total fatty acids vs time receiving placebo or DHA supplementation for participants in the modified intent-to-treat cohort. Error bars indicate standard error; dashed line, mean RBC DHA level (mean [SE], 2.91% [0.15%]) from 29 age-matched individuals with normal visual function; and shaded area, 95% confidence interval.
Primary Outcome Measure
A main effect of time was identified for light-adapted cone 31-Hz ERG amplitudes (P < .001), with the XLRP population showing significant progression. A main effect of DHA treatment assignment was not identified (P = .30) (Table 2) and no interaction between assignment group and year was evident (P = .62).
Table 2.
Baseline Characteristics and Yearly Rates of Change in ERG Outcome Measures for Modified Intent-to-Treat Cohorta
| Outcome Measure | Baseline | Yearly Rate of Change |
P Value for Effect of Treatment |
||
|---|---|---|---|---|---|
| Placebo (n = 27) |
DHA (n = 33) |
Placebo (n = 27) |
DHA (n = 33) |
||
| Fundamental harmonic of FFT LA 31-Hz cone ERG amplitudeb | |||||
| Log µV, mean (SE) [range] | 0.693 (0.084) [−0.32 to 1.33] | 0.761 (0.093) [−0.51 to 1.53] | −0.022 (0.002) | −0.028 (0.001) | .30 |
| µV, mean (SE) | 7.1 (1.2) | 10.5 (1.2) | −0.95 (1.00) | −0.94 (1.00) | |
| Annual % loss | 4.9 | 6.2 | |||
| Rod ERG amplitudeb,c | |||||
| Log µV, mean (SE) [range] | 0.828 (0.080) [0.500 to 2.012] | 0.813 (0.082) [0.477 to 2.105] | −0.023 (0.001) | −0.010 (0.001) | .27 |
| µV, mean (SE) | 6.73 (1.20) | 6.50 (1.21) | −0.95 (1.00) | −0.98 (1.00) | |
| Annual % loss | 6.0 | 2.3 | |||
| Maximal ERG amplitudeb | |||||
| Log µV, mean (SE) [range] | 1.253 (0.094) [0.362 to 2.359] | 1.300 (0.089) [0.491 to 2.359] | −0.036 (0.001) | −0.042 (0.001) | .65 |
| µV, mean (SE) | 17.8 (1.2) | 20.0 (1.2) | −0.92 (1.00) | −0.91 (1.00) | |
| Annual % loss | 8.0 | 9.2 | |||
| Cone ERG implicit time, mean (SE) [range], ms | 38.7 (0.9) [25 to 46] | 38.9 (0.7) [30 to 46] | 0.12 (0.02) [no change over 4 y] | .77 | |
Abbreviations: DHA, docosahexaenoic acid; ERG; electroretinography; FFT, fast Fourier transform; LA, light-adapted.
The mean (SE) or lower limit normative values28 (n = 229) are as follows for the outcome measures: mean (SE) cone ERG amplitude, 63.1 (1.0) µV or 1.80 (0.01) log µV; mean (SE) rod ERG amplitude, 134.9 (1.0) µV or 2.13 (0.01) log µV; lower-limit normative value for maximal ERG amplitude, greater than 190 µV or greater than 2.28 log µV; and mean (SE) cone ERG implicit time, 29.2 (0.1) milliseconds.
The ERG amplitudes were obtained in microvolts and converted to log microvolts for statistical analysis.
Includes data sets with nondetectable amplitudes (ie, ≤3.0 µV).
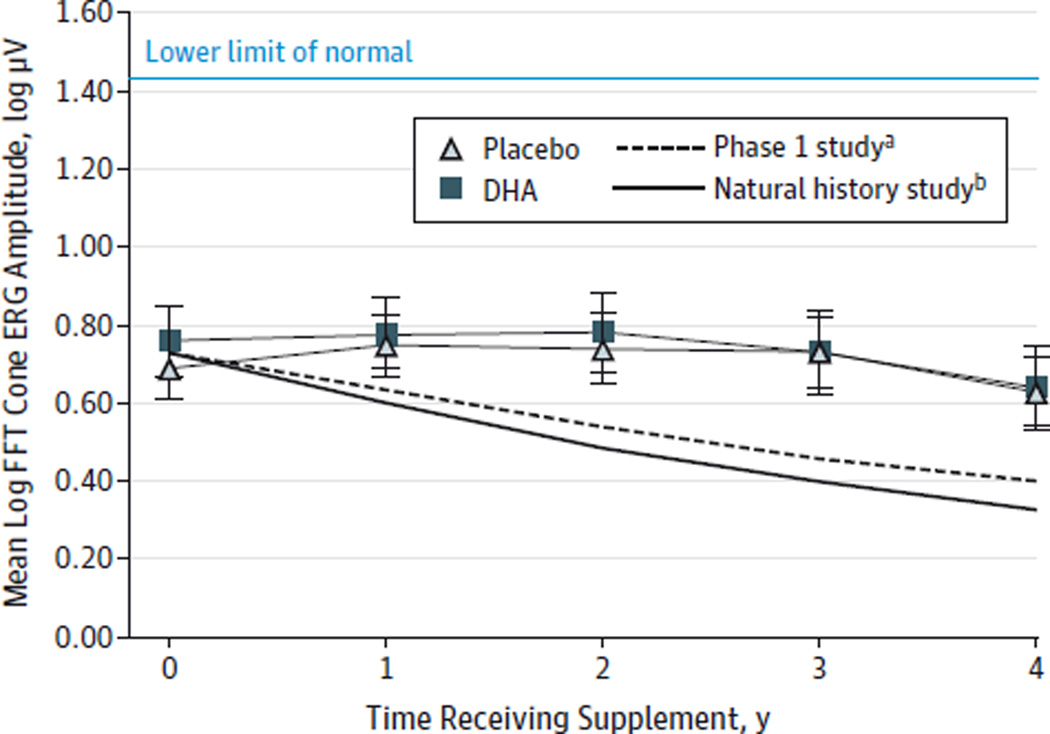
As shown in Figure 3, the rate of cone ERG loss was low in both groups during the 4-year study period. The rate of cone loss in the placebo group (0.022 log µV/y; 4.9%) was substantially less than the 14% and 18% rates found in a previous phase 1 study19 and a natural history study.28
Figure 3.
Fast Fourier Transform (FFT) Cone Electroretinography (ERG) 31-Hz Amplitudes as a Function of Time for Participants in the Modified Intent-to-Treat Cohort
Mean log µV values for each trial year in participants receiving placebo or docosahexaenoic acid (DHA) supplementation. For placebo vs DHA groups, P = .58 at year 1; P = .34 at year 2; P = .21 at year 3; and P = .34 at year 4. The lower limit of normal FFT cone amplitudes is 1.43 log µV (27.0 µV). Error bars indicate standard error.
aThere was a 14% event rate for the placebo group in the phase 1 X-linked retinitis pigmentosa clinical trial.19
bThere was an 18% event rate for patients with X-linked retinitis pigmentosa in the natural history study.28
Secondary ERG Functions
No difference between the placebo and DHA groups was evident for the rates of rod or maximal ERG functional loss in the mITT cohort (P = .27 and .65, respectively) (Table 2). Similarly, cone ERG flicker implicit time demonstrated no differences with assignment group or time (Table 2).
Supplemental Vitamin A Intake
Four participants in the placebo group and 5 in the DHA group had a daily intake of 7500 to 15 000 IU of vitamin A throughout the 4-year trial (Table 1). Not surprisingly with these small numbers, the rates of change in cone ERG amplitude and secondary ERG outcomes were similar to participants taking vitamin A or not in both the placebo group (P > .12) and the DHA group (P > .12).
Associations Between ERG Function and RBC DHA Level
No association was evident between the rate of change in cone ERG amplitude and RBC DHA level (Pearson correlation r = 0.19; P = .18). The RBC DHA level was not correlated with rod and maximal ERG amplitudes or cone implicit times (P > .10).
Per Protocol Analysis
Participants in the DHA group of the per protocol cohort (n = 29) had a mean (SE) 6-month to 4-year RBC DHA level of 11.1% (0.2%) (range, 8.9%–13.9%). The distribution of average RBC DHA levels among these participants was as follows: 8.0% to 9.9% DHA, n = 5; 10.0% to 10.9% DHA, n = 9; 11.0% to 11.9% DHA, n = 7; 12.0% to 12.9% DHA, n = 7; and 13.0% to 13.9% DHA, n = 1. The level of DHA in RBCs of the placebo groups for the per protocol and mITT cohorts were identical. Mixed-model analysis of the annual rates of change in cone, rod, or maximal ERG amplitudes or cone ERG implicit times in the per protocol cohort did not demonstrate DHA vs placebo group differences or show marked variation from those in the mITT cohort (data not shown).
Genetic Homogeneity
Mutations in RPGR were found in 25 participants (93%) in the placebo group and 29 participants (88%) in the DHA group (Table 1). The frequencies of RPGR and RP2 in our population are in agreement with published literature.29,30 No DHA treatment effect was found for cone ERG flicker responses for only participants with mutations in RPGR (P = .19).
Treatment-Emergent Adverse Events
Of 78 enrolled participants, 51 had no TEAEs. Twenty-seven participants had a total of 42 related or possibly related TEAEs (22 in the DHA group and 20 in the placebo group). Self-reported and blood chemistry TEAEs were sporadic with no identifiable trends; the exception was 1 participant with a family history of Crohn disease who was sensitive to DHA supplementation. No severe TEAEs requiring hospitalization were reported in the 4-year trial interval. Further details addressing safety and metabolism in the DHAX trial are available (D.K.H.-W.,D.G.B., G.E.F., R.S.,N.S.P.,A.T.,D.R.H., unpublished data, August 2005 to May 2012).
Discussion
No effect was found on the annual rate of decline in the primary outcome measure, cone ERG amplitude, by 30 mg/kg/d of DHA supplementation during the 4-year DHAX trial (P = .30) despite greater than a 3-fold elevation in the RBC DHA level. The annual rate of loss of cone ERG function, the event rate, in this cohort of participants was lower than that found in previous studies demonstrating the superiority of a placebo-control trial design over a historical-control trial design. In a 4-year natural history study,28 the loss of cone ERG function in patients with XLRP averaged 0.085 log µV/y (18% loss per year). In a 4-year phase 1 clinical trial,19 cone ERG amplitude in the placebo group decreased by 0.065 log µV/y (14% loss per year). In the present trial, the event rate in the mITT placebo group was only 0.022 log µV/y (4.9% loss per year) (Table 2). Participants receiving DHA had a rate of cone loss of 0.028 log µV/y (6.2% loss per year), which by comparison with previous studies would have represented a marked reduction in ERG functional loss. Similarly, the event rate for rod ERG function was reduced in the current trial. In our phase 1 trial, participants in the placebo arm lost 0.079 log µV/y (16.6% loss per year) compared with a loss of 0.023 log µV/y (6.0% loss per year) in the DHAX trial. It is not clear why the event rates in the placebo group were lower than in previous studies. It was not due to the use of fast Fourier transform to derive the fundamental frequency, since the time-domain measure of 31-Hz amplitude similarly declined (0.024 log µV/y). Systematic changes in equipment are unlikely to be the cause as calibrations were performed monthly. Genetic makeup may affect the rate of progression because a retrospective analysis in patients with RPGR mutations reported a mean rate of decline of 7.1% for cone ERG amplitude.31 Age of participants with XLRP is unlikely to be responsible because the age range is similar to that in previous studies. It seems unlikely that ingredients in the corn/soy oil placebo influenced the event rate. However, all participants received a multivitamin to support systemic antioxidant levels; this supplement may have slowed the rate of loss relative to previous studies. It is also possible that improvements in general health and awareness of diet during the past 20 years have influenced the rate of progression.
Trial participants receiving DHA at a dosage of 30 mg/kg/d had an average 4-fold elevation in the RBC DHA level compared with those receiving placebo. For a single participant, the highest average RBC DHA level was 13.9%. The target of 30 mg/kg/d falls within the range of DHA that infants receive in breast milk. Worldwide, breastfeeding provides between 3 and 84 mg/kg/d of DHA depending on maternal diet.32–34 In other studies, the mean (SE) RBC DHA level increased over 6 months from 2.8% (0.1%) to 11.1% (0.3%) in children with cystic fibrosis who received DHA supplementation at a dosage of 50 mg/kg/d; the RBC DHA level in 2 patients reached 13.1%.35 In children with peroxisomal diseases receiving DHA at a dosage of 100 mg/kg/d, the mean (SE) plasma DHA level increased 5-fold in 1 year from 5.3 (2.7) µg/mL to 26.3 (7.4) µg/mL.36 It remains uncertain whether a maximum ceiling for blood DHA level was attained in the DHAX trial or in recent DHA supplementation trials. No correlations were found between RBC DHA level and rates of ERG decline.
In previous trials in hereditary retinal disease, 14 children with long-chain 3-hydroxyacyl–coenzyme A dehydrogenase deficiency receiving DHA at a dosage of 65 to 130 mg/d for at least 2 years had moderate benefits in visual acuity and retinal function as measured by visual evoked potentials and ERG, respectively.37 Children with peroxisomal disorders (n = 23) receiving DHA at a dosage of 200 mg/d had improvements in nystagmus, visual acuity, and ERG function.38 In a placebo-controlled randomized trial of patients with peroxisomal disorders receiving placebo (n = 11) or DHA at a dosage of 100 mg/kg/d (n = 8) for 1 year, no group differences in ERG function were found.36 In 20 patients with Stargardt macular degeneration receiving DHA at a dosage of 840 mg/d for 6 months, no improvements in visual acuity or multifocal ERG amplitudes were found.39 In a crossover study of DHA at a dosage of 20 mg/kg/d, 8 patients with Best macular dystrophy had 2- to 3-fold elevations in plasma DHA level and improvements in multifocal ERGs.40
In a phase 1 trial in which patients with XLRP received DHA at a dosage of 400 mg/d, biochemical safety was not compromised by supplementation for 4 years.20 Similarly, adults with retinitis pigmentosa receiving DHA at a dosage of 1200 mg/d for 4 years displayed no adverse effects.17 The occurrence of TEAEs in the DHAX trial was consistent with these safety outcomes, although it would be prudent to closely monitor patients choosing to take high doses of DHA, particularly those prone to or with a family history of gastrointestinal problems.
The strengths of the DHAX trial include genetic homogeneity, use of a single study site, a long intervention period, RBC DHA monitoring of compliance, and close adherence to protocol. A lower-than-expected event rate and an underpowered trial due to participant dropout were primary trial limitations. A larger sample size, longer trial, and attainment of target blood DHA levels of 13% would have been desirable.
Conclusions
Elevated DHA levels did not effectively slow disease progression as measured by cone and rod ERG function. However, the event rate for this trial was unexpectedly less than predicted from previous studies in XLRP. Although there is a strong rationale for DHA supplementation, the DHAX trial along with previous trials using ω-3 fatty acids have yet to demonstrate compelling evidence of efficacy.
Supplementary Material
Acknowledgments
Funding/Support: Dr Hoffman was supported by grant 5RO1FD002543 from the Orphan Products Development program of the US Food and Drug Administration. Dr Birch was supported by grant C-TX02-0704-0274 from the Foundation Fighting Blindness. DSM Nutritional Products provided DHA and placebo capsules gratis.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DSM Nutritional Products, which provided DHA and placebo capsules gratis, supports a separate infant nutrition clinical trial currently in progress at the Retina Foundation of the Southwest.
We thank retinal specialists and ophthalmologists across the United States for referrals of patients with this rare orphan disease. Robert Koenekoop, MD, McGill University Health Centre, Montreal, Quebec, Canada, and his staff provided recruitment efforts and assisted with Canadian customs issues; they did not receive compensation for these contributions. Hemaxi Patel, MS, Retina Foundation of the Southwest, Dallas, Texas, assisted with visual function testing and provided attention to patient care; she received compensation as an employee.
Footnotes
Author Contributions: Dr Hoffman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hoffman, Hughbanks-Wheaton, Birch.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Hoffman, Hughbanks-Wheaton, Birch.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Hoffman, Pearson.
Obtained funding: Hoffman, Birch.
Administrative, technical, or material support: Hoffman, Hughbanks-Wheaton, Pearson, Takacs, Klein, Locke.
Study supervision: Hoffman, Hughbanks-Wheaton, Birch.
Conflict of Interest: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Disclosures: No other disclosures were reported.
Additional Contributions: The following were members of the data and safety monitoring committee: Gerald Fishman, MD (chair), Lighthouse for the Blind and Visually Impaired, Chicago, Illinois; Norman Salem, PhD (until 2007), National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health until 2007, then DSM Nutritional Products, Columbia, Maryland; Barbara Philippon, RN, MS, independent consultant, Dallas, Texas; Kenneth Alexander, PhD, University of Illinois at Chicago, Chicago; Robert J. Anderson, PhD, University of Illinois at Chicago, Chicago; Martha Neuringer, PhD, Oregon Health & Science University, Beaverton; and John Paul SanGiovanni, ScD, National Eye Institute, National Institutes of Health, Bethesda, Maryland. Committee members received annual compensation from the US Food and Drug Administration (Drs Fishman, Alexander, Anderson, and Neuringer and Ms Philippon) or the Foundation Fighting Blindness (Drs Salem and SanGiovanni).
Supplemental content at jamaophthalmology.com
REFERENCES
- 1.Bunker CH, Berson EL, Bromley WC, Hayes RP, Roderick TH. Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol. 1984;97(3):357–365. doi: 10.1016/0002-9394(84)90636-6. [DOI] [PubMed] [Google Scholar]
- 2.Wheaton DH, Daiger SP, Birch DG. The Southwest Eye Registry: distribution of disease types and mutations. In: Anderson RA, LaVail M, Hollyfield JG, editors. New Insights Into Retinal Degenerative Diseases. New York, NY: Klewer-Plenum; 2001. pp. 339–345. [Google Scholar]
- 3.Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Dryja TP. Ocular findings in patients with autosomal dominant retinitis pigmentosa and rhodopsin, proline-347-leucine. Am J Ophthalmol. 1991;111(5):614–623. doi: 10.1016/s0002-9394(14)73708-0. [DOI] [PubMed] [Google Scholar]
- 4.Ayuso C, Trujillo MJ, Robledo M, et al. Novel rhodopsin mutation in an autosomal dominant retinitis pigmentosa family: phenotypic variation in both heterozygote and homozygote Val137Met mutant patients. Hum Genet. 1996;98(1):51–54. doi: 10.1007/s004390050158. [DOI] [PubMed] [Google Scholar]
- 5.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22(2):79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 6.de Turco EB, Jackson FR, Parkins N, Gordon WC. Strong association of unesterified [3H]docosahexaenoic acid and [3H-docosahexaenoyl]phosphatidate to rhodopsin during in vivo labeling of frog retinal rod outer segments. Neurochem Res. 2000;25(5):695–703. doi: 10.1023/a:1007571305987. [DOI] [PubMed] [Google Scholar]
- 7.Dratz EA, Deese AJ. The role of docosahexaenoic acid (22: 6 ω-3) in biological membranes: examples from photoreceptors and model membrane bilayers. In: Simopoulos AP, Kifer RR, Martin RE, editors. Health Effects of Polyunsaturated Fatty Acids in Seafoods. New York, NY: Academy Press; 1986. pp. 319–351. [Google Scholar]
- 8.Rotstein NP, Aveldaño MI, Barrantes FJ, Politi LE. Docosahexaenoic acid is required for the survival of rat retinal photoreceptors in vitro. J Neurochem. 1996;66(5):1851–1859. doi: 10.1046/j.1471-4159.1996.66051851.x. [DOI] [PubMed] [Google Scholar]
- 9.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31(8):321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas CV, Martínez JI, Flores I, Hoffman DR, Uauy R. Gene expression analysis in human fetal retinal explants treated with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2003;44(7):3170–3177. doi: 10.1167/iovs.02-1138. [DOI] [PubMed] [Google Scholar]
- 11.Gong J, Rosner B, Rees DG, Berson EL, Weigel-DiFranco CA, Schaefer EJ. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1992;33(9):2596–2602. [PubMed] [Google Scholar]
- 12.Hoffman DR, Birch DG. Docosahexaenoic acid in red blood cells of patients with X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1995;36(6):1009–1018. [PubMed] [Google Scholar]
- 13.Sarkadi-Nagy E, Wijendran V, Diau GY, et al. The influence of prematurity and long chain polyunsaturate supplementation in 4-week adjusted age baboon neonate brain and related tissues. Pediatr Res. 2003;54(2):244–252. doi: 10.1203/01.PDR.0000072795.38990.F2. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman DR, Birch DG. Omega 3 fatty acid status in patients with retinitis pigmentosa. World Rev Nutr Diet. 1998;83:52–60. doi: 10.1159/000059653. [DOI] [PubMed] [Google Scholar]
- 15.Birch DG, Birch EE, Hoffman DR, Uauy RD. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. Invest Ophthalmol Vis Sci. 1992;33(8):2365–2376. [PubMed] [Google Scholar]
- 16.Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(6):761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 17.Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch Ophthalmol. 2004;122(9):1297–1305. doi: 10.1001/archopht.122.9.1297. [DOI] [PubMed] [Google Scholar]
- 18.Berson EL, Rosner B, Sandberg MA, et al. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment: subgroup analyses. Arch Ophthalmol. 2004;122(9):1306–1314. doi: 10.1001/archopht.122.9.1306. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman DR, Locke KG, Wheaton DH, Fish GE, Spencer R, Birch DG. A randomized, placebo-controlled clinical trial of docosahexaenoic acid supplementation for X-linked retinitis pigmentosa. Am J Ophthalmol. 2004;137(4):704–718. doi: 10.1016/j.ajo.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 20.Wheaton DH, Hoffman DR, Locke KG, Watkins RB, Birch DG. Biological safety assessment of docosahexaenoic acid supplementation in a randomized clinical trial for X-linked retinitis pigmentosa. Arch Ophthalmol. 2003;121(9):1269–1278. doi: 10.1001/archopht.121.9.1269. [DOI] [PubMed] [Google Scholar]
- 21.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. International Society for Clinical Electrophysiology of Vision. Standard for clinical electroretinography (2004 update) Doc Ophthalmol. 2004;108(2):107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 22.Alexander KR, Raghuram A, McAnany JJ. Comparison of spectral measures of period doubling in the cone flicker electroretinogram. Doc Ophthalmol. 2008;117(3):197–203. doi: 10.1007/s10633-008-9123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman DR, Birch EE, Birch DG, et al. Impact of early dietary intake and blood lipid composition of long-chain polyunsaturated fatty acids on later visual development. J Pediatr Gastroenterol Nutr. 2000;31(5):540–553. doi: 10.1097/00005176-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Division of AIDS, National Institutes of Health. Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0. [Accessed April 3, 2014];2004 Dec; clarification August 2009. http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf. [Google Scholar]
- 25.Churchill JD, Bowne SJ, Sullivan LS, et al. Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54(2):1411–1416. doi: 10.1167/iovs.12-11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahim AT, Bowne SJ, Sullivan LS, et al. Polymorphic variation of RPGRIP1L and IQCB1 as modifiers of X-linked retinitis pigmentosa caused by mutations in RPGR. Adv Exp Med Biol. 2012;723:313–320. doi: 10.1007/978-1-4614-0631-0_41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosner B. Fundamentals of Biostatistics. 3rd. Boston, MA: Duxbury Press; 1990. pp. 419–430. [Google Scholar]
- 28.Birch DG, Anderson JL, Fish GE. Yearly rates of rod and cone functional loss in retinitis pigmentosa and cone-rod dystrophy. Ophthalmology. 1999;106(2):258–268. doi: 10.1016/S0161-6420(99)90064-7. [DOI] [PubMed] [Google Scholar]
- 29.Vervoort R, Lennon A, Bird AC, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet. 2000;25(4):462–466. doi: 10.1038/78182. [DOI] [PubMed] [Google Scholar]
- 30.Breuer DK, Yashar BM, Filippova E, et al. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am J Hum Genet. 2002;70(6):1545–1554. doi: 10.1086/340848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007;48(3):1298–1304. doi: 10.1167/iovs.06-0971. [DOI] [PubMed] [Google Scholar]
- 32.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85(6):1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman DR, Theuer RC, Castañeda YS, et al. Maturation of visual acuity is accelerated in breast-fed term infants fed baby food containing DHA-enriched egg yolk. J Nutr. 2004;134(9):2307–2313. doi: 10.1093/jn/134.9.2307. [DOI] [PubMed] [Google Scholar]
- 34.Lapillonne A, Groh-Wargo S, Gonzalez CHL, Uauy R. Lipid needs of preterm infants: updated recommendations. J Pediatr. 2013;162((3)(suppl)):S37–S47. doi: 10.1016/j.jpeds.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd-Still JD, Powers CA, Hoffman DR, et al. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition. 2006;22(1):36–46. doi: 10.1016/j.nut.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Paker AM, Sunness JS, Brereton NH, et al. Docosahexaenoic acid therapy in peroxisomal diseases: results of a double-blind, randomized trial. Neurology. 2010;75(9):826–830. doi: 10.1212/WNL.0b013e3181f07061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillingham MB, Weleber RG, Neuringer M, et al. Effect of optimal dietary therapy upon visual function in children with long-chain 3-hydroxyacyl CoA dehydrogenase and trifunctional protein deficiency. Mol Genet Metab. 2005;86(1–2):124–133. doi: 10.1016/j.ymgme.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noguer MT, Martinez M. Visual follow-up in peroxisomal-disorder patients treated with docosahexaenoic acid ethyl ester. Invest Ophthalmol Vis Sci. 2010;51(4):2277–2285. doi: 10.1167/iovs.09-4020. [DOI] [PubMed] [Google Scholar]
- 39.Querques G, Benlian P, Chanu B, et al. DHA supplementation for late onset Stargardt disease: NAT-3 study. Clin Ophthalmol. 2010;4:575–580. doi: 10.2147/opth.s10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee TK, Clandinin MT, Hèbert M, MacDonald IM. Effect of docosahexaenoic acid supplementation on the macular function of patients with Best vitelliform macular dystrophy: randomized clinical trial. Can J Ophthalmol. 2010;45(5):514–519. doi: 10.3129/i10-028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.