Abstract
Population ecology has classically focused on pairwise species interactions, hindering the description of general patterns and processes of population abundance at large spatial scales. Here we use the metabolic theory of ecology as a framework to formulate and test a model that yields predictions linking population density to the physiological constraints of body size and temperature on individual metabolism, and the ecological constraints of trophic structure and species richness on energy partitioning among species. Our model was tested by applying Bayesian quantile regression to a comprehensive reef-fish community database, from which we extracted density data for 5609 populations spread across 49 sites around the world. Our results indicate that population density declines markedly with increases in community species richness and that, after accounting for richness, energetic constraints are manifested most strongly for the most abundant species, which generally are of small body size and occupy lower trophic groups. Overall, our findings suggest that, at the global scale, factors associated with community species richness are the major drivers of variation in population density. Given that populations of species-rich tropical systems exhibit markedly lower maximum densities, they may be particularly susceptible to stochastic extinction.
Keywords: energetic equivalence, ecosystem function, metabolic theory of ecology, food web, trophic efficiency, evenness
1. Introduction
The abundance of any given species population is influenced by myriad factors including, but not limited to, interspecific competition, habitat suitability and disturbance regime. Nevertheless, population abundance is ultimately constrained by the availability of energy and resources in the environment [1–4]. Still, it remains unclear to what extent these energetic constraints can be used to predict abundances of particular species populations at particular sites [5].
Body size is often a focus of this debate because of its primary role in determining metabolic rates, and hence resource demands, of individuals [3]. The influence of body size on population density (expressed as individuals per unit area or volume) has been investigated using two distinct approaches [6]: (i) global size–density relationships (GSDRs) among multiple species and sites, (ii) local size–density relationships (LSDRs) among multiple species at the same site. White et al. [6] note that GSDRs often exhibit stronger correlations than LSDRs. This discrepancy could reflect the fact that GSDRs are typically derived from population-level studies [6], which may focus predominantly on sites where the focal species are relatively abundant [7].
A useful point of departure for investigating the role of body size in constraining population density is the energetic equivalence rule (EER) [8]. The EER is an empirical generalization, based primarily on GSDRs [6,8], that population density per unit area, Dp (individuals ha−1), often varies with individual body mass, Mi, as 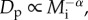 where α ≈ 3/4. Given that individual metabolic rate scales as
where α ≈ 3/4. Given that individual metabolic rate scales as 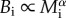 for multicellular organisms [2,3,9,10], the EER is so named because it implies that population-level energy flux, DpBi, is independent of body size, i.e.
for multicellular organisms [2,3,9,10], the EER is so named because it implies that population-level energy flux, DpBi, is independent of body size, i.e. 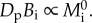 Evidence for and against the EER has been presented [8,11,12], which raises more general questions about the extent to which energetic constraints on individuals can be used to predict population densities.
Evidence for and against the EER has been presented [8,11,12], which raises more general questions about the extent to which energetic constraints on individuals can be used to predict population densities.
Trophic level may also constrain population density because only a fraction of the energy assimilated at one trophic level (approx. 10%) is transferable to higher trophic levels owing to energy losses through respiration and other processes [1]. Thus, in closed systems, total abundances are expected to be higher for herbivores than for secondary and tertiary consumers if other variables such as body size are held constant. This expectation is consistent with data from some pelagic food webs (e.g. [13,14]). However, in open systems, trophic-level effects may be obscured by external energy subsidies. For example, on reefs, subsidies to pelagic consumers [15,16] may help explain why total abundances of piscivorous and other carnivorous fish, relative to herbivores, are far higher than would be predicted given expected energy losses between trophic levels [10].
In some food webs, particularly pelagic communities, trophic level may be determined largely by body size, rather than by species identity [17]. In such systems, frequency distributions of body size for all individuals comprising communities, f(Mi), often adhere to power-function probability distributions with scaling exponents, s, that are steeper than that of metabolic rate (i.e. 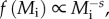 where s > α) [17–19]. For such size ‘spectra’ [17], theory predicts that s ≈ α + 1/4 if there is a 10% energy transfer efficiency between trophic levels, and predators consume prey that are four orders of magnitude smaller in size [16,20]. However, a key assumption of size-spectrum theory—that body size is tightly and positively correlated with trophic level—is questionable for some communities, such as reef fishes (see the electronic supplementary material, figure S1). For example, in the Indo-Malaysian Archipelago, the benthic herbivorous fish Bolbometopon muricatum is 59-fold larger than the piscivore Synodus variegatus (46 kg versus 780 g). Size-spectrum theory also assumes a closed system, and therefore does not account for the fact that reef-fish communities consume two distinct classes of resources (benthic, pelagic), the latter of which may be heavily subsidized by external sources (e.g. [10,15,16]).
where s > α) [17–19]. For such size ‘spectra’ [17], theory predicts that s ≈ α + 1/4 if there is a 10% energy transfer efficiency between trophic levels, and predators consume prey that are four orders of magnitude smaller in size [16,20]. However, a key assumption of size-spectrum theory—that body size is tightly and positively correlated with trophic level—is questionable for some communities, such as reef fishes (see the electronic supplementary material, figure S1). For example, in the Indo-Malaysian Archipelago, the benthic herbivorous fish Bolbometopon muricatum is 59-fold larger than the piscivore Synodus variegatus (46 kg versus 780 g). Size-spectrum theory also assumes a closed system, and therefore does not account for the fact that reef-fish communities consume two distinct classes of resources (benthic, pelagic), the latter of which may be heavily subsidized by external sources (e.g. [10,15,16]).
Another potential constraint on population density is community species richness, which exhibits broad-scale correlations with indices of environmental energy availability, particularly temperature and ecosystem primary production [21]. Most biological communities comprise relatively few abundant species and many rare species, with maximum abundance per species and variation in abundance among species generally decreasing with increasing species richness [22]. Theoretical explanations for this pattern involve some combination of deterministic (e.g. resource partition, species interactions) and stochastic processes [22]. Regardless of the underlying mechanisms, if total community abundance is held constant at some carrying capacity dictated by total energy availability in the environment [4], average density per species must decline with increasing species richness [12].
In this study, we assess the relative importance of individual- (body size), population- (trophic group) and community- and ecosystem-level attributes (temperature, species richness, area) in determining population densities of both rare and abundant species in communities. In so doing, we evaluate the general hypothesis that energetic constraints on population density are manifested most strongly for the most abundant species because they garner the largest fractions of the energy and resources used by the community [23] and are therefore most likely to be limited by energy and resource availability [6,24]. Our approach is timely given the increasing recognition that abundant taxa represent only a small fraction of all species present in a community, yet account for a large fraction of the biomass and energy turnover in many ecosystems [25–27]. Using the metabolic theory as a framework [3], we first derive null expectations for population density under the assumption of energetic equivalence with respect to multiple variables, including body size, and then use these null expectations as quantitative benchmarks for comparison.
We evaluate these null expectations using one of the most comprehensive datasets of reef-fish community structure currently available [10]. Our approach explicitly bridges the gap between the GSDR and LSDR approaches because we analyse local-scale community-level data for a global collection of sites using quantile regression [28], thereby allowing us to separately characterize density trends for rare and abundant taxa. Reef fishes are ideal study organisms because they encompass high total species richness (more than 6000 species) and variation in richness among sites (approx. 50 for temperate reefs to approx. 3000 for some tropical reefs) [29], they can occupy diverse habitats and they vary substantially in body mass (more than six orders of magnitude: approx. 0.1 g to approx. 500 kg), trophic mode and thermal regime (approx. 17–30°C minimum monthly average SST) [29,30].
2. Material and methods
(a). Predictions under energetic equivalence
The relationship of individual metabolic rate, Bi (g C d−1), to body mass, Mi (g), is generally described by a power function of the form [2,3,9]
| 2.1 |
where Bo is a metabolic normalization (g C g−α d−1) that varies among taxa and environments [3], and with other variables, particularly temperature [10,31]. Among fishes, the dimensionless scaling exponent α is approximately 0.75 [10], which is similar in magnitude to values observed for other multicellular taxa [2,9]. Recent work [10] indicates that, for fishes, the temperature dependence of Bo can be characterized as follows:
| 2.2 |
where bo is the value of the metabolic normalization at some arbitrary absolute temperature for standardization, Ts (K),
| 2.3 |
and k is Boltzmann's constant (8.62 × 10−5 eV K−1). In equation (2.3), the temperature dependence of kinetics, K(T), is the product of two terms: an exponential function that characterizes temperature-induced enhancement of rates below the temperature optimum, Topt (K), using an activation energy, Er (eV), and a second term in squared brackets that characterizes declines in rates above Topt using an inactivation energy, Ei [32]. For fishes, Er, Ei and Topt vary significantly among taxa, with respective family-level averages of about 0.6 eV, 2 eV and 33°C [10].
The EER is typically characterized in terms of body mass as 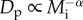 under the assumptions that temperature is held constant, and that body size, Mi, and hence metabolic rate, Bi, are similar for all individuals comprising the population. Here we relax both of these assumptions by extending equations (2.2) and (2.3) to derive an alternative expression for the EER
under the assumptions that temperature is held constant, and that body size, Mi, and hence metabolic rate, Bi, are similar for all individuals comprising the population. Here we relax both of these assumptions by extending equations (2.2) and (2.3) to derive an alternative expression for the EER
| 2.4 |
where Bp is the average annual respiratory flux for an individual (see the electronic supplementary material, equation S2), and Rp is the annual respiratory flux of the population (g C ha−1 yr−1) (electronic supplementary material, equation S1), which is assumed to be independent of Bp, following the EER. Equation (2.4) accounts for variation in mass among the Jp individuals using a population-level average for body mass, 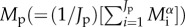 [10,33], and accounts for temperature variation, T(t), through time, t, over a time interval of τ = 1 year using a community-level average for temperature kinetics,
[10,33], and accounts for temperature variation, T(t), through time, t, over a time interval of τ = 1 year using a community-level average for temperature kinetics, 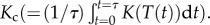 While the EER is typically evaluated using raw arithmetic averages for body mass [6], this approach entails an approximation that becomes less accurate as variation in body size increases [34] (see the electronic supplementary material, figure S2). Equation (2.4), by contrast, does not entail this approximation, and is therefore preferable for evaluating the EER, if there exists substantial variation in body size and size-frequency data are available [12]. Both terms in equation (2.4) take scaling exponents of −1 because population density will decline inversely with increases in per-individual metabolic demands under the assumption of EER. Thus, these −1 values represent benchmarks for assessing if populations that differ in their body-size distributions and temperature kinetics are equivalent with respect to energy use.
While the EER is typically evaluated using raw arithmetic averages for body mass [6], this approach entails an approximation that becomes less accurate as variation in body size increases [34] (see the electronic supplementary material, figure S2). Equation (2.4), by contrast, does not entail this approximation, and is therefore preferable for evaluating the EER, if there exists substantial variation in body size and size-frequency data are available [12]. Both terms in equation (2.4) take scaling exponents of −1 because population density will decline inversely with increases in per-individual metabolic demands under the assumption of EER. Thus, these −1 values represent benchmarks for assessing if populations that differ in their body-size distributions and temperature kinetics are equivalent with respect to energy use.
Equation (2.4) is derived based solely on effects of individual energetics on population density; however, such effects may be modified by other variables. For example, in a closed system at steady-state, population density may differ among trophic groups, g, even after controlling for body size, because total energy availability is lower at higher trophic levels [1]. Density estimates are also expected to vary with community species richness and area, Sc and Ac, because average population density is equal to Jc/(AcSc) for a community comprised Jc individuals and Sc species [12], and Sc increases nonlinearly with Ac [35,36]. Here, we statistically assess the effects of these variables by fitting the following expression:
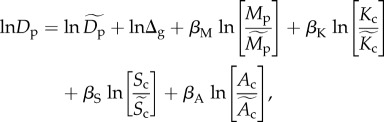 |
2.5 |
which assumes power-function scaling relations for the effects of average size-corrected body mass, temperature kinetics, richness and area (respectively, quantified by the scaling exponents βM, βK, βS and βA). Treatment of richness and area effects in this way is consistent with species–area relationships, which are often characterized using scaling exponents z as 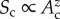 [35]; however, such functions are only approximations because z varies among systems and with spatial scale [36]. A diagnostic plot of the model residuals suggests that the model, taken as a whole, provides a reasonable fit to the data (figure S3). For our analysis, effects of trophic group are standardized by separately estimating Δg for each group (g) subject to the constraint that the product of the estimates ΠΔg = 1. Effects of other variables are standardized using the median estimate of average size-corrected body mass for the 5609 populations included in our analysis (
[35]; however, such functions are only approximations because z varies among systems and with spatial scale [36]. A diagnostic plot of the model residuals suggests that the model, taken as a whole, provides a reasonable fit to the data (figure S3). For our analysis, effects of trophic group are standardized by separately estimating Δg for each group (g) subject to the constraint that the product of the estimates ΠΔg = 1. Effects of other variables are standardized using the median estimate of average size-corrected body mass for the 5609 populations included in our analysis (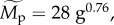 corresponding to body mass of
corresponding to body mass of 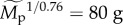 ), and the median estimates of temperature kinetics (
), and the median estimates of temperature kinetics (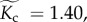 where Ts = 20°C), community species richness (
where Ts = 20°C), community species richness (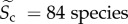 ) and sampling area (
) and sampling area (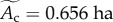 ) for the 49 communities included in our analysis. Consequently, the normalized density,
) for the 49 communities included in our analysis. Consequently, the normalized density, 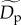 (individuals ha−1), in equation (2.5) corresponds to the estimated population density for a typical trophic group at these standardized values.
(individuals ha−1), in equation (2.5) corresponds to the estimated population density for a typical trophic group at these standardized values.
These equations provide a useful framework for assessing energetic equivalence (or lack thereof) among populations with respect to multiple variables, as demonstrated by combining our expressions for population density, Dp (equation (2.5)), and time-averaged individual metabolic rate, Bp (electronic supplementary material, equation S2), to characterize annual respiratory flux
| 2.6 |
Following the above equation, energetic equivalence with respect to trophic group for reef fishes would be indicated by identical estimates of Δg = 1 for herbivores, invertivores, omnivores, piscivores and planktivores. Energetic equivalence with respect to body mass and temperature would be indicated by values of −1 for βM and βK, respectively, following equation (2.4). Thus, values more than −1 for one or both of these fitted parameters would indicate that larger bodied (and/or warmer) populations flux relatively more energy. By contrast, energetic equivalence with respect to species richness and area would be indicated by slopes of 0 for βS and βA, respectively.
(b). Model fitting procedure
We fit equation (2.5) to empirical data using quantile regression, a flexible and robust technique that entails few statistical assumptions [28]. Here we use mixed-effects quantile regression, which is widely known in statistics and economics, but which has thus far been used in only a handful of ecology studies (e.g. [37,38]). We implement this regression technique using a hierarchical Bayesian procedure [39,40] in order to determine posterior distributions and associated 95% credible intervals (CIs) for the fitted parameters. Analyses were conducted using JAGS v. 3.4.0 and the R package R2jags version 0.5–6 [41] in R v. 3.2.1 [42] (see the electronic supplementary material for detailed explanation and JAGS code).
We adopt this mixed-effects methodology in order to allow the normalized density, 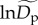 in equation (2.5), to vary among sites by treating it as the sum of two parameters
in equation (2.5), to vary among sites by treating it as the sum of two parameters
| 2.7 |
a fixed effect, 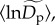 corresponding to an average among communities for the normalized density, and a random effect,
corresponding to an average among communities for the normalized density, and a random effect, 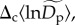 corresponding to a community-level deviation from this average. In our model, community-level random effects,
corresponding to a community-level deviation from this average. In our model, community-level random effects, 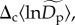 are assumed to be normally distributed with a mean of 0. Treating
are assumed to be normally distributed with a mean of 0. Treating 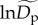 in this way allows us to control for the potential effects of other unmeasured variables (e.g. sampling protocol, ecosystem productivity, habitat quality) that might otherwise lead to correlated residuals at the community level. All of the other fitted parameters—lnΔg, βM, βK, βS and βA—were treated as having only fixed effects for the model presented in the main text.
in this way allows us to control for the potential effects of other unmeasured variables (e.g. sampling protocol, ecosystem productivity, habitat quality) that might otherwise lead to correlated residuals at the community level. All of the other fitted parameters—lnΔg, βM, βK, βS and βA—were treated as having only fixed effects for the model presented in the main text.
In order to assess whether determinants of population density varied with density, we fit a series of 30 quantile regression models, corresponding to 30 different quantiles, q. Together these models yield predictions that encompass rare (q = 0.15) to abundant species (q = 0.95). For example, setting q = 0.95, the fitted quantile regression model yields predictions for a density threshold that is exceeded by only 5% of species. Note that, because the normalized density is allowed to vary among communities in our analysis, following equations (2.5) and (2.7), this threshold corresponds to 5% of species at a given site. Quantile regression is useful for modelling heteroscedastic (e.g. constrained) relationships among variables because parameter estimates are allowed to vary by quantile, perhaps due to the competing effects of different processes [28]. If, for example, energetic constraints on population density were greater for more abundant taxa, we would expect the slopes βM and βK to become more negative at high values of q.
We analysed community-level data collected from 49 communities (islands, atolls and coastal contiguous reefs) using standardized belt transects in which divers tallied the numbers, species identities and body lengths of all fishes (electronic supplementary material) [10]. Body masses were inferred from body lengths by estimating the wet weights of individuals using length–weight conversion formulae. Each species was assigned to one of five trophic groups (herbivores, omnivores, planktivores, invertivores and piscivores) as described in [10]. Community-level estimates of temperature kinetics, Kc, were calculated based on weekly satellite estimates of mean annual sea surface temperature [43]. Community-level estimates of richness, Sc, were calculated as the total numbers of species sampled over the entire sampling area, Ac.
3. Results
Quantile regression analyses indicate that population density varies markedly among taxa within communities, as indicated by an 191-fold increase in the average normalized density (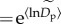 ) from about 3 ind. ha−1 for relatively rare species (q = 0.15) to 481 ind. ha−1 for relatively abundant species (q = 0.95) (figure 1a). An increase in
) from about 3 ind. ha−1 for relatively rare species (q = 0.15) to 481 ind. ha−1 for relatively abundant species (q = 0.95) (figure 1a). An increase in 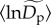 with q is expected, on a mathematical basis, because higher quantiles correspond to more abundant taxa, and the parameter
with q is expected, on a mathematical basis, because higher quantiles correspond to more abundant taxa, and the parameter 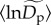 represents the intercept of the fitted model (equations (2.5) and (2.7)). Our mixed-model approach also characterizes deviations in normalized densities from the average,
represents the intercept of the fitted model (equations (2.5) and (2.7)). Our mixed-model approach also characterizes deviations in normalized densities from the average, 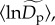 as random effects,
as random effects, 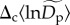 (equations (2.5) and (2.7)). The estimated standard deviation of these random effects,
(equations (2.5) and (2.7)). The estimated standard deviation of these random effects, 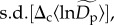 implies that normalized densities vary on average about 1.58-fold (≈e2×0.23) among communities for rare species (
implies that normalized densities vary on average about 1.58-fold (≈e2×0.23) among communities for rare species (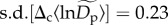 at q = 0.15) and about 4.48-fold (≈e2×0.75) among communities for abundant species (
at q = 0.15) and about 4.48-fold (≈e2×0.75) among communities for abundant species (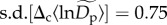 at q = 0.95) (electronic supplementary material, figure S4).
at q = 0.95) (electronic supplementary material, figure S4).
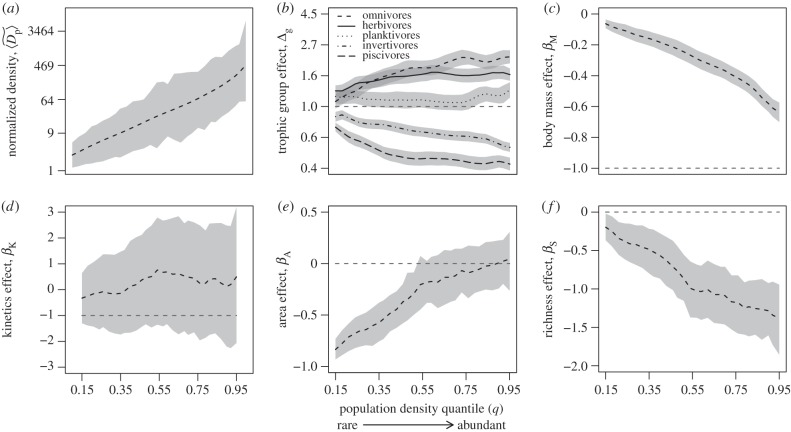
Figure 1.
Relationships of parameter estimates (from equations (2.5) and (2.7)) to density quantiles for parameters used to characterize effects of (a) normalized population density, (b) trophic group, (c) size-corrected body mass, (d) temperature kinetics, (e) sampling area and (f) species richness. Dashed lines represent averages of posterior distributions, and shading represents 95% credible intervals.
Importantly, all of the parameters used to characterize the effects of predictor variables (with the exception of temperature kinetics) vary significantly between rare (q = 0.15) and abundant (q = 0.95) species (figure 1b–f). These findings indicate that determinants of population density vary significantly with density. Thus, they support our use of quantile regression over more traditional statistical methods that assume homoscedastic relationships among variables.
Our analysis yields two lines of evidence in support of the hypothesis that energetic constraints on population density are most pronounced for the most abundant species. First, differences in the normalized densities among trophic groups are not statistically significant for rare species (lower quantiles; figure 1b), but become highly significant for abundant species judging by the non-overlapping 95% CIs for the estimates of differences at larger quantiles (grey areas of the figure). Second, the body-size effect, represented by the slope βM, becomes steeper moving towards more abundant species (higher quantiles; figure 1c), indicating a constrained (i.e. wedge-shaped) relationship of body size to abundance (figure 2).
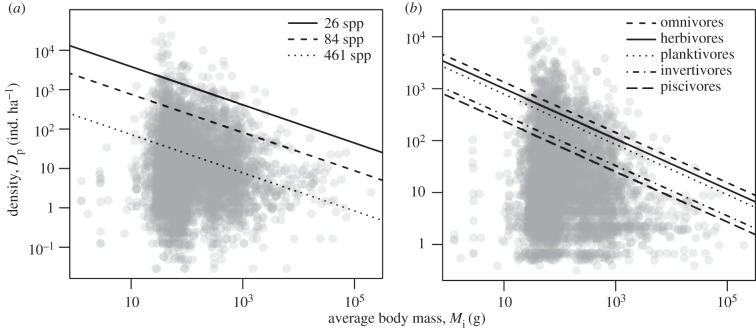
Figure 2.
Relationship of standardized population density to body mass (grey circles), along with predicted effects (lines) at the highest density quantile (q = 0.95) for (a) species richness and (b) trophic groups. Population density values have been standardized differently in (a,b) to graphically depict partial effects of variables of interest after accounting for temperature (standardized to median temperature in a and b), sampling area (standardized to median area in a and b), trophic group (standardized in a), and species richness (standardized to median species richness in b) (see Methods for median values). Average size-corrected body mass, Mp, has been transformed (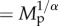 ) into body mass units (g) for plotting.
) into body mass units (g) for plotting.
Despite some evidence of energetic constraints, our analysis yields no evidence of energetic equivalence. First, regarding trophic group, the observed differences in the density normalizations (characterized by Δg) imply that population densities (and hence energy fluxes, following electronic supplementary material, equation S1) are greater for omnivores, herbivores and planktivores (in that order) than for invertivores and piscivores, after controlling for other variables (figure 1b). Regarding average size-corrected body mass, even for the most abundant species (q = 0.95), the fitted slope is far shallower than −1 (−0.64; 95% CI: −0.70 to −0.57; figure 1c). Similar results are obtained if size–density relationships are allowed to vary among communities (electronic supplementary material, figure S5). Thus, despite the fact that population density declines with increasing size-corrected body mass (figure 3a), population energy flux actually increases with body size (figure 3b). Regarding temperature kinetics, the 95% CIs for the slope overlap the values of both −1 and 0 at all density quantiles (figure 1d). Thus, for the reef communities considered here, which encompass a predicted increase of 1.37-fold in temperature kinetics moving from warm temperate (mean annual sea surface temperature of 22°C) to tropical communities (mean annual sea surface temperature of 28°C), population densities appear to be essentially independent of thermal regime after accounting for other variables.
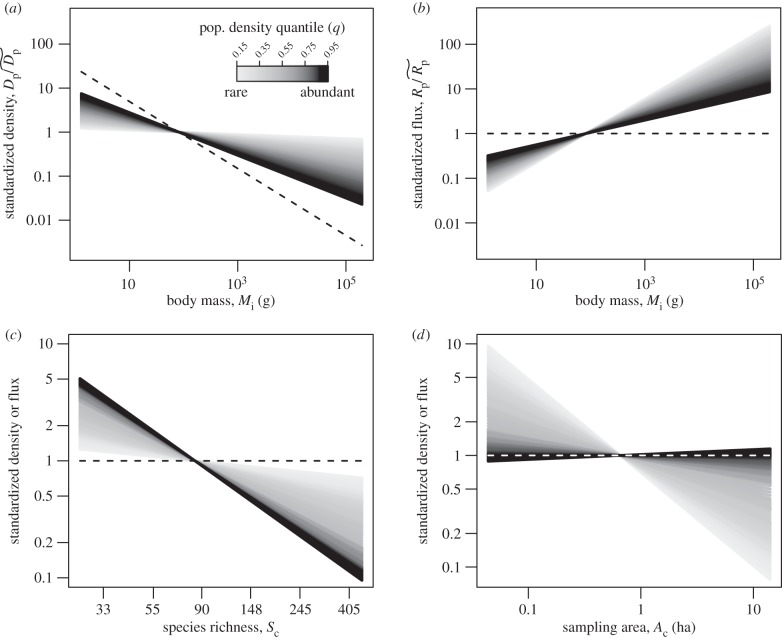
Figure 3.
Estimated effects of (a,b) size-corrected body mass, (c) species richness, and (d) sampling area on population density (equation (2.5)) and energy flux (electronic supplementary material, equation S1). Dashed lines (black in a–c, white in d) represent expectations based on the assumption of energetic equivalence. Grey-scale lines represent predictions of quantile regression models fitted to different population density quantiles, q, that encompass rare (light grey, q = 0.15) to abundant species (black, q = 0.95) (see figure 1 for parameter estimates of quantile regression models at different q-values). Population densities, Dp (equation (2.5)), and fluxes, Rp (electronic supplementary material, equation S1), have been standardized as 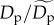 and
and 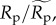 respectively. Therefore, the y-axes represent n-fold deviations from
respectively. Therefore, the y-axes represent n-fold deviations from 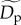 and/or
and/or 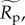 both of which were estimated from the quantile regression models based on median values for size-corrected body mass (
both of which were estimated from the quantile regression models based on median values for size-corrected body mass (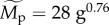 ), temperature kinetics (
), temperature kinetics (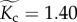 ), community species richness (
), community species richness (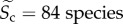 ) and sampling area (
) and sampling area (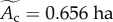 ) (see Methods). Average size-corrected body mass, Mp, has been transformed (
) (see Methods). Average size-corrected body mass, Mp, has been transformed (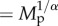 ) into body mass units (g) for plotting.
) into body mass units (g) for plotting.
Our findings indicate that ecological constraints of species richness (i.e. competition) on population density were also strongest on the most abundant species. Specifically, after accounting for the variables described above (trophic group, body size or temperature; figure 1b–d), and for sampling area (figure 1e), species richness had a pronounced negative effect on population density (characterized by βS; figures 1f and 3c), particularly for the most abundant species (βS at q = 0.95: −1.38; 95% CI: −1.85 to −0.94). The magnitude of this slope implies an approximately 53-fold decline in population density 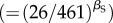 attributable to species richness moving from the lowest- to the highest-richness community (26–461 species). This effect of richness on population densities of abundant species (q = 0.95) is substantially greater than the approximately 18-fold effect of average size-corrected body mass
attributable to species richness moving from the lowest- to the highest-richness community (26–461 species). This effect of richness on population densities of abundant species (q = 0.95) is substantially greater than the approximately 18-fold effect of average size-corrected body mass 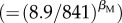 over a range encompassing 99% of the Mp values (8.9–841 gα) (figure 2a) and the 5.7-fold variation attributable to trophic group
over a range encompassing 99% of the Mp values (8.9–841 gα) (figure 2a) and the 5.7-fold variation attributable to trophic group 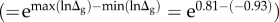 (figures 1b and 2b).
(figures 1b and 2b).
4. Discussion
Overall, results of our statistical analysis—which simultaneously assesses individual-, population- and community-level determinants of population density for both rare and abundant species—indicate that there are many ways to achieve rarity [44], but that high population density is associated with a particular combination of energetic and ecological factors. The highest densities are achieved by populations of organisms that are small bodied, and that occur at lower trophic levels in communities with low species richness. With respect to energetics, our results provide some support for effects on population density attributable to trophic group, which constrains the total energy available at different trophic levels [1], and to body size, which may constrain density through its effects on energetic demands of individuals [3,8,12]. Importantly, however, the magnitudes of these effects are inconsistent with energetic equivalence, in agreement with other recent studies (e.g. [11,19]). In particular, our results indicate that, on average, energy fluxes of abundant taxa (q = 0.95) increase with body size (figure 3b). Our findings also indicate that the strength of energetic constraints varies considerably with relative density, as indexed by the density quantile (figure 3). Overall, the energetic variables considered here appear to be of limited utility for predicting the abundances of most species.
While trophic group was found to be an important determinant of population density, the arrangement of trophic groups was not as expected based on simple Lindeman efficiency arguments. In particular, omnivore populations, rather than herbivore populations, achieved the highest densities, as indexed by Δg (figures 1b and 2b). Moreover, among abundant taxa (q = 0.95), densities of piscivore populations were only about fourfold lower than those of herbivores, and not two orders of magnitude lower, as would be predicted based on a 10% Lindeman efficiency if piscivores were separated from herbivores by two trophic levels (i.e. 0.102 = 100-fold). In this respect, the population-level findings presented here reinforce the results of a recent community-level analysis conducted using these reef-fish data [10], and thus lend further support to the argument that piscivores receive substantial energy subsidies from outside the reef [10,16]. Overall, these findings highlight that trophic constraints most likely operate at spatial scales encompassing both the reef and its surroundings, and at taxonomic scales encompassing not only fishes, but also invertebrates and unicells.
When interpreting our findings regarding trophic groups, it is important to note that our analysis assigns each species to one trophic group, regardless of size and therefore does not account for any ontogenetic shifts in resource use. Although our analysis encompasses only juveniles and adults more than 10 cm length—stages at which dietary shifts may occur primarily through shifts in prey size rather than prey type, e.g. [45]—we cannot discount the possibility that ontogenetic shifts in resource use influence the observed effects of trophic groups on population density. It is also important to note that our simplified energetic approach yields abundance predictions by assuming that populations are at or near carrying capacity with respect to the availability of limiting resources. In reality, reef-fish populations may be regulated not only by competition and resource limitation, but also by rates of recruitment and/or predation [46]. Predation rates, in turn, are influenced not only by the densities of predators and prey, but also by other factors such as the availability of refugia for prey populations. In light of such complexities, it is perhaps not surprising that individual energetics, as indexed by body mass, accounts for only a modest fraction of the variation in population abundance (figure 2). Still, it is important to note that the population-level analyses conducted here, along with complementary community-level analyses of reef-fish data [10,16], suggest that energy fluxes of larger bodied reef fishes are far higher than would be predicted by size-spectrum theory [17]. While these findings do not by themselves contradict a key basic assumption of size-spectrum theory—that body size plays a key role in mediating trophic interactions—they do suggest that one or more of the assumptions in current models (e.g. closed-system assumption, common resource-pool assumption) must be relaxed to account for the complexity of trophic interactions in reef systems.
Comparison of the population-level results presented here with those of the community-level analysis using the same data [10] highlights important differences between population- and community-level trends. For instance, invertivores were found to be the most abundant trophic group at the community level [10], but were significantly less abundant than herbivores, omnivores and planktivores at the population level (figure 1b). These seemingly disparate findings can be reconciled by noting that invertivores are generally the most diverse trophic group of fishes in reef ecosystems [29].
Our findings highlight that estimates of population density are sensitive to the spatial scale of sampling, and that the magnitude of this sampling effect is substantially greater for rare than abundant taxa (figures 1e and 3d). Abundant species are expected to be relatively ubiquitous across space, so estimates of their densities are expected and observed to be relatively insensitive to sampling area. By contrast, rare species are more likely to be spatially restricted, so one expects to encounter more of them, each with progressively lower estimates of density (Dp), as community sampling area, Ac, increases [35]. Here we address this issue statistically, by explicitly incorporating area and its differential effects on density estimates of abundant versus rare species, using quantile regression. We view our approach as complementary to that of Damuth [8], which involves estimating ecological densities of populations, i.e. abundances of populations in areas comprised entirely of suitable habitat. Our method of estimating population density implicitly assumes that all sampled hard-bottom reef area is suitable habitat, and thus may underestimate ecological densities, particularly for some microhabitat-specific species, such as gobies and clownfishes. Applying the ecological density concept in reef systems would be challenging because tropical reef fishes often exhibit a high degree of specialization in terms of resource use, and resources availability often exhibits substantial fine-scale spatial heterogeneity [47].
Remarkably, our results indicate that effects of species richness on densities of the abundant taxa are of comparable or even greater magnitude than those attributable to individuals energetics, as indexed by body size (figure 2). While richness appeared to significantly constrain the densities of rare taxa (e.g. q = 0.15) as well, its effects were relatively weak (figures 1f and 3c). Undoubtedly, these findings reflect a nearly ubiquitous feature of species abundance distributions [23]: as species richness goes up, abundances of taxa become more equitable, due in part to reductions in the abundances of abundant taxa (electronic supplementary material, figure S6) [48,49]. Still, it is noteworthy that our analysis indicates that effects of species richness on population density are of equal or greater magnitude than those of body size given that size varies by more than six orders of magnitude (0.05 g to 192 kg) for the species included in our analysis. These findings suggest that, at the broadest spatial scales, the population densities of reef fishes are, to a large extent, governed by broad-scale factors associated with species richness rather than by local-scale ecological factors and intrinsic attributes of species. Given that species-rich reef-fish communities exhibit substantially lower maximum populations densities (figure 2a), they may be more susceptible to local stochastic extinction [50]. More generally, our findings could therefore be viewed as consistent with a key prediction of neutral biodiversity theory that increases in metacommunity diversity are associated with elevated rates of speciation and extinction [22,51]. Thus, establishing links between broad-scale gradients in reef biodiversity, speciation rates and extinction rates represents an important research challenge.
5. Conclusion
Here we assess the relative roles of energetics and ecology in influencing population density at broad spatial scales. Our results indicate that rarity may be achieved in many ways, but there are very few ways for a species to be abundant (figure 2). These results were obtained by separately assessing determinants of population density of rare and abundant species using a quantile regression approach (figure 1). Although our results identify energetics as an important determinant of density for abundant species, we find no evidence for energetic equivalence among different reef-fish populations (figure 3), and community species richness appears to be the key variable explaining differences in densities of abundant taxa at broad spatial scales. These findings are broadly consistent with empirical findings from other communities such as plants and mammals [48,49,52], and highlight the need for further theoretical work that explicitly links population abundance to community species richness and macroevolutionary dynamics in marine ecosystems (e.g. [51]). Further work will be necessary to incorporate effects of other ecological variables, such as overfishing, habitat destruction and pollution, which are likely contributing to changes in abundance, and which may differentially affect species that vary in size and occur at different trophic levels.
Supplementary Material
Acknowledgements
We would like to thank three anonymous referees for helping us improve this article. The project was supported by Macquarie University (PhD scholarship to D.R.B.), Australian Research Council's Discovery Projects funding scheme (DP0987218 to A.P.A.), CESAB-FRB (Fondation pour la Recherche en Biodiversité), through the GASPAR programme, SISBIOTA-Mar (CNPq 563276/2010-0 and FAPESC 6308/2011-8 to S.R.F.), CAPES, Marinha do Brasil, Instituto Laje Viva and the National Geographic Society.
Data accessibility
Supporting data necessary to reproduce all analyses in this manuscript is available from Dryad http://dx.doi.org/10.5061/dryad.j2qr4.
Competing interests
We have no competing interests.
Funding
D.R.B.: iMQRES PhD scholarship from Macquarie University; M.K.: Institut de recherche pour le développement (IRD—France), ZONECO programme (EUR$60 000), Demecofish programme (funded by the MacArthur Foundation, US$250 000) and the Typatoll programme. S.R.F.: Pro-Africa project (CNPq grant 490531/2007-5), the National Geographic Society (grant 7937-05), SISBIOTA-Mar network (CNPq 563276/2010-0 and FAPESC 6308/2011-8). A.M.F.: National Geographic Society (Pristine Seas Project), NSF. A.P.A.: Australian Research Council's Discovery Projects funding scheme (DP0987218).
References
- 1.Lindeman RL. 1942. The trophic-dynamic aspect of ecology. Ecology 23, 399–417. ( 10.2307/1930126) [DOI] [Google Scholar]
- 2.Peters RH. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 4.Allen AP, Gillooly JF, Brown JH. 2005. Linking the global carbon cycle to individual metabolism. Funct. Ecol. 19, 202–213. ( 10.1111/j.1365-2435.2005.00952.x) [DOI] [Google Scholar]
- 5.Tilman D, HilleRisLambers J, Harpole S, Dybzinski R, Fargione J, Clark C, Lehman C. 2004. Does metabolic theory apply to community ecology? It's a matter of scale. Ecology 85, 1797–1799. ( 10.1890/03-0725) [DOI] [Google Scholar]
- 6.White EP, Ernest SM, Kerkhoff AJ, Enquist BJ. 2007. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 22, 323–330. ( 10.1016/j.tree.2007.03.007) [DOI] [PubMed] [Google Scholar]
- 7.Cotgreave P. 1993. The relationship between body size and population abundance in animals. Trends Ecol. Evol. 8, 244–248. ( 10.1016/0169-5347(93)90199-Y) [DOI] [PubMed] [Google Scholar]
- 8.Damuth J. 1987. Interspecific allometry of population density in mammals and other animals: the independence of body mass and population energy-use. Biol. J. Linnean Soc. 31, 193–246. ( 10.1111/j.1095-8312.1987.tb01990.x) [DOI] [Google Scholar]
- 9.Savage VM, Gillooly JF, Woodruff WH, West GB, Allen AP, Enquist BJ, Brown JH. 2004. The predominance of quarter-power scaling in biology. Funct. Ecol. 18, 257–282. ( 10.1111/j.0269-8463.2004.00856.x) [DOI] [Google Scholar]
- 10.Barneche DR, Kulbicki M, Floeter SR, Friedlander AM, Maina J, Allen AP. 2014. Scaling metabolism from individuals to reef-fish communities at broad spatial scales. Ecol. Lett. 17, 1067–1076. ( 10.1111/ele.12309) [DOI] [PubMed] [Google Scholar]
- 11.Isaac NJB, Storch D, Carbone C. 2013. The paradox of energy equivalence. Glob. Ecol. Biogeogr. 22, 1–5. ( 10.1111/j.1466-8238.2012.00782.x) [DOI] [Google Scholar]
- 12.Allen AP, Brown JH, Gillooly JF. 2002. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–1548. ( 10.1126/science.1072380) [DOI] [PubMed] [Google Scholar]
- 13.Sheldon RW, Sutcliffe WH Jr, Paranjape MA. 1977. Structure of pelagic food chain and relationship between plankton and fish production. J. Fish. Res. Board Can. 34, 2344–2353. ( 10.1139/f77-314) [DOI] [Google Scholar]
- 14.Pauly D, Christensen V. 1995. Primary production required to sustain global fisheries. Nature 374, 255–257. ( 10.1038/374255a0) [DOI] [Google Scholar]
- 15.Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams DM. 1988. Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bull. Mar. Sci. 42, 459–479. [Google Scholar]
- 16.Trebilco R, Baum JK, Salomon AK, Dulvy NK. 2013. Ecosystem ecology: size-based constraints on the pyramids of life. Trends Ecol. Evol. 28 423–431. ( 10.1016/j.tree.2013.03.008) [DOI] [PubMed] [Google Scholar]
- 17.Kerr SR, Dickie LM. 2001. The biomass spectrum: a predator–prey theory of aquatic production. New York, NY: Columbia University Press. [Google Scholar]
- 18.Jennings S, Mackinson S. 2003. Abundance–body mass relationships in size-structured food webs. Ecol. Lett. 6, 971–974. ( 10.1046/j.1461-0248.2003.00529.x) [DOI] [Google Scholar]
- 19.Reuman DC, et al. 2009. Allometry of body size and abundance in 166 food webs. Adv. Ecol. Res. 41, 1–44. ( 10.1016/S0065-2504(09)00401-2) [DOI] [Google Scholar]
- 20.Brown JH, Gillooly JF. 2003. Ecological food webs: high-quality data facilitate theoretical unification. Proc. Natl Acad. Sci. USA 100, 1467–1468. ( 10.1073/pnas.0630310100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currie DJ, et al. 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134. ( 10.1111/j.1461-0248.2004.00671.x) [DOI] [Google Scholar]
- 22.Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 23.McGill BJ, et al. 2007. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10, 995–1015. ( 10.1111/j.1461-0248.2007.01094.x) [DOI] [PubMed] [Google Scholar]
- 24.Blackburn TM, Lawton JH, Perry JN. 1992. A method of estimating the slope of upper bounds of plots of body size and abundance in natural animal assemblages. Oikos 65, 107–112. ( 10.2307/3544892) [DOI] [Google Scholar]
- 25.Gaston KJ. 2010. Valuing common species. Science 327, 154–155. ( 10.1126/science.1182818) [DOI] [PubMed] [Google Scholar]
- 26.Gaston KJ. 2011. Common ecology. BioScience 61, 354–362. ( 10.1525/bio.2011.61.5.4) [DOI] [Google Scholar]
- 27.ter Steege H, et al. 2013. Hyperdominance in the Amazonian tree flora. Science 342, 1243092 ( 10.1126/science.1243092) [DOI] [PubMed] [Google Scholar]
- 28.Cade BS, Noon BR. 2003. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 1, 412–420. ( 10.1890/1540-9295(2003)001%5B0412:AGITQR%5D2.0.CO;2) [DOI] [Google Scholar]
- 29.Parravicini V, et al. 2013. Global patterns and predictors of tropical reef fish species richness. Ecography 36, 1254–1262. ( 10.1111/j.1600-0587.2013.00291.x) [DOI] [Google Scholar]
- 30.Froese R, Pauly D. 2012. FishBase. World Wide Web electronic publication. See http://www.fishbase.org, (Version 12/2012) (accessed 20 February 2015).
- 31.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251. ( 10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 32.Schoolfield R, Sharpe P, Magnuson C. 1981. Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. J. Theor. Biol. 88, 719–731. ( 10.1016/0022-5193(81)90246-0) [DOI] [PubMed] [Google Scholar]
- 33.Yvon-Durocher G, Allen AP. 2012. Linking community size structure and ecosystem functioning using metabolic theory. Phil. Trans. R. Soc. B 367, 2998–3007. ( 10.1098/rstb.2012.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage VM. 2004. Improved approximations to scaling relationships for species, populations, and ecosystems across latitudinal and elevational gradients. J. Theor. Biol. 227, 525–534. ( 10.1016/j.jtbi.2003.11.030) [DOI] [PubMed] [Google Scholar]
- 35.Rosenzweig ML. 1995. Species diversity in space and time. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Harte J, Smith AB, Storch D. 2009. Biodiversity scales from plots to biomes with a universal species–area curve. Ecol. Lett. 12, 789–797. ( 10.1111/j.1461-0248.2009.01328.x) [DOI] [PubMed] [Google Scholar]
- 37.Alhamzawi R, Yu K, Vinciotti V, Tucker A. 2011. Prior elicitation for mixed quantile regression with an allometric model. Environmetrics 22, 911–920. ( 10.1002/env.1118) [DOI] [Google Scholar]
- 38.Fornaroli R, Cabrini R, Sartori L, Marazzi F, Vracevic D, Mezzanotte V, Annala M, Canobbio S. 2015. Predicting the constraint effect of environmental characteristics on macroinvertebrate density and diversity using quantile regression mixed model. Hydrobiologia 742, 153–167. ( 10.1007/s10750-014-1974-6) [DOI] [Google Scholar]
- 39.Yu K, Moyeed RA. 2001. Bayesian quantile regression. Stat. Probab. Lett. 54, 437–447. ( 10.1016/S0167-7152(01)00124-9) [DOI] [Google Scholar]
- 40.Geraci M, Bottai M. 2007. Quantile regression for longitudinal data using the asymmetric laplace distribution. Biostatistics 8, 140–154. ( 10.1093/biostatistics/kxj039) [DOI] [PubMed] [Google Scholar]
- 41.Su Y-S, Yajima M. 2015. R2jags: A package for running ‘JAGS’ from R. R package version 0.03-08. See http://CRAN.R-project.org/package=R2jags. [Google Scholar]
- 42.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 43.Selig ER, Casey KS, Bruno JF. 2010. New insights into global patterns of ocean temperature anomalies: implications for coral reef health and management. Glob. Ecol. Biogeogr. 19, 397–411. ( 10.1111/j.1466-8238.2009.00522.x) [DOI] [Google Scholar]
- 44.Gaston KJ. 1994. Rarity. California, CA: Chapman & Hall. [Google Scholar]
- 45.McCormick MI. 1998. Ontogeny of diet shifts by a microcarnivorous fish, Cheilodactylus spectabilis: relationship between feeding mechanics, microhabitat selection and growth. Mar. Biol. 132, 9–20. ( 10.1007/s002270050367) [DOI] [Google Scholar]
- 46.Hixon MA, Jones GP. 2005. Competition, predation, and density-dependent mortality in demersal marine fishes. Ecology 86, 2847–2859. ( 10.1890/04-1455) [DOI] [Google Scholar]
- 47.Belmaker J. 2009. Species richness of resident and transient coral-dwelling fish responds differentially to regional diversity. Glob. Ecol. Biogeogr. 18, 426–436. ( 10.1111/J.1466-8238.2009.00456.X) [DOI] [Google Scholar]
- 48.Currie DJ, Fritz JT. 1993. Global patterns of animal abundance and species energy use. Oikos 67, 56–68. ( 10.2307/3545095) [DOI] [Google Scholar]
- 49.Niklas KJ, Midgley JJ, Rand RH. 2003. Size-dependent species richness: trends within plant communities and across latitude. Ecol. Lett. 6, 631–636. ( 10.1046/j.1461-0248.2003.00473.x) [DOI] [Google Scholar]
- 50.Lande R, Engen S, Saether B-E. 2003. Stochastic population dynamics in ecology and conservation. Oxford Series in Ecology and Evolution; New York, NY: Oxford University Press. [Google Scholar]
- 51.Reuman DC, Gislason H, Barnes C, Mélin F, Jennings S. 2014. The marine diversity spectrum. J. Anim. Ecol. 83, 963–979. ( 10.1111/1365-2656.12194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson CN. 1998. Rarity in the tropics: latitudinal gradients in distribution and abundance in australian mammals. J. Anim. Ecol. 67, 689–698. ( 10.1046/j.1365-2656.1998.00232.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data necessary to reproduce all analyses in this manuscript is available from Dryad http://dx.doi.org/10.5061/dryad.j2qr4.