Abstract
Environmental signals can induce phenotypic changes that span multiple generations. Along with phenotypic responses that occur during development (i.e. ‘within-generation’ plasticity), such ‘transgenerational plasticity’ (TGP) has been documented in a diverse array of taxa spanning many environmental perturbations. New theory predicts that temporal stability is a key driver of the evolution of TGP. We tested this prediction using natural populations of zooplankton from lakes in Connecticut that span a large gradient in the temporal dynamics of predator-induced mortality. We reared more than 120 clones of Daphnia ambigua from nine lakes for multiple generations in the presence/absence of predator cues. We found that temporal variation in mortality selects for within-generation plasticity while consistently strong (or weak) mortality selects for increased TGP. Such results provide us the first evidence for local adaptation in TGP and argue that divergent ecological conditions select for phenotypic responses within and across generations.
Keywords: ecological epigenetics, life-history evolution, maternal effects, phenotypic plasticity
1. Introduction
Much research performed over the past two decades has shown that the environment can induce phenotype changes that persist for multiple generations [1–3]. This ‘transgenerational plasticity’ (TGP) is a generalization of the well-studied ‘maternal effect’ and occurs whenever the environment experienced by parents modifies the phenotypes of subsequent generations without altering genotypes [4]. TGP has been documented in organisms spanning the tree of life [1] and provides us an additional mechanism by which organisms may respond to environmental stressors such as rising temperatures [5], food shortages [6], and canopy shading [7]. While there exist numerous examples of TGP that are assumed to be adaptive and thus shaped by natural selection [5,7–15], tests of the ecological conditions that favour evolutionary shifts in TGP remain conspicuously absent.
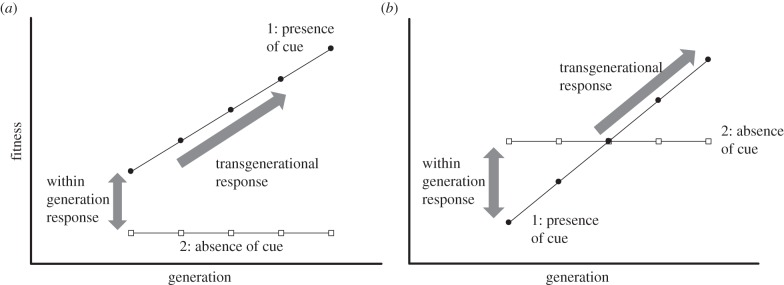
Many theoretical frameworks predict that similar conditions favour the evolution of phenotypic responses that occur ‘within’ (i.e. during development) and ‘across’ generations (i.e. TGP) [16–24]. Varying environmental conditions that are consistent between parent and offspring generations are expected to favour simultaneous increases in phenotypic responses within and across generations or ‘local coadaptation’ between both forms of plasticity (figure 1) [22,23]. That is, within- and across-generation responses will respond in a similar fashion to environmental signals to improve fitness. Only recently, however, has theory identified the ecological selective pressures that may act independently on within- and across-generation responses [25–27]. In particular, within-generation plasticity is favoured when there exists high temporal variability in the environment, whereas low temporal variability and a slow rate of environmental change are predicted to favour across-generation responses (figure 1) [25]. This is because strong transgenerational responses allow parent and offspring phenotypes to be matched to current conditions when the environment changes infrequently relative to the generation time of the organism [26]. Such a scenario leads to an ‘antagonistic’ trajectory of evolution whereby divergent patterns of selection favour within- or across-generation plasticity but not both (figure 1). The extent to which within- and across-generation responses evolve in concert or in opposition, however, remains unknown.
Figure 1.
Predictions from theory for the evolution of within- and across-generation phenotypic plasticity. The line with closed circles represents expectations for organisms reared in the presence of the environmental cue. The line with open squares represents the expectations for organisms not exposed to the environmental cue. (a) Local co-adaptation: high environmental (temporal) variation is expected to favour increased within- and across-generation plasticity [22,23]. (b) Antagonistic adaptation: divergent conditions select for within- versus across-generation plasticity [25–27]. Low temporal variation favours increased transgenerational plasticity.
Daphnia have long served as a model organism for ecological and evolutionary research [28]. This is because they are a ubiquitous feature of aquatic habitats with clearly defined roles as grazers on phytoplankton [29], and they exhibit many characteristics that make them well suited for laboratory study. Daphnia are also well known to respond phenotypically to the presence of predators by producing morphological defences (head and tail spines) and altering life-history traits [30,31]. We recently used this interplay between Daphnia and their fish predators to quantify patterns of TGP in Daphnia [15]. We found that Daphnia exposed to predator cues programmed future generations for faster development [15]. More importantly, this work revealed an extensive genetic variation in the direction and magnitude of phenotypic responses to predator cues within and across generations. Such results lead to the prediction that divergent ecological conditions may have the potential to drive evolutionary shifts in TGP. The key next step is to test whether contrasting ecological conditions, such as habitat stability [25–27], does indeed have the ability to drive evolutionary changes in TGP.
In New England, Daphnia are found across a mosaic of lakes that differ in the intensity and duration of predation by a dominant fish predator, the alewife (Alosa pseudoharengus) [32–34]. This includes lakes with (i) anadromous alewife that migrate seasonally between marine and freshwater, (ii) landlocked alewife that are permanent freshwater residents, and (iii) no alewife. Populations of anadromous and landlocked alewives differ in the time they occupy particular lakes, which influences the abundances of zooplankton. Adult anadromous alewives immigrate into lakes very predictably each spring (approx. March–May), and young-of-the-year (YOY) alewives emigrate from these lakes each fall. As a result, Daphnia attain very high abundances in the spring in lakes with anadromous alewife, but are entirely removed from the water column by YOY alewife predation by early summer [34]. Conversely, in lakes with landlocked alewives, Daphnia are rare year-round because of intense predation by resident alewife [34]. In lakes without alewife, Daphnia are preyed upon by several other fishes (e.g. bluegill, pumpkinseed, etc.) [33,34], but rates of predator-induced mortality are much lower in these lakes (Daphnia are very common during the spring and summer months) [34]. Our focal lakes collectively differ in the temporal dynamics of mortality as rates of predation are consistently intense in lakes with landlocked alewife, but vary strongly in lakes with anadromous alewife. This study system thus provides us all of the raw materials to test for the evolution of TGP [22,23,25–27].
Here, we assessed predator-induced TGP in Daphnia ambigua from ‘anadromous’, ‘landlocked’, and ‘no alewife’ lakes. We reared third-generation laboratory-born Daphnia in the presence and absence of fish predator cues and measured within- (in generation 1) and across-generation (in generation 2) life-history responses for all populations (see experimental design in the electronic supplementary material, figure S1). We focused on life-history traits, because predator-induced morphological responses are minor in D. ambigua and thus variation in development rates and reproductive investment offer a strong proxy for relative fitness. Our previous research has demonstrated that Daphnia from anadromous lakes exhibit stronger within-generation responses to predator cues (i.e. divergent patterns of plasticity across population) [35] and that Daphnia programme future generations for faster development and increased reproductive outputs when exposed to predators (i.e. existence of TGP) [15] (figure 1). Thus, if within- and across-generation plasticity evolve in concert, then we expect that such transgenerational responses will also be stronger in Daphnia from anadromous lakes. Conversely, if the evolution of TGP is driven by high temporal stability, then we expect increased TGP in Daphnia from landlocked and no alewife lakes (figure 1). Based on our experimental design, divergent patterns of TGP among our focal populations should manifest as significant statistical interactions between predator treatment, generation, and lake type (i.e. anadromous, landlocked, no alewife).
2. Material and methods
(a). Focal populations
This study used clones of D. ambigua from three lakes with anadromous alewife (Bride, Dodge, Gorton), three lakes with landlocked alewife (Amos, Long, Quonnipaug), and three lakes with no alewife (Black, Gardner, Wyassup) [33,34]. It is important to note that Daphnia are exposed to fish predation in all lakes as they all contain a variety of generalist planktivorous fish predators such as bluegill (Lepomis macrochirus), pumpkinseed (Lepomis gibbosus), redbreast sunfish (Lepomis auritus), and white perch (Morone americana) [33,34]. As described above, the key difference in predator communities across lakes is the duration of alewife predation: seasonally intense in anadromous lakes, always present in landlocked lakes, and absent in no alewife lakes. We have previously shown that these lakes do not differ in potential covarying environmental parameters such as in size, depth, productivity, or alewife biomass (for landlocked and anadromous lakes only) [34,36].
(b). Experiment overview
We tested Daphnia for divergent transgenerational responses to predator cues by rearing clones in a common garden setting. We reared all clones in the presence and absence of predator cues in generation 1 and quantified life-history responses in generation 2 (see electronic supplementary material, figure S1). This experiment included 15 clones per lake for all except three lakes; 13, 14, and eight clones were reared from Dodge, Quonnipaug, and Gorton, respectively. These lakes contained fewer clones, because fewer sexual eggs hatched in the laboratory.
(c). Experimental protocols
We established populations of Daphnia by hatching resting eggs (ephippia) from lake sediments collected via Ekman grab. The majority of sediment samples were collected in August–September 2009 except for the sample from Gorton Pond, which was collected in March 2012. The first laboratory generation consisted of a single post-ephippial female that was reared in a 90 ml jar containing COMBO medium [37] and fed a non-limiting supply of green algae (species: Scenedesmus obliquus; concentration: approx. 1.0 mg carbon (C) l−1 d−1; photoperiod 14 light (L) : 10 dark (D); 13°C). Daphnia were transferred to jars containing fresh media and algae every other day throughout the duration of the experiment. Because the resting eggs represent the product of sexual reproduction, we assumed that each clone is distinct. For the second laboratory generation, two neonates taken from the second clutch of each clone were reared under the same conditions (i.e. size of container, photoperiod, temperature) as the previous generation.
We evaluated patterns of within- and across-generation plasticity using third-generation laboratory reared clones of Daphnia from all focal populations. We collected six individuals per clone (less than 12 h old) and randomly assigned each individual to one of two treatments: (i) predator exposure in the first generation (Gen. 1 = P, Gen. 2 = PN) and (ii) no predator exposure (Gen. 1 = N, Gen. 2 = NN; see electronic supplementary material, figure S1). The experiment was run for two experimental generations. We exposed Daphnia to the presence of predators in the first generation only, because our previous work showed that across-generation responses are similar in magnitude when Daphnia are continually exposed to predator cues for multiple generations or when Daphnia are only reared in the presence of the cue for the first generation [15]. Our predator treatment included filtered lake water conditioned by alewife (see Kairomone collection below) as well as the presence of ‘alarm cues’ released by crushed conspecifics (added at a concentration of 50 Daphnia l–1) [38]. All individuals received specified quantities of algae (S. obliquus concentration: 0.8 mg C l−1 d−1) and experienced the same temperature (13°C) and photoperiod (14 L : 10 D) as the previous generations. Each clone was replicated three times per combination of treatment and generation for a total sample size of 1 500 jars (125 clones across all lakes × 3 replicates per treatment × 2 treatments × 2 generations).
In this experiment, we quantified age at maturation (defined as the release of the first clutch into the brood chamber), size at maturation, and the size of the first two clutches of offspring. We monitored all individuals for maturation twice daily beginning on day 5. When the release of the first clutch was confirmed, age at maturation was recorded, and each individual was photographed for estimates of size and fecundity (using ImageJ). After maturation, all individuals were examined every day for the production of clutches 2 and 3. To initiate the second experimental generation, we collected newly born (less than 12 h) individuals from the second clutch of each jar and placed them into a new jar containing fresh media and algae. In the predator treatment, second-generation individuals were exposed to predator cues during embryonic development and very early life-history stages.
(d). Kairomone collection
Approximately 200 YOY anadromous alewife were collected on 12 August 2013 from Bride Lake and transported to a laboratory facility at Linsley Pond in North Branford CT (see [34] for lake locations). All alewife were placed in a large outdoor circular tank that contained approximately 750 l of lake water from Linsley pond (fish density = 0.27 fish l−1). Each morning, we added the contents of 15 plankton tows from Linsley pond to the tank containing alewife. The collection of predator-conditioned water was initiated 24 h following the first day of alewife feeding. Predator-conditioned water was collected daily for 3 days, filtered using membrane filters (47 mm diameter, 0.2 µm, Millipore Corporation), and then stored at −20°C for several months prior to use [38]. The concentration of alewife kairomones in the predator treatments equalled 0.0132 fish l−1.
(e). Statistical analyses
We examined our focal populations for divergent patterns of plasticity using linear-mixed models implemented with restricted maximum-likelihood estimation (SPSS v. 21). We characterized the presence of TGP as a significant interaction between predator cue and generation. In addition, we expect that local adaptation in TGP will manifest as a third-order interaction between lake type, predator cue, and generation. We entered lake type (anadromous, landlocked, no alewife), predator treatment (presence, absence), generation, and all interactions among these factors as fixed effects. Of course, the lakes differ from each other in ways that are not accounted for in our simple characterization of lake type. To account for this, we entered lake (nested within lake type) as a random effect. Similarly, we control for unexplained variation among clones from each lake by treating clone (nested within lake) also as a random effect. When performing our analyses, we initially evaluated the significance and explanatory power of the interactions between the nested random effects and all fixed effects. Importantly, the significance of the fixed effects did not depend upon the presence (or removal) of any random effect terms. Our final models retained the lake and clone effects and any significant interactions between the nested random effects and the fixed effects. These analyses used Satterthwaite approximations as the denominator degrees of freedom. Data for age at maturation were log-transformed, whereas clutch size data were square-root transformed to improve fits with normality and homogeneity of variances.
(f). Intrinsic rate of increase
We combined our estimates of age at maturation and clutch size with published estimates of interclutch interval [36] to calculate intrinsic rates (r) for each clone from each lake [39]. We calculated r as r = ln(R0)/G, where R0 is the net reproductive rate (sum of fecundity × survivorship) and G is generation time (average age of the parents of all offspring produced by a single cohort). Differences in r were evaluated using a linear-mixed model, with lake type, predator treatment, and generation as fixed effects, and lake (nested within lake type) entered as a random effect.
3. Results
(a). Age at maturation
Our results revealed divergent within- and across-generation (i.e. TGP) responses to predator cues that are correlated with contrasting fish predator communities (table 1 and electronic supplementary material, S1 and figure 2). This is most clearly represented by the highly significant (p < 0.01) ‘predator × generation × lake type’ interaction for age at maturation (table 1 and figure 2; electronic supplementary material, S2). In generation 1, Daphnia from lakes with anadromous alewife matured earlier in the presence than in the absence of predator cues (figure 2a). Daphnia from landlocked lakes exhibited the opposite pattern (i.e. delayed maturation in the presence of predator cues; figure 2b), whereas there were no differences between predator and non-predator treatments for Daphnia from no alewife lakes in generation 1 (figure 2c). The differences in the timing of maturation among the focal populations in generation 1 were strong; Daphnia from lakes with anadromous alewife matured 1.0 d and 0.6 d earlier than Daphnia from landlocked and no alewife lakes in the presence of predator cues, respectively. Such differences among lake types then disappeared in generation 2, because the Daphnia populations exhibited contrasting responses to predator cues across generations. Daphnia from landlocked and no alewife lakes responded to the presence of predator cues in generation 1 by accelerating rates of development in the subsequent generation (figure 2). As a result, Daphnia from landlocked and no alewife lakes matured earlier in the presence compared with the absence of predator cues in generation 2 (figure 2b,c). Daphnia from anadromous lakes exhibited the opposite response across generations; exposure to predator cues in generation 1 yielded a slower rate of development in generation 2 (figure 2a).
Table 1.
Analyses of life-history traits. Linear-mixed models were used with lake type, predator treatment, and generation entered as fixed effects. Lake (nested within lake type) and clone (nested within lake) (and ‘lake × treatment’ and ‘clone × treatment’ interactions) were entered as random effects. The denominator degrees of freedom are displayed after each F-value. Significant (p < 0.05) results are displayed in italics.
| d.f. | age at maturation F (d.f.) | size at maturation F (d.f.) | clutch size F (d.f.) | intrinsic rate of increase (r) F (d.f.) | |
|---|---|---|---|---|---|
| fixed effects | |||||
| generation | 1 | 3.79+ (11.9) | 18.59*** (1 269) | 6.67* (1 242) | 0.14n.s. (12.5) |
| lake type | 2 | 0.02n.s. (15.3) | 1.01n.s. (6) | 0.98n.s. (6) | 1.92n.s. (12.5) |
| predator | 1 | 4.58* (49.2) | 10.1** (1 270) | 7.26** (1 243) | 1.39n.s. (475) |
| generation × lake type | 2 | 2.8+ (10.4) | 0.01n.s. (1 269) | 2.26n.s. (1 242) | 2.85+ (12.5) |
| generation × predator | 1 | 1.08n.s. (49.1) | 7.54** (1 269) | 0.77n.s. (1 243) | 1.27n.s. (475) |
| predator × lake type | 2 | 2.94+ (1 273) | 2.92+ (1 270) | 0.77n.s. (1 243) | 1.23n.s. (475) |
| generation × lake type × predator | 2 | 5.17** (1 272) | 1.16n.s. (1 269) | 1.14n.s. (1 243) | 2.98+ (475) |
| random effects | |||||
| lake (lake type) | 1 | 1.16n.s. | 1.27n.s. | 1.03n.s. | 0.9n.s. |
| clone (lake type) | 1 | 3.53*** | 5.82*** | 4.07*** | — |
| lake (lake type) × predator | 1 | 1.09n.s. | 0.06n.s. | 1.56n.s. | 0.44n.s. |
| lake (lake type) × generation | 1 | 1.69+ | 0.52n.s. | 1.58n.s. | 1.34n.s. |
| lake (lake type) × predator × generation | 1 | 1.5n.s. | 0.54n.s. | 1.43n.s. | 1.07n.s. |
| clone (lake) × predator | 1 | 0.32n.s. | 0.76n.s. | 0.9n.s. | — |
| clone (lake) × generation | 1 | 1.55n.s. | 0.1n.s. | 0.96n.s. | — |
| clone (lake) × predator × generation | 1 | 1.22n.s. | 1.76+ | 0.45n.s. | — |
±0.05 < p < 0.1.
*p < 0.05.
**p < 0.01.
***p < 0.001.
non-significant (n.s.) – p > 0.05.
Figure 2.
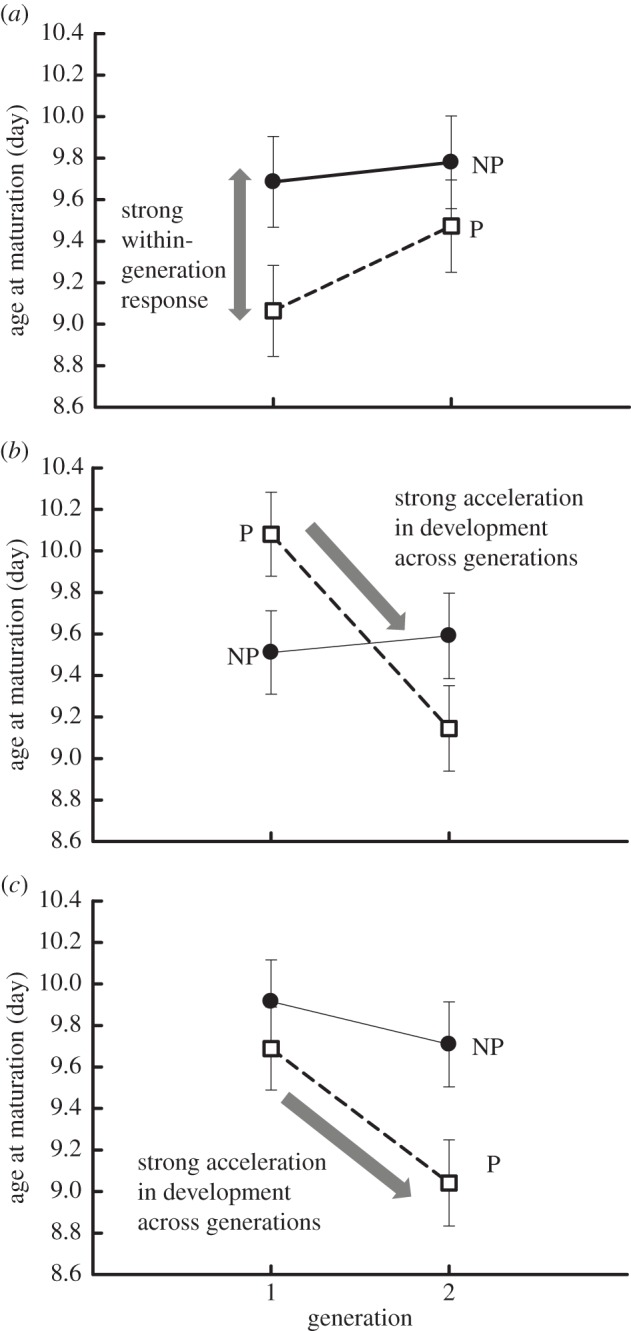
TGP in development rate depends upon predation regime. (a) Anadromous lakes, (b) landlocked lakes, and (c) no alewife lakes. P, predator treatment; NP, non-predator treatment. Exposure to predator cues in generation 1 yielded strong transgenerational responses in Daphnia from landlocked and no alewife lakes (acceleration in development across generations) but weak or absent TGP in Daphnia from anadromous lakes (delayed development across generations). The lake type × generation × predator treatment interaction was significant (p < 0.01). Error bars =±1 standard error (s.e.).
(b). Size at maturation and clutch size
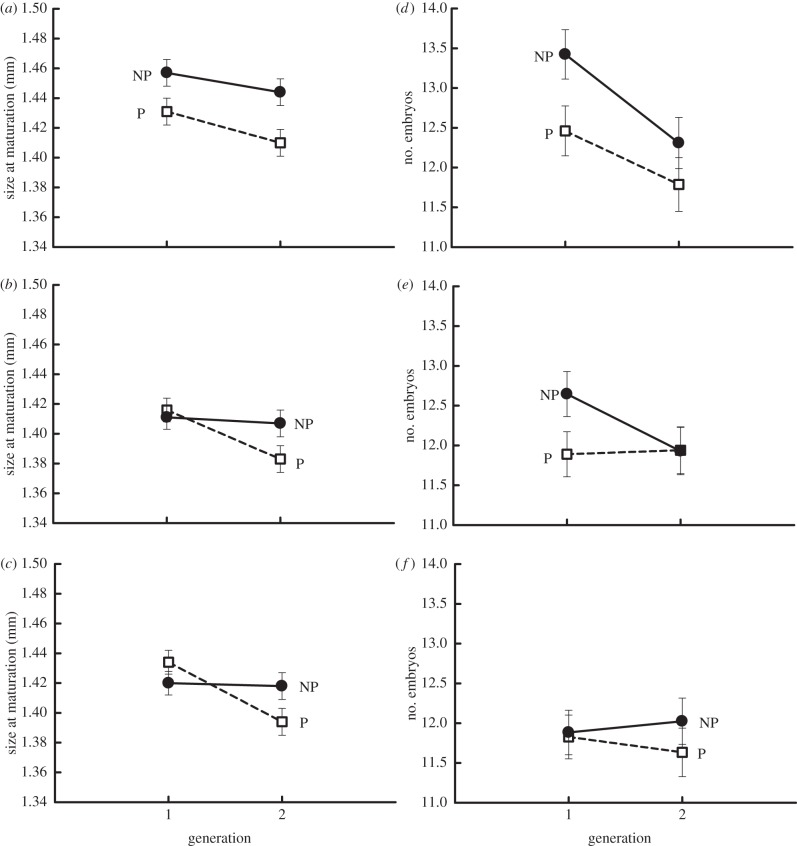
We did not observe significant ‘predator × generation × lake type’ interactions for size at maturation or clutch size (table 1, figure 3). We did however observe a transgenerational response for size at maturation as the interaction between generation and predator treatment was significant for this trait (table 1). Small differences were observed between generation 1 and 2 for the non-predator treatment, but size at maturation declined between generation 1 and 2 for the predator treatment. We did not observe an across-generation response for clutch size (i.e. non-significant ‘predator × generation’ interactions), but clutch size did vary significantly (p < 0.05) across generations and predator treatments (table 1). Average clutch sizes declined by 3.5% between generation 1 and 2.
Figure 3.
Transgenerational responses for size at maturation (a–c) and clutch size (d–f). (a,d) Anadromous lakes, (b,e) landlocked lakes, (c,f) no alewife lakes. P, predator treatment; NP, non-predator treatment. The lake type × generation × predator treatment interaction was not significant for either trait (p > 0.05). Error bars = ±1 s.e.
(c). Intrinsic rate of increase
Contrasting responses to predator cues (within and across generations) for the timing of maturation among Daphnia from anadromous, landlocked, and no alewife lakes was associated with a marginally non-significant (p = 0.052) ‘lake type × predator × generation’ interaction for intrinsic rate of increase (table 1 and figure 3). Daphnia from lakes with anadromous alewife exhibited higher (approx. 3%) intrinsic rates of increase in the presence versus the absence of predator cues in generation 1 (figure 3). Such differences then disappeared in generation 2 (figure 3). Conversely, Daphnia from lakes with landlocked alewife responded to predator cues by strongly increasing intrinsic rates of increase across generations. For this population, intrinsic rates of increases in the presence of predators were approximately 6% lower when compared with the non-predator treatments in generation 1, but such trends were then reversed in generation 2 (r was 4% higher in predator versus non-predator treatments in generation 2; figure 3).
(d). Lake and clone effects
We also observed significant variation among clones from each lake for all life-history traits (table 1). Although we did not find evidence for variance among lakes within each lake type, nor did we find ‘lake × predator × generation’ or ‘lake × predator’ interactions (table 1). Our results therefore suggest that responses within lake types are consistent.
4. Discussion
Our results provide exciting evidence for locally adapted differences in TGP in Daphnia from lakes that experience contrasting fish predator communities (figures 2 and 3). Exposure to predator cues during generation 1 facilitated stronger within-generation responses in Daphnia from lakes with anadromous alewife as these populations matured earlier than Daphnia from landlocked and no alewife lakes (figure 2; see also [35,36,40–42]). Consistent with recent theory suggesting that the evolution of within- versus among-generation plasticity can be decoupled [25–27], we observed the opposite responses across generations. Daphnia from landlocked and no alewife lakes strongly programmed offspring for faster development in the second experimental generation, whereas these transgenerational responses were weak in Daphnia from lakes with anadromous alewife (figures 2 and 4). As a result, life-history differences among lake types observed in generation 1 were eliminated in generation 2. Given that population-level differences in life-history traits, such as age at maturation, can significantly alter consumer–resource dynamics and primary production [42], these evolved differences in TGP are likely to be ecologically relevant. While there exist many examples of TGP [5,7–13] including work showing that closely related species can differ in TGP [12] and that epigenetic markers can vary across populations [43], this is the first study documenting interpopulation divergence in TGP.
Figure 4.
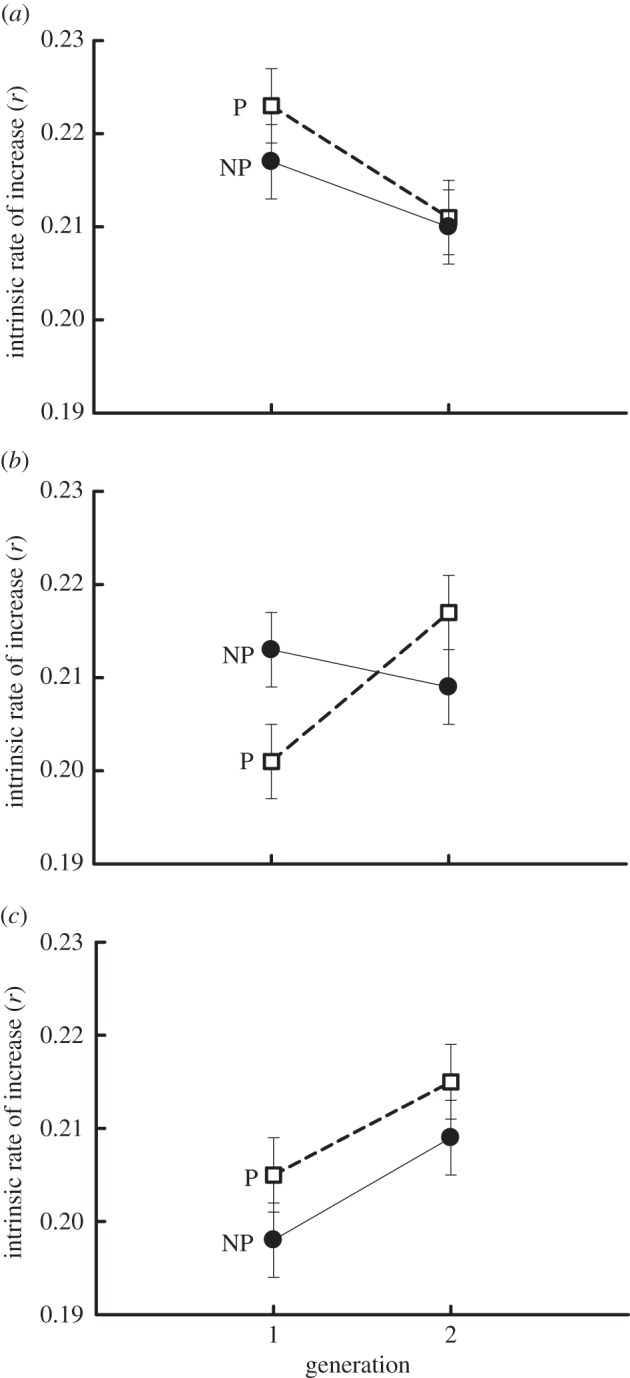
Contrasting transgenerational patterns for intrinsic rates of increase. (a) Anadromous lakes, (b) landlocked lakes, and (c) no alewife lakes. P, predator treatment; NP, non-predator treatment. The lake type × generation × predator treatment interaction was marginally non-significant (p = 0.052). Error bars =±1 s.e.
The results of this study provide new insights into the ecological conditions that drive the evolution of TGP. Our results are inconsistent with the hypothesis that selection will promote simultaneous or coupled increases in within- and across-generation plasticity [22,23]. Instead, our results provide empirical evidence that within- and across-generation plasticity can evolve along independent trajectories in nature. Strong seasonal pulses in predation are associated with the evolution of within-generation plasticity [35], whereas low temporal variability in predator-induced mortality is correlated with increased TGP (see conceptual figure electronic supplementary material, figure S3). Recent theory [25–27] identified environmental stability as one of the key determinants of selection on transgenerational inheritance, with high temporal stability favouring the evolution of increased TGP. This is because TGP decreases the likelihood of environmentally mismatched phenotypes (between parent and offspring) when the rate of environmental change is low compared with generation time. Considering the variation in the temporal dynamics of predation in this system [33,34], differences in TGP among Daphnia from lakes with anadromous, landlocked, and no alewife generally support this novel theory for age at maturation [25,26]. However, we did not observe divergent patterns of TGP among lake types for size at maturation and clutch size (table 1). In general, transgenerational responses for these latter traits were weak in magnitude when compared with very strong transgenerational responses observed for age at maturation (in landlocked and no alewife lakes; figures 2 and 4). Possible explanations for the lack of response observed in these latter traits are that there are trade-offs associated with TGP in development rate [44] or that selection acts more strongly on traits that occur early in life (see also [15]) or on traits with close connections to population growth, such as age at maturation. It is also possible that constraints imposed by the genetic architecture underlying particular traits or the pleiotropic impacts of modifying particular traits (e.g. age at maturation versus clutch size) may restrict what traits are likely to respond to selection for TGP [45].
In lakes with anadromous alewife, Daphnia responded to the presence of predator cues in generation 1 by maturing faster when compared with the non-predator treatments but then slowed their rate of development between generation 1 and 2 (figure 2). For these populations, exposure to a predator cue essentially forecasts the presence of a predator for the immediate future. The observed within-generation response in this population aligns then with theoretical expectations for organisms inhabiting seasonal environments [22–27]. Given that the presence of a predator varies in these lakes, parental Daphnia are not expected to adaptively alter the phenotypes of future generations. The extent to which the observed delay in development rate across generations is maladaptive or simply reflects trade-offs associated with responses within and across generations is unclear ([15] and see also [44]). It is also important to note that the induction of TGP in a temporally varying environment depends upon the rate of environmental change in relation to the generation length of the organism [22–27]. TGP is expected to evolve when the environment changes infrequently compared with the generation length of the organism. On average, it seems likely that the generation time of Daphnia (approx. one week) is shorter than the period of time that Daphnia are susceptible to predation by YOY alewife (approx. three to four weeks). Our results thus signal that the duration of an environmental stimulus may need to exceed the generation length of the organism by more than four times to favour increased TGP.
Our results also revealed similar transgenerational responses in Daphnia from landlocked and no alewife lakes (for age at maturation; figure 2) despite drastic differences in the intensity of fish predation in these two lake types [34]. Daphnia from lakes with landlocked alewife are consistently under strong predation threat and are always rare in the water column, whereas Daphnia in lakes without alewife are consistently abundant [34]. In contrast with some aspects of theory [27], consistent patterns of TGP in Daphnia from landlocked and no alewife lakes indicate that selection on TGP does not depend upon the overall magnitude of mortality, but instead on the temporal dynamics of mortality in relation to generation time (see [27]). Our results collectively serve as a valuable guide to further refine and test existing theory, and to test this theory in other natural systems.
Context-dependent patterns of trait variation (i.e. phenotypic plasticity) have been appreciated for at least 100 years [46]. Interest in TGP developed more slowly, but there now exist numerous examples of environmentally induced responses that span multiple generations (1–3). To the best of our knowledge, no studies have explored natural populations for evolved differences in TGP (but see [12] for interspecific differences). We therefore expect that ecologically driven divergence in TGP is likely to be more common than is currently appreciated. Environmental factors that have been shown to drive genetic shifts in within-generation plasticity [47–50] represent a promising target to experimentally test when and how TGP evolves in nature. Divergent patterns of plasticity may have far-reaching implications for population dynamics [51], community interactions, as well as the long hypothesized link between plasticity and evolutionary processes [3,52–54]. Accordingly, a greater understanding of the prevalence and drivers of evolutionary shifts in TGP has profound implications for predicting the resistance of species and populations to environmental change.
5. Conclusion
Here we leveraged variation in the intensity and duration of a dominant fish predator [34] to test for locally adapted differences in TGP. In agreement with new theory ([25,26], see also [27]), our results provide the first empirical evidence supporting environmental stability as a key selective force driving the evolution of TGP [25,26], with consistently strong (or weak) predator-induced mortality being associated with the evolution of increased TGP (figures 2 and 3). As a result, our results illustrate that the ecological forces that select for increased within- and across-generation plasticity can be fundamentally distinct in a natural system. Considering the diverse importance of understating the abilities of organisms to respond to environmental change, understanding the extent to which divergent ecological conditions drive divergent patterns of TGP in other environments and organisms is an important priority for future research.
Supplementary Material
Acknowledgements
We thank Nathan Campbell, Deirdre Whittington, Jennifer Nguyen, Mina Wilson, Ishrat Durdana for help in the laboratory and Andrew Jones and John Park for help in the field. We thank several anonymous reviewers for comments that greatly improved this manuscript. M.W. and T.C. thank a UTA Research Enhancement Programme grant for funding, whereas S.M. and D.P. thank NSF for funding.
Data accessibility
All data associated with this paper can be found at the designated Dryad depository: doi:10.5061/dryad.794d4.
Authors' contributions
M.R.W., S.M., D.P. designed the study. M.R.W., J.H., M.P., K.B., M.J.W., D.P. performed the experiments. M.R.W., S.M. analysed all data. M.R.W., S.M., D.P. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Jablonka E, Raz G. 2009. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol 84, 131–176. ( 10.1086/598822) [DOI] [PubMed] [Google Scholar]
- 2.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438. ( 10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 3.Bonduriansky R, Crean AJ, Day T. 2012. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl 5, 192–201. ( 10.1111/j.1752-4571.2011.00213.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox CW, Mousseau TA. 1998. Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. In Maternal effects as adaptations (eds Mousseau TA, Fox CW), pp. 159–177. New York, NY: Oxford University Press. [Google Scholar]
- 5.Salinas S, Munch SB. 2012. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett 15, 159–163. ( 10.1111/j.1461-0248.2011.01721.x) [DOI] [PubMed] [Google Scholar]
- 6.Bashey F. 2006. Cross-generational environmental effects and the evolution of offspring size in the Trinidadian guppy Poecilia reticulata. Evolution 60, 348–361. ( 10.1111/j.0014-3820.2006.tb01111.x) [DOI] [PubMed] [Google Scholar]
- 7.Galloway LF, Etterson JR. 2007. Transgenerational plasticity is adaptive in the wild. Science 318, 1134–1136. ( 10.1126/science.1148766) [DOI] [PubMed] [Google Scholar]
- 8.Schmitt J. 1993. Reaction norms of morphological and life-history traits to light availability in Impatiens capensis. Evolution 1993, 1654–1668. ( 10.2307/2410210) [DOI] [PubMed] [Google Scholar]
- 9.Thiede DA. 1998. Maternal inheritance and its effect on adaptive evolution: a quantitative genetic analysis of maternal effects in a natural plant population. Evolution 1998, 998–1015. ( 10.2307/2411232) [DOI] [PubMed] [Google Scholar]
- 10.Galloway LF. 2005. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 166, 93–100. ( 10.1111/j.1469-8137.2004.01314.x) [DOI] [PubMed] [Google Scholar]
- 11.Galloway LF, Etterson JR. 2009. Plasticity to canopy shade in a monocarpic herb: within- and between-generation effects. New Phytol 182, 1003–1012. ( 10.1111/j.1469-8137.2009.02803.x) [DOI] [PubMed] [Google Scholar]
- 12.Sultan SE, Barton K, Wilczek AM. 2009. Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 90, 1831–1839. ( 10.1890/08-1064.1) [DOI] [PubMed] [Google Scholar]
- 13.Dyer AR, Brown CS, Espeland EK, McKay JK, Meimberg H, Rice KJ. 2010. SYNTHESIS: the role of adaptive trans-generational plasticity in biological invasions of plants. Evol. Appl. 3, 179–192. ( 10.1111/j.1752-4571.2010.00118.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SM, Galloway LF. 2010. Environmental context determines within-and potential between-generation consequences of herbivory. Oecologia 163, 911–920. ( 10.1007/s00442-010-1634-0) [DOI] [PubMed] [Google Scholar]
- 15.Walsh MR, Cooley F, Biles K, Munch SB. 2015. Predator-induced phenotypic plasticity within-and across-generations: a challenge for theory? Proc. R. Soc. B 282, 20142205 ( 10.1098/rspb.2014.2205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levins R. 1968. Evolution in changing environments. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Jablonka E, Lachmann M, Lamb MJ. 1992. Evidence, mechanisms and models for the inheritance of acquired traits. J. Theor. Biol. 158, 245–268. ( 10.1016/S0022-5193(05)80722-2) [DOI] [Google Scholar]
- 18.Scheiner SM. 1993. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Evol. S 24, 35–68. ( 10.1146/annurev.es.24.110193.000343) [DOI] [Google Scholar]
- 19.Day T, Bonduriansky R. 2011. A unified approach to the evolutionary consequences of genetic and nongenetic inheritance. Am. Nat 178, E18–E36. ( 10.1086/660911) [DOI] [PubMed] [Google Scholar]
- 20.Fischer B, Taborsky B, Kokko H. 2011. How to balance the offspring quality–quantity tradeoff when environmental cues are unreliable. Oikos 120, 258–270. ( 10.1111/j.1600-0706.2010.18642.x) [DOI] [Google Scholar]
- 21.Shea N, Pen I, Uller T. 2011. Three epigenetic information channels and their different roles in evolution. J. Evol. Biol. 24, 1178–1187. ( 10.1111/j.1420-9101.2011.02235.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyle RB, Ezard TH. 2012. The benefits of maternal effects in novel and in stable environments. J. R. Soc. Interface 9, 2403–2413. ( 10.1098/rsif.2012.0183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezard TH, Prizak R, Hoyle RB. 2014. The fitness costs of adaptation via phenotypic plasticity and maternal effects. Funct. Ecol. 28, 693–701. ( 10.1111/1365-2435.12207) [DOI] [Google Scholar]
- 24.Kuijper B, Johnstone RA, Townley S. 2014. The evolution of multivariate maternal effects. PLoS Comput. Biol. 10, e1003550 ( 10.1371/journal.pcbi.1003550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leimar O, McNamara JM. 2015. The evolution of transgenerational integration of information in heterogeneous environments. Am. Nat. 185, E55–E69. ( 10.1086/679575) [DOI] [PubMed] [Google Scholar]
- 26.Uller T, English S, Pen I. 2015. When is incomplete epigenetic resetting in germ cells favoured by natural selection? Proc. R. Soc. B 282, 20150682 ( 10.1098/rspb.2015.0682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuijper B, Hoyle RB. 2015. When to rely on maternal effects and when on phenotypic plasticity? Evolution 69, 950–968. ( 10.1111/evo.12635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miner BE, De Meester L, Pfrender ME, Lampert W, Hairston NG. 2012. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc. R. Soc. B 279, 1873–1882. ( 10.1098/rspb.2011.2404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter SR, Cottingham KL, Schindler DE. 1992. Biotic feedbacks in lake phosphorus cycles. Trends Ecol. Evol. 7, 332–336. ( 10.1016/0169-5347(92)90125-U) [DOI] [PubMed] [Google Scholar]
- 30.Riessen HP. 1999. Predator-induced life history shifts in Daphnia: a synthesis of studies using meta-analysis. Can. J. Fish. Aquat. Sci. 56, 2487–2494. ( 10.1139/f99-155) [DOI] [Google Scholar]
- 31.Stibor H. 1992. Predator induced life history shifts in a freshwater cladoceran. Oecologia 92, 162–165. ( 10.1007/BF00317358) [DOI] [PubMed] [Google Scholar]
- 32.Brooks JL, Dodson SI. 1965. Predation, body size, and the composition of plankton. Science 150, 28–35. ( 10.1126/science.150.3692.28) [DOI] [PubMed] [Google Scholar]
- 33.Palkovacs EP, Post DM. 2008. Eco-evolutionary interactions between predators and prey: can predator-induced changes to prey communities feedback to shape predator foraging traits? Evol. Ecol. Res. 10, 699–720. [Google Scholar]
- 34.Post DM, Palkovacs EP, Schielke EG, Dodson SI. 2008. Intraspecific variation in a predator affects community structure and cascading trophic interactions. Ecology 89, 2019–2032. ( 10.1890/07-1216.1) [DOI] [PubMed] [Google Scholar]
- 35.Walsh MR, Post DM. 2012. The impact of intraspecific variation in a fish predator on the evolution of phenotypic plasticity and investment in sex in Daphnia ambigua. J. Evol. Biol. 25, 80–89. ( 10.1111/j.1420-9101.2011.02403.x) [DOI] [PubMed] [Google Scholar]
- 36.Walsh MR, Post DM. 2011. Interpopulation variation in a fish predator drives evolutionary divergence in prey in lakes. Proc. R. Soc. B 278, 2628–2637. ( 10.1098/rspb.2010.2634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L. 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377, 147–159. ( 10.1023/A:1003231628456) [DOI] [Google Scholar]
- 38.Laforsch C, Beccara L, Tollrian R. 2006. Inducible defenses: the relevance of chemical alarm cues in Daphnia. Limnol. Oceanogr. 51, 1466–1472. ( 10.4319/lo.2006.51.3.1466) [DOI] [Google Scholar]
- 39.Gotelli NJ. 1998. A primer of ecology, 4th edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 40.Walsh MR, La Pierre KJ, Post DM. 2014. Phytoplankton composition modifies predator-driven life history evolution in Daphnia. Evol. Ecol. 28, 397–411. ( 10.1007/s10682-013-9666-7) [DOI] [Google Scholar]
- 41.Walsh MR, Whittington D, Walsh MJ. 2014. Does variation in the intensity and severity of predation drive evolutionary changes in reproductive life span and senescence? J. Anim. Ecol. 83, 1279–1288. ( 10.1111/1365-2656.12247) [DOI] [PubMed] [Google Scholar]
- 42.Walsh MR, DeLong JP, Hanley TC, Post DM. 2012. A cascade of evolutionary change alters consumer-resource dynamics and ecosystem function. Proc. R. Soc. B 279, 3184–3192. ( 10.1098/rspb.2012.0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrera CM, Bazaga P. 2010. Epigenetic differentiation and relationship to adaptive genetic divergence in discrete populations of the violet Viola cazorlensis. New Phytol. 187, 867–876. ( 10.1111/j.1469-8137.2010.03298.x) [DOI] [PubMed] [Google Scholar]
- 44.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roff D. 2012. Evolutionary quantitative genetics. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 46.Baldwin JM. 1896. A new factor in evolution. Am. Nat. 30, 536–553. ( 10.1086/276428) [DOI] [Google Scholar]
- 47.Donohue K, Messiqua D, Hammond Pyle E, Heschel MS, Schmitt J. 2000. Evidence of adaptive divergence in plasticity: density- and site-dependent selection on shade-avoidance responses in Impatiens capensis. Evolution 54, 1956–1968. ( 10.1111/j.0014-3820.2000.tb01240.x) [DOI] [PubMed] [Google Scholar]
- 48.Lind MI, Persbo F, Johansson F. 2007. Pool desiccation and developmental thresholds in the common frog, Rana temporaria. Proc. R. Soc. B 275, 1073–1080. ( 10.1098/rspb.2007.1737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollander J. 2008. Testing the grain size model for the evolution of phenotypic plasticity. Evolution 62, 1381–1389. ( 10.1111/j.1558-5646.2008.00365.x) [DOI] [PubMed] [Google Scholar]
- 50.Baythavong BS. 2011. Linking the spatial scale of environmental variation and the evolution of phenotypic plasticity: selection favors adaptive plasticity in fine-grained environments. Am. Nat 178, 75–87. ( 10.1086/660281) [DOI] [PubMed] [Google Scholar]
- 51.Rossiter M. 1996. Incidence and consequences of inherited environmental effects. Annu. Rev. Ecol. Syst. 27, 451–476. ( 10.1146/annurev.ecolsys.27.1.451) [DOI] [Google Scholar]
- 52.Richerson PJ, Boyd R. 2005. Not by genes alone: how culture transforms human evolution. Chicago, IL, USA: University of Chicago Press. [Google Scholar]
- 53.Crispo E. 2007. The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61, 2469–2479. ( 10.1111/j.1558-5646.2007.00203.x) [DOI] [PubMed] [Google Scholar]
- 54.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this paper can be found at the designated Dryad depository: doi:10.5061/dryad.794d4.