Abstract
Lekking is a rare, but iconic mating system where polygynous males aggregate and perform group displays to attract females. Existing theory postulates that demographic and environmental stability are required for lekking to be an evolutionarily viable reproductive strategy. However, we lack empirical tests for the hypotheses that lek stability is facilitated by age-specific variation in demographic rates, and by predictable, abundant resources. To address this knowledge gap, we use multistate models to examine how two demographic elements of lek stability—male survival and recruitment—vary with age, social status and phase of the El Niño Southern Oscillation (ENSO) in a Neotropical frugivorous bird, the wire-tailed manakin (Pipra filicauda). Our results show that demographic and environmental conditions were related to lek stability in the Ecuadorean Amazon. Apparent annual survival probability of territorial males was higher than that of non-territorial floaters, and recruitment probability increased as males progressed in an age-graded queue. Moreover, annual survival of territorial males and body condition of both floaters and territory holders were higher following years with El Niño conditions, associated with reduced rainfall and probably higher fruit production in the northern Neotropics, and lower after years with wet, La Niña conditions that predominated our study. Recruitment probabilities varied annually, independent of ENSO phase, and increased over our study period, but the annual mean number of territorial males per lek declined. Our results provide empirical support for hypothesized demographic and environmental drivers of lek dynamics. This study also suggests that climate-mediated changes in resource availability can affect demography and subsequent lek stability in a relatively buffered, lowland rainforest.
Keywords: survival, recruitment, pipridae, lek stability, El Niño Southern Oscillation
1. Introduction
Lekking, a form of male-dominance polygyny, is a reproductive strategy in which males are spatially aggregated and perform group displays to attract and mate with females. Consistent with evolutionary theory, leks are thought to form because individuals cannot profitably control or monopolize the resources essential for successful mating [1,2]. Leks of most species also exhibit remarkable spatial and temporal stability [2–4]. At the population level, lek stability is defined by spatial site persistence and temporal continuity in lek size; at the individual level, it is defined by strong site fidelity and lifetime tenure at leks. This stability facilitates male fitness benefits when females show site fidelity [5,6] or when lower ranking males can appropriate copulations from long-tenured, dominant males [7,8]. Existing theory postulates that demographic and environmental stability are required for lekking to be an evolutionarily viable reproductive strategy [3,9,10]. The demographic processes that enable lek stability are ultimately shaped by spatial and temporal patterns of resource availability. However, empirical studies have rarely been designed to elucidate the demographic [11] and environmental [12] mechanisms that facilitate lek stability, information that is essential for understanding how climatic variation will affect the population dynamics of lekking species.
One condition for spatial and temporal stability of leks is related to life history and demographic structure. Intense sexual selection and reproductive skew characterize lek-mating systems, and have been hypothesized to cause age-specific variation in demographic rates [9]. Demographic stability of leks requires a long-term balance between the mortality of territorial males and recruitment of non-territorial ‘floater’ males into the breeding population. For most lekking vertebrates, this stability is generated by multi-year site persistence (i.e. high annual survival rates) of territorial males and delayed recruitment of floater males into leks. Conspicuous lekking displays have been hypothesized to increase predation risk [13,14], but studies in a number of taxa document low predation risk on leks [2] and high apparent annual survival of territorial, lekking males (e.g. family Pipridae, 0.66–0.97; [15–17]). Limited opportunities for recruitment in lek social systems make behavioural dominance, social status, and in some species, social partnerships prerequisites for territoriality. Moreover, delayed male recruitment and age-specific queuing for breeding status maintain dominance hierarchies, social cohesion and spatial lek persistence [11,18–21]. The relationships between survival and recruitment are therefore key to our understanding of lek stability, but few studies have simultaneously measured these demographic parameters while controlling for age and status.
A second condition for lek stability is that resources are more reliable at lek sites (i.e. environmental hotspots) relative to other locations in the landscape [12,22]. At the ultimate level, dietary reliance on fruit is often linked to the evolution of lekking behaviour in birds (approx. 75% of the 97 avian lekking species are frugivorous; [23]). At the proximate level, the distribution and availability of resources, such as fruit, can influence lek placement, size and density [12,24,25]. Lekking display behaviour is also energetically demanding [26,27], leading to the hypothesis that resource availability should be positively correlated with male survival and recruitment, and therefore with lek stability. Despite this apparent causal link, no studies have explicitly examined how environmental factors influence demographic processes at leks. One posible challenge to such a test is the difficulty of quantifying long-term spatial and temporal variation in food availability (e.g. for frugivorous species that consume fruits from more than 100 plant species).
Here, we use 11 years of capture–recapture data to examine how two demographic elements of lek stability—survival and recruitment—vary with age, social status and phase of the El Niño Southern Oscillation (ENSO) in a Neotropical frugivorous bird, the wire-tailed manakin (Pipra filicauda). ENSO is the greatest source of climate variation in the equatorial tropics [28,29] and can serve as a reliable proxy for local weather and resource availability [30]. El Niño events tend to increase solar irradiance and decrease precipitation in the northern Neotropics, whereas La Niña events produce the opposite effects [29,31]. El Niño droughts synchronize flowering and increase fruit production, and positively affect the abundance of frugivorous vertebrates [32,33]. El Niño conditions were infrequent during our study, whereas La Nina conditions dominated the later half of the dataset.
We test three predictions derived from the hypothesized demographic and environmental conditions that facilitate lek stability. First, apparent annual survival of territory holders should be high relative to floaters. Annual survival probability in our analyses represents the complement of mortality and permanent emigration, and therefore estimates male site persistence. Lower apparent survival/site persistence for P. filicauda floater males is expected because some individuals will fail to establish the social partnerships required for territorial recruitment and will permanently emigrate from the study area. Second, the probability of floaters ascending to territorial status should be positively correlated with position in the age-graded queue. When recruitment is constrained by age-graded queues, stable dominance hierarchies and coalition partnerships, floater settlement will facilitate spatial lek site persistence and temporal continuity in lek size [11,20]. Third, manakin survival and body condition should be higher following years characterized by the warm, El Niño phase of the Southern Oscillation. As a corollary of these predictions, we examined annual variation in mean lek size over the study period to test if recruitment was sufficient to maintain temporal lek stability. This study design allows us to link environmental variation with demography to provide much needed data on the effects of climate variation on tropical birds [34,35], while concurrently advancing our understanding of the ecological mechanisms that influence spatial and temporal stability of leks.
2. Material and methods
(a). Study species
Wire-tailed manakins are a small, understory frugivore broadly distributed across the northern Amazon basin, and have been the focus of detailed demographic and behavioural monitoring in Ecuador since 2001. Breeding males form exploded leks and defend territories where individuals are within auditory, but not visual range [36]. Territorial males form stable, male–male display coalitions with both territorial and non-territorial floater males [21]. Floaters follow age-graded queues for territorial status [20,21]. Like other manakins, territorial males spend as much as 90% of daylight hours within lek sites. Territorial males only leave their territories for short foraging bouts (less than 100 m; T.B. Ryder 2003, personal observation), in part because P. filicauda leks are in locations with relatively abundant and diverse fruit resources (i.e. environmental hotspots; [12]). By contrast, floaters range over larger areas, often visiting coalition partners at contiguous leks [20]. The combination of high site fidelity and stable coalition partnerships greatly facilitate demographic monitoring and the examination of temporal changes in status and lek size.
(b). Field sampling
We conducted the study during November—March 2003–2013 at the 650 ha Tiputini Biodiversity Station within the Yasuní Biosphere Reserve, Orellana province (0°38′ S 76°08′ W). The site is dominated by lowland, terra firme forest, but also includes some floodplain, varzea habitat [12]. Climate at Tiputini is largely aseasonal, but peak rainfall occurs from May to August, with November to March being the driest months (average rainfall = 2676 mm yr−1; n = 6). Phenological data from the adjacent Yasuni Biological Station shows that the onset of dry weather from November to March increases flowering synchrony followed by peak fruit abundance from April to June [37]. Peak reproductive activity for wire-tailed manakins occurs in the driest months [38], but varies with annual precipitation patterns.
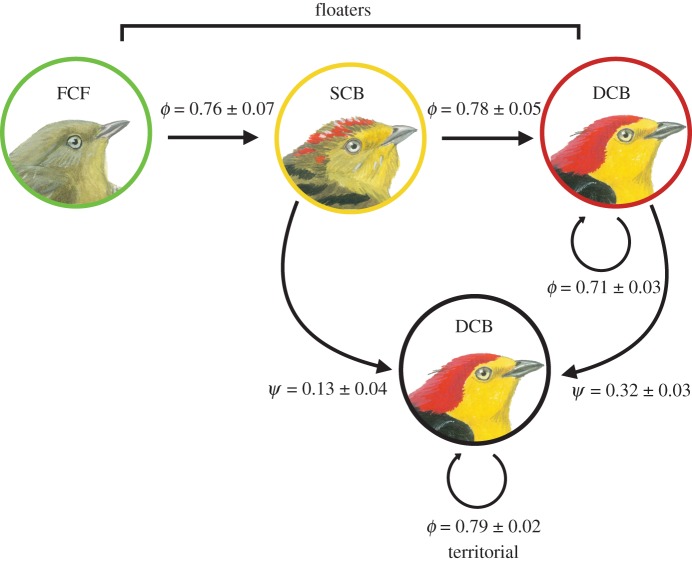
We studied eight leks on two 100 ha study plots and along 30 km of trail. The study area expanded from 2003 to 2004 to ensure that floater males displaying away from the focal leks were detected, but mark–resight sampling remained fixed thereafter. Leks consisted of eight to 10 male territories and 10–12 floater males that formed display partnerships with territory holders (see below). At the start of each field season, we used mist nets to systematically capture any unbanded males on territories and within 50–75 m of leks. Each captured bird was sexed, aged, marked with a unique combination of aluminium and coloured plastic leg rings, had its right tarsus measured to the nearest 0.01 mm with digital callipers and weighed to the nearest 0.1 g with a digital scale. Wire-tailed manakins have delayed plumage maturation and can be accurately aged into their third year of life ([39]; figure 1). Following the aging system of Wolfe et al. [40], we used moult limits and plumage criteria to classify males as first-cycle formative (FCF; less than 1 year old), second-cycle basic (SCB; 2 years old) or definitive-cycle basic (DCB; more than or equal to 3 years old).
Figure 1.
Apparent annual survival of male wire-tailed manakins at Tiputini Biodiversity Station, Ecuador, varied with social status, but not with age. Territory holders had higher estimated survival than all age-classes of floaters. Recruitment probability increased with male age; older (DCB) males had a higher probability of obtaining a territory than younger (SCB) males.
We visited all leks five to 10 times per field season to resight colour-marked individuals and to survey the number of floater and territorial males associated with each lek. During these visits, we used plumage and behaviour [20,21,41] to classify status (floater versus territory holder) for every male detected. A bird was considered present in a given season if it was detected during at least one survey. The stability of male–male display coalitions partnerships enabled us to resight a large proportion of the floater population annually. We assessed changes in male status by annually monitoring territorial turnover and status changes among floater males. Definitive plumage (i.e. DCB) is a prerequisite for territorial status: social ascendancy only occurs when a definitive floater obtains a vacant territory on a lek site [20]. Therefore, age, plumage and territorial status are partially confounded in P. filicauda. Changes in male status from floater to territory holder were always maintained within and across years; we detected no status reversals.
(c). Data analyses
We estimated annual survival, recruitment and recapture probabilities with, multistate mark–recapture models implemented in program MARK (v. 7.1; [42]). Our 11 year encounter dataset was constructed from captures of 273 unique males, 608 resightings of these individuals, and had an effective sample size of 835. A key assumption of multistate models is within-season population closure [42]. Here, we define the population as all leks (n = 12) within the larger geographical study area. Several attributes of the manakin system allowed us to meet this assumption. First, territorial males have high within- and between-year site fidelity, and are rarely missed during resighting surveys (see Results). Second, floaters establish stable coalition partnerships with territorial males each breeding season, making within-season, temporary emigration from our study site highly unlikely. Finally, all 12 leks at the 650 ha Tiputini reserve have been mapped, and floaters frequently visit territorial, coalition partners at contiguous leks [20]. Although eight leks formed the basis for our analysis, the remaining four were monitored annually for movement and recruitment of marked males. The demographic closure of our study population within each capture interval permitted a relaxation of the general assumption of equal capture probability [43]. We accounted for temporal variation in effort by allowing detection probability to be time-specific (see below).
We used a two-stage modelling approach, described below. Candidate model sets were balanced, or symmetrical, with respect to all predictors [44]. Models were fitted using a logit link function and ranked by Akaike's information criterion, corrected for small sample sizes (AICc); relative likelihood of each model i was estimated with AICc weights (wi; [45]). We assessed the importance of individual predictor variables by summing the wi of relevant candidate models [45,46]. Results are given as a parameter estimate ±1 s.e.
The first candidate model set tested the predictions that annual survival and recruitment probabilities increased with age and status. The set included six parametrizations of survival probability (ϕ): (i) constant, (ii) year-specific, (iii) conditional on social status (floater, lek territory holder), (iv) an interaction between age class, represented by plumage category, and status (1st year floater FCF, 2nd year floater SCB, 3+ year floater DCB and territorial DCB), and (v–vi) additive and multiplicative interactions between year and social status. See the electronic supplementary material, table S1, for full model details. We were limited to three age categories because many unmarked males were first captured in DCB plumage. Our data were also too sparse to model interactions between age, status and year. Detection probability (ρ) in all models was year-specific for floaters because field effort varied among years, and constant for territory holders because only four of 80 territorial capture histories contained interior zeros. Given that territoriality is a prerequisite for breeding in this system [41], we define recruitment as the process by which floater males obtain territories, rather than simply entering the adult population. Recruitment probability (ψ) from floater to territory holder was modelled in four ways: (i) constant, (ii) conditional on age class for SCB and DCB floaters, and (iii–iv) as additive and multiplicative interactions between age class and year. We fixed ψ at 0 for FCF males because definitive plumage is a prerequisite to hold a territory, and for all territorial individuals because status transitions were unidirectional from floater to territory holder. Recruitment probability can be modelled for SCB plumage males because some individuals attain territories shortly after moulting into definitive plumage early in their third year of life. The complete first set contained 24 candidate models (electronic supplementary material, table S2).
The second model set tested if ϕ and ψ varied with ENSO phase, our proxy for fruit resource availability at Tiputini. We used annual mean monthly values of the standardized Southern Oscillation Index (SOI) to represent ENSO conditions for each calendar year. Data were downloaded from the NOAA Climate Prediction Center (www.cpc.ncep.noaa.gov/data/indices/soi). High, positive values of SOI indicate La Niña conditions and low, negative values indicate El Niño conditions ([29]; electronic supplementary material, figure S1a). We used a 1 year time lag where mean, monthly SOI from year y was used to predict manakin survival and recruitment to year y + 1. The first model set, described above, provided strong support for the hypothesis that survival and recruitment varied by social status (see Results). Therefore, we used a subset of those candidate models as the basis for testing if ϕ and ψ were correlated with SOI. The second candidate set included five parametrizations of ϕ: (i) conditional on social status (floater, lek territory holder), (ii–iii) additive and multiplicative interactions between social status and year, and (iv–v) additive and multiplicative interactions between social status and the previous year's SOI value. Detection probability (ρ) in all models was year-specific for floaters and constant for territory holders. We modelled ψ from floater to territory holder in five ways: (i) conditional on age class for SCB and DCB floaters, (ii–iii) as additive and multiplicative interactions between age class and year, (iv–v) as additive and multiplicative interactions between age class and the previous year's SOI value. We fixed ψ at 0 for both FCF males and all territorial individuals. The complete second set contained 27 candidate models (electronic supplementary material, table S3).
We used program U-CARE [47] and the JollyMove model (JMV; [48]) to confirm multistate model goodness of fit and lack of over-dispersion while allowing encounter and survival probabilities to vary by state. Our data met model assumptions under a fully time-saturated model. Tests for state differences in re-encounter probability (transience) (3G.SR; all p > 0.35), state-dependent recapture probability (3G.SM; all p > 0.41) and global goodness of fit the JMV model (all p > 0.16) did not indicate departure from expected frequencies.  , a measure of variance inflation to assess over-dispersion between general and saturated models, were close to 1 for all age-classes
, a measure of variance inflation to assess over-dispersion between general and saturated models, were close to 1 for all age-classes  , indicating a lack over-dispersion for our global model.
, indicating a lack over-dispersion for our global model.
Given the hypothesized link between resources and survival, we tested if male body condition varied with SOI by calculating a scaled mass index for each individual capture. The index uses a scaling exponent bSMA calculated by dividing the slope of the ordinary least-squares (OLS) regression (mass versus tarsus) by the Pearson's correlation coefficient of those two morphological variables [49]. This scaled mass index is a better measure of relative energy reserves and body condition than standard OLS regression residuals [49]. Although we captured some territorial males during coalition displays at leks, annual banding efforts were largely focused on unmarked floaters. Because the vast majority of males did not have repeat condition measures, we chose not to use this index as an individual covariate in our multistate models. Scaled condition indices were based on an average of 30 males yr−1. We used a Pearson correlation coefficient to assess the relationship between SOI values in year y and the body mass index in year y + 1.
Finally, we used a generalized linear mixed model to test if lek size changed over time. Lek size was modelled as a function of year and year2, and lek identity (ID) was included as a random effect. Because year and SOI are collinear, we did not model the effects of ENSO phase on lek size. We also excluded 2003, the first year of the study, because not all territories had been found and mapped. We tested for the effects of year on lek size by iteratively dropping terms and comparing models with likelihood ratio tests. The simplest model included lek size, the random effect of lek ID and an intercept. Models were fitted with program R and the lme4 package [50].
3. Results
Our results support the prediction that territorial males have high annual survival probability, and hence site persistence, relative to floater males (table 1 and figure 1; best-fit model: ϕterritorial = 0.79 ± 0.02; ϕfloater = 0.74 ± 0.02). The strong and predictable site persistence of territorial males was further evidenced by nearly perfect detection (0.98 ± 0.01), whereas ρ varied annually for floaters (electronic supplementary material, figure S2a). Based on Σwi, models with status-specific ϕ had 4.4 times more support than models with ϕ differing by age × status classes (see the electronic supplementary material, table S2). However, our age × status models had some statistical support (Σwi = 0.142), suggesting that DCB floaters had either lower annual survival or higher emigration rates than DCB territory holders and the younger FCF and SCB floaters (figure 1; electronic supplementary material, table S2). Models that specified ϕ as a function of year received little statistical support (table 1).
Table 1.
Model selection rankings for the top 10 general models examining the influence of age and status on wire-tailed manakins survival and recruitment probability at Tiputini Biodiversity Station, Ecuador from 2003 to 2013. (Model structure includes notation for male status (F, floater; T, territorial) and three age-classes (FCF = 1st year, SCB = 2nd year, DCB = 3+ years). The influence of age on survival is only possible for floaters because of age-related plumage maturation (i.e. all territorial males are in definitive plumage). Transitions in status from floater to territorial are unidirectional (T to F fixed at 0) and territories are typically obtained after the 2nd to 3rd year transition in the age-graded queue.)
| model |
||||||
|---|---|---|---|---|---|---|
| ϕ | ρ | ψ | Ka | deviance | ΔAICc | wib |
| F[.] × T [.] | F[yr] T[.] | SCB[yr] + DCB[yr] | 23 | 681.42 | 0.00 | 0.404 |
| F[.] × T [.] | F[yr] T[.] | SCB[yr] × DCB[yr] | 31 | 665.50 | 1.20 | 0.222 |
| [.] | F[yr] T[.] | SCB[yr] + DCB[yr] | 22 | 685.60 | 2.07 | 0.144 |
| F[age] × T [.] | F[yr] T[.] | SCB[yr] + DCB[yr] | 25 | 680.13 | 2.96 | 0.092 |
| [.] | F[yr] T[.] | SCB[yr] × DCB[yr] | 30 | 669.72 | 3.25 | 0.080 |
| F[age] × T [.] | F[yr] T[.] | SCB[yr] × DCB[yr] | 33 | 664.17 | 4.19 | 0.050 |
| F[yr] + T [yr] | F[yr] T[.] | SCB[yr] + DCB[yr] | 31 | 672.73 | 8.42 | 0.006 |
| F[yr] + T [yr] | F[yr] T[.] | SCB[yr] × DCB[yr] | 40 | 656.73 | 12.08 | 0.000 |
| [yr] | F[yr] T[.] | SCB[yr] + DCB[yr] | 31 | 677.28 | 12.97 | 0.000 |
| [yr] | F[yr] T[.] | SCB[yr] × DCB[yr] | 39 | 661.31 | 14.45 | 0.000 |
aNumber of identifiable parameters.
bModel weight.
Recruitment probability was positively correlated with floater position in the age-graded queue, as predicted (figure 1). Our results also indicated that ψ increased annually for both SCB and DCB floaters (figure 2; electronic supplementary material, table S2). An additive effect of age class and year (i.e. an equal slope for the year effect) on ψ had 1.8 times more support than a multiplicative effect (Σwi = 0.64; see the electronic supplementary material, table S3).
Figure 2.
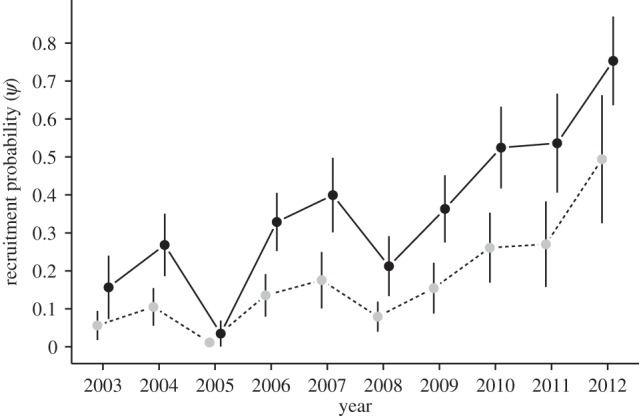
Recruitment probabilities of floater wire-tailed manakins into the breeding population at Tiputini Biodiversity Station, Ecuador varied among years. DCB birds (black dots and solid line) are at least 3 years of age and second-cycle birds (SCB; grey dots and dashed line) are 2 years of age.
Male survival and body condition varied with ENSO phase. The best-fit multistate model included a negative correlation between SOI and ϕ of territorial, but not floater males (slope parameter, βterritorial = −0.42, 95% confidence interval (CI) = −0.80, −0.05; table 2). Annual survival of territorial males was approximately 10% higher when the preceding year was characterized by the negative, El Niño phase of the Southern Oscillation and lower following positive-phase, La Niña years (figure 3a). An unequal slope model with SOI affecting ϕ of both territorial and floater males also received substantial support, but the slope for floaters was not statistically different from zero (βfloaters = 0.37, 95% CI = −0.14, 0.87; table 2). Models that included the effect of SOI on survival had six times more support than those did not (Σwi = 0.86; see the electronic supplementary material, table S3). Similarly, male body condition was negatively correlated with SOI (r = −0.82, p = 0.007; figure 3b) and declined over time (electronic supplementary material, figure S2b) because positive-phase, La Niña years predominated during the later half of our study (electronic supplementary material, figure S1).
Table 2.
Model selection rankings for the top 10 models examining the effect of El Niño phase, represented by the SOI, on wire-tailed manakin survival and recruitment probability at Tiputini Biodiversity Station, Ecuador from 2003 to 2013. (Model structure includes notations for male status (F, floater; T, territorial) and age (SCB = 2nd year, DCB = 3+ years).)
| model |
||||||
|---|---|---|---|---|---|---|
| ϕ | ρ | ψ | Ka | deviance | ΔAICc | wib |
| F[.] × T [SOI] | F[yr] T[.] | SCB[yr] + DCB[yr] | 24 | 676.69 | 0.00 | 0.324 |
| F[SOI] × T [SOI] | F[yr] T[.] | SCB[yr] + DCB[yr] | 25 | 674.58 | 0.02 | 0.322 |
| F[.] × T [.] | F[yr] T[.] | SCB[yr] + DCB[yr] | 23 | 681.42 | 2.60 | 0.088 |
| F[SOI] × T [.] | F[yr] T[.] | SCB[yr] + DCB[yr] | 24 | 679.31 | 2.62 | 0.087 |
| F[SOI] × T [SOI] | F[yr] T[.] | SCB[yr] × DCB[yr] | 34 | 658.62 | 3.43 | 0.059 |
| F[.] × T [.] | F[yr] T[.] | SCB[yr] × DCB[yr] | 31 | 665.50 | 3.80 | 0.049 |
| F[SOI] + T [SOI] | F[yr] T[.] | SCB[yr] + DCB[yr] | 24 | 680.80 | 4.11 | 0.042 |
| F[SOI] + T [SOI] | F[yr] T[.] | SCB[yr] × DCB[yr] | 32 | 664.90 | 5.36 | 0.022 |
| F[SOI] × T [SOI] | F[yr] T[.] | SCB[SOI] + DCB[SOI] | 18 | 698.84 | 9.50 | 0.003 |
| F[SOI] × T [SOI] | F[yr] T[.] | SCB [SOI] × DCB[SOI] | 19 | 697.94 | 10.70 | 0.002 |
aNumber of identifiable parameters.
bModel weight.
Figure 3.
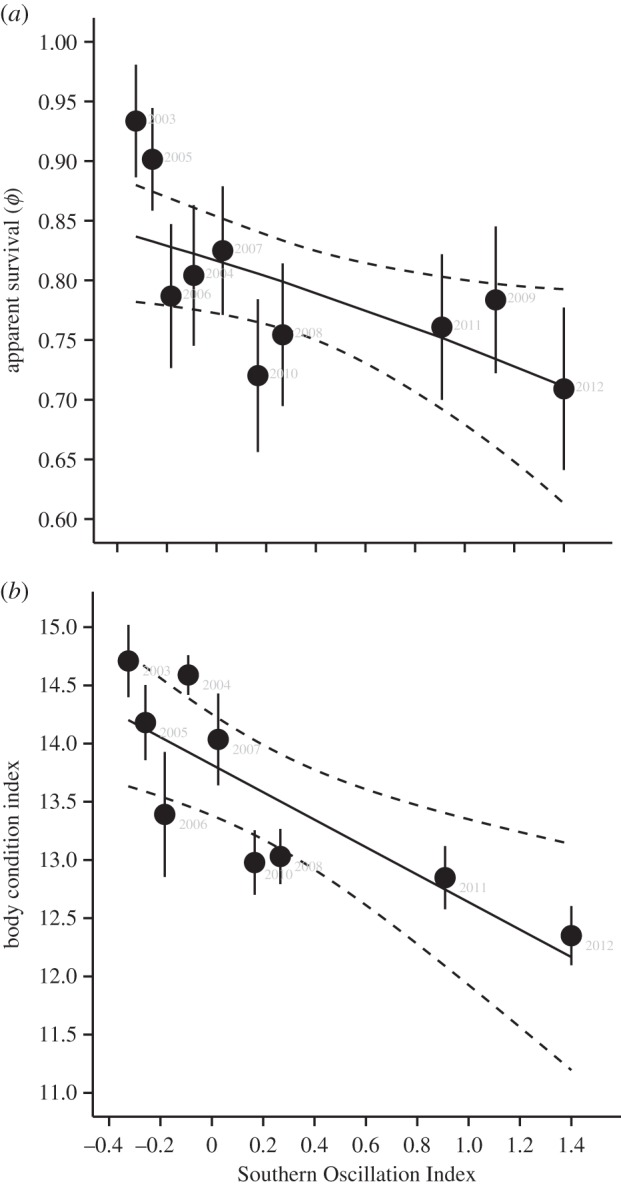
Survival and body condition of male wire-tailed manakins varied with phase of the ENSO as measured by the SOI. (a) Annual survival probability was higher survival following El Niño years and lower following La Niña years. Points and error bars (s.e.) are annual survival estimates for territorial males. The solid line represents apparent survival estimated using beta parameters from the best-fit model incorporating SOI. Dashed lines represent upper and lower 95% confidence intervals for estimated survival probability. (b) Body condition was also higher following El Niño years and lower following La Niña years. Points and error bars (s.e.) are mean male body condition estimates. One year with insufficient data on body condition (2009) is excluded. Estimated mean body condition (solid line) and the 95% CI (dashed lines) were estimated using beta parameters from a model incorporating the influence of SOI on male condition.
Despite an increase in recruitment probability over time (figure 2), the number of male territories per lek size declined over our study period for five of the six leks monitored. Models that included a year and year2 effect received strong support relative to the null model, and the model with quadratic effect of year on lek size had more than three times the support as the model with only a linear effect of year (electronic supplementary material, table S4). The decline in lek size was nonlinear, with the largest decreases occurring after the 2010 breeding season (electronic supplementary material, figure S3).
4. Discussion
Extensive research has documented the remarkable spatial and temporal stability of leks despite male turnover [3,4,12,51], yet the relative importance of demographic and environmental factors in maintaining this stability have remained unclear. Multiple ecological factors and their interactions have been hypothesized to influence lek dynamics, including high survival of territorial males, delayed recruitment into the breeding population and predictable, abundant resources. Despite the importance of lek stability for the viability of this reproductive strategy, no studies have explicitly tested these predictions. Our results for an Amazonian lekking bird show that male apparent survival varies with status (territorial versus floater) and that recruitment probability increases as non-territorial males progress in an age-graded queue. We also demonstrate that annual survival of territorial males and body condition are correlated with the SOI, a proxy for rainfall pattern and fruit availability in the northern Neotropics. Below, we discuss these findings in the context of hypothesized demographic and environmental prerequisites of lek stability. We also consider how climate-mediated resource limitation affects lek stability, and how this mechanism informs our ability to predict consequences of future environmental change.
(a). The benefits of territoriality
Our best-supported multistate models indicate that territorial male P. filicauda have a higher annual survival probability than floater males. To our knowledge, this is the first study to examine status-specific survival differences for manakins. Territory holders also showed extremely high site fidelity, with detection probabilities close to 1 (ρ = 0.98) and an average territory tenure of 5+ years, both of which should facilitate stable lek sizes over time. Our survival estimates for territorial males are higher than those published previously for P. filicauda [15], but within the ranges reported for other Pipridae [16,17]. Previous work has shown that leks are located in food-rich areas [12,25] and that resource availability can influence lek size and population density [24]. Therefore, lek placement in environmental hotspots could influence survival of territory holders directly [12] or indirectly if these males use public information to increase foraging efficiency [52].
In contrast to our results, two studies of lekking grouse (Phasianidae) found that male survival did not significantly vary with breeding status or effort [53,54]. The survival estimates for floater males we report here could be biased low if these individuals are more likely than territory holders to permanently disperse from a study area. Floater P. filicauda follow an age-graded queue for territorial status. Fidelity to lek sites has clear benefits to these individuals because the probability of territorial inheritance increases with number and stability of coalition partnerships with territorial males [20]. Floaters, however, wait up to 6 years to obtain a territory. Thus, some individuals, particularly definitive plumage floaters (ϕDCB floater = 0.71 ± 0.03; figure 1), may be more likely to permanently emigrate from the population if they fail to establish stable coalition partnerships early in life. If this hypothesis is true, reduced floater survival is indicative of lower site persistence, and further underscores the importance of social partnerships on recruitment dynamics and subsequent lek stability. Additional research on other lekking species is needed to confirm the generality of our survival results and to better understand how age-graded queues influence dispersal decisions and recruitment processes.
(b). Orderly queues for status
Territorial ascension or turnovers occur when a territorial male disappears and one of his stable coalition partners inherits the territory. Breeding males, on average, have three stable coalition partners [20]; however, we know little about how a coalition partner inherits a vacant territory. The data presented here show that floaters in definitive plumage (more than 3 years old) have a higher recruitment probability than young floaters (less than or equal to 3 years old; figure 1). Given that coalition stability increases with partnership length [21] and that male age is a good proxy for dominance status, we hypothesize that the probability of territorial inheritance is driven by partnership tenure and position in the dominance hierarchy. Age-graded queuing for status is a common characteristic of lekking social systems [18,55,56] and the orderly nature of those queues in P. filicauda is probably key to both the demographic and social stability of this lek-mating system. Viewed cumulatively, age-specific patterns of survival and recruitment highlight the costs and benefits of status, and suggest that territorial and floater males may be subject to different demographic selection pressures.
(c). Drivers of individual condition and demography
Resource availability and vertebrate vital rates are affected by large-scale climate cycles, such as ENSO, across a range of species and habitat types [57–60]. Vertebrate frugivores are thought to be particularly sensitive to bottom-up trophic instability resulting from climatic variation. In the northern Neotropics, where ENSO effects rainfall patterns, El Niño droughts have been shown to induce synchronized flowering and increased seed set that subsequently increases the abundance of frugivorous vertebrates [32,33]. More broadly, a number of empirical studies have highlighted negative effects of fruit scarcity on body condition [61], breeding phenology [24] and population dynamics [32,62]. We found that territorial male P. filicauda had both higher survival and individual body condition following El Niño years, associated with presumably greater fruit abundance, and lower condition and survival following La Niña years.
We hypothesize that the correlation between ENSO phase and survival of territorial males was owing to the energetic costs of lekking display and space-use constraints. Acrobatic displays by male P. filicauda are physically demanding [27], and male manakins often loose mass over the breeding season [63]. Territorial males also display at significantly higher rates than floater males [21]. Moreover, territorial males must maximize both lek residency time to increase fitness and foraging efficiency to maintain breeding season body mass. By contrast, floaters regularly move among leks. The combination of reduced energetic demands (i.e. lower display rates) and larger home ranges of floaters may make them less vulnerable to local food depletion and better able to exploit patchy food resources in years with low fruit availability. Changes in fruit availability may decrease male body condition, but are unlikely to cause male P. filicauda mortality via starvation. Ultimately, other direct causes of mortality, such as predation, probably interact with resource abundance and individual condition to influence temporal patterns of survival.
(d). Lek stability
Understanding how climate-related mechanisms limit resource availability and lek stability will help us predict the demographic responses of lek-breeding species to environment perturbations. Our results show that the mean number of territories per lek declined for a common Amazonian bird during consecutive years with La Niña conditions. Although the losses of male territories we documented (range 1–4) may seem inconsequential, the P. filicauda social system is driven by age-graded queues, and vacancies represent the sole access to mating opportunities [41]. Even loss of a single territory suggests either an insufficient number of young recruits, inadequate fruit availability at leks, or a combination of the two, to maintain stable lek sizes through time. Climatic effects on female survival and/or fecundity could be one potential cause of insufficient recruitment. We hypothesize that food limitation related to successive, wet La Niña years is the mechanism responsible for the declines in lek size. Alternatively, changes in habitat structure associated with lek sites, such as increased frequency of treefall gaps and concomitant effects on the understory light environment, could have influenced territory placement and the number of territories per lek [64]. Understory habitat at Tiputini is dynamic, with new treefall gaps forming and existing gaps becoming overgrown. However, our 12 monitored leks and their constituent territories did not move or change configuration, even though some gaps formed near lek sites. Therefore, we suggest that habitat change is not responsible for the patterns we present here. Additional research is needed to test if climate-mediated variation in food availability is a general mechanism that affects lek dynamics. Regardless of the mechanism, changes in lek size could create a negative feedback loop, given that social facilitation and stable coalition partnerships are known to influence lek activity and recruitment [20,21,41].
In conclusion, this study provides empirical support for hypothesized demographic and environmental conditions that favour stable vertebrate leks over space and time. Our results also implicate a climate-based mechanism that affects resource availability, demographic rates and lek stability in lowland rainforest. Specifically, the finding that survival is correlated with ENSO raises questions about how changing environmental conditions might affect population dynamics of lek-breeding species in the Amazon. Intact Amazonian forests and the species therein are typically thought to be resilient or buffered against climatic change [65], although few empirical studies have corroborated this idea [34]. However, niche modelling predicts that manakin species limited to lowland Amazonian habitats are likely to be affected by climate change [66]. This study appears to support this idea. Our results, combined with projections for increased rainfall in the western Amazon during the December–February dry season [67], suggest that survival of territorial males and lek sizes of P. filicauda will decline in the future. Further research is needed to determine if and how synergies between climate, food availability and deforestation could reduce lek sizes below some key social threshold (i.e. Allee effects) and jeopardize long-term persistence of frugivorous, lekking species.
Supplementary Material
Acknowledgements
We thank our field technicians, J. Blake and B. Loiselle, and S. Converse and J. Hines for assistance with multistate models and GOF testing. D. C. Romo, K. Swing and D. Mosquera provided logistical support. Jeff Brawn, Daniel Cadena and one anonymous reviewer provided feedback that improved the manuscript.
Ethics
This research was conducted with permission from the Ministerio de Ambiente, Tena, Ecuador. All protocols followed International Animal Care and Use Committees guidelines for the University of Missouri–St. Louis (UMSL) and the Smithsonian's National Zoological Park.
Data accessibility
Data are available on Data Dryad.
Authors' contribution
T.B.R. designed and conducted fieldwork. T.B.R. and T.S.S. conducted the statistical analyses and wrote the manuscript. Both authors gave approval for publication.
Competing interests
We have no competing interests.
Funding
This research was funded by the Harris World Ecology Center, National Science Foundation (IOB 0508189 and IOS 1353085) and the Smithsonian Migratory Bird Center.
References
- 1.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 2.Hoglund J, Alatalo RV. 1995. Leks, p. 248 Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Bradbury JW, Vehrencamp SL, Gibson RM. 1989. Dispersion of displaying male sage grouse I. Patterns of temporal variation. Behav. Ecol. Sociobiol. 24, 1–14. ( 10.1007/bf00300112) [DOI] [Google Scholar]
- 4.Berres ME. 2002. Long-term persistence of white-bearded manakin (Manacus manacus) leks in the Arima valley of Trinidad, West Indies. In Studies in Trinidad and Tobago ornithology honouring Richard French (eds Hayes FE, Temple SA), pp. 131–137. St. Augustine, West Indies: Department of Life Sciences, University of West Indies. [Google Scholar]
- 5.Gibson RM, Bradbury JW, Vehrencamp SL. 1991. Mate choice in lekking sage grouse revisited: the roles of vocal display, female site fidelity, and copying. Behav. Ecol. 2, 165–180. ( 10.1093/beheco/2.2.165) [DOI] [Google Scholar]
- 6.Jiguet F, Bretagnolle V. 2006. Manipulating lek size and composition using decoys: an experimental investigation of lek evolution models. Am. Nat. 168, 758–768. ( 10.1086/508808) [DOI] [PubMed] [Google Scholar]
- 7.Rintamaki PT, Alatalo RV, Hoglund J, Lundberg A. 1995. Male territoriality and female choice on black grouse leks. Anim. Behav. 49, 759–767. ( 10.1016/0003-3472(95)80208-8) [DOI] [Google Scholar]
- 8.Lanctot RB, Weatherhead PJ, Kempenaers B, Scribner KT. 1998. Male traits, mating tactics and reproductive success in the buff-breasted sandpiper, Tryngites subruficollis. Anim. Behav. 56, 419–432. ( 10.1006/anbe.1998.0841) [DOI] [PubMed] [Google Scholar]
- 9.McDonald DB. 1993. Demographic consequences of sexual selection in the long-tailed manakin. Behav. Ecol. 4, 297–309. ( 10.1093/beheco/4.4.297) [DOI] [Google Scholar]
- 10.Kokko H. 1997. Evolutionary stable strategies of age-dependent sexual advertisement. Behav. Ecol. Sociobiol. 41, 99–107. ( 10.1007/s002650050369) [DOI] [Google Scholar]
- 11.Duraes R, Loiselle BA, Blake JG. 2008. Spatial and temporal dynamics at manakin leks: reconciling lek traditionality with male turnover. Behav. Ecol. Sociobiol. 62, 1947–1957. ( 10.1007/s00265-008-0626-0) [DOI] [Google Scholar]
- 12.Ryder TB, Blake JG, Loiselle BA. 2006. A test of the hotspot hypothesis for three species of manakins (Aves: Pipridae) in lowland wet forests of Ecuador. Auk 123, 247–258. ( 10.1642/0004-8038(2006)1230247:ATOTEH%5D2.0.CO;2) [DOI] [Google Scholar]
- 13.Ryan MJ. 1985. The Tungara frog: a study of sexual selection and communication. Chicago, IL: University of Chicago Press. [Google Scholar]
- 14.Trail PW. 1987. Predation and antipredator behavior at Guiana cock-of-the rock leks. Auk 104, 496–507. ( 10.2307/4087549) [DOI] [Google Scholar]
- 15.Blake JG, Loiselle BA. 2008. Estimates of apparent survival rates for forest birds in eastern Ecuador. Biotropica 40, 485–493. ( 10.1111/j.1744-7429.2007.00395.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce-Higgins JW, Brace RC, Hornbuckle J. 2007. Survival of band-tailed manakins. Condor 109, 167–172. ( 10.1650/0010-5422(2007)109167:sobm%5D2.0.co;2) [DOI] [Google Scholar]
- 17.Ruiz-Gutiérrez V, et al. 2012. Survival of resident neotropical birds: considerations for sampling and analysis based on 20 years of bird-banding efforts in Mexico. Auk 129, 500–509. ( 10.1525/auk.2012.1117) [DOI] [Google Scholar]
- 18.Kokko H, Lindstrom J, Alatalo RV, Rintamaki PT. 1998. Queuing for territory positions in the lekking black grouse (Tetrao tetrix). Behav. Ecol. 9, 376–383. ( 10.1093/beheco/9.4.376) [DOI] [Google Scholar]
- 19.McDonald DB. 1989. Cooperation under sexual selection: age graded changes in a lekking bird. Am. Nat. 134, 709–730. ( 10.1086/285007) [DOI] [Google Scholar]
- 20.Ryder TB, McDonald DB, Blake JG, Parker PG, Loiselle BA. 2008. Social networks in the lek-mating wire-tailed manakin (Pipra filicauda). Proc. R. Soc. B 275, 1367–1374. ( 10.1098/rspb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryder TB, Blake JG, Loiselle BA, Parker PG. 2011. The composition, stability and kinship of reproductive coalitions in a lekking bird. Behav. Ecol. 22, 282–290. ( 10.1093/beheco/arq213) [DOI] [Google Scholar]
- 22.Bradbury JW. 1981. The evolution of leks. In Natural selection and social behavior (eds Alexander RD, Twinkle DW), pp. 139–169. New York, NY: Chiron Press. [Google Scholar]
- 23.Snow DW. 1971. Evolutionary aspects of fruit eating birds. Ibis 113, 194–202. ( 10.1111/j.1474-919X.1971.tb05144.x) [DOI] [Google Scholar]
- 24.Worthington A. 1982. Population size and breeding rhythms of two species of manakins in relation to food supply. In The ecology of a tropical forest (eds Leigh E, Rand AS, Windsor D), pp. 213–226. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 25.Westcott DA. 1994. Leks of leks: a role for hotspots in lek evolution. Proc. R. Soc. Lond. B 258, 281–286. ( 10.1098/rspb.1994.0174) [DOI] [Google Scholar]
- 26.Vehrencamp SL, Bradbury JW, Gibson RM. 1989. The energetic cost of display in male sage grouse. Anim. Behav. 38, 885–896. ( 10.1016/s0003-3472(89)80120-4) [DOI] [Google Scholar]
- 27.Barske J, Fusani L, Wikelski M, Feng NY, Santos M, Schlinger BA. 2014. Energetics of the acrobatic courtship in male golden-collared manakins (Manacus vitellinus). Proc. R. Soc. B 281, 20132482 ( 10.1098/rspb.2013.2482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiladis GN, Diaz HF. 1989. Global climatic anomalies associated with extremes in the Southern Oscillation. J. Clim. 2, 1069–1090. () [DOI] [Google Scholar]
- 29.Malhi Y, Wright J. 2004. Spatial patterns and recent trends in the climate of tropical rainforest regions. Phil. Trans. R. Soc. Lond. B 359, 311–329. ( 10.1098/rstb.2003.1433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenseth NC, Ottersen G, Hurrell JW, Mysterud A, Lima M, Chan KS, Yoccoz NG, Ådlandsvik B. 2003. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc. R. Soc. Lond. B 270, 2087–2096. ( 10.1098/rspb.2003.2415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward FI, Lomas MR, Quaife T. 2008. Global responses of terrestrial productivity to contemporary climatic oscillations. Phil. Trans. R. Soc. B 363, 2779–2785. ( 10.1098/rstb.2008.0017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright SJ, Carrasco C, Calderon O, Paton S. 1999. The El Nino Southern Oscillation, variable fruit production, and famine in a tropical forest. Ecology 80, 1632–1647. ( 10.1890/0012-9658(1999)0801632:TENOSO%5D2.0.CO;2) [DOI] [Google Scholar]
- 33.Wright SJ, Calderón O. 2006. Seasonal, El Niño and longer term changes in flower and seed production in a moist tropical forest. Ecol. Lett. 9, 35–44. ( 10.1111/j.1461-0248.2005.00851.x) [DOI] [PubMed] [Google Scholar]
- 34.Berton J, Harris C, Sekercioglu CH, Sodhi NS, Fordham DA, Paton DC, Brook BW. 2011. The tropical frontier in avian climate impact research. Ibis 153, 877–882. ( 10.1111/j.1474-919X.2011.01166.x) [DOI] [Google Scholar]
- 35.Sekercioglu CH, Primack RB, Wormworth J. 2012. The effects of climate change on tropical birds. Biol. Conserv. 148, 1–18. ( 10.1016/j.biocon.2011.10.019) [DOI] [Google Scholar]
- 36.Heindl M. 2002. Social organization on leks of the wire-tailed manakin in southern Venezuela. Condor 104, 772–779. ( 10.1650/0010-5422(2002)1040772:SOOLOT%5D2.0.CO;2) [DOI] [Google Scholar]
- 37.Castañeda P, Javier A, Saltos R, Geovanny H, Rentería H, Bethsabe C, Renato L, Reyes V. 2014. Arboles emblemáticos de Yasuní, Ecuador. Quito, Ecuador: Pontificia Universidad Catolica Del Ecuador. [Google Scholar]
- 38.Ryder TB, Duraes R, Tori WP, Hidalgo JR, Loiselle BA, Blake JG. 2008. Nest survival for two species of manakins (Pipridae) in lowland Ecuador. J. Avian Biol. 39, 355–358. ( 10.1111/j.2008.0908-8857.04290.x) [DOI] [Google Scholar]
- 39.Ryder TB, Durães R. 2005. It's not easy being green: using molt limits to age and sex green plumage manakins (Aves: Pipridae). Ornithol. Neotrop. 16, 481–491. [Google Scholar]
- 40.Wolfe JD, Ryder TB, Pyle P. 2009. Using molt cycles to categorize the age of tropical birds: an integrative new system. J. Field Ornithol. 81, 186–194. ( 10.1111/j.1557-9263.2010.00276.x) [DOI] [Google Scholar]
- 41.Ryder TB, Parker PG, Blake JG, Loiselle BA. 2009. It takes two to tango: reproductive skew and social correlates of male mating success in a lek-breeding bird. Proc. R. Soc. B 276, 2377–2384. ( 10.1098/rspb.2009.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White GC, Kendall WL, Barker RJ. 2006. Multistate survival models and their extensions in program MARK. J. Wildl. Manage. 70, 1521–1529. ( 10.2193/0022-541x(2006)701521:msmate%5D2.0.co;2) [DOI] [Google Scholar]
- 43.Burnham KP, Overton WS. 1979. Robust estimation of population size when capture probabilities vary among animals. Ecology 60, 927–936. ( 10.2307/1936861) [DOI] [Google Scholar]
- 44.Doherty PF Jr, White GC, Burnham KP. 2012. Comparison of model building and selection strategies. J. Ornithol. 152, S317–S323. ( 10.1007/s10336-010-0598-5) [DOI] [Google Scholar]
- 45.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 46.Arnold TW. 2010. Uninformative parameters and model selection using Akaike's information criterion. J. Wildl. Manage. 74, 1175–1178. ( 10.2193/2009-367) [DOI] [Google Scholar]
- 47.Cloquet R, Reboulet AM, Lebreton JD, Gimenez O, Pradel R. 2005. U-CARE 2.2 user's manual. Montpellier, France: CEFE. [Google Scholar]
- 48.Pradel R, Wintrebert CMA, Gimenez O. 2003. A proposal for a goodness-of-fit test to the Arnason-Schwarz multisite capture–recapture model. Biometrics 59, 43–53. ( 10.1111/1541-0420.00006) [DOI] [PubMed] [Google Scholar]
- 49.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 50.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package v. 1.1–7. See https://cran.r-project.org/web/packages/lme4/index.html.
- 51.Hovi M, Alatalo RV, Hoglund J, Lundberg A. 1996. Traditionality of black grouse Tetrao tetrix leks. Ornis Fennica 73, 119–123. [Google Scholar]
- 52.Wagner RH, Danchin E. 2003. Conspecific copying: a general mechanism for social aggregation. Anim. Behav. 65, 405–408. ( 10.1006/anbe.2003.2037) [DOI] [Google Scholar]
- 53.Nooker JK, Sandercock BK. 2008. Phenotypic correlates and survival consequences of male mating success in lek-mating greater prairie-chickens (Tympanuchus cupido). Behav. Ecol. Sociobiol. 62, 1377–1388. ( 10.1007/s00265-008-0566-8) [DOI] [Google Scholar]
- 54.Kervinen M, Alatalo RV, Lebigre C, Siitari H, Soulsbury CD. 2012. Determinants of yearling male lekking effort and mating success in black grouse (Tetrao tetrix). Behav. Ecol. 23, 1209–1217. ( 10.1093/beheco/ars104) [DOI] [Google Scholar]
- 55.McDonald DB. 1993. Delayed plumage maturation and orderly queues for status: a manakin mannequin experiment. Ethology 94, 31–45. ( 10.1111/j.1439-0310.1993.tb00545.x) [DOI] [Google Scholar]
- 56.East ML, Hofer H. 2001. Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav. Ecol. 12, 558–568. ( 10.1093/beheco/12.5.558) [DOI] [Google Scholar]
- 57.Sillett TS, Holmes RT, Sherry TW. 2000. Impacts of a global climate cycle on population dynamics of a migratory songbird. Science 288, 2040–2042. ( 10.1126/science.288.5473.2040) [DOI] [PubMed] [Google Scholar]
- 58.Grosbois V, Gimenez O, Gaillard JM, Pradel R, Barbraud C, Clobert J, Moller AP, Weimerskirch H. 2008. Assessing the impact of climate variation on survival in vertebrate populations. Biol. Rev. 83, 357–399. ( 10.1111/j.1469-185X.2008.00047.x) [DOI] [PubMed] [Google Scholar]
- 59.Robinson RA, Baillie SR, Crick HQP. 2007. Weather-dependent survival: implications of climate change for passerine population processes. Ibis 149, 357–364. ( 10.1111/j.1474-919X.2006.00648.x) [DOI] [Google Scholar]
- 60.Wolfe JD, Ralph CJ, Elizondo P. 2015. Changes in the apparent survival of a tropical bird in response to the El Nino Southern Oscillation in mature and young forests in Costa Rica. Oecologia 174, 715–721. ( 10.1007/s00442-015-3256-z) [DOI] [PubMed] [Google Scholar]
- 61.Pereira R, Marques MJ, Tiago J, Palmeirim JM. 2010. Ecological responses of frugivorous bats to seasonal fluctuation in fruit availability in Amazonian forests. Biotropica 42, 680–687. ( 10.1111/j.1744-7429.2010.00635.x) [DOI] [Google Scholar]
- 62.Haugaasen T, Peres CA. 2007. Vertebrate responses to fruit production in Amazonian flooded and unflooded forests. Biodivers. Conserv. 16, 4165–4190. ( 10.1007/s10531-007-9217-z) [DOI] [Google Scholar]
- 63.McDonald DB. 1989. Correlates of male mating success in a leking bird with male-male cooperation. Anim. Behav. 37, 1007–1022. ( 10.1016/0003-3472(89)90145-0) [DOI] [Google Scholar]
- 64.Heindl M, Winkler H. 2003. Interacting effects of ambient light and plumage color patterns in displaying wire-tailed manakins (Aves, Pipridae). Behav. Ecol. Sociobiol. 53, 153–162. ( 10.1007/s00265-002-0562-3) [DOI] [Google Scholar]
- 65.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 66.Anciaes M, Peterson AT. 2006. Climate change effects on Neotropical manakin diversity based on ecological niche modeling. Condor 108, 778–791. ( 10.1650/0010-5422(2006)108%5B778:CCEONM%5D2.0.CO;2) [DOI] [Google Scholar]
- 67.Malhi Y, Roberts JT, Betts RA, Killeen TJ, Li W, Nobre CA. 2008. Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172. ( 10.1126/science.1146961) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on Data Dryad.