Abstract
The societies of ants, bees and wasps are genetically closed systems where queens only mate during a brief mating episode prior to their eusocial life and males therefore provide queens with a lifetime supply of high-quality sperm. These ejaculates also contain a number of defence proteins that have been detected in the seminal fluid but their function and efficiency have never been investigated in great detail. Here, we used the honeybee Apis mellifera and quantified whether seminal fluid is able to combat infections of the fungal pathogen Nosema apis, a widespread honeybee parasite that is also sexually transmitted. We provide the first empirical evidence that seminal fluid has a remarkable antimicrobial activity against N. apis spores and that antimicrobial seminal fluid components kill spores in multiple ways. The protein fraction of seminal fluid induces extracellular spore germination, which disrupts the life cycle of N. apis, whereas the non-protein fraction of seminal fluid induces a direct viability loss of intact spores. We conclude that males provide their ejaculates with efficient antimicrobial molecules that are able to kill N. apis spores and thereby reduce the risk of disease transmission during mating. Our findings could be of broader significance to master honeybee diseases in managed honeybee stock in the future.
Keywords: antimicrobial activity, flow cytometry, spore germination, host parasite interaction
1. Introduction
Sex is the most widespread mode of reproduction in animals and has a number of well-documented advantages compared with clonal reproduction [1,2]. However, it can also incur substantial costs, such as for example the need for a species to produce two types of sexes [3]. Furthermore, in species engaging in reproductive behaviours involving close physical contact such as copulation, parasites can hitchhike on the mating process and be transmitted between sexes or to offspring [4]. To achieve this, sexually transmitted diseases contaminate ejaculates and use them as vectors for the transfer to females [4]. To counter this, males are expected to evolve adaptations to reduce the risk of infecting their mates or offspring and ejaculates are indeed known to contain molecules with antimicrobial activity [5,6]. Host–parasite interactions in reproductive tissues and secretions has received relatively little scientific attention in insects but have been studied in vertebrates [7], where it is suggested that proteins with antimicrobial functions within the ejaculate are of central importance for male fitness because they suppress microbial growth [8,9] and affect sperm motility [10]. Antimicrobial proteins in ejaculates have also been reported in insects [5,6,11–13], and antimicrobial activity of seminal fluid in the bedbug Cimex lectularius reduces sperm mortality in vitro [13,14]. However, the functioning of antimicrobial molecules in ejaculates or in the female's sexual tract or their influence on male and female reproductive success in insects have not been studied in detail.
The males of hymenopteran social insects (the eusocial ants, bees and wasps) are under strong selection to provide females (queens) with high-quality ejaculates [12,15,16], and components such as seminal fluid [5] have been identified as the key determinants of male fertility and fitness [17–19]. Proteomic analyses of seminal fluid have provided detailed insights into the complex biochemical networks that both support sperm [6,20] and target ejaculates of rival males to reduce their sperm viability and reproductive success [17]. These studies have also identified a number of antimicrobial and defence-related proteins that could counter potentially sexually transmitted diseases [6].
Male social insects are known to be more susceptible to parasites because their immunity is often reduced compared with workers and queens [21–23]. Two different explanations have been put forward to explain compromised male immunity in hymenopteran social insects. The haplodiploid susceptibility hypothesis states that haploidy in social insect males reduces genetic variation in their immune system genes and consequently increases their disease susceptibility [24]. Alternatively, the trade-off hypothesis states that social insect males reallocate immunity-related investments to maximize fertility in order to satisfy the exceptionally high demands on sperm numbers and quality [25]. In this case, antimicrobial and defence proteins within the ejaculate represent key long-term investments of males into high-quality ejaculates, as it is not in the interest of an infected male to transfer diseases to a queen. The two hypotheses therefore make different predictions about the efficiency of antimicrobial systems within ejaculates. The haplodiploid susceptibility hypothesis predicts that social insect males have very limited opportunities to compensate for a genetically determined inferiority of their antimicrobial defence. On the other hand, the trade-off hypothesis predicts that males invest into efficient antimicrobial defence in their ejaculates to minimize infection and fitness costs to their mates and offspring. However, to date, empirical work has been lacking that quantifies the biological activity, efficiency or the molecular mechanism of these defences.
Here, we used the European honeybee Apis mellifera, where queens mate with 25 or more males [26,27] and thereby increase a queen's risk of acquiring pathogens through ejaculates [4]. A number of honeybee pathogens have been detected both in ejaculates and in queens that were artificially inseminated with infected semen; for example, acute bee paralysis virus and deformed wing virus [28–30]. Furthermore, spores of the widespread fungal pathogens Nosema apis and Nosema ceranae have recently been identified in honeybee semen and are able to infect queens if transferred during mating [31,32]. Because some of the antimicrobial and defence-related proteins identified in the seminal fluid of honeybees have predicted antifungal activities [6], we hypothesized that these proteins reduce the viability of Nosema spores and the risk of disease transfer during mating.
2. Material and methods
(a). Seminal fluid collection
Mature A. mellifera drones were collected at the entrance of hives while returning from their daily mating flights in an apiary at the University of Western Australia. Up to 200 drones were placed in wooden cages and kept in foster colonies prior to the experiment. To collect ejaculates, we used a method developed earlier to artificially inseminate honeybees [33]. In short, we allowed drones to fly in a cage for 10 min before inducing ejaculation by anaesthetizing them with chloroform. We manually squeezed the males' abdomen between two fingers and collected ejaculates appearing at the tip of the endophallus with a pipette. Our previous work indicated that this mode of semen collection does not result in major contaminations, as indicated by the absence of highly abundant tissue or haemolymph proteins in our seminal fluid samples [6]. To separate sperm from seminal fluid, we used a previously developed protocol [6,20]. In brief, we centrifuged pooled semen samples for 25 min at 18 500g at 4°C. The supernatant containing the seminal fluid was collected and centrifuged again for 10 min at 18 500g in 4°C to remove remaining sperm and was then stored at −80°C.
(b). Nosema apis spore collection
We took advantage of the fact that Western Australia is currently free of N. ceranae, a pathogen that only recently switched from its original host, the Asian honeybee (Apis ceranae) to European honeybees [34]. Consequently, we were able to study interactions of antimicrobial molecules in the honeybee seminal fluid with N. apis in the absence of possible confounding effects of N. ceranae. To collect N. apis spores, we used a previously developed technique [31]. In brief, we caught honeybee workers at the entrance of hives with confirmed N. apis infections and killed them at −20°C. We dissected and pooled the intestines of 20 workers, added 1 ml of distilled water and homogenized the sample by adding a 3 mm tungsten bead (Qiagen, Australia). After manually shaking each sample for 2 min, we used 0.5 ml of the homogenate and layered it onto 1.5 ml of 100% Percoll (Sigma-Aldrich, Australia), followed by centrifugation at 18 000g for 60 min at 4°C. The pellet containing N. apis spores was washed with 1.5 ml DDI water, briefly vortexed and centrifuged again at 20 700g for 5 min at 4°C. This procedure was repeated three times before the spore pellet was finally resuspended in 100 µl of DDI water and the spore concentration quantified as described by Cantwell [35]. The final sample was diluted to a concentration of 109 spores ml−1, before freezing it at −80°C prior to any further experiments. Our previous experiments have confirmed that this method of collection and freezing does not impact the viability of the spores [36].
(c). Nosema apis spore viability in seminal fluid
To quantify the effect of seminal fluid on N. apis, we incubated spores in undiluted seminal fluid or seminal fluid diluted 1 : 2, 1 : 10, 1 : 100 and 1 : 1000, as well as in Hayes semen diluent (0.15 M NaCl, 1.80 mM CaCl2, 2.68 mM KCl, 1.19 mM NaHCO3) as a control. To do this, we used 106 spores in 10 µl of each treatment and incubated all samples at room temperature for 5 min, 4, 6, 8 and 24 h in the dark. For each treatment and incubation time, we used three independent biological replicates of seminal fluid that we collected prior to the experiment as described above. Spore viability was quantified using a flow cytometry method developed earlier [36]. In brief, we used fluorescent nucleic acid dyes and stained spore samples for 90 min in the dark on ice with 5 µM SYTO (16) green and 0.02 µM SYTOX red (Invitrogen, USA), which allowed us to distinguish live and dead spores [36]. To increase spore concentration in samples, we centrifuged them at 20 800g for 10 min at 4°C and discarded 680 µl of the supernatant before resuspending the spores in the remaining supernatant. We then used a BD FASCalibur flow cytometer with CellQuest Pro v. 5.1™ (Becton Dickinson, USA) to quantify N. apis spore viability in our samples. A 488 nm (blue) laser was used to excite SYTO green (emission collected using 530/30 BP filter) and a 635 nm (red) laser was used to excite SYTOX red (emission collected using 670/30 BP filter). All parameters were recorded using logarithmic amplifications. In the forward scatter (FSC) and side scatter (SSC), we set the flow cytometer to capture 10 000 spores, but smaller particles were also gated that we will referred to as debris. We used FlowJo v. 10 for Windows (TreeStar, USA) for gating and analysis of the raw data. We used spore solutions with known viabilities to set live and dead spore gates. Within the spore gate, the spores were sub-gated into live and dead spores within the FL1 and FL4 channels and the percentages of live (Q4 + Q3) and dead (Q1 + Q2) spores were calculated for each sample. The debris was sub-gated into general and fluorescent debris, which is made up of particles exceeding a fluorescent intensity of 5 in SYTO green-stained particles. The fluorescent debris was quantified as a ratio of the total count of particles passing the flow cytometer. Each biological sample was measured twice and averages of technical replicates were used for statistical analyses.
(d). Nosema apis spore germination
Our microscopic and flow cytometry work indicated that seminal fluid induces germination-like rupture of N. apis spores, which is the first step to establish an infection and characterized by the protrusion of a polar tube that will penetrate a host cell to transfer nuclear material of the parasite [37]. To confirm that some of the debris observed in our flow cytometry data resembles that derived from germinated N. apis spores, we artificially induced spore germination as described by De Graaf [37]. To do this we used 106 N. apis spores and added 0.5 M sodium chloride, 0.5 M sodium hydrogen carbonate, pH 6.0 (0.1 M orthophosphoric acid) and incubated the sample for 15 min at 37°C. Microscopic confirmation of successful germination was carried out using an Olympus BX53 microscope with a PlanN (UIS2) lens under DIC. Digital photos were taken using the UC50 camera and on the LabSens software (Olympus, Japan). Spore viability and debris were then measured using flow cytometry as described above.
(e). Size exclusion separation to isolate the active component in seminal fluid
To measure the biological activity of different fractions of seminal fluid, we split seminal fluid samples into a protein fraction, containing molecules more than 3 kDa, and a non-protein fraction, containing small molecules less than 3 kDa, using Amicon® Ultra-0.5 ml 3 kDa molecular weight cut-off centrifugal filters (Millipore Corporation, USA). After centrifugation of 100 µl of seminal fluid for 20 min at 14 000g at 4°C, the flow through (FT) was recovered and 100 µl of Hayes was added to the protein fraction of seminal fluid and centrifuged again for 20 min at 14 000g at 4°C. This procedure was repeated five times to ensure maximal removal of the non-protein part of seminal fluid before the protein fraction was collected by reversing the cartridge and centrifuging for 10 min at 1000g in 4°C. Both fractions were reconstituted back to the original volume of 100 µl using Hayes solution; the washes were kept separately. We collected a total of four independent biological replicates of seminal fluid for this experiment and quantified the antimicrobial activity of both fractions on N. apis spores using flow cytometry, as described above. To visualize the success of our separation, we used SDS-PAGE and ran 30 µl of the non-protein and 3 µl of the protein fraction, as well as 3 µl of non-fractionated seminal fluid and 30 µl Hayes as controls. To do this, we used a 4–20% Mini-PROTEAN® TGX™ Precast Protein Gel (Bio-Rad), run at constant 200 V, running a Dual Xtra LMW standard (250-2 kDa) (Bio-Rad). The gel was stained with Coomassie. As shown in the electronic supplementary material, figure S1, the SDS-PAGE protein separation showed comparable protein banding patterns for the protein fraction and non-fractionated seminal fluid. As expected, no protein or peptide bands are visible in the non-protein fraction and the Hayes control lane. Consequently, our centrifugation protocol successfully separated seminal fluid into a protein and non-protein fraction, which is consistent with results we published earlier [19].
The fractions were furthermore visualized by spectrophotometry, using a Nano-drop (Thermo Scientific, Wilmington, USA). To do this, we analysed 2 µl in protein mode and visualized absorbance between 220 and 350 nm.
(f). Solid-phase extraction and protein separation of seminal fluid samples
To further quantify the biological activity of different protein fractions of seminal fluid, we used solid-phase extraction (SPE) C18 Macro SpinColumns (Nest Group, USA) according to the manufacturer's instructions. Four biological replicates were used for separation, 300 µl of sample (40 µl of the seminal fluid added to 260 µl of DDI water with 0.1% trifluoroacetic acid (TFA)) was loaded onto the cartridge, then the FT, wash and 20, 30, 40, 60 and 80% acetonitrile (ACN) elutions were collected by centrifugation. Fractions were dried in a vacuum centrifuge (Acid Resistant CentriVap Concentrator, Labconco, USA) until dryness and reconstituted in 20 µl of Hayes. In order to further determine the nature of the active compounds, the protein fractions (more than 3 kDa) were reconstituted to 300 µl using 0.1% TFA in DDI water and separated using C18 SPE-cartridges (as described above) and eluted with 0, 30 or 80% ACN by centrifugation. Because of the very small amounts of material that became available, we were not able to visualize samples on SDS-PAGE and no chromatograms were available for the individual fractions.
(g). Zone of inhibition assays
To quantify antimicrobial activity of seminal fluid in bacteria, instead of N. apis, we performed zone of inhibition assays, using Arthrobacter globiformis. Bacteria were grown at 30°C either in Luria-Bertani (LB) broth or on LB agar plates (15 g l−1 agar). An overnight culture of A. globiformis was prepared in 10 ml of LB broth and incubated overnight at 30°C with shaking at 80 r.p.m. We then layered 150 µl of the bacterial culture onto LB plates. Droplets of 1 µl of seminal fluid or ampicillin as a positive control were added to LB plates and incubated for 48 h at 30°C. We then checked the plates for signs of reduced bacterial growth, indicated by clear circles around areas where seminal fluid or ampicillin samples had been applied. These assays were performed three times.
(h). Statistics
Statistical analyses were performed using Rx64 2.14.0 software for Window v. 7 and SPSS for Macintosh. Prior to statistical analysis, we calculated linear regression residuals to determine whether data were normally distributed. ANOVA and Tukey's Honestly Significant Difference post hoc or Tukey's HSD tests were used to test for differences between treatments and to identify significant differences within treatments. For datasets that we were not normally distributed, we used Generalized Linear Models, Kruskal–Wallis and Mann–Whitney U-tests.
3. Results
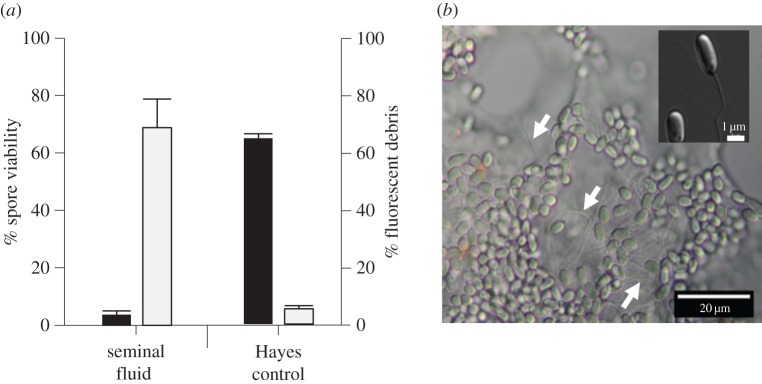
We found that seminal fluid of honeybees is remarkably efficient in killing N. apis by significantly reducing spore viability compared with the control treatment (figure 1, Wald-χ2 = 1857.934, d.f. = 5, p < 0.001). Furthermore, incubation time had no significant effect on N. apis spore viability (Wald-χ2 = 0.416, d.f. = 1, p = 0.416). Consequently, the antimicrobial effect of seminal fluid occurred rapidly and did not alter spore viabilities afterwards for up to 24 h. We therefore performed all further experiments using an incubation time of 5 min. Post hoc tests revealed that the spore killing effect was most pronounced in pure seminal fluid, but statistically significant reductions in spore viability were also found in samples diluted up to 10 times (figure 1). When we analysed our flow cytometry data in more detail (figure 2a), we found that the amount of fluorescent debris increased significantly in samples with higher concentrations of seminal fluid (Wald-χ2 = 178.598, d.f. = 5, p < 0.001) but was not influenced by incubation time (Wald-χ2 = 0.068, d.f. = 1, p = 0.795). When we inspected these samples microscopically, we found that exposure to seminal fluid resulted in the clumping and apparent germination of N. apis spores (figure 2b), while control spores did not show any signs of germination-like rupture and clumping.
Figure 1.
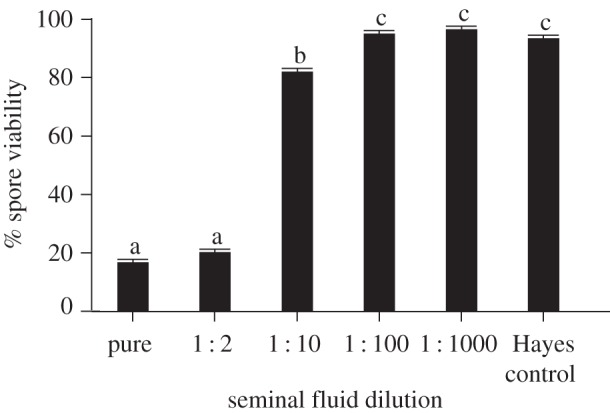
The seminal fluid of honeybees (A. mellifera) is highly efficient in killing spores of the fungal pathogen N. apis. Flow cytometry identified two distinct populations of spores (for methodological details, see [36]), consisting of live and dead spores. Spore viability was consequently calculated as the percentage of live spores within the total amount of spores counted. Letters above columns indicate significant differences from post hoc tests between treatments. See main text for statistical details. Data are presented as means and standard errors of means.
Figure 2.
(a) The effect of seminal fluid and Hayes control solution on the viability of N. apis spores (black columns) and the fluorescent debris detected in samples by flow cytometry (grey columns). Germination resulted in the release of nuclear material from spores and consequently increased the amount of fluorescent debris detected by flow cytometry. (b) Differential Interference Contrast image of N. apis spores exposed to complete seminal fluid, showing the clumping of spores and filaments protruding from germinated spores (white arrows; see insert for a magnified view of a single germinated spore and its filament). Data are presented as means and standard errors of means. (Online version in colour.)
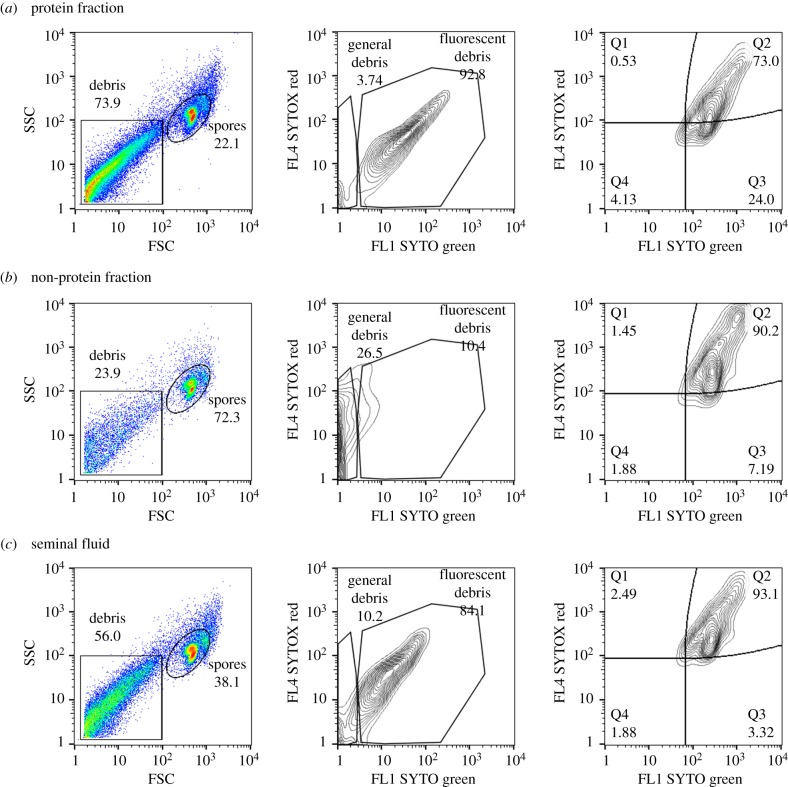
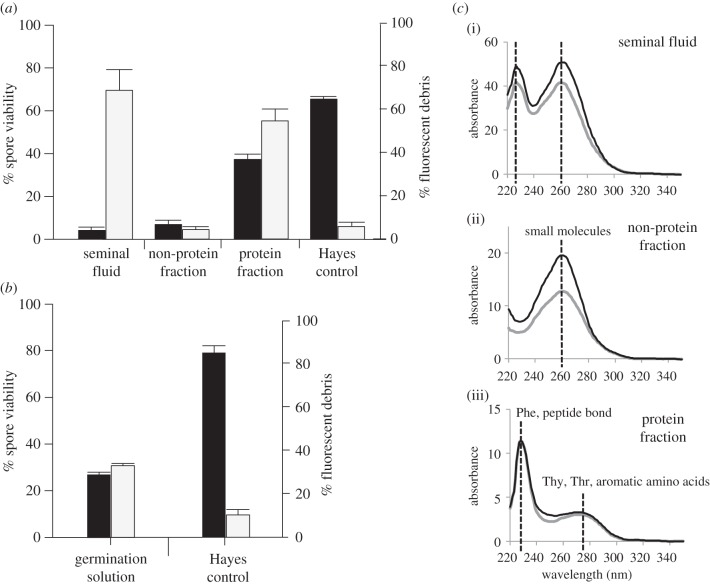
When we used flow cytometry to visualize the antimicrobial effect of pure seminal fluid, the protein fraction and the non-protein fraction on N. apis spores (see figure 3 for examples of flow cytometry dot plots), we found fluorescent debris only in samples where spores were exposed to undiluted and the protein fraction of seminal fluid. When we consequently quantified the antimicrobial effect of the non-protein and protein fractions on N. apis, we found that both fractions significantly reduced spore viability compared with the control treatment (figure 4a, ANOVA, F = 209.2, d,f, = 3, p < 0.001). Post hoc tests revealed that the potency of the non-protein fraction in killing spores was not significantly different from complete seminal fluid (Tukey's HSD, p = 0.790), but the protein fraction killed significantly fewer spores compared with complete seminal fluid (ANOVA, F = 209.2, d.f. = 3, p < 0.001). As expected from our visual observations, the amount of fluorescent debris differed significantly between treatments (figure 4a, ANOVA, F = 11.82, d.f. = 3, p < 0.001) and was lower in the non-protein fraction compared with the protein fraction (Tukey's HSD, p = 0.005) or seminal fluid (Tukey's HSD, p = 0.024). There was no significant difference in the amount of fluorescent debris between the protein fraction and complete seminal fluid (Tukey's HSD, p = 0.806) or between the non-protein fraction and the control sample (figure 4a, Tukey's HSD, p = 0.924).
Figure 3.
Dot plots from light scatter (FSC/SSC) analysis of N. apis-treated spores with the protein fraction (a), the non-protein fraction (b) and seminal fluid (c). Dot plots were gated into two regions: spores and debris as high FSC/high SSC and low FSC/low SSC. The debris was further sub-gated into general and fluorescent debris (containing nucleic acid material). The spores were further sub-gated into two populations, dead spores (Q1 + Q2) and live spores (Q4 + Q3). (Online version in colour.)
Figure 4.
(a) The antimicrobial effect of pure seminal fluid, as well as the non-protein and protein fractions of seminal fluid on N. apis spore viability (black columns) and fluorescent debris (grey columns). All seminal fluid samples significantly decreased spore viability compared with the Hayes control treatment, but only complete seminal fluid and the protein fraction of seminal fluid resulted in a significant increase in the amount of fluorescent debris. (b) The effect of germination solution (see [37] for details) on N. apis was comparable to that of the protein fraction as seen in (a). Germination solution significantly decreased N. apis viability and increased the amount of fluorescent debris measured by flow cytometry. (c) Absorbance spectra of unfractionated seminal fluid (i), the non-protein (ii) and protein fraction (iii) of seminal fluid. The protein fraction of seminal fluid shows a peak for the aromatic amino acids and peptide bond, whereas the non-protein fraction shows a different peak at 260 nm. The black and grey lines show data from two biological replicates. Data are presented as means and standard errors of means.
When we analysed N. apis spores exposed to germination solution, we found a significant reduction in spore viability (Mann–Whitney U-test: N = 8, p = 0.029) and a significant increase of fluorescent debris (Mann–Whitney U-test: N = 8, p = 0.029) compared with the Hayes control (figure 4b). Because these findings were similar to those obtained from spores exposed to the protein fraction of seminal fluid (figure 4a), we conclude that molecules in the protein fraction induce germination-like rupture of the Nosema spore wall, whereas the non-protein fraction killed spores without spore wall rupture.
Furthermore, the absorbance spectra of the different fractions showed three distinct peaks (figure 4c). Non-fractionated seminal fluid showed two peaks, one at 220–235 nm and another at 245–275 nm. When the seminal fluid was separated into the non-protein and protein fractions, the non-protein fraction contained small molecules (less than 3 kDa) that have an absorbance at 245–275 nm, while a peak consistent with a peptide bond or phenylalanine at 220–235 nm is not visible. The protein fraction, on the other hand, contained molecules that have an absorbance consistent with proteins, showing a peak for aromatic amino acids, such as threonine and tyrosine at 275–290 nm and a peak at 220–235 nm consistent with the peptide bond.
When we analysed the effect of different reverse-phase C18 SPE separation fractions of seminal fluid, we found an overall significant reduction in N. apis spore viability (Kruskal Wallis, χ2 = 25.79, d.f. = 7, p < 0.001, figure 5). Post hoc comparison revealed significant reductions in spore viability in the FT, 40%, 60% and 80% (v/v) ACN fractions compared with the Hayes control solution. Furthermore, only the fractions eluted with 40% and 60% ACN produced significantly more fluorescent debris compared with the Hayes control. The FT fraction killed spores but did not produce more fluorescent debris than the Hayes control. The detected activity of the FT as well as proteins that bind to the stationary phase therefore provide further evidence for the presence of at least two types of molecules that are capable to kill N. apis spores.
Figure 5.
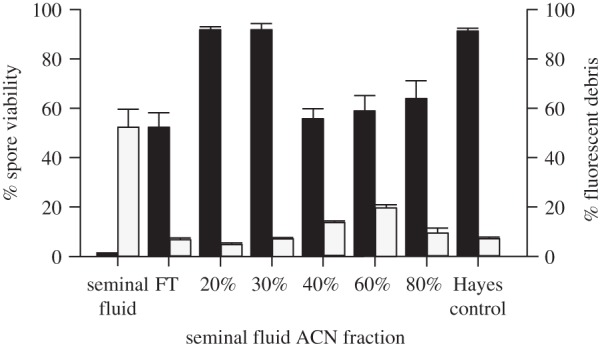
Antimicrobial effect of seminal fluid fractions after C18 (reverse-phase) SPE-separation on N. apis spore viability (black columns) and fluorescent debris (grey columns). Apart from complete seminal fluid, we also found the FT, and the 40%, 60% and 80% fractions to significantly reduce N. apis spore viability, compared with the Hayes control. This coincided with a significant increase in fluorescent debris detected in complete seminal fluid and the 40% and 60% fractions compared with the Hayes control but not the FT. Data are presented as means and standard errors of means.
A further separation of the protein fraction (more than 3 kDa) by C18 reverse-phase showed that the molecules retained killed N. apis spores and produced fluorescent debris using the fraction eluted with 80% ACN. We therefore confirmed that a protein fraction compound induced spore wall rupture, whereas a non-protein fraction compound had a direct killing effect on N. apis spores. Finally, we found that the two biologically active compounds must have substantially different biochemical properties, given the different affinity to the C18 reverse-phase solid-phase that complements the evidence of different absorbance spectra from the protein and non-protein fractions (figure 4c).
Finally, we found that seminal fluid had no measureable effects on the growth of A. globiformins compared with the ampicillin control treatment, as no measurable zones of inhibition were observed in any of the seminal fluid samples tested. Follow-up work using two additional microbial species (Escherichia coli and Saccharomyces cerevisiae) provided the same results.
4. Discussion
We provide empirical evidence that seminal fluid of honeybees has a remarkable anti-microsporidial activity and is able to reduce spore viability of the sexually transmitted pathogen N. apis by over 80% (figures 1a, 2a, 4a and 5). Our findings imply that males are able to efficiently protect their ejaculates in order to reduce the risk of sexually transmitting the pathogen to the queen during mating. Our findings therefore provide support for the trade-off hypothesis, implying that males maximize immunity in their germ line at the cost of their somatic tissue. This adds further empirical evidence for the trade-off hypothesis to what has already been published in ants and bees [15,25,38]. Our results are also in line with our previous work, where we found males to be highly susceptible to N. apis [31], but able to suppress the spread of the infection to their reproductive organs. Contaminations of the ejaculate seem to occur as a consequence of dysentery and/or the rupturing of tissue during the ejaculation process and the disease can consequently be transmitted during mating from the male to the queen [32]. Our findings therefore imply that the anti-microsporidial activity of seminal fluid provides an additional male adaptation to further reduce the risk of sexual transmission, but does not provide complete protection. As we show, seminal fluid is highly efficient in reducing the viability of N. apis spores (figures 1a, 2a, 4a, 5), but a small fraction of spores are able to survive exposure to seminal fluid for up to 24 h. Experimental follow-up work confirmed that these spores are still capable of infecting worker bees (B. Baer, J. Grassl, Y. Peng, A. Mittra, C. Browne, 2015, unpublished data). It is therefore likely that the N. apis spore samples used for our experiments contained a mixture of spores from several individual strains, as we collected them from workers of different colonies. Consequently, surviving spores might represent strains with some level of resistance against the antimicrobial activity within the seminal fluid. More experimental work is required to test the idea that the survival of spores in seminal fluid samples is indeed non-random, and whether seminal fluids of different genotypes of honeybees vary in their effectiveness to kill individual N. apis strains. If this is the case, studying antimicrobial activity of seminal fluid against N. apis spores offers a new opportunity to unravel host–parasite interactions and the underlying genotype × genotype interactions at the proteomic level.
Our experiments provide further insights into a remarkable complexity of antimicrobial activity within an insect ejaculate. First, from our analyses of the fluorescent debris data (figures 3 and 4a), microscopic observations (figure 2b) spectrophotometric absorbance (figure 4c) and the comparison of antimicrobial activity in different subfractions of seminal fluid (figure 5), we conclude that seminal fluid components kill N. apis spores in at least two distinctly different ways. The protein fraction of the seminal fluid induces germination-like rupture of the spore wall, whereas small molecules in the non-protein fraction of seminal fluid kills N. apis spores directly, implying some form of redundancy within the defence system of the honeybees ejaculate. Second, we found that the defence response against N. apis also shows a degree of specificity, as our zone of inhibition experiments detected no antimicrobial activity of seminal fluid towards a number of other microorganisms. Such a specific antimicrobial response to a pathogen in the ejaculate has so far only been described for vertebrates [7,9]. Consequently, finding redundancy and specificity of an immune protection in an insect is quite surprising and requires further research to unravel the genetic and biochemical mechanisms that underlie the phenotypic interaction between the honeybee's antimicrobial defence and the N. apis pathogen, using novel approaches such as evolutionary proteomics [39].
The antimicrobial activity that we found in the protein fraction of seminal fluid is consistent with a protein or proteins being responsible for the observed antimicrobial effect, based on their observed size, absorbance and chemical properties. Interestingly, we found that the protein fraction of seminal fluid induced the germination of N. apis spores. Germination of microsporidia, such as Nosema, can be caused by a number of factors such as changes in pH, temperature, ionic concentrations or exposure of dehydrated spores to water [37,40–42]. Regardless of the trigger, spore germination results from an increase of osmotic pressure inside the spore that eventually triggers the expulsion of the polar tube [41–43], but the exact mechanisms are still unknown. Here, we conclude that the protein fraction also plays a role in spore germination and thereby killing the microsporidia. Seminal fluid of insects contains a range of proteins with predicted antimicrobial activity including proteases, peptidases [6,20,44–46] and chitinase in honeybees [6]. Fungal cell walls contain a chitin/protein matrix; rupture requires chitinase and proteases and this is a normal part of the germination process in the life cycle of fungi that produce spores [47]. Significant work has been undertaken on fungal chitinase and proteases that would act from inside of the spore in model fungi [47]. Premature weakening of the wall by exogenous proteases and chitinases from the seminal fluid could therefore lead to rupture of the spore wall due to turgor pressure from the cell and expansion of the spore cell, which would also expose the fungal cell to other forms of antimicrobial attack by the insect defence machinery [48,49]. The specificity of the effect we detected in our samples is consistent with the known properties of chitinases that appear to be optimized for chitin/protein matrices in different species and to act synergistically with a complex network of proteases [50–53]. More work is required to specifically study whether the chitinases and/or proteases present in the seminal fluid are indeed involved in triggering spore germination in N. apis. Spore germination ultimately results in the expulsion of genomic material from the spore, and in the case of seminal fluid-treated spores, we find this material to accumulate in the fluorescence debris. Successful N. apis infections depend on the parasite delivering its sporoplasm into the cytosol of a host cell [42,54]. The protein fraction of the seminal fluid therefore provides an efficient pathway to interrupt the life cycle of N. apis spores and indirectly killing the spores before they could propagate.
Based on the SDS-PAGE gel (electronic supplementary material, figure S1), the absorbance spectra (figure 4c) and the chemical properties of active fractions in their interaction with C18, we can conclude that the biologically active molecule in the non-protein fraction of seminal fluid is unlikely to be either a protein or a peptide. Nevertheless, the identity of the antimicrobial molecule(s) and its biological mode of activity will need to be studied in more detail in the future. The non-protein fraction of seminal fluid is able to decrease spore viability without cell wall rupture and does not cause spore germination, implying it can enter the spore directly. Analysis of seminal fluid-treated spores and/or biochemical dissection of seminal fluid samples, using the bioassays of Nosema spore viability or rupture as developed here, could be used in the future to identify the compounds responsible.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the honeybee keepers of Western Australia for providing necessary honeybee material for this study.
Data accessibility
The datasets supporting this article are accessible at http://dx.doi.org/10.5061/dryad.18bv9. There is one supplementary figure attached to this paper that can be downloaded as a part of the electronic supplementary material.
Authors' contributions
Y.P. and B.B. designed and Y.P. and J.G. conducted the experimental work presented in this paper, all authors contributed to the analyses of data and the writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We were supported by the Australian Research Council (ARC) through a Queen Elizabeth II Fellowship and a Future Fellowship to B.B., a Future Fellowship to A.H.M., an ARC Linkage Project to B.B. and the facilities of the ARC Centre of Excellence in Plant Energy Biology.
References
- 1.Hamilton WD. 1980. Sex versus non-sex versus parasite. Oikos 35, 282–290. ( 10.2307/3544435) [DOI] [Google Scholar]
- 2.Hamilton WD, Axelrod R, Tanese R. 1990. Sexual reproduction as an adaptation to resist parasites: a review. Proc. Natl Acad. Sci. USA 87, 3566–3573. ( 10.1073/pnas.87.9.3566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maynard Smith J. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Knell RJ, Webberley KM. 2004. Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol. Rev. 79, 557–581. ( 10.1017/s1464793103006365) [DOI] [PubMed] [Google Scholar]
- 5.Poiani A. 2006. Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 60, 289–310. ( 10.1007/s00265-006-0178-0) [DOI] [Google Scholar]
- 6.Baer B, Heazlewood JL, Taylor NL, Eubel H, Millar AH. 2009. The seminal fluid proteome of the honeybee Apis mellifera. Proteomics 9, 2085–2097. ( 10.1002/pmic.200800708) [DOI] [PubMed] [Google Scholar]
- 7.Stamey TA, Fair WR, Timothy MM, Chung HK. 1968. Antibacterial nature of prostatic fluid. Nature 218, 444–447. ( 10.1038/218444a0) [DOI] [PubMed] [Google Scholar]
- 8.Edstrom AML, Malm J, Frohm B, Martellini JA, Giwercman A, Morgelin M, Cole AM, Sorensen OE. 2008. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J. Immunol. 181, 3413–3421. ( 10.4049/jimmunol.181.5.3413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mardh PA, Colleen S. 1975. Antimicrobial activity of human seminal fluid. Scand. J. Urol. Nephrol. 9, 17–23. ( 10.3109/00365597509139907) [DOI] [PubMed] [Google Scholar]
- 10.Narciandi F, Lloyd A, Meade KG, O'Farrelly C. 2014. A novel subclass of bovine β-defensins links reproduction and immunology. Reprod. Fertil. Dev. 26, 769–777. ( 10.1071/rd13153) [DOI] [PubMed] [Google Scholar]
- 11.Lung O, Kuo L, Wolfner MF. 2001. Drosophila males transfer antibacterial proteins from their accessory glands and ejaculatory duct to their mates. J. Insect Physiol. 47, 617–622. ( 10.1016/S0022-1910(00)00151-7) [DOI] [PubMed] [Google Scholar]
- 12.Zareie R, Eubel H, Millar AH, Baer B. 2013. Long-term survival of high quality sperm: insights into the sperm proteome of the honeybee Apis mellifera. J. Proteome Res. 12, 5180–5188. ( 10.1021/pr4004773) [DOI] [PubMed] [Google Scholar]
- 13.Otti O, Naylor RA, Siva-Jothy MT, Reinhardt K. 2009. Bacteriolytic activity in the ejaculate of an insect. Am. Nat. 174, 292–295. ( 10.1086/600099) [DOI] [PubMed] [Google Scholar]
- 14.Otti O, McTighe AP, Reinhardt K. 2013. In vitro antimicrobial sperm protection by an ejaculate-like substance. Funct. Ecol. 27, 219–226. ( 10.1111/1365-2435.12025) [DOI] [Google Scholar]
- 15.Sturup M, Baer-Imhoof B, Nash DR, Boomsma JJ, Baer B. 2013. When every sperm counts: factors affecting male fertility in the honeybee Apis mellifera. Behav. Ecol. Sociobiol. 24, 1192–1198. ( 10.1093/beheco/art049) [DOI] [Google Scholar]
- 16.Hunter FM, Birkhead TR. 2002. Sperm viability and sperm competition in insects. Curr. Biol. 12, 121–123. ( 10.1016/S0960-9822(01)00647-9) [DOI] [PubMed] [Google Scholar]
- 17.den Boer SPA, Baer B, Boomsma JJ. 2010. Seminal fluid mediates ejaculate competition in social insects. Science 327, 1506–1509. ( 10.1126/science.1184709) [DOI] [PubMed] [Google Scholar]
- 18.den Boer SPA, Boomsma JJ, Baer B. 2008. Seminal fluid enhances sperm viability in the leafcutter ant Atta colombica. Behav. Ecol. Sociobiol. 62, 1843–1849. ( 10.1007/s00265-008-0613-5) [DOI] [Google Scholar]
- 19.King M, Eubel H, Millar AH, Baer B. 2011. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J. Insect Physiol. 57, 409–414. ( 10.1016/j.jinsphys.2010.12.011) [DOI] [PubMed] [Google Scholar]
- 20.Baer B, Zareie R, Paynter E, Poland V, Millar AH. 2012. Seminal fluid proteins differ in abundance between genetic lineages of honeybees. J. Proteomics 75, 5646–5653. ( 10.1016/j.jprot.2012.08.002) [DOI] [PubMed] [Google Scholar]
- 21.Gerloff CU, Ottmer BK, Schmid Hempel P. 2003. Effects of inbreeding on immune response and body size in a social insect, Bombus terrestris. Funct. Ecol. 17, 582–589. ( 10.1046/j.1365-2435.2003.00769.x) [DOI] [Google Scholar]
- 22.Baer B, Krug A, Boomsma JJ, Hughes WOH. 2005. Examination of the immune responses of males and workers of the leaf-cutting ant Acromyrmex echinatior and the effect of infection. Insectes Soc. 52, 298–303. ( 10.1007/s00040-005-0809-x) [DOI] [Google Scholar]
- 23.Vainio L, Hakkarainen H, Rantala MJ, Sorvari J. 2004. Individual variation in immune function in the ant Formica exsecta; effects of the nest, body size and sex. Evol. Ecol. 18, 75–84. ( 10.1023/B:EVEC.0000017726.73906.b2) [DOI] [Google Scholar]
- 24.O'Donnell S, Beshers SN. 2004. The role of male disease susceptibility in the evolution of haplodiploid insect societies. Proc. R. Soc. Lond. B 271, 979–983. ( 10.1098/rspb.2004.2685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturup M, Baer B, Boomsma JJ. 2014. Short independent lives and selection for maximal sperm survival make investment in immune defences unprofitable for leaf-cutting ant males. Behav. Ecol. Sociobiol. 68, 947–955. ( 10.1007/s00265-014-1707-x) [DOI] [Google Scholar]
- 26.Schlüns H, Moritz RFA, Neumann P, Kryger P, Koeniger G. 2005. Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim. Behav. 70, 125–131. ( 10.1016/j.anbehav.2004.11.005) [DOI] [Google Scholar]
- 27.Baer B. 2005. Sexual selection in Apis bees. Apidologie 36, 187–200. ( 10.1051/apido:2005013) [DOI] [Google Scholar]
- 28.de Miranda JR, Fries I. 2008. Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invertebr. Pathol. 98, 184–189. ( 10.1016/j.jip.2008.02.004) [DOI] [PubMed] [Google Scholar]
- 29.Yue C, Schroder M, Gisder S, Genersch E. 2007. Vertical-transmission routes for deformed wing virus of honeybees (Apis mellifera). J. Gen. Virol. 88, 2329–2336. ( 10.1099/vir.0.83101-0) [DOI] [PubMed] [Google Scholar]
- 30.Yue C, Schroder M, Bienefeld K, Genersch E. 2006. Detection of viral sequences in semen of honeybees (Apis mellifera): evidence for vertical transmission of viruses through drones. J. Invertebr. Pathol. 92, 105–108. ( 10.1016/j.jip.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 31.Peng Y, Imhoof-Baer B, Millar AH, Baer B. 2015. Consequences of Nosema apis infection for male honey bees and their fertility. Sci. Rep. 5, 1–5. ( 10.1038/srep10565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts K, Evison S, Baer B, Hughes WOH. 2015. The cost of promiscuity: sexual transmission of Nosema microsporidian parasites in polyandrous honey bees. Sci. Rep. 5, 1–7. ( 10.1038/srep10982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schley P. 1987. Einführung in die Technik der instrumentellen Besamung von Bienenköniginnen, 2nd edn, 103 p; Giessen, Germany: Köhler Offset KG. [Google Scholar]
- 34.Williams GR, Shutler D, Little CM, Burgher-MacLellan KL, Rogers REL. 2011. The microsporidian Nosema ceranae, the antibiotic Fumagilin-B (R), and western honey bee (Apis mellifera) colony strength. Apidologie 42, 15–22. ( 10.1051/apido/2010030) [DOI] [Google Scholar]
- 35.Cantwell GE. 1970. Standard methods for counting Nosema spores. Am. Bee J. 110, 223. [Google Scholar]
- 36.Peng Y, Lee-Pullen TF, Heel K, Millar AH, Baer B. 2014. Quantifying spore viability of the honey bee pathogen Nosema apis using flow cytometry. Cytom. Part A 85, 454–462. ( 10.1002/cyto.a.22428) [DOI] [PubMed] [Google Scholar]
- 37.De Graaf DC, Masschelein G, Vandergeynst F, Debrabander HF, Jacobs FJ. 1993. In vitro germination of Nosema apis (Microsporidia, Nosematidae) spores and its efftect on their αα-trehalose/D-glucose ratio. J. Invertebr. Pathol. 62, 220–225. ( 10.1006/jipa.1993.1103) [DOI] [Google Scholar]
- 38.Sturup M, den Boer SPA, Nash DR, Boomsma JJ, Baer B. 2011. Variation in male body size and reproductive allocation in the leafcutter ant Atta colombica: estimating variance components and possible trade-offs. Insectes Soc. 58, 47–55. ( 10.1007/s00040-010-0115-0) [DOI] [Google Scholar]
- 39.Baer B, Millar AH. 2016. Proteomics in evolutionary ecology. J. Proteomics. ( 10.1016/j.jprot.2015.09.031) [DOI] [PubMed] [Google Scholar]
- 40.Leitch GJ, Ceballos C. 2008. Effects of host temperature and gastric and duodenal environments on microsporidia spore germination and infectivity of intestinal epithelial cells. Parasitol. Res. 104, 35–42. ( 10.1007/s00436-008-1156-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Undeen AH, Vander Meer RK. 1999. Microsporidian intrasporal sugars and their role in germination. J. Invertebr. Pathol. 73, 294–302. ( 10.1006/jipa.1998.4834) [DOI] [PubMed] [Google Scholar]
- 42.Frixione E, Ruiz L, Cerbon J, Undeen AH. 1997. Germination of Nosema algerae (Microspora) spores: conditional inhibition by D2O, ethanol and Hg2+ suggests dependence of water influx upon membrane hydration and specific transmembrane pathways. J. Eukaryot. Microbiol. 44, 109–116. ( 10.1111/j.1550-7408.1997.tb05946.x) [DOI] [PubMed] [Google Scholar]
- 43.Undeen AH, Frixione E. 1990. The role of osmotic pressure in the germination of Nosema algerae spores. J. Protozool. 37, 561–567. ( 10.1111/j.1550-7408.1990.tb01265.x) [DOI] [PubMed] [Google Scholar]
- 44.Chapman T. 2001. Seminal fluid-mediated fitness traits in Drosophila. Heredity 87, 511–521. ( 10.1046/j.1365-2540.2001.00961.x) [DOI] [PubMed] [Google Scholar]
- 45.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56, 21–40. ( 10.1146/annurev-ento-120709-144823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirot LK, Hardstone MC, Helinski MEH, Ribeiro JMC, Kimura M, Deewatthanawong P, Wolfner MF, Harrington LC. 2011. Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Neglect. Trop. Dis. 5, e989 ( 10.1371/journal.pntd.0000989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartl L, Zach S, Seidl-Seiboth V. 2011. Fungal chitinases: diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 93, 533–543. ( 10.1007/s00253-011-3723-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer KJ, Subbaratnam M. 1997. Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem. Mol. Biol. 11, 887–900. ( 10.1016/S0965-1748(97)00078-7) [DOI] [PubMed] [Google Scholar]
- 49.Arakane Y, Muthukrishnan S. 2010. Insect chitinase and chitinase-like proteins. Cell Mol. Life Sci. 67, 201–216. ( 10.1007/s00018-009-0161-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giri AP, Harsulkar AM, Patankar AG, Gupta VS, Sainani MN, Deshpande VV, Ranjekar PK. 1998. Association of induction of protease and chitinase in chickpea roots with resistance to Fusarium oxysporum f.sp. ciceri. Plant Pathol. 47, 693–699. ( 10.1046/j.1365-3059.1998.00299.x) [DOI] [Google Scholar]
- 51.Machinandiarena M, Castillo M, Olivieri F, Daleo G, Oliva C. 2001. Protease inhibitor activity is associated to a basic chitinase from potato but not to an acidic one. Potato Res. 44, 187–195. ( 10.1007/BF02410105) [DOI] [Google Scholar]
- 52.Hodgson JJ, Arif BM, Krell PJ. 2011. Interaction of Autographa californica multiple nucleopolyhedrovirus cathepsin protease progenitor (proV-CATH) with insect Baculovirus chitinase as a mechanism for proV-CATH cellular retention. J. Virol. 85, 3918–3929. ( 10.1128/JVI.02165-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leger RJS, Charnley AK, Cooper RM. 1985. Cuticle-degrading enzymes of entomopathogenic fungi: mechanisms of interaction between pathogen enzymes and insect cuticle. J. Invertebr. Pathol. 47, 295–302. ( 10.1016/0022-2011(86)90099-6) [DOI] [Google Scholar]
- 54.De Graaf DC, Raes H, Sabbe G, De Rycke PH, Jacobs FJ. 1994. Early development of Nosema apis (Microspora: Nosematidae) in the midgut epithelium of the honeybee (Apis mellifera). J. Invertebr. Pathol. 63, 74–81. ( 10.1006/jipa.1994.1012) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are accessible at http://dx.doi.org/10.5061/dryad.18bv9. There is one supplementary figure attached to this paper that can be downloaded as a part of the electronic supplementary material.