Highlights
-
•
The uncinate fasciculus (UF) is a limbic fiber tract.
-
•
It has a protracted developmental timecourse.
-
•
It has been implicated in several developmental disorders including conduct disorder and autism.
-
•
UF perturbation may affect memory retrieval, linking reward/punishments to memory.
Keywords: Diffusion tensor imaging, White matter, Orbitofrontal cortex, Autism, Conduct disorder, Anti-social personality disorder
Abstract
The uncinate fasciculus (UF) is a long-range white matter tract that connects limbic regions in the temporal lobe to the frontal lobe. The UF is one of the latest developing tracts, and continues maturing into the third decade of life. As such, individual differences in the maturational profile of the UF may serve to explain differences in behavior. Indeed, atypical macrostructure and microstructure of the UF have been reported in numerous studies of individuals with developmental and psychiatric disorders such as social deprivation and maltreatment, autism spectrum disorders, conduct disorder, risk taking, and substance abuse. The present review evaluates what we currently know about the UF's developmental trajectory and reviews the literature relating UF abnormalities to specific disorders. Additionally, we take a dimensional approach and critically examine symptoms and behavioral impairments that have been demonstrated to cluster with UF aberrations, in an effort to relate these impairments to our speculations regarding the functionality of the UF. We suggest that developmental disorders with core problems relating to memory retrieval, reward and valuation computation, and impulsive decision making may be linked to aberrations in uncinate microstructure.
The human brain follows a distinct spatial and temporal pattern of maturation that begins with phylogenetically older posterior and inferior regions and then progressively extends to more anterior and superior regions (for reviews, see Durston et al., 2001, Lenroot and Giedd, 2006, Toga et al., 2006). Postmortem (Huttenlocher et al., 1994, Yakovlel and Lecours, 1967), MRI (Courchesne et al., 2000, Durston et al., 2001, Giedd et al., 1999a, Giedd et al., 1999b, Gogtay et al., 2004, Good et al., 2001, Matsuzawa et al., 2001, Paus et al., 1999, Sowell et al., 2003), and diffusion tensor imaging (DTI) studies (Barnea-Goraly et al., 2005, Bava et al., 2010, Giorgio et al., 2008, Hasan et al., 2007, Lebel and Beaulieu, 2011, Lebel et al., 2012, McKinstry et al., 2002, Mukherjee and McKinstry, 2006, Mukherjee et al., 2001, Schmithorst and Yuan, 2010, Schneider et al., 2004, Snook et al., 2007, Yap et al., 2013) suggest that concurrent changes in gray and white matter are two important events that follow distinct developmental trajectories that significantly contribute to brain maturation.
The increase in global gray matter volume during development follows an inverted U-shaped curve that increases and peaks during adolescence and then decreases until early adulthood, whereas global white matter increases steadily throughout childhood into adulthood (Giedd et al., 1999a). If maturational changes are examined over the course of the entire lifespan, global white matter volume also follows an inverted U-shaped trajectory, with white matter volume reaching its peak around 40 years of age (Hasan et al., 2007, Lebel et al., 2012). It is believed that the function of cortical regions is based on the intrinsic properties and extrinsic pattern of white matter input and output and that the information transmission properties of a given white matter tract can be predicted by the functions of the cortical regions it connects (Passingham et al., 2002, Van Essen and Maunsell, 1983). Although the function of the uncinate fasciculus (UF) is still largely unclear, its location and connectivity often associate it with the limbic system and its functions (e.g., emotion, episodic memory, etc.), making it a likely candidate for disruption in disorders affecting personality, emotion, and episodic memory. The extended development of this white matter tract into the third decade of life might also make it more susceptible to disruptions in function and could help explain why the UF has been implicated in several developmental and psychiatric disorders.
We previously reviewed the human adult and non-human primate literature on the UF (Von Der Heide et al., 2013). The purpose of the present literature review is to integrate current knowledge about the UF's developmental trajectory with the relevant literature on developmental disorders, while placing them in the theoretical context of our findings on the adult UF. We believe that this review will be of interest to both clinicians and cognitive scientists, and we aim to link the UF to clinical disorders, as well as to normal cognition, as we believe that one informs the other.
1. Anatomy and maturation of the uncinate fasciculus
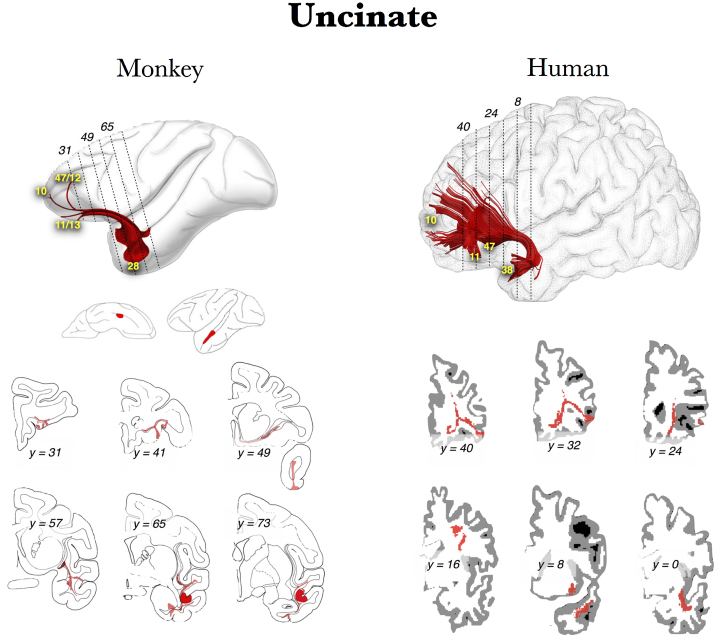
We described the anatomy of the uncinate fasciculus previously (Von Der Heide et al., 2013). In brief, it is a long-range association pathway that creates a monosynaptic pathway between the anterior temporal lobes (BA 38 including perirhinal cortex and portions of the anterior parahippocampal gyrus) and amygdala to the lateral orbitofrontal cortex (OFC; BA 11, 47/12) and BA 10. It has a distinctive hook shape, arcing around the Sylvian fissure into the frontal lobe (see Fig. 1; Schmahmann and Pandya, 2006, Thiebaut de Schotten et al., 2012). It is frequently damaged in epilepsy resection surgery, as well as blunt-force trauma affecting the frontal lobes.
Fig. 1.
Reconstructions of the uncinate fasciculus: comparison between post-mortem axonal tracing in monkey and human in vivo Spherical Deconvolution (SD) tractography suggests simian-human similarities (Thiebaut de Schotten et al., 2012). Used with permission.
The UF is one of the last white matter tracts to reach its maturational peak, with its developmental time course extending throughout adolescence, young adulthood, and peaking beyond the age of 30 (see Fig. 2; Lebel et al., 2012, Lebel et al., 2008). Although the basic characteristics of UF macrostructure (e.g., volume, length, shape) have been reported in adult studies (Hasan et al., 2009, Malykhin et al., 2008, Taoka et al., 2006, Wakana et al., 2007), little is known about the trajectories of these characteristics across development. Until recently, when developmental DTI studies began to fill in gaps of knowledge, relatively little has also been known about maturational changes in the microstructural characteristics of the UF.
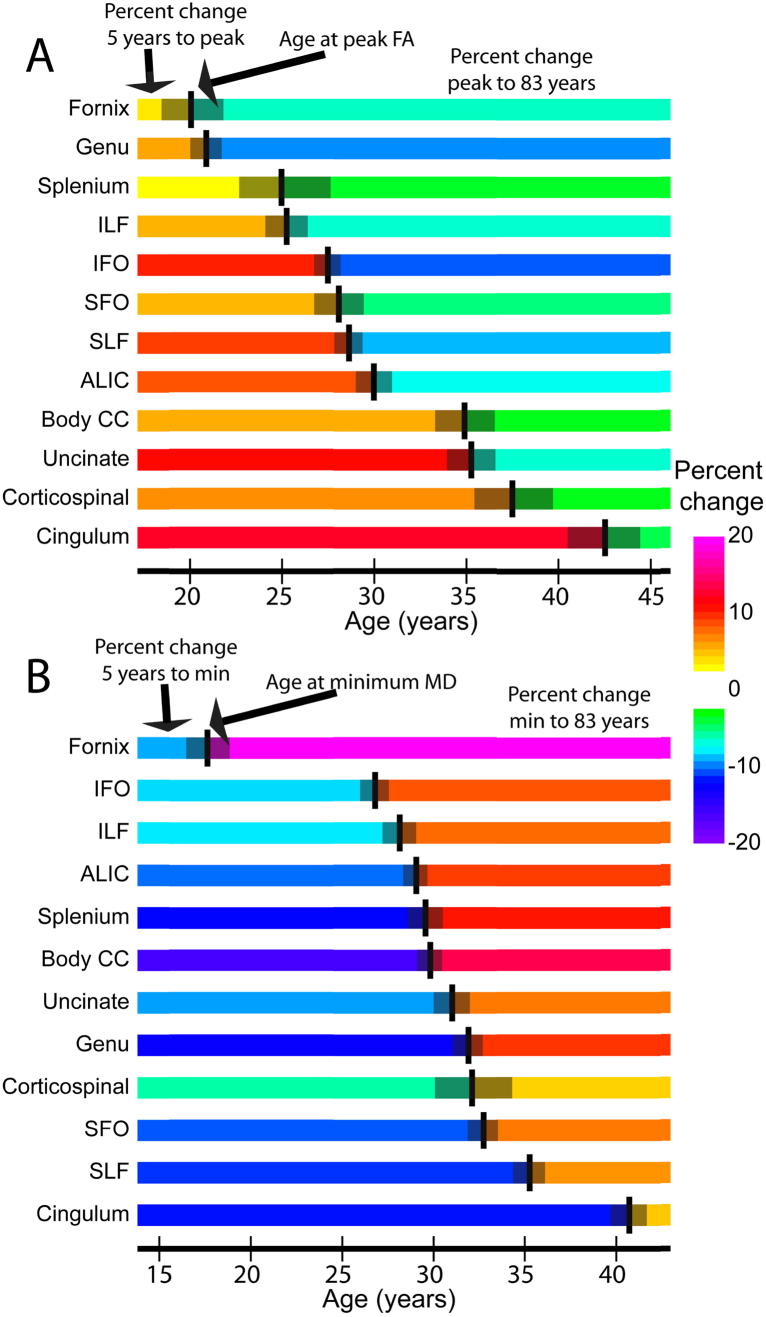
Fig. 2.
Magnitude of DTI parameter changes as a function of age. The age of (A) peak fractional anisotropy, FA; and (B) minimum mean diffusivity, MD. For each tract, the location of the black vertical line represents the age at peak FA or minimum MD. The gray bar to each side represents the standard error of the age estimate (based on the standard error of the fitting parameters). The color of the bars represents the magnitude of change from 5 years to the peak/minimum (left) and from the peak/minimum to 83 years (right). Note that the age scale is different for FA and MD, but the color bar is the same. Of note, the uncinate peaks in the early to mid 30's. ILF: inferior longitudinal fasciculus; IFO: inferior fronto-occipital fasciculus; SFO: superior fronto-occipital fasciculus; SLF: superior longitudinal fasciculus; ALIC: anterior limb of the internal capsule; CC: corpus callosum (Lebel et al., 2012). Used with permission.
Recent developmental DTI studies report that from childhood to adulthood, fractional anisotropy (FA) values, which are thought to reflect myelination, white matter organization, and the density of the fiber tracts, continue to increase with age (Giorgio et al., 2010), whereas measures of local diffusion such as mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) of the UF significantly decrease (Eluvathingal et al., 2007). A similar pattern of development has also been documented for other white matter tracts (see Lebel et al., 2012; L. T. Westlye et al., 2010). However, unlike other white matter association tracts, the FA values of the UF are some of the last to reach their peak maturity between 28 and 35 years of age. UF measures of axial and radial diffusivity are also slow to reach their minima during this developmental time frame (Lebel et al., 2012).
While many studies have now characterized the pattern of white matter changes over the course of development using age, very few have considered examining changes that coalesce with pubertal changes, rather than chronological age. One study (Asato et al., 2010) found that the vast majority of white matter tracts, including the uncinate, are still immature during mid-puberty, as measured by RD. Their findings suggest that the UF is still immature during adolescence when measured using both chronological age and pubertal status. Another recent study (Menzies et al., 2015) demonstrated significant decreases in MD from early puberty to late puberty in a number of tracts, including the UF. All participants were male, and the study demonstrated a significant negative relationship between testosterone levels and MD averaged across the white matter regions that showed a pubertal status effect during early and late puberty.
There is some evidence suggesting asymmetry of the left and right uncinate; however, inconsistencies exist with respect to the direction of the asymmetry (i.e. left hemisphere greater than right vs. right hemisphere greater than left) depending on the specific measure examined. Studies using post-mortem dissection techniques, a method that measures white matter macrostructure and is believed to be more reliable than DTI, have reported that the right uncinate is larger than the left uncinate in approximately 80% of the brains studied (Highley et al., 2002, Park et al., 2004). In contrast, DTI studies, which measure white matter microstructure, have reported inconsistent results with some studies reporting relatively higher FA values in the left UF (e.g., Diehl et al., 2008, Eluvathingal et al., 2007; K. Hasan et al., 2009) while other studies have reported relatively higher FA values in the right UF (Rodrigo et al., 2007) or no asymmetry in FA values at all (Lebel et al., 2008, Rodrigo et al., 2007, Taoka et al., 2006). It should be noted that although many assume that the relationship between macrostructure and microstructure is straightforward, the small number of studies that have explicitly looked at this have reported only weak correlations (Fjell et al., 2008). In regards to developmental changes in asymmetry, evidence is scant due to the fact that participants in DTI studies are usually over 6 years in age. The data that does exist also conflicts (Geng et al., 2012, Johnson et al., 2014).
2. Clinical disorders
In this section, we review evidence for the involvement of the uncinate in three disorders: social deprivation and maltreatment, autism spectrum disorders, and conduct disorder. These disorders were chosen because our earlier review of the diffusion imaging literature revealed that the UF is frequently mentioned in the contexts of these developmental disorders (Von Der Heide et al., 2013). When possible, we ground these findings in basic research on the functionality of the UF.
2.1. Effects of deprivation, maltreatment, and stress on the uncinate fasciculus
Previously institutionalized children who have been exposed to severe socio-emotional deprivation often show deficits in global cognitive function and significant developmental delays at the time of adoption (Judge, 2003, Rutter et al., 2001, Rutter, 1998). Long-term neurocognitive and behavioral deficits appear to persist in a significant proportion of these children (Behen et al., 2008, Chugani et al., 2001, Tottenham et al., 2011). These deficits include prolonged impairments in executive functions, language, and memory (Behen et al., 2008). They also include social and emotional problems, such as indiscriminate friendliness, problems with regulating emotions, and difficulties interpreting facial expressions (Tottenham et al., 2011).
Both gray and white matter differences have been documented in previously institutionalized children and these neurological changes are thought to underlie the reported deficits in socio-emotional functioning. The gray matter differences are most apparent in limbic regions such as the amygdala (Tottenham et al., 2010). Likewise, compared to non-institutionalized children, previously institutionalized children show structural differences in the left (Eluvathingal et al., 2006) and bilateral UF (Govindan et al., 2010). White matter differences are by no means limited to the UF, being found throughout the cortex. These finding have led to the “prolonged stress hypothesis,” suggesting that limbic regions are especially vulnerable to prolonged stress during early development.
Studies of social deprivation can be subsumed by a broader category of studies that investigate the effects of childhood maltreatment. Childhood maltreatment refers to verbal, physical, emotional, or sexual abuse or neglect, and various types of maltreatment are known to have a significant effect on the behavior, cognitive functions, and gray and white matter structure and function of children and adults (Hart & Rubia, 2012). Only two studies have investigated differences in white matter connectivity in this population, and only one of these studies (Eluvathingal et al., 2006) reported differences in the UF. The other study (Choi et al., 2009), which tested subjects exposed to verbal abuse, reported abnormalities in other white matter association tracts. Although childhood may be a time particularly vulnerable to prolonged stress during development, recent evidence suggests that prolonged stress can have a significant impact during late adolescence/young adulthood as well. Admon and colleagues (2013) collected MRI, fMRI, and DTI measures of 18-year old males prior to being recruited as a combat paramedics and then again, 18 months later. They found that individuals that had a maladaptive response to stressful military service not only showed a reduction in hippocampal volume, but also in the microstructural properties of the UF (significantly lower FA values; Admon et al., 2013).
It is difficult to draw strong conclusions about this literature as it is quite small. Moreover, the sample sizes in the DTI studies tends to be quite small, possibly due to difficulties in recruiting and testing this population. This population is also difficult to analyze in regards to brain-behavior difference since they are known to have heterogeneous cognitive and behavioral deficits. That being said, our review of the current literature suggests that early and severe social deprivation is associated with abnormalities in the structure, function, and structural connectivity of limbic regions, including the UF. It is known that limbic regions are acutely sensitive to chronic stress due to excitotoxicity from glucocorticoids (Conrad, 2008). It is plausible that stress-induced cell death and cell damage in limbic regions causes concomitant white matter retraction, scaring, and degradation in the UF, as well as other limbic fiber tracts. Future researchers should search for behavioral correlations between the UF and individuals who have experienced prolonged stress. Potential areas of investigation can be found in Section 3 of this paper. Of special interest in the question of whether there is a critical age range during which the effects of stress are especially deleterious on white matter microstructure and whether there can be later recovery.
2.2. Autism
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders characterized by significant impairments in social interaction and communication and restricted repetitive patterns of behavior (American Psychiatric Association, 2013). Recent studies have revealed that core deficits of autism and autistic traits in healthy humans are associated with atypical connectivity and decreased communication between brain regions (Brock et al., 2002, Di Martino et al., 2011, Dinstein et al., 2011, Iidaka et al., 2012, Just et al., 2004, Schipul et al., 2011). Several laboratories have reported the presence of macrostructural abnormalities in individuals with ASD, such as differences in the overall volume, length, shape and density of the UF (Bigler et al., 2003, Neeley et al., 2007, Kumar et al., 2010, Sundaram et al., 2008). Some studies have reported an asymmetry, such that the volume of the UF in individuals with ASD is greater in the left versus right hemisphere (Pugliese et al., 2009, Thomas et al., 2010).
White matter microstructural differences have also been reported such as group differences in FA, MD, and RD values as well as reports of reduced leftward lateralization in the FA values of the UF in individuals with autism (Lo et al., 2011). A study by Wolff and colleagues (2012) tested the siblings of children with autism, who were considered high risk for ASD. They found that FA values in association tracts of siblings with autism were higher than the control group at 3 months, and then followed a significantly smaller rate of change, resulting in significantly lower FA values in the UF and other white matter association tracts by 24 months (Wolff et al., 2012).
Overall, results from these studies suggest that abnormalities of the UF are present in individuals with ASD that could contribute to the characteristic deficits seen in socio-emotional functioning as well as cognitive functioning. However, a recent review by Travers et al. (2012) suggests several outstanding points that future research needs to address. First, there has been conflicting evidence of microstructural atypicalities in the UF in individuals with autism (Travers et al., 2012). Although several studies have reported that individuals with ASD have decreased FA in the left (Cheon et al., 2011) or bilateral UF (Poustka et al., 2012), other studies have reported increases in FA (Sahyoun et al., 2010) or no group differences in FA (Ameis et al., 2011, Pugliese et al., 2009, Shukla et al., 2011). It is possible that different patterns can be associated with differences in symptoms or behavioral characteristics of ASD.
Second, there are widespread white matter abnormalities ASD; the UF is not uniquely aberrant (Bloeman et al., 2010, Fletcher et al., 2010, Kumar et al., 2010, Müller, 2007, Pardini et al., 2009, Pugliese et al., 2009, Shukla et al., 2011). This makes it critical for future studies to be able to isolate differences specific to the UF and the relationship of these differences to behavior, symptoms, and clinical outcomes in individuals with ASD. Research in typically developing populations might help isolate these relationships. For example, a recent study of 6 month-old typically developing infants found that FA of the right UF specifically predicted joint attention (i.e., nonverbal social communication of shared interest) but not receptive language ability at 9 months of age (Elison et al., 2013). Infants with autism often fail to use social gaze in joint attention tasks (Charman et al., 1997), and it would be informative to know if the UF might be associated with these specific deficits and if therapeutic improvements in joint attention ability might relate to changes in the structure and integrity of the UF.
Finally, reported disruptions in white matter can be exaggerated during imaging experiments by head movements. A recent study of children with ASD reported widespread changes in white matter that mostly disappeared once head motion was controlled for in the analysis (Yendiki et al., 2014).
In sum, there is some evidence for the UF's involvement in ASD but it is difficult to ascertain more specific brain-behavior relationships on the basis of the existing literature. Fruitful areas to investigate include episodic future thinking (which might include theory of mind), social reward sensitivity and learning, as well as value-based updating of memory representations (see Section 3).
2.3. Conduct disorder and psychopathic traits
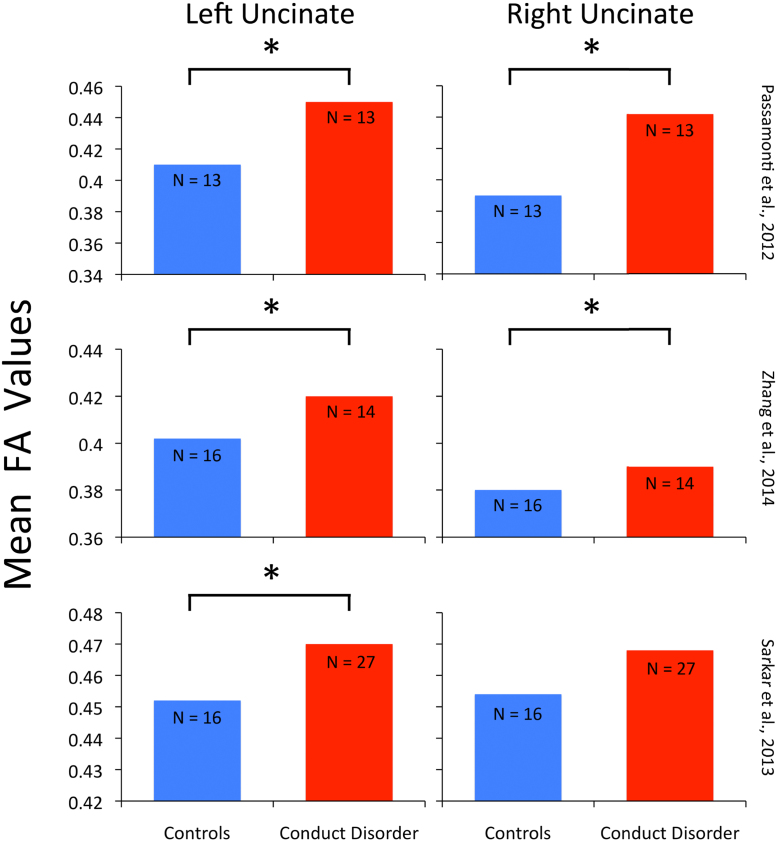
Conduct disorder (CD) and childhood psychopathy are characterized by aggression, impulsivity, and remorseless behavior that often violates the rules or the rights of others (Zhang et al., 2014). The DTI literature investigating anti-social traits in children and adolescents consistently shows alterations in UF microstructure (Passamonti et al., 2012, Sarkar et al., 2013, Zhang et al., 2014). We summarize these findings in Fig. 3.
Fig. 3.
Alterations in fractional anisotropy (FA) values in conduct disorder (red bars) and matched controls (blue bars) across three laboratories (Passamonti et al., 2012, Sarkar et al., 2013, Zhang et al., 2014). Error bars represent the standard error of the mean. Asterisks indicate significant differences between groups.
The adolescent studies tend to report group differences in fractional anisotropy in the left, and sometimes right UF. In some instances, microstructural differences have been linked to specific behaviors, such as the finding reported by Sarkar et al. (2013) that antisocial behaviors correlated with FA values in the left uncinate. Another study reported an interaction of gender (CD is more prevalent in males), conduct disorder, and UF microstructure (Zhang et al., 2014). Our enthusiasm for this small literature is bolstered by the fact that the DTI literature on adults with anti-social traits and incarceration also consistently reports alterations in UF microstructure (reviewed in Von Der Heide et al., 2013).
There are several ways in which differences in the UF might be linked to psychopathic traits and behavior. First, Passamonti et al. (2012) proposed that there may be problems with a maturational clock, meaning that increased FA in adolescents and young adults with CD could be the result of early maturation of the UF, leading to white matter degradation during development and a transition into full-blown psychopathy as adolescents mature into adults. Alternatively, we have proposed that alterations in UF microstructure may lead to impaired communication between the frontal lobe and medial/anterior temporal lobe, which may serve to form a flexible mental representation of episodes associated with reward or punishment (Ramnani et al., 2004, Von Der Heide et al., 2013). This might explain how deficits in reversal learning (discussed later) connect to impaired decision-making in children with developmental psychopathic traits. More specifically, it is possible that differences in the UF might lead to the perseveration of antisocial behavior due to deficits updating and adapting responses to aversive emotional stimuli (Herpertz et al., 2008). Further, gender differences in the integrity of the UF in individuals with CD might account for larger deficits in reversal learning for CD males and perhaps, even offer some explanation as to why there are higher rates of conduct disorder in males rather than females. A similar account might also be applicable for children diagnosed with the hyperactive subtype of attention-deficit hyperactive disorder who also show deficits in reversal learning, increased impulsivity, and elevated levels of socially unacceptable behavior.
2.4. Functional anatomy: proposed functions of the uncinate fasciculus
There is a small but growing movement in psychiatry that advocates for a dimensional approach to psychiatric disorders. By this view, the current taxonomic system as found in the Diagnostic and Statistical Manual of Mental Disorders is disregarded in favor of a view focused on symptoms, such as deficits in social processing or cognitive control, which may cut across traditional diagnostic categories (Ledford, 2013). In this section, we turn to behavioral traits that appear to correlate more clearly with variation in UF structure, as compared to specific clinical disorders.
2.5. Reversal learning and impulsive responding
Impulsive responding and decision making are a hallmark of problems with cognitive control, a deficit found in several psychiatric disorders. In the laboratory, cognitive flexibility and is frequently measured using reversal learning paradigms. During reversal learning tasks, participants are first trained to associate various stimuli with either rewards or penalties. Once these associations are adequately learned, the corresponding reward contingencies are reversed, and participants must learn to respond to the newly rewarded stimulus, while inhibiting responses to the previously rewarded stimulus (Izquierdo & Jentsch, 2012). However, participants are not immediately aware of this reversal; therefore, the initially rewarding stimulus remains pre-potent, and participants often exhibit perseverative behaviors toward the previously rewarded stimulus. The ease with which participants adapt their choices after contingency reversal serves as an index of cognitive flexibility. Individual differences in the degree of perseveration after reversal reveal important differences in the propensity to make impulsive decisions (Clark et al., 2004). Reversal learning deficits are found in several developmental disorders characterized by impulsiveness, including conduct disorder (Budhani and Blair, 2005, Finger et al., 2008), attention deficit hyperactivity disorder (Itami & Uno, 2002), and pediatric bipolar disorder (Gorrindo et al., 2005).
A large body of research has demonstrated that portions of the OFC play a key role in reversal learning. Evidence suggests that specific reward contingencies are encoded in the OFC, which is also responsible for maintaining and updating the reward history associated with particular stimuli (Fellows, 2011). Across rodents, monkeys, and human patients, OFC damage leads to severe reversal learning impairments, even on exceedingly simple tasks (Fellows and Farah, 2003, Fellows, 2011, Ragozzino, 2007, Rudebeck and Murray, 2011). Recently, it has been proposed that damage to the white matter connecting the OFC to the temporal lobes may be the source of reversal learning deficits in macaques, rather than damage to the OFC gray matter (Rudebeck et al., 2013). Indeed, our laboratory has shown that within a population of neurologically normal young adults, individual variability in the microstructure of the UF predicts reversal learning performance (Alm et al., 2015, Alm et al., in press, accepted pending revisions).
2.6. Sensitivity to reward and punishment
The UF is considered a limbic fiber pathway and researchers have speculated that it may play an important role in emotional regulation (reviewed in Von Der Heide et al., 2013). While some theories about the UF's role in emotion have not been supported by sufficient evidence, there is a literature specifically related to reward processing. Recall that the OFC is thought to be critical for encoding specific reward values, as well as for updating and maintaining patterns of reward history (Fellows, 2011). Thus, we can presume that some of the white matter emerging from neurons in the OFC would also play a role in relaying information about rewards and punishments to temporal lobe structures.
A small, albeit growing literature has implicated the UF in reward processing in healthy adults. For instance, Camara and colleagues (2010) employed a combined fMRI/DTI approach in order to investigate the relationship between BOLD activation, microstructural indices of white matter, and processing of rewards and punishments. They found that for a simple two-choice gambling task, individual differences in reward related activation in the ventral striatum, a region implicated in reward processing, correlated positively with FA values within a cluster corresponding to the uncinate (Camara et al., 2010). Participants with greater BOLD percent signal change in the ventral striatum on loss versus gain trials also had higher uncinate FA values. A positive correlation was also found between uncinate FA and a measure indexing sensitivity to punishment. The authors argue that these findings suggest that differences in reward processing are associated with the microstructural properties of anatomical connections between reward regions.
This hypothesis was bolstered by a study conducted by Bjornbekk and colleagues (2012). In this study, a self-report measure of reward dependence from the Temperament and Character Inventory (TCI; Cloninger et al., 1993) was administered to a large cohort (N = 263) of adults. Reward dependence, defined as the tendency to depend on or react strongly to various forms of reinforcement, correlated negatively FA in a cluster consistent with the uncinate (Bjørnebekk et al., 2012). Individuals more dependent on rewarding stimuli or social cues tended to have lower FA values within the uncinate cluster. Since the age range in this study was so large (20-85 years), the researchers also performed the analysis on the youngest portion of their cohort after a median split. The negative correlation between reward dependence and FA remained significant in this sample.
Again, we must note the inconsistency with respect to directionality of FA correlations across DTI studies. We speculate that this difference is likely due to the specific reward indices measured. Keeping in mind that these speculations are only based on two studies, it is conceivable that rewards and punishments lie on opposite ends of the same continuum, and they may interact in a “seesaw” fashion such that if an individual is highly sensitive to one dimension, he or she will be less sensitive to the other. This framework is consistent with the directionality differences found by these studies, suggesting that if higher FA values are associated with greater loss-specific BOLD activation (and thus more sensitivity to punishments), then higher FA values would in turn be linked to lower reward dependence.
In our review of the literature, only one study has included children or adolescents in their study cohort; however, this study did not directly address the relationship between white matter integrity and reward processing within the children or adolescent participants. Instead, Olson and colleagues (2009) investigated this relationship across the entire study sample. Specifically, they tested a sample of participants ranging from 9 to 23 years of age on a delay discounting task. This paradigm assesses participants’ abilities to delay gratification by assessing preference for delayed over immediate rewards (Ohmura et al., 2006). Steeper discounting rates reflect the preference for immediate over delayed rewards, while less steep discounting rates reflect the selection of delayed rewards. The results revealed a positive relationship between the ability to delay gratification and uncinate FA, controlling for IQ. In other words, less steep discounting rates, and therefore “greater tolerances for delayed rewards”, were associated with increased FA in the UF (Olson et al., 2009). Despite the overall relationship found between FA and discount rate, this correlation did not survive after controlling for age. It is well known that the ability to selected delayed over immediate rewards develops with age (e.g. Steinberg et al., 2009); therefore, it is unclear whether this effect would be upheld in a strictly developmental cohort.
Although there is very little research that is specifically related to structural connectivity and reward processing in developmental populations, a large body of research has centered around the development of neural regions responsible for reward processing. The development of the so-called neural “reward network” follows a trajectory similar to an inverted U, with reward sensitivity reaching its peak during adolescence. This trajectory leaves the adolescent population vulnerable to heightened risk taking and reward seeking behaviors (Steinberg, 2010). Such behaviors are associated with substance use, which is often initiated during adolescence (Jacobus et al., 2013). A meta-analysis reviewing the DTI literature related to adolescent substance use (Baker et al., 2013) found no alterations specific to UF microstructure in adolescent substance users; however, two more recent studies have, in fact, found effects in the UF. In one longitudinal study (Luciana et al., 2013), researchers examined the effects of initiation of alcohol use within a cohort ranging in age from 14 to 19 years old at baseline. For this particular study, participants included only individuals who had never ingested an alcoholic beverage at baseline. At two year follow-up, 30 of the 55 participants reported the initiation of alcohol use during the interval between baseline and follow-up. Luciana and colleagues (2013) found that compared to alcohol users, non-users exhibited greater FA increases in the right UF from baseline to the two year follow-up.
Another study (Jacobus et al., 2013) also took a longitudinal approach investigating white matter differences in binge drinking and cannabis use. Their cohort of adolescents ranged from 16 – 19 years of age at baseline and consisted of three groups based on self-report at a three year follow-up: those who engaged in at least three episodes of binge drinking only (for females, defined as more than four drinks on one occasion; for males, more than five drinks on one occasion), those who engaged in binge drinking plus marijuana use, and those who consistently self-reported minimal alcohol and marijuana use over the three year period (controls). At three year follow-up, the researchers found a number of clusters, including both left and right UF, that had higher FA values in controls than each of the user groups.
2.7. Long-term memory retrieval
Of all the reviewed findings on the UF, the most robust literature is the one linking the UF to various types of long-term memory. Findings in the adult literature provide a potential framework for studies that investigate the developmental time course of this link; thus, we review them here. Studies of adult populations have linked the left UF to semantic memory, the type of memory used for remembering facts and concepts, divorced from the time and place in which they were encoded. It is the type of memory frequently assessed in school (e.g. What is the capital of Michigan?) and is closely aligned with core language processes. The left UF connects regions of the brain that have putative functions in language: the anterior temporal lobes and portions of the frontal lobes, both of which have variously been proposed to encode, store, and retrieve semantic knowledge (Catani and Mesulam, 2008, Grossman et al., 2004).
Two studies examined individuals with left hemispheric lesions and aphasia and found correlations between left UF microstructural properties and the retrieval of object names (Catani et al., 2013, Meinzer et al., 2010). For instance, Harvey and colleagues (2013) (Harvey, Wei et al., 2013) tested 10 individuals with aphasia due to large left hemisphere lesions on two semantic tasks, including Pyramids and Palm Trees (Howard & Patterson, 1992). They found that task performance strongly correlated with FA values in the left UF (Harvey et al., 2013). The authors’ interpretation is that the UF is involved in semantic control, although other interpretations are also plausible. Other investigators have reported that patients with semantic dementia have decreased UF FA values as compared to matched controls (Agosta et al., 2010). However, not all studies have found a relationship between the UF and semantic memory (Duffau et al., 2009). Indeed, unilateral transection of the UF, due to surgery, leads to only mild problems in semantic memory retrieval, although it is common to observe problems with one specific category of semantics: people's names (Papagno et al., 2011). Difficulties with proper name retrieval are commonly observed after anterior temporal lobe resection as well (Drane et al., 2008), which necessarily damages the UF.
The evidence linking the UF to episodic memory is more consistent. Episodic memory is a record of a person's experience that includes time and place information (Tulving, 1983). The formation of new episodic memories is known to rely on a hippocampal circuit as well as frontal control regions. The most compelling findings on this topic can be found in the non-human primate literature. The one type of learning that is consistently impaired after UF dissection is conditional rule learning (CRL) in which the monkey learns to associate a particular object with a particular choice location that is rewarded (Bussey et al., 2002; E. A. Gaffan et al., 1988, Gutnikov et al., 1997, Parker and Gaffan, 1998). However, UF disconnection has little to no effect on several types of memory, such as the ability to learn about visual objects or learn the associations between objects and locations, as well as configural learning and delayed matching-to-sample performance (Browning and Gaffan, 2008, Eacott and Gaffan, 1992; D. Gaffan and Eacott, 1995, Gutnikov et al., 1997).
Likewise, in the small but growing human DTI literature, only some types of memory appear to be linked to UF function (see Table 1). Performance on verbal memory tasks with strong recollection demands, such as the California Verbal Learning Task (CVLT) or the auditory immediate and delayed memory subtests from the Weschler Memory Scale (WMS), is significantly correlated with uncinate FA, regardless of the population studied (e.g. individuals with temporal lobe epilepsy, schizophrenia, or mild cognitive impairment; Mabbott et al., 2009, McDonald et al., 2008, Wendelken et al., 2014). In contrast, significant correlations between uncinate FA and performance on visual memory tasks are observed more sporadically, with several studies finding no relationship (McDonald et al., 2008, Metzler-Baddeley et al., 2011). This could either reflect something meaningful – that the UF plays only a minor role in visual memory – or it could reflect the fact that the literature is small and the tasks used to test visual memory have been suboptimal. Our sentiments are with the second option.
Table 1.
Diffusion imaging studies of long-term forms of memory and their findings in regards to the uncinate fasciculus (UF). Only studies that used standard DTI measures (Fractional Anisotropy (FA), Mean Diffusivity (MD), Axial Diffusivity (AD), and Apparent Diffusion Coefficient (ADC) are included. Studies that examined populations with known white matter pathology (e.g. multiple sclerosis) were excluded. A ‘0’ in the right-most columns indicates that no significant correlation was observed. When a significant effect was observed, it is noted for the left uncinate (L UF), right uncinate (R UF), or combined (L+R). Abbreviations: Alz = Alzheimer's Disease; BNT = Boston Naming Task; CVLT = California Verbal Learning Task; MMSE = Mini-Mental State Examination; MCI = mild cognitive impairment; TL = temporal lobe; WAIS = Weschler Adult Intelligence Task; WMS = Weschler Memory Scale.
| Author | Control population (mean age) | Special population (M age) | Tasks | L UF | R UF | L + R |
|---|---|---|---|---|---|---|
| Correlations with Verbal Episodic Memory | ||||||
| Mabbott et al. (2009) | – | Children (M = 12) | CVLT | FA | 0 | – |
| Wendelken et al. (2014) | HC (M = 19) | Children (M = 10) | Selective Memory Encoding Task | FA | FA | – |
| McDonald et al. (2008) | HC (M = 39) | TL Epilepsy (M = 34) |
1. WMS - auditory immediate and delayed memory; 2. BNT | FA | 0 | – |
| Diehl et al. (2008) | HC | TL Epilepsy (M = 40) | WMS - auditory immediate and delayed memory | FA/ADC | 0 | – |
| Nestor et al. (2008) | HC (M = 41) | Schizophrenia (M = 39) | WMS - complete | 0 | 0 | – |
| Fujie et al. (2008) | HC (M = 71) | MCI (M = 72) | WMS – logical memory | FA | 0 | – |
| Lockhart et al. (2012) | HC (M = 24) |
Low or High White Matter Hyperintensity Adults (M=76; 77) | Common object-color source memory task - 2 hour delay | – | – | FA |
| Correlations with Visual Episodic Memory | ||||||
| Niogi et al. (2008) | HC (M = 30) |
Traumatic Brain Injury (M = 32) | 1.Attentional Network Test; 2. CVLT | 0 | FA | |
| Hanlon et al. (2012) | HC (M = 38) |
Schizophrenia (M = 33) |
Virtual Morris Water Maze | – | – | FA |
| McDonald et al. (2008) | HC (M = 39) | TL Epilepsy (M = 34) | WMS – visual, immediate and delayed memory | 0 | 0 | – |
|
Diehl et al. (2008) |
HC | TL Epilepsy (M = 40) | WMS - visual immediate and delayed memory | 0 | ADC | – |
| Sasson et al. (2010) | HC (M = 51) | – | 1. Benton Visual Retention Test; 2. Brief Visuospatial Memory Test; 3.Finger tapping task | AD, FA, AD, RD | 0 | – |
| Hirni et al. (2013) | Older HC (M=72) | MCI (M=73); Alz (M=67) | 1. CVLT (no effect); 2. Rey-Osterrieth; 3. BNT; 4. Category Verbal Fluency -Animals | – | – | FA |
| Metzler-Baddeley et al. (2011) | – | Older adults (M = 68) | 1. Object location; 2. Doors and People (no effect) | – |
– |
FA |
| Metzler-Baddeley et al. (2012) | Older HC (M = 74) | Mild Cognitive Impairment (M = 77) | 1.Doors and People; 2. Face recognition and confidence | AD, RD |
AD, RD |
– |
| Other measures of memory | ||||||
| Kiuchi et al. (2009) | Older HC (M = 72) | MCI (M = 73); Alz (M = 75) | 1. MMSE; 2. Alz Assessment Scale | ADC | FA, ADC | ADC |
Turning to the small developmental literature, Mabbott and colleagues (2009) tested 22 normally developing children and adolescents and found that left UF FA values correlated with both free and cued recall on the CVLT. There was no correlation between performance on a visual episodic memory task and microstructural properties of the UF. A large-scale study by Wendelken and colleagues (2014) examined the relationship between performance on a mnemonic control task and microstructure of the UF in a population of adolescents and young adults. Participants were asked to actively attend to or ignore scenes that were displayed for 3 s; they were then probed to determine if a displayed image was novel or had been presented previously, in an attempt to measure the extent to which attention modulation during encoding affected long-term retrieval. Independent of task performance, there was a significant difference in FA within the left UF between adults and adolescents. Among adolescents, there was a significant relationship between age and FA in the left UF (Wendelken et al., 2014). Along with the increase in UF FA came improved performance in the “attended” trials of the task, demonstrating top-down enhancement of recall accuracy in attended trials compared to competing trials.
We propose that one plausible role for the UF in episodic memory may be to adjudicate between competing memory representations. Going back to the monkey studies, performance in the conditional rule learning task was impaired after UF transection (Bussey et al., 2002; E. A. Gaffan et al., 1988, Gutnikov et al., 1997, Parker and Gaffan, 1998). In this task, many similar target objects are presented, and the monkeys are only rewarded if they correctly recall which one of the four choice locations was paired with each target. These locations are repeated each trial so there is a great deal of retrieval competition. Likewise, in many verbal recall tasks, there is a great deal of competition from other word stimuli. In contrast, some of the visual episodic memory tasks commonly tested in this literature, such as the Rey-Osterrieth (Hirni et al., 2013, Mabbott et al., 2009), rely heavily on mental imagery with little retrieval competition.
If our speculation is correct, it would predict that the UF is essential in tasks in which a high degree of competition between representations must be resolved to make a memory decision, regardless of the type of memory being tested. Several findings support this hypothesis. First, we recently tested a population of normal young adults on a task in which face-name associations had to be learned through reward and punishment feedback. Our results show that variability in UF microstructure predicted one's ability to learn face-name pairs (Hower et al., 2014). A similar finding using face-scene pairings was earlier reported by Thomas and colleagues (Thomas et al., 2015). Second, as mentioned earlier, the studies on conditional rule learning in macaques support this idea. Third, the findings by Wendelken and colleagues (2014) showing that improved performance in the attended trials of the task correlated with uncinate FA again hint that the UF is most important when competing representations require adjudication. Last, we note that the one semantic memory task that is consistently linked to the UF is proper name retrieval, which has severe retrieval competition, given the arbitrary nature of name-face pairings such that different people, who have nothing in common, such as Diana Ross and Princess Diana, can have the same first name. Last, rapid and flexible memory retrieval is a key ingredient of episodic future thinking, as well as allowing one to form expectations about future outcomes, which is essential in reinforcement learning. Thus the role of the UF in memory retrieval may be exceedingly far reaching.
3. Conclusions
There is growing interest in understanding developmental changes in structural connectivity and the implications for behavior. Our review of the literature revealed several limitations and issues to consider that are important for conducting this type of research (see Box 1).
Box 1. Issues to consider when conducting a developmental DTI study.
1. A sufficient sample size is critically important. This will help ensure significant power to find an effect if it exists and will allow one to control for multiple comparisons.
2. A longitudinal design is optimal. Most studies investigating maturation of the UF have been cross sectional, a design that is quicker and cheaper to execute than a longitudinal study. Longitudinal studies, however, control for between-subjects variance by testing the same participants at different developmental time points and, as a result, have greater inferential value. The few existing longitudinal studies in this literature are valuable (e.g. Lebel and Beaulieu, 2011, Taddei et al., 2012).
3. Head movement is a significant problem in DTI since the dependent measures are also measures of movement, albeit at a microscopic scale. It is likely that some of the positive effects reported in the literature are in fact, movement artifacts (see Yendiki et al., 2014). This is particularly important when studying a developmental cohort, as children and special populations (e.g. individuals with ASD, ADHD, etc.) are more likely to exhibit problematic movement during diffusion imaging acquisition, compromising the quality of the data. Training children to lie still in a mock-scanner with software that provides movement feedback can help with this problem. Additional cushioning around the face and head are also helpful. As a last resort, movement can be dealt with during the analysis phase.
4. The issue of crossing fibers is also a problem in traditional DTI techniques. The diffusion tensor model used by such techniques is insufficient at modeling areas where two or more fiber pathways may cross paths. This is especially an issue for tracts with high anatomical curvature, such as the uncinate. Therefore, technical precautions must be taken and consideration should be given to the possibility of a more powerful technique, such as probabilistic DTI.
5. Potential gender differences in white matter microstructure must be considered, even if gender is not a variable of direct interest. A recent DTI study (Ingalhalikar et al., 2014) found notable gender differences in white matter in a large cohort of participants ranging in age from 8 to 22. Similarly, we have found significant gender differences in UF microstructural indices in a smaller sample of healthy young adults (Alm et al., 2015, Alm et al., in press accepted pending revisions). Developmental studies should control for gender and/or cranial volume (which tends to correlate with gender).
6. One should carefully consider how development is indexed: by chronological age or by sexual maturation.
The focus of this review was the development of the limbic white matter structure, the uncinate fasciculus. The UF exhibits a very late maturational profile, with microstructural development spanning into the 30 s, thus it is plausible that aberrations in its developmental trajectory could underlie certain psychopathologies. Although the literature is sprinkled with inconsistent findings, there is evidence linking uncinate abnormalities to child maltreatment, ASD, and conduct disorder and psychopathic traits. However, our review of the DTI literature revealed the disappointing conclusion that the traditional approach of using DSM-V categories generally had low levels of reproducibility. It is unclear whether these inconsistent findings are due to technical and statistical problems (see Box 1) or variability in the sample population. The one exception to this was antisocial personality disorder and conduct disorder, which have shown consistent correlations with UF microstructure (see Fig. 3).
We emphasize the utility of taking a dimensional approach to psychiatric disorders, one focused on symptoms/behavior and the examination of white matter structure-function relationships, rather than focusing on traditional diagnostic categories. We are enthusiastic about using this approach in diffusion imaging studies of the developing brain. In our previous review of the adult literature, we hypothesized that the UF allows information about temporal-lobe based mnemonic associations (for instance, a person's name + face + biographical information) to modify behavior by interacting with systems in the lateral OFC that are instrumental for making associations between stimuli and rewards, and ultimately, decision making. The bidirectionality of UF information flow would ensure that temporal lobe representations of objects and people reflect up-to-date reward/punishment history (Von Der Heide et al., 2013). We list more specific predictions below and they can also be found in Table 2.
Table 2.
Possible behavioral deficits linked to information transmission properties of the uncinate fasciculus and populations in which one might observe such deficits.
| Behavioral deficit | Where it might be observed | Evidence for relationship |
|---|---|---|
| Deficient memory retrieval | Children with poor scholastic achievement (found in many clinical groups including previously institutionalized children); medial temporal lobe epilepsy; possibly linked to deficits in episodic future thinking and theory of mind | Strong |
| Impulsive decision making/problems learning from errors | Conduct disorder; ADHD; substance abuse | Modest |
| Disordered sensitivity to reward and punishment | Bipolar disorder and conduct disorder; sensation seeking; substance abuse; anhedonia; sensitivity to social rewards in autism spectrum disorder | Emerging |
First, problems with memory retrieval, which may be evidenced by poor scholastic achievement, might be linked to variability in UF microstructure. Children who suffer from disorders characterized by memory impairments, such as medial temporal lobe epilepsy, likely have both macro and microstructural perturbation of the UF (reviewed in Von Der Heide et al., 2013). In our laboratory, we have found a robust relationship between associative memory performance and UF microstructure in a population with limited behavioral variability – college students. Moreover, we found that individual differences in this tract also predicted memory performance after a lengthy delay (45 min), exactly the sort of memory tested in a scholastic setting (Alm et al., 2015, Alm et al., in press).
Second, individuals with developmental disorders characterized by impulsive decision making in which memory and knowledge should be used to alter behavior but are not successfully employed, should have predictable alterations in UF microstructure. Disorders that fall into this category include conduct disorder, attention deficit hyperactivity disorder (ADHD), and substance abuse. We note that reversal learning deficits have been reported in CD (Budhani & Blair, 2005), and ADHD (Itami & Uno, 2002), as well as certain types of substance abuse (Izquierdo & Jentsch, 2012). More generally, damage or alterations in UF microstructure could impair fronto-temporal communication required for learning from errors (Ramnani et al., 2004) as well as episodic future thinking that guides decision making. This in turn could lead to deficits in decision making tasks that rely on long-term memory, since these tasks require the integration of past knowledge about rewards and punishments with one's current state. If true, this would predict that variation in UF microstructure should index individual differences in decision making. Our findings in an adult population show an association between UF microstructure and reversal learning in normal young adults (Alm et al., submitted). We predict these findings will generalize to children and be especially robust in developmental cohorts that exhibit impulsive decision making (e.g. CD and ADHD).
Third, there is emerging evidence that the UF may play some role in sensitivity to reward and punishment. This is an interesting construct in regards to substance abuse and risk taking. Moving forward, it will be important to examine the specificity of changes in UF microstructure, and we should aim to directly link such alterations to addictive patterns and behaviors. For instance, perhaps individual differences in laboratory measures of sensation seeking, risk taking, and impulsivity would predict differences in UF microstructure during adolescence. We speculate that differences within UF microstructure of adolescents would predict differences in reward sensitivity, such that a more developed reward system would correspond to more highly developed microstructure of the UF. It is also possible that tendencies specifically related to drug dependence, e.g. craving symptoms, could predict similar UF alterations. Future research should seek to explore white matter alterations at this higher level of specificity.
Importantly, given the protracted development of the UF, we expect that performance on UF-dependent tasks will vary with age until approximately 30 years of age. To our knowledge, this has not yet been examined.
Acknowledgements
This work was supported by a National Institute of Health grant to I. Olson [RO1 MH091113]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health
References
- Admon R., Leykin D., Lubin G., Engert V., Andrews J., Pruessner J., Hendler T. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum. Brain Mapp. 2013;34:2808–2816. doi: 10.1002/hbm.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Henry R.G., Migliaccio R., Neuhaus J., Miller B.L., Dronkers N.F., Gorno-Tempini M.L. Language networks in semantic dementia. Brain. 2010;133(1):286–299. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm K.H., Rolheiser T., Mohamed F.B., Olson I.R. Fronto-temporal white matter connectivity predicts reversal learning errors. Front. Hum. Neurosci. 2015;9:343. doi: 10.3389/fnhum.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm K.H., Unger A., Nugiel T., Zhang H.R., Rolheiser T., Troiani V., Olson I.R. Individual differences in associative learning and delayed retrieval predicted by white matter connectivity. Annual Meeting of the Cognitive Neuroscience Society; San Francisco, CA; 2015. (in press) [Google Scholar]
- Ameis S.H., Fan J., Rockel C., Voineskos A.N., Lobaugh N.J., Soorya L., Anagnostou E. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: A diffusion tensor imaging study. PLOS One. 2011;6(11):e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: A DTI study. Cereb. Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A.P. Fifth Edition. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Baker S.T.E., Yücel M., Fornito A., Allen N.B., Lubman D.I. A systematic review of diffusion weighted MRI studies of white matter microstructure in adolescent substance users. Neurosci. Biobehav. Rev. 2013;37(8):1713–1723. doi: 10.1016/j.neubiorev.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Menon V., Eckert M., Tamm L., Bammer R., Karchemskiy A., …, Reiss A.L. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bava S., Thayer R., Jacobus J., Ward M., Jernigan T.L., Tapert S.F. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behen M.E., Helder E., Rothermel R., Solomon K., Chugani H.T. Incidence of specific absolute neurocognitive impairment in globally intact children with histories of early severe deprivation. Child Neuropsychol. 2008;14:452–469. doi: 10.1080/09297040802244136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D., Tate D.F., Neeley E.S., Wolfson L.J., Miller M.J., Rice S.A., Lainhart J.E. Temporal lobe, autism, and macrocephaly. Am. J. Neuroradiol. 2003;24:2066–2076. [PMC free article] [PubMed] [Google Scholar]
- Bjørnebekk A., Westlye L.T., Fjell A.M., Grydeland H., Walhovd K.B. Social reward dependence and brain white matter microstructure. Cereb. Cortex. 2012;22(11):2672–2679. doi: 10.1093/cercor/bhr345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloeman O.J.N., Deeley Q., Sundram F., Daly E.M., Barker G.J., Jones D.K., Murphy D.G.M. White matter integrity in Asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res. 2010;3(5):203–213. doi: 10.1002/aur.146. [DOI] [PubMed] [Google Scholar]
- Brock J., Brown C.C., Boucher J., Rippon G. The temporal binding deficit hypothesis of autism. Dev. Psychopathol. 2002;14(2):209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Browning P.G.F., Gaffan D. Impairment in object-in-place scene learning after uncinate fascicle section in macaque monkeys. Behav. Neurosci. 2008;122(2):477–482. doi: 10.1037/0735-7044.122.2.477. [DOI] [PubMed] [Google Scholar]
- Budhani S., Blair R.J.R. Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J. Child Psychol. Psychiatry. 2005;46(9):972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- Bussey T.J., Wise S.P., Murray E.A. Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behav. Neurosci. 2002;116(4):703–715. [PubMed] [Google Scholar]
- Camara E., Rodriguez-Fornells A., Münte T.F. Microstructural brain differences predict functional hemodynamic responses in a reward processing task. J. Neurosci. 2010;30(34):11398–11402. doi: 10.1523/JNEUROSCI.0111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44:953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E., Malik F., Martersteck A., Wieneke C., Rogalski E. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136(Pt 8):2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T., Swettenham J., Baron-Cohen S., Cox A., Baird G., Drew A. Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Dev. Psychol. 1997;33(5):781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Cheon K.-A., Kim Y.-S., Oh S.-H., Park S.-Y., Yoon H.-W., Herrington J., Schultz R.T. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: a Diffusion Tensor Imaging study. Brain Res. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Choi J., Jeong B., Rohan M.L., Polcari A.M., Teicher M.H. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatry. 2009;65(3):227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani H.T., Behen M.E., Muzik O., Juha ´sz C., Nagy F., Chugani C. Local brain funcional activity following early deprivation: A study of postinstitutionalized Romanian orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Clark L., Cools R., Robbins T.W. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55(1):41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Cloninger R.C., Svrakic D.M., Przybeck T.R. A psychobiological model of temperament and character. Arch. Gen. Psychiatry. 1993;50(12):975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Conrad C.D. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Chisum H.J., Townsend J., Cowles A., Covington J., Egaas B., Press G.A. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Kelly C., Grzadzinski R., Zuo X.-N., Mennes M., Mairena M.A., Milham M.P. Aberrant striatal functional connectivity in children with autism. Biol. Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl B., Busch R.M., Duncan J.S., Piao Z., Tkach J., Lüders H.O. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49(8):1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Dinstein I., Pierce K., Eyler L., Solso S., Malach R., Behrmann M., Courchesne E. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70(6):1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane D.L., Ojemann G.a, Aylward E., Ojemann J.G., Johnson L.C., Silbergeld D.L., Tranel D. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46(5):1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H., Gatignol P., Moritz-Gasser S., Mandonnet E. Is the left uncinate fasciculus essential for language?. A cerebral stimulation study. J. Neurol. 2009;256(3):382–389. doi: 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Durston S., Hulshoff Pol H.E., Casey B.J., Gledd J.N., Buitelaar J.K., van Engeland H. Anatomical MRI of the developing human brain: What have we learned? J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Eacott M.J., Gaffan D. Inferotemporal-frontal disconnection: The uncinate fascicle and visual associative learning in monkeys. Eur. J. Neurosci. 1992;4:1320–1332. doi: 10.1111/j.1460-9568.1992.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Elison J.T., Wolff J.J., Heimer D.C., Paterson S.J., Gu H., Hazlett H.C., Piven J. Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Dev. Sci. 2013;16(2):186–197. doi: 10.1111/desc.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal T.J., Chugani H.T., Behen M.E., Juha ´sz C., Muzik O., Maqbool M., Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Eluvathingal T.J., Hasan K.M., Kramer L., Fletcher J.M., Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb. Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows L.K. Orbitofrontal contributions to value-based decision making: evidence from humans with frontal lobe damage. Ann. N. Y. Acad. Sci. 2011;1239:51–58. doi: 10.1111/j.1749-6632.2011.06229.x. [DOI] [PubMed] [Google Scholar]
- Fellows L.K., Farah M.J. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Finger E.C., Marsh A.A., Mitchell D.G., Reid M.E. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch. Gen. Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Greve D.N., Fischl B., Benner T., van der Kouwe A.J.W., Walhovd K.B. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. NeuroImage. 2008;42(4):1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.T., Whitaker R.T., Tao R., DuBray M.B., Froehlich A., Ravichandran C., Lainhart J.E. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage. 2010:1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujie S., Namiki C., Nishi H., Yamada M., Miyata J., Sakata D., Murai T. The role of the uncinate fasciculus in memory and emotional recognition in mild cognitive impairment. Dement. Geriatr. Cogn. Disorders. 2008;26(5):432–439. doi: 10.1159/000165381. [DOI] [PubMed] [Google Scholar]
- Gaffan D., Eacott M.J. Visual learning for an auditory secondary reinforcer by macaques is intact after uncinate fascicle section: Indirect evidence for the involvement of the corpus striatum. Eur. J. Neurosci. 1995;7(9):1866–1871. doi: 10.1111/j.1460-9568.1995.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Gaffan E.A., Gaffan D., Harrison S. Disconnection of the amygdala from visual association cortex impairs visual reward-association learning in monkeys. J. Neurosci. 1988;8(9):3144–3150. doi: 10.1523/JNEUROSCI.08-09-03144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Prom-Wormley E.C., Perez J., Kubarych T., Styner M., Lin W., Gilmore J.H. White matter heritability using diffusion tensor imaging in neonatal brains. Twin Res. Hum. Genet. 2012;15(03):336–350. doi: 10.1017/thg.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Rapoport J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Rajapakse J.C., Vaituzis A.C., Liu H., Castellanos F.X. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1999;23(4):571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Chadwick M., James S., Winmill L., Douaud G., James A.C. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Douaud G., James A.C., James S., De Stefano N., Johansen-Berg H. Changes in white matter microstructure during adolescence. NeuroImage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I., Ashburner J., Hensen R.N., Friston K.J., Frackowiak R.S. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gorrindo T., Blair R.J.R., Budhani S., Dickstein D.P., Pine D.S., Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am. J. Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Govindan R.M., Behen M.E., Helder E., Makki M.I., Chugani H.T. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cereb. Cortex. 2010;20:561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., McMillan C., Moore P., Ding L., Glosser G., Work M., Gee J. What's in a name: voxel based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(3):628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Gutnikov S.A., Ma Y.-Y., Buckley M.J., Gaffan D. Monkeys can associate visual stimuli with reward delayed by 1 s even after perirhinal cortex ablation, uncinate fascicle section or amygdalectomy. Behav. Brain Res. 1997;87(1):85–96. doi: 10.1016/s0166-4328(96)02259-0. [DOI] [PubMed] [Google Scholar]
- Hanlon F.M., Houck J.M., Klimaj S.D., Caprihan A., Mayer A.R., Weisend M.P., Tesche C.D. Frontotemporal anatomical connectivity and working-relational memory performance predict everyday functioning in schizophrenia. Psychophysiology. 2012;49:1340–1352. doi: 10.1111/j.1469-8986.2012.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Rubia K. Neuroimaging of child abuse: A critical review. Front. Hum. Neurosci. 2012;6:1–24. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D.Y., Wei T., Ellmore T.M., Hamilton aC., Schnur T.T. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia. 2013;51(5):789–801. doi: 10.1016/j.neuropsychologia.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Hasan K.M., Iftikhar A., Kamali A., Kramer L.A., Ashtari M., Cirino P.T., Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K.M., Sankar A., Halphen C., Kramer L.A., Brandt M.E., Fletcher J.M., Paul T. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18(16):1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- Herpertz S.C., Huebner T., Marx I., Vloet T.D., Fink G.R., Stoecker T., Herpertz-Dahlmann B. Emotional processing in male adolescents with childhood-onset conduct disorder. J. Child Psychol. Psychiatry. 2008;49(7):781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Highley J.R., Walker M.A., Esiri M.M., Crow T.J., Harrison P.J. Asymmetry of the uncinate fasciculus: A post-mortem study of normal subjects and patients with schizophrenia. Cereb. Cortex. 2002;12(11):1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Hirni D.I., Kivisaari S.L., Monsch A.U., Taylor K.I. Distinct neuroanatomical bases of episodic and semantic memory performance in Alzheimer's disease. Neuropsychologia. 2013;51(5):930–937. doi: 10.1016/j.neuropsychologia.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Howard D., Patterson K. Thames Valley Publishing Co; Bury St. Edmunds: 1992. The pyramid and palm trees test: A test of semantic access from words and pictures. [Google Scholar]
- Hower K.H., Rolheiser T., Olson I.R. Fronto-temporal white matter connectivity predicts learning from rewards and punishments. Annual Meeting of the Cognitive Neuroscience Society; Boston, MA; 2014. [Google Scholar]
- Huttenlocher P.R., Taravath S., Mojtahedi S. Periventricular heterotopia and epilepsy. Neurology. 1994;44(1):51–55. doi: 10.1212/wnl.44.1.51. [DOI] [PubMed] [Google Scholar]
- Iidaka T., Miyakoshi M., Harada T., Nakai T. White matter connectivity between superior temporal sulcus and amygdala is associated with autistic traits in healthy humans. Neurosci. Lett. 2012;510:2154–2158. doi: 10.1016/j.neulet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M., Smith A., Parker D., Satterthwaite T.D., Elliott M.A., Ruparel K., Verma R. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. USA. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itami S., Uno H. Orbitofrontal cortex dysfunction in attention-defcit hyperactivity disorder revealed by reversal and extinction tasks. NeuroReport. 2002;13(18):2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Jentsch J.D. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219(2):607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L.M., Bava S., Tapert S.F. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: A 3-year investigation. Psychiatry Res. 2013;214(3):374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.T., Yeatman J.D., Wandell B.A., Buonocore M.H., Amaral D.G., Nordahl C.W. Diffusion properties of major white matter tracts in young, typically developing children. NeuroImage. 2014;88:143–154. doi: 10.1016/j.neuroimage.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge S. Developmental recovery and deficit in children adopted from Eastern European orphanages. Child Psychiatry Hum. Dev. 2003;34(1):49–62. doi: 10.1023/a:1025302025694. [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Minshew N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127(8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kiuchi K., Morikawa M., Taoka T., Nagashima T., Yamauchi T., Makinodan M., Kichikawa K. Abnormalities of the uncinate fasciculus and posterior cingulate fasciculus in mild cognitive impairment and early Alzheimer's disease: A diffusion tensor tractography study. Brain Res. 2009;1287:184–191. doi: 10.1016/j.brainres.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Kumar A., Sundaram S.K., Sivaswamy L., Behen m., Makki E., Ager M.I., Chugani J.D.C. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb. Cortex. 2010;20:2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Ledford H. Psychiatry framework seeks reform diagnostic doctrine. Nature News. 2013:718–729. [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Behav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lo Y.-C., Soong W.-T., Gau S.S.-F., Wu Y.-Y., Lai M.-C., Yeh F.-C., Tseng W.-Y.I. The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: A study using diffusion spectrum imaging tractography. Psychiatric Res. 2011;192:60–66. doi: 10.1016/j.pscychresns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Lockhart S.N., Mayda A.B., Roach V., Fletcher A.E., Carmichael E., Maillard O., DeCarli P.C. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front. Hum. Neurosci. 2012;6:1–12. doi: 10.3389/fnhum.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Collins P.F., Muetzel, Ryan L., Lim K.O. Effects of alcohol use initiation on brain structure in typically developing adolescents Monica. Am. J. Drug Alcohol Abuse. 2013;39(6):345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott D.J., Rovet J., Noseworthy M.D., Smith M.L., Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Malykhin N., Concha L., Seres P., Beaulieu C., Coupland N.J. Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatric Res. 2008;164:132–142. doi: 10.1016/j.pscychresns.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J., Matsui M., Konishi T., Noguchi K., Gur R.C., Bilker W., Miyawaki T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb. Cortex. 2001;11(4):335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- McDonald C.R., Ahmadi M.E., Hagler D.J., Tecoma E.S., Iragui V.J., Gharapetian L., Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71(23):1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry R.C., Mathur A., Miller J.H., Ozcan A., Snyder A.Z., Schefft G.L., Neil J.J. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb. Cortex. 2002;12(12):1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- Meinzer M., Mohammadi S., Kugel H., Schiffbauer H., Flöel A., Albers J., Deppe M. Integrity of the hippocampus and surrounding white matter is correlated with language training success in aphasia. NeuroImage. 2010;53(1):283–290. doi: 10.1016/j.neuroimage.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Menzies L., Goddings A.-L., Whitaker K.J., Blakemore S.-J., Viner R.M. The effects of puberty on white matter development in boys. Dev. Cogn. Neurosci. 2015;11:116–128. doi: 10.1016/j.dcn.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C., Hunt S., Jones D.K., Leemans A., Aggleton J.P. Temporal association tracts and the breakdown of episodic memory in mild cognitive impairment. Neurology. 2012;79(23):2233–2240. doi: 10.1212/WNL.0b013e31827689e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C., Jones D.K., Belaroussi B., Aggleton J.P., O'Sullivan M.J. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J. Neurosci. 2011;31(37):13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P., McKinstry R.C. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin. N. Am. 2006;16(1):19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Miller J.H., Shimony J.S., Conturo S.J.S., Lee B.C., Almli C.R., McKinstry R.C. Normal brain maturation during childhood: Developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Müller R.-A. The study of autism as a distributed disorder. Ment. Retard. Dev. Disabil. Res. Rev. 2007;13(1):85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeley E.S., Bigler E.D., Krasn L., Ozonoff S., McMahon W.M., Lainhart J.E. Quantitative temporal lobe differences: Autism distinguished from controls using classification and regression tree analysis. Brain Dev. 2007;29:389–399. doi: 10.1016/j.braindev.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor P.G., Kubicki M., Niznikiewicz M., Gurrera R.J., McCarley R.W., Shenton M.E. Neuropsychological disturbance in schizophrenia: A diffusion tensor imaging study. Neuropsychology. 2008;22(2):246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P., Ghajar J., Johnson C.E., Kolster R., Lee H., McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131(Pt 12):3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Ohmura Y., Takahashi T., Kitamura N., Wehr P. Three-month stability of delay and probability discounting measures. Exp. Clin. Psychopharmacol. 2006;14(3):318–328. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]
- Olson E.A., Collins P.F., Hooper C.J., Muetzel R., Lim K.O., Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J. Cogn. Neurosci. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno C., Miracapillo C., Casarotti A., Romero Lauro L.J., Castellano A., Falini A., Bello L. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134(Pt 2):405–414. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- Pardini M., Garaci F.G., Bonzano L., Roccatagliata L., Palmieri M.G., Pompili E., Gialloreti E. White matter reduced streamline coherence in young men with autism and mental retardation. Eur. J. Neurol. 2009;16:1185–1190. doi: 10.1111/j.1468-1331.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- Park H.J., Westin C.F., Kubicki M., Maier S.E., Niznikiewicz M., Baer A., Shenton M.E. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: A diffusion tensor MRI study. Neuroimage. 2004;23(1):213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A., Gaffan D. Memory after frontal/temporal disconnection in monkeys: Conditional and non-conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia. 1998;36:259–271. doi: 10.1016/s0028-3932(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Passamonti L., Fairchild G., Fornito A., Goodyer I.M., Nimmo-Smith I., Hagan C.C., Calder A.J. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PloS One. 2012;7(11):e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R.E., Stephan K.E., Kötter R. The anatomical basis of functional localization in the cortex. Nat. Rev. Neurosci. 2002;3(8):606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Paus T., Zijdenbos A., Worsely K., Collins D.L., Blumenthal J., Giedd J.N., Evans A.C. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Poustka L., Jennen-Steinmetz C., Henze R., Vomstein K., Haffner J., Sieltjes B. Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J. Biol. Psychiatry. 2012;13(4):269–280. doi: 10.3109/15622975.2011.591824. [DOI] [PubMed] [Google Scholar]
- Pugliese L., Catani M., Ameis S., Dell’Acqua F., de Schotten M.T., Murphy C., Murphy D.G. The anatomy of extended limbic pathways in Asperger syndrome: A preliminary diffusion tensor imaging tractography study. NeuroImage. 2009;47:427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Ragozzino M.E. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann. N. Y. Acad. Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ramnani N., Elliott R., Athwal B.S., Passingham R.E. Prediction error for free monetary reward in the human prefrontal cortex. NeuroImage. 2004;23(3):777–786. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Rodrigo S., Oppenheim C., Chassoux F., Golestani N., Cointepas Y., Poupon C., Meder J.F. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur. Radiol. 2007;17(7):1663–1668. doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- Rudebeck P.H., Murray E.A. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J. Neurosci. 2011;31(29):10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck P.H., Saunders R.C., Prescott A.T., Chau L.S., Murray E.A. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat. Neurosci. 2013;16(8):1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M.L. Developmental catch-up, and deficit, following adoption after severe global early privation. J. Child Psychol. Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- Rutter M.L., Kreppner J.M., O’Connor T.G. Specificity and heterogeneity in children's responses to profound institutional privation. Br. J. Psychiatry. 2001;179:97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- Sahyoun C.P., Belliveau J.W., Soulières I., Schwartz S., Mody M. Neuroimaging of the functional and structural networks underlying visuospatial versus linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Craig M.C., Catani M., Dell’acqua F., Fahy T., Deeley Q., Murphy D.G.M. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol. Med. 2013;43(2):401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Sasson E., Doniger G.M., Pasternak O., Assaf Y. Structural correlates of memory performance with diffusion tensor imaging. NeuroImage. 2010;50(3):1231–1242. doi: 10.1016/j.neuroimage.2009.12.079. [DOI] [PubMed] [Google Scholar]
- Schipul S.E., Keller T.A., Just M.A. Inter-regional brain communication and its disturbance in autism. Front. Syst. Neurosci. 2011;5:1–11. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J., Pandya D. Oxford University Press; New York: 2006. Fiber Pathways of the Brain. [Google Scholar]
- Schmithorst V.J., Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72(1):16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schneider J.F.L., Il’yasov K.A., Hennig J., Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46(4):258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Smylie D.M., Müller R.-A. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49(5):1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L., Plewes C., Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. NeuroImage. 2007;34:243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human lifespan. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Graham S., O’Brien L., Woolard J., Cauffman E., Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Sundaram S.K., Kumar A., Makki M.I., Behen M.E., Chugani H.T., Chugani D.C. Diffusion tensor imaging of frontal lobe in autism specturm disorder. Cereb. Cortex. 2008;18(11):2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei M., Tettlamanti M., Zanoni A., Cappa S., Battaglia M. Brain white matter organisation in adolescence is related to childhood cerebral responses to facial expressions and harm avoidance. NeuroImage. 2012;61(4):1394–1401. doi: 10.1016/j.neuroimage.2012.03.062. [DOI] [PubMed] [Google Scholar]
- Taoka T., Iwasaki S., Sakamoto M., Nakagawa H., Fukusumi A., Myochin K., Kichikawa K. Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer Disease: Evaluation of the “tract of interest” by diffusion tensor tractography. Am. J. Neuroradiol. 2006;27:1040–1045. [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Dell’Acqua F., Valabregue R., Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Thomas C., Humphreys K., Jung K.-J., Minshew N., Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: A DTI tractography study. Cortex. 2010;47(4):863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Avram A., Pierpaoli C., Baker C. Diffusion MRI properties of the human uncinate fasciculus correlate with the ability to learn visual associations. Cortex. 2015 doi: 10.1016/j.cortex.2015.01.023. [Epub] [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Casey B.J. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front. Psychol. 2011;2:1–9. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Millner A., Gilhooly T., Zevin J.D., Casey B.J. Elevated amygdala response to faces following early deprivation. Dev.Sci. 2010;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers B.G., Adluru N., Ennis C., Tromp D.P.M., Destiche D., Doran S., Alexander A.L. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5(5):289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Oxford University Press; New York: 1983. Elements of Episodic Memory. [Google Scholar]
- Van Essen D.C., Maunsell J.H.R. Hierarchical organization and functional streams in the visual cortex. Trends Neurosci. 1983;6:370–375. [Google Scholar]
- Von Der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(Pt 6):1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C., Lee J.K., Pospisil J., Sastre M., Ross J.M., Bunge S.A., Ghetti S. White matter tracts connected to the medial temporal lobe support the development of mnemonic control. Cereb. Cortex. 2014:1–10. doi: 10.1093/cercor/bhu059. http://www.ncbi.nlm.nih.gov/pubmed/24675870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjørnerud A., Due-Tønnessen P., Engvig A., Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wolff J.J., Gu H., Grerig G., Elison J.T., Styner M., Gouttard S., Piven J. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlel P.A., Lecours I.R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain in Early Life. Oxford; Blackwell: 1967. [Google Scholar]
- Yap Q.J., Teh I., Fusar-Poli P., Sum M.Y., Kuswanto C., Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J. Neural Transm. 2013;120:1369–1395. doi: 10.1007/s00702-013-0971-7. [DOI] [PubMed] [Google Scholar]
- Yendiki A., Koldewyn K., Kakunoori S., Kanwisher N., Fischl B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Gao J., Shi H., Huang B., Wang X., Situ W., Yao S. Sex differences of uncinate fasciculus structural connectivity in individuals with conduct disorder. BioMed Res. Int. 2014;2014:1–9. doi: 10.1155/2014/673165. [DOI] [PMC free article] [PubMed] [Google Scholar]